Abstract
Background
HIV-infected patients may be at a greater risk of Hospital-Acquired Infections (HAIs) but risks factors for HAIs have not been well described in this population.
Objective
The aim of this study was to examine the incidence, temporal trends and risk factors of HAIs among adult HIV positive patients.
Methods
This was a retrospective cohort study carried out in an academic health system in New York City which included four hospitals over a 9-year period from 2006 to 2014. Simple and multiple logistic regression models were built to determine risk factors associated with site-specific HAIs such as Urinary Tract Infections (UTIs), Pneumonia (PNUs) and Bloodstream Infections (BSIs).
Findings
There were 10,575 HIV positive discharges and 1,328 had HAIs: 697 UTIs, 555 BSIs and 192 PNUs. The incidence rate of HAIs decreased from 19.8 to 15.1 new infections per 1000 person-days between 2006 and 2014 (p value<0.001). In addition to the expected risk factors of urinary catheter use for UTI and central venous line use for BSI, symptomatic HIV and renal failure were significant risk factors for both UTIs (95% CI OR: (1.24, 2.27) and (1.46, 2.11) respectively) and BSIs (95% CIs OR: (2.28, 4.18) and (1.81, 2.71) respectively).
Conclusion
HIV-infected patients had similar risk factors for HAIs as HIV-uninfected patients. Further research is required to address how patients’ CD4 counts and viral loads affect their susceptibility to HAIs.
Keywords: HIV, hospital-acquired infections, incidence rate, risk factors
INTRODUCTION
Hospital-acquired infections (HAIs), also referred to as nosocomial infections, constitute a serious public health and health care problem in the United States. According to the Centers for Disease Control and Prevention (CDC), 1.7 million of hospitalized patients acquire HAIs annually while being treated for other conditions and approximately 99 000 HAI-related deaths occur annually in the US [1]. While HAIs and prevention strategies have been well described for some patient populations, little is known about risk factors for HAI among people living with the Human Immunodeficiency Virus (HIV) who are admitted to the hospital [2–5].
It is possible that HIV-infected patients may be at a greater risk of HAIs than HIV-negative patients [6]. First, the immunosuppression observed in HIV-infected patients makes them more susceptible to infections [7]. In addition, because of their deficient immune system, HIV-infected patients may be hospitalized more and for longer periods of time when compared to other individuals [7, 8]. Given that there were about 1.2 million of people living with HIV in the US in 2014, the potential burden of HAI in this population may be considerable [9] since inpatient mortality among HIV-positive persons who develop an HAI is greater than that among HIV-patients who do not [8–10]. Hence, assessing risk factors of HAIs in HIV-infected patients may contribute to the identification of potential prevention strategies in this vulnerable population.
Recent studies suggest that acute renal failure and CD4 are associated with HAIs in HIV-infected patients [10, 11]. In addition, Mitha et al. previously reported that steroid use was associated with Urinary Tract Infections (UTIs), Blood Stream Infection (BSI) and Pneumonia (PNU) among HIV positive patients. However, that study included only a short period of time and a small sample size [10]. Furthermore, more information is needed about the epidemiology of types of HAIs among HIV-infected patients as well as the causative organisms associated with those infections. Therefore, the aim of this study was to examine the incidence, temporal trends and risk factors of HAIs among adult HIV positive patients from 2006 to 2014 in four urban New York City hospitals.
METHODS
Sample and Settings
Data for this study were collected from an NIH-funded database (Health Information Technology to Reduce Healthcare-Associated Infection, HIT-HAI, R01NR010822) compiled from multiple electronic sources from four hospitals in a large academic medical system in New York City– -a pediatric hospital, a community hospital and two tertiary/quaternary hospitals providing care to a diverse population. The data were collected between 2006 and 2014 and 965,642 discharges were reported during this period. For this study, only HIV positive patients who were aged 18 years old or older were included in the statistical analysis. After excluding all HIV-negative patients, there were 10,525 hospital discharges with positive HIV infection.
Data Collection
The study was approved by the Columbia University Medical Center Institutional Review Board. The data extracted from the database included demographics; laboratory reports including microbiological laboratory results from urine, blood and respiratory specimens; procedures received by the patients including urinary and central catheterization as well as mechanical ventilation; patient medical history; drug use; total length of stay as inpatient; admission to intensive care unit and death.
Study Definitions
HAIs included in the analysis were UTIs, BSIs, and PNUs which developed at least 48 hours after hospital admission. UTIs, BSIs, and PNUs were defined according to a set of algorithms adapted by a group of expert clinicians and researchers from the surveillance definitions from the Centers for Disease Control and Prevention National Healthcare Safety Network (NHSN, http://www.cdc.gov/nhsn/about.html) for HAIs [12].
The six organisms which represented the most common causes of HAI in the study institutions were examined in this study: Acinetobacter baumanii, Enterococcus faecalis/faecium, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pneumoniae.
Statistical Analysis
The incidence rate of HAIs among HIV positive patients was calculated by dividing the total number of HAIs per year by the total number of person-days for each year. A Cochrane-Armitage trend test was performed to assess temporal trends in HAI incidence and the change in length of stay over the 9-year study period [13]. When performing a Cochrane-Armitage test, the Somers’ D(R|C) coefficient assessing the association between row variable (incidence rate) and column variable (year) was calculated [14].
Descriptive statistics were summarized and then chi-squared tests were performed to assess the association between HAIs and other categorical variables such as sex, hospital, prior hospitalization and antibiotic use. Independent-sample t-test was performed to assess whether there was any difference in age, Charlson co-morbidity Index [15] score, or length of stay between patients who developed HAIs and those who did not. Separate simple logistic regression analyses for UTI, BSI and PNU were carried out using logistic regression models for each independent predictor. Variables significant in simple logistic regression analyses were included in a multivariable logistic regression model. Odds ratios, p-values as well as 95% Confidence Intervals (CI) were determined to assess if these predictors were significant.
Variables assessed as separate predictors were: symptomatic HIV (defined by the ICD-9-CM code V08 among patients who did not have any HIV-related condition and had not yet been diagnosed with any HIV-related illness), age, sex, length of stay, ICU stay prior to infections, stay in skilled nursing facility, renal failure, presence of malignancy, diabetes mellitus, Charlson Co-morbidity Index, prior hospitalizations. In addition, immunosuppressive and chemotherapeutic agents association with HAIs was also assessed in simple logistic regression models. In addition, device-related variables known to increase rise of HAI, including Central Venous (CV) lines for BSI, urinary catheterization for UTI, and mechanical ventilation for PNU were also assessed in multiple logistic regression analyses and included in the respective multivariable logistic regression analyses a priori.
Finally, we assessed changes in average of length of stay among HIV-positive patients over time. The analyses were conducted using SAS statistical software, version 9.4 (SAS Institute, Inc., Cary, North Carolina) and a p value <0.05 was considered significant.
RESULTS
There were 10,575 discharges with HIV positive infection (or as HIV infected patients) and 1,328 had HAIs (12.6%) from 2006 to 2014, about three-fourths of which occurred in men. The mean age among patients with HAIs was 48.5 years vs. 47.6 years for patients who did not develop any HAIs. Patients who developed an HAI stayed on average of twice as long in the hospital compared to those who did not develop any infection (10 days vs. 5 days respectively). Table 1 below summarizes the main characteristics among HIV-positive patients with and without HAIs.
Table 1.
Characteristics of hospitalized HIV positive-patients with and without a HAI.
| Patients with HAIs | Patients without HAIs | p-value* | |
|---|---|---|---|
| N | 1328 | 9247 | |
| Female (%) | 529 (39.8) | 3055 (33) | <0.0001 |
| Mean age (years, sd) | 48.5 (11.88) | 47.6 (11.87) | 0.01 |
|
Hospital (%) Tertiary hospital 1 Pediatric hospital Community hospital Tertiary Hospital 2 |
10 756 (56.9) 9 (0.67) 114 (8.58) 449 (33.85) |
12 4336 (46.9) 260 (2.81) 814 (8.80) 3837 (41.49) |
0<0.0001 |
| Admitted from a skilled nursing facility (N, %) | 40 (3.0) | 124 (1.3) | <0.0001 |
| Renal failure (%) | 524 (39.5) | 1862 (20.1) | <0.0001 |
| Presence of malignancy (%) | 158 (11.9) | 957 (10.4) | 0.09 |
| Diabetes mellitus (%) | 210 (15.8) | 1337 (14.5) | 0.19 |
| Antibiotic use* (%) | 1070 (80.6) | 4469 (48.3) | <0.0001 |
| Median length of stay (IQR, days) | 10 (14) | 5 (5) | <0.0001 |
| Median Charlson_score | 8 | 6 | <0.0001 |
| Prior hospitalization (%) | 519 (39.1) | 3568 (38.6) | 0.73 |
| Symptomatic HIV (%) | 971 (73.1) | 5026 (54.4) | <0.0001 |
For patients with an HAI, antibiotic use prior to infection; for those not developing an HAI, antibiotic use throughout hospitalization.
In total, there were 697 UTIs, 555 BSIs and 192 PNUs. There were 75 patients with both a UTI and a BSI, 35 patients who developed both a BSI and a PNU, and 40 patients who had both a UTI and PNU. Acinetobacter baumannii, Enterococcus faecalis, Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus accounted for about 40% of UTIs. These same organisms in addition to Streptococcus pneumoniae accounted for about 39% of BSIs and about 79% of PNUs.
Temporal Trends of HAIs from 2006 to 2014
The incidence rate for HAIs was 19.8 new infections per 1000 person-days in 2006 vs. 15.1 new infections per 1000 person-days in 2014 (p<0.001). The Somers’ D(R|C) coefficient was −0.014 (95% CI: −0.204, −0.0081). These results indicate that over time there was a slow decrease in incidence rate as shown in Fig. (1). In order to assess whether these findings were due to the fact that the length of stay of inpatients decreased each year, we compared the average of length of stay among HIV-patients who developed an HAI for each year; there was no significant difference in average length of stay of patients between years (p-value =0.06, results not shown).
Fig. (1).
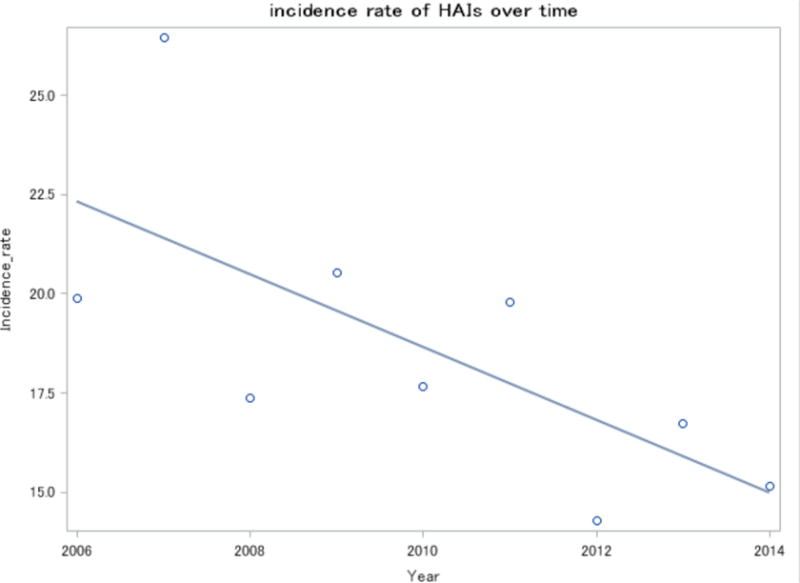
Incidence rate (new infections per 100 discharges) of HAIs over time.
Risk Factors for HAIs Among HIV-Infected Patients
Tables 2–4 summarize risk factors for UTIs, BSIs and PNUs when assessed in simple and multiple logistic analyses. Symptomatic HIV, male sex, renal failure, and ICU stay were predictors associated with UTIs and BSIs in univariate analysis. In addition, urinary catheter use and the presence of a central venous line were associated with an increased risk in UTI and BSI, respectively, among HIV-positive patients.
Table 2.
Risk factors associated with UTIs among HIV positive patients. Unadjusted odds ratio and adjusted odds ratio*
| Variables | Crude Odds Ratio (95% CI) | Adjusted Odds Ratio*(95% CI) |
|---|---|---|
| Urinary catheter use before UTI | 2.86 (2.44, 3.35) | 2.27 (1.90, 2.70) |
| Symptomatic HIV | 1.63 (1.38, 1.92) | 1.68 (1.24, 2.27) |
| Sex (female) | 2.27 (1.95, 2.65) | 2. 38 (2.03, 2.80) |
| Diabetes mellitus | 1.36 (1.11, 1.65) | 1.21 (0.97, 1.51) |
| Prior hospitalization in past 30 days | 1.18 (1.01, 1.38) | 1.02 (0.87, 1.20) |
| Renal failure | 2.18 (1.86, 2.57) | 1.76 (1.46, 2.11) |
| ICU stay | 1.55 (1.3, 1.84) | 1.53 (1.23, 1.89) |
| Stay in skilled nursing facility | 2.61 (1.69, 4.02) | 2.06 (1.31, 3.25) |
| Charlson Co-morbidity Score | 1.08 (1.06, 1.1) | 0.99 (0.95, 1.03) |
| Age | 1.01 (1, 1.02) | 1.06 (1, 1.02) |
Results from the multivariable logistic regression model with all significant risk factors in simple logistic regression analysis.
Table 4.
Risk factors associated with PNUs among HIV positive patients. Unadjusted odds ratio and adjusted odds ratio*.
| Variables | Crude Odds Ratio (95% CI) | Adjusted Odds Ratio*(95% CI) |
|---|---|---|
| Mechanical ventilator use before PNU | 19.25 (14.02,26.43) | 6.07 (3.76, 9.78) |
| Symptomatic HIV | 5.74 (3.7, 8.89) | 6.18 (3.47, 10.98) |
| Renal failure | 2.73 (2.05, 3.65) | 1.36 (0.96,1.92) |
| ICU stay | 7.65 (5.72, 10.22) | 2.12 (1.35, 3.33) |
| Stay in skilled nursing facility | 2.84 (1.38, 5.89) | 2.16 (0.98 4.72) |
| Charlson Co-morbidity Score | 1.143 (1.10, 1.19) | 0.93 (0.87, 1) |
| Immunosuppressive and chemotherapeutic agents | 5.42 (4.05, 7.25) | 2.08(2.03, 3.86) |
Results from the multivariable logistic regression model with all significant risk factors in simple logistic regression analysis.
After adjusting for all the significant individual predictors in a multivariable logistic regression model, urinary catheter use, symptomatic HIV, female sex, renal failure, ICU stay and previous stay in skilled nursing facility were all associated with an increased risk of UTI (Table 2). Similarly, adjusting for all the significant individual predictors in a multivariable logistic regression model to assess risk factors for BSIs, central venous line, symptomatic HIV infection, renal failure, ICU stay, high risk medications and presence of a malignancy remained positive predictors for BSIs.
There was no interaction between malignancy and administration of these immunosuppressive and chemotherapeutic agents and therefore both variables were included in the multivariable logistic regression models if there was a significant association in our simple logistic regression models.
Symptomatic HIV (adjusted OR=1.68 and 95% CI (1.24, 2.27) for UTI, adjusted OR=3.09 and 95% CI (2.28, 4.18) for BSI), renal failure (adjusted OR=1.76 and 95% CI (1.46, 2.11) for UTI, adjusted OR=2.21 and 95% CI (1.81, 2.71) for BSI) were both predictors of UTIs and BSIs.
Symptomatic HIV, mechanical ventilation, renal failure, stay in skilled nursing facility, ICU stay and high risk medications were individual predictors associated with PNUs in univariate logistic regression analyses.
Finally, mechanical ventilation, symptomatic HIV, ICU stay and high risk medications were significantly associated with an increased risk of PNUs after adjusting for all significant predictors in a multivariable logistic regression model assessing risk factors for PNUs (Table 3).
Table 3.
Risk factors associated with BSIs among HIV positive patients. Unadjusted odds ratio and adjusted odds ratio*.
| Variables | Crude Odds Ratio (95% CI) | Adjusted Odds Ratio*(95% CI) |
|---|---|---|
| Central Venous line use before BSI | 4.93 (4.03, 6.09) | 2.65 (2.07, 3.4) |
| Symptomatic HIV | 2.97 (2.41, 3.65) | 3.09 (2.28, 4.18) |
| Sex (female) | 0.74 (0.61, 0.89) | 0.80 (0.66, 0.98) |
| Prior hospitalization | 0.83 (0.70, 1) | 0.72 (0.60, 0.87) |
| Renal failure | 3.05 (2.56, 3.63) | 2.21 (1.81, 2.71) |
| ICU stay | 3.64 (2.99, 4.42) | 1.74(1.36, 2.22) |
| Stay in skilled nursing facility | 1.84 (1.08, 3.15) | 1.48 (0.85, 2.6) |
| Charlson Co-morbidity Score | 1.13 (1.11, 1.16) | 0.97 (0.93, 1.01) |
| Malignancy | 1.63 (1.28, 2.06) | 1.41 (1.04, 1.64) |
| Immunosuppressive and chemotherapeutic agents | 1.98 (1.64, 2.36) | 1.22 (1, 1.50) |
Results from the multivariable logistic regression model with all significant risk factors in simple logistic regression analysis.
DISCUSSION
To our knowledge, this is the first large study to identify risk factors for HAIs and to provide insights for infection control as well as case management in HIV-positive patients. In this study, the incidence rates of HAIs decreased from 19 to about 15 infections per 1000 patients-days between 2006 and 2014.These estimates are higher than what is reported in HIV-uninfected patients [16–18].
In our current study, UTIs were the most prevalent type of infections among HIV-infected patients followed by BSIs and PNUs. Similarly, in two previous studies carried out in the US, UTIs were the most common infections identified in HIV-infected patients whereas in a prospective study carried out in Italy, BSIs were the most common [8, 10, 19].
Our findings are consistent with previous studies carried out in HIV-uninfected patients. It has been established that patients with renal failure are susceptible to HAIs [20, 21].
In a retrospective study assessing the infection burden in 433 HIV-uninfected patients with renal disease from 1992 to 2000, 20 and 10% of those patients had PNUs and UTIs respectively. In another cohort study assessing BSI incidence in HIV-uninfected patients with renal failure in the ICU in a Belgian hospital, the incidence of nosocomial BSIs was 9% higher in patients with renal failure when compared to patients without renal failure [22]. In the same study, HIV-uninfected patients with renal failure had a 10% incidence in UTIs. However, in that particular study the increased incidence did not lead to an increase in mortality and length of stay among those patients [22]. In two other multicenter prospective cohort studies carried out in HIV-uninfected patients, renal failure was also shown to be associated with higher incidence of PNUs and UTIs [23, 24]. These studies suggest that association between renal failure and HAIs also observed in our current study is not unique to HIV-infected patients. One explanation that has been suggested is that patients with renal failure have an impaired microflora due to their long-term use of antibiotics [21] which subsequently renders them more susceptible to infections.
Further, stay in skilled nursing facility and ICU stay as well as the presence of malignancy has also been previously described in the general population as risk factors for HAI [25–28]. In a three -year prevalence study carried out in nursing homes of a specific region in the Netherlands from 2007 to 2009, the prevalence of HAIs increased from 6.7% in 2007 to 7.6% in 2009 [29]. Although no information was provided on the HIV status of the patients in that study, HIV prevalence in that region is very low [29]. Similar to our findings, UTIs were also the most common infections diagnosed in those nursing home residents [29]. In another study assessing the prevalence of HAIs in 133 Veteran Affairs nursing home care units in the United States, the prevalence of HAIs was estimated at 5.2% and UTIs were also the most common types of HAIs [30]. As confirmed in a recent study of longitudinal trends of HAIs in 14 000 nursing homes in the United States from 2006 to 2011, HAIs infection rates are rising [31].
The old age of nursing home residents and the shortage of staff are potential factors that could explain the high burden of HAIs in those facilities [25]. In addition, in an earlier study analyzing the prevalence of HAIs in US hospital using the National Nosocomial Infections surveillance system (NNIS) database, more than 30% of HAIs in the US occurs in ICU patients [32].
Also consistent with the literature on HAIs, we found urinary catheter use and central venous line to be associated with an increased risk in UTIs and BSIs respectively [33–35]. Hence reducing inappropriate use of urinary catheters and their rapid removal are key strategies to reduce the risk for UTI in patients [35, 36]. In addition, it has been shown that adequate training of staff as well as the use of antiseptic-impregnated central venous catheter in select high risk patients can reduce the risk of BSIs [36, 37].
Similar to national trends regarding overall rates of HAI, we also observed a decrease in HAIs incidence overtime from 2006 to 2014 [32]. In the national and state HAI progress report published in 2014, there was a 50% decrease in BSIs, a 17% decrease in SSIs from 2008 to 2014 [38], and in non-ICU settings there was also a decrease in UTIs during the same period [38].This suggests that with time there may be better implementation of infection control strategies by health care personnel [38], but among HIV-positive patients, this could also be due to improved access to antiretroviral therapy and linkage to care among newly diagnosed HIV-infected individuals in New York City. According to New York City Department of Health and Mental Hygiene (NY-CDOHMH), the number of newly diagnosed HIV patients linked to care in NYC increased from 63% in 2010 to 72% in 2014 [39]. During the same period there was also a 13% increase in the number of HIV patients who achieved viral suppression [39]. In our dataset, we also observed a decrease in the percentage of admissions of patients with symptomatic HIV from 60% in 2006 to 53% in 2014.
There are some limitations associated with this study. Because it was retrospective and used only electronically available data, it is possible that some infections may have been missed. Further, 60% of infections observed in this study were caused by organisms other than the six specific species we analyzed. Because patients included in this study were from an urban academic health system, our findings may not be generalizable to other health care settings. In addition, we did not have measures of viral load and CD4 counts which are important prognostic markers for HIV [40]. In the current study, we used symptomatic HIV as a proxy to assess how well the patients were doing.
CONCLUSION
To our knowledge, this study is the first large study to demonstrate that although rates of HAI among HIV-infected patients are likely to be higher than among HIV-uninfected patients, the risk factors are similar. Therefore, when designing strategies to prevent nosocomial infections among HIV-patients, the same control measures used in HIV-uninfected patients should also apply to HIV-infected patients [2–4]. Further research is needed to assess how CD4+ counts and viral loads affect incidence of HAIs among HIV-infected patients.
Acknowledgments
Declared none.
Biography

Christophe T. Tchakoute
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
PATIENT CONSENT
Declared none.
References
- 1.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007 Mar;1:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Contr Hosp Epidemiol. 2014 Sep 1;35(S2):S89–107. [PubMed] [Google Scholar]
- 3.Anderson DJ, Podgorny K, Berríos-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Contr Hosp Epidemiol. 2014 Jun 1;35(06):605–27. doi: 10.1086/676022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Contr Hosp Epidemiol. 2011 Feb 1;32(02):101–14. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 5.Sydnor ER, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. 2011 Jan 1;24(1):141–73. doi: 10.1128/CMR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panis C, Matsuo T, Reiche EM. Nosocomial infections in human immunodeficiency virus type 1 (HIV-1) infected and AIDS patients: major microorganisms and immunological profile. Braz J Microbiol. 2009 Mar;40(1):155–62. doi: 10.1590/S1517-838220090001000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damasceno LS, Girao ES, de Queiroz RF, Perdigao RL, Damasceno AS, Tavora LG. Healthcare Associated Infections in Intensive Care Units HIV Positive Patients. Amer J Infect Dis. 2011 Apr 1;7(2):51. [Google Scholar]
- 8.Petrosillo N, Viale P, Nicastri E, et al. Nosocomial bloodstream infections among human immunodeficiency virus-infected patients: incidence and risk factors. Clin Infect Dis. 2002 Mar 1;34(5):677–85. doi: 10.1086/338813. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease control and Prevention. HIV/AIDS Surveillance data 2014. accessed at http://www.cdc.gov/hiv/statistics/overview/ataglance.html on december 10th 2015.
- 10.Mitha M, Furuya EY, Larson E. Risk of healthcare associated infections in HIV positive patients. J Infect Prevent. 2014 Nov 1;15(6):214–20. doi: 10.1177/1757177414548694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega M, Almela M, Soriano A, et al. Bloodstream infections among human immunodeficiency virus-infected adult patients: epidemiology and risk factors for mortality. Eur J Clin Microbiol Infect Dis. 2008 Oct 1;27(10):969–76. doi: 10.1007/s10096-008-0531-5. [DOI] [PubMed] [Google Scholar]
- 12.Apte M, Neidell M, Yoko Furuya E, Caplan D, Glied S, Larson E. Using electronically available inpatient hospital data for research. Clin Translat Sci. 2011 Oct 1;4(5):338–45. doi: 10.1111/j.1752-8062.2011.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuhäuser M, Hothorn LA. An exact Cochran-Armitage test for trend when dose-response shapes are a priori unknown. Comput Statis Data Anal. 1999 Jun 28;30(4):403–12. [Google Scholar]
- 14.SAS Institute Inc. Base SAS® 9.2 Procedures Guide: Statistical Procedures. Third. Cary, NC: SAS Institute Inc; 2010. [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun 1;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Cohen B, Choi YJ, Hyman S, Furuya EY, Neidell M, Larson E. Gender differences in risk of bloodstream and surgical site infections. J Gen Int Med. 2013 Oct 1;28(10):1318–25. doi: 10.1007/s11606-013-2421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004 Aug 1;39(3):309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 18.Pittet D, Allegranzi B, Storr J, et al. Infection control as a major World Health Organization priority for developing countries. J Hosp Infect. 2008 Apr 30;68(4):285–92. doi: 10.1016/j.jhin.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Goetz AM, Squier C, Wegener MM, Muder RR. Nowosocomial infections in the human immunodeficiency virus-infected patient: A two-year survey. Amer J Infect Contr. 1994 Dec 31;22(6):334–9. doi: 10.1016/0196-6553(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 20.Berman SJ, Johnson EW, Nakatsu C, Alkan M, Chen R, LeDuc J. Burden of infection in patients with end-stage renal disease requiring long-term dialysis. Clin Infect Dis. 2004 Dec 15;39(12):1747–53. doi: 10.1086/424516. [DOI] [PubMed] [Google Scholar]
- 21.Berman SJ. Infections in patients with end-stage renal disease: an overview. Infect Dis Clin North Amer. 2001 Sep 1;15(3):709–20. doi: 10.1016/s0891-5520(05)70168-9. [DOI] [PubMed] [Google Scholar]
- 22.Hoste EA, Blot SI, Lameire NH, Vanholder RC, De Bacquer D, Colardyn FA. Effect of nosocomial bloodstream infection on the outcome of critically ill patients with acute renal failure treated with renal replacement therapy. J Amer Soc Nephrol. 2004 Feb 1;15(2):454–62. doi: 10.1097/01.asn.0000110182.14608.0c. [DOI] [PubMed] [Google Scholar]
- 23.Venditti M, Falcone M, Corrao S, Licata G, Serra P. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Inter Med. 2009 Jan 6;150(1):19–26. doi: 10.7326/0003-4819-150-1-200901060-00005. [DOI] [PubMed] [Google Scholar]
- 24.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009 Dec 2;302(21):2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 25.Montoya A, Mody L. Common infections in nursing homes: a review of current issues and challenges. Aging Health. 2011 Dec;7(6):889–99. doi: 10.2217/AHE.11.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnussen MH, Robb SS. Nosocomial infections in a long-term care facility. Amer J Infect Cont. 1980 Feb 29;8(1):12–7. doi: 10.1016/s0196-6553(80)80073-3. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis WR, Edwards JR, Culver DH, et al. Nosocomial infection rates in adult and pediatric intensive care units in the United States. Amer J Med. 1991 Sep 16;91(3):S185–91. doi: 10.1016/0002-9343(91)90367-7. [DOI] [PubMed] [Google Scholar]
- 28.Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Amer J Med. 1993 Mar 31;94(3):281–8. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 29.Eikelenboom-Boskamp A, Cox-Claessens JH, Boom-Poels PG, Drabbe MI, Koopmans RT, Voss A. Three-year prevalence of healthcare-associated infections in Dutch nursing homes. J Hosp Infect. 2011 May 31;78(1):59–62. doi: 10.1016/j.jhin.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Tsan L, Davis C, Langberg R, et al. Prevalence of nursing home-associated infections in the Department of Veterans Affairs nursing home care units. Amer J Infect Cont. 2008 Apr 30;36(3):173–9. doi: 10.1016/j.ajic.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Herzig C, Dick A, Sorbero M, Pogorzelska-Maziarz M, Cohen CC, Stone P. In open forum infectious diseases. suppl 1. Vol. 1. Oxford University Press; 2014. Dec 1, Longitudinal trends in infection rates in us nursing homes, 2006–2011; pp. S258–S259. [Google Scholar]
- 32.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Pub Health Rep. 2007 Mar 1;15:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2013 Sep 27;2012:1544. doi: 10.1136/bmjqs-2012-001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Contr Hosp Epidemiol. 2010 Apr 1;31(04):319–26. doi: 10.1086/651091. [DOI] [PubMed] [Google Scholar]
- 35.Safdar N, Maki DG. Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005 Aug 1;128(2):489–95. doi: 10.1378/chest.128.2.489. [DOI] [PubMed] [Google Scholar]
- 36.Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA. 1999 Jan 20;281(3):261–7. doi: 10.1001/jama.281.3.261. [DOI] [PubMed] [Google Scholar]
- 37.Fridkin SK, Pear SM, Williamson TH, Galgiani JN, Jarvis WR. The role of understaffing in central venous catheter-associated bloodstream infection. Infect Contr Hosp Epidemiol. 1996 Mar 1;17(03):150–8. doi: 10.1086/647262. [DOI] [PubMed] [Google Scholar]
- 38.Arnold K, Avery L, Bennett R. National and state healthcare-associated infections progress report [Google Scholar]
- 39.New York City Department of Health and Mental Hygiene (NY-CDOHMH), HIV surveillance report 2014.
- 40.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Inter Med. 1997 Jun 15;126(12):946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]


