Abstract
Background
The B cell receptor TACI is important for T-independent antibody responses. One in 200 blood donors are heterozygous for the TACI A181E mutation.
Objective
To investigate the impact on B cell function of TACI A181E heterozygosity in reportedly healthy subjects and of the corresponding TACI A144E mutation in mice.
Methods
Nuclear factor kappa B (NFκB) activation was measured by luciferase assay in 293T cells co-transfected with wild-type (WT) and mutant TACI. TACI driven proliferation, isotype switching and antibody responses were measured in B cells from heterozygous TACI A144E knock-in mice. Mouse mortality was monitored after intranasal pneumococcal challenge.
Results
The levels of natural antibodies to the pneumococcal polysaccharide component phosphocholine (PC) were significantly lower in A181E heterozygous than TACI sufficient Swedish blood donors never immunized with pneumococcal antigens. While overexpressed hTACI A181E and mTACI A144E acted as a dominant negative in transfectants, homozygosity for A144E in mice resulted in absent TACI expression in B cells, indicating that the mutant protein is unstable when naturally expressed. A144E heterozygous mice, like TACI+/− mice, expressed half the normal level of TACI on their B cells and exhibited similar defects in APRIL-driven B cell activation, antibody responses to TNP-Ficoll, production of natural antibodies to PC, and survival following intranasal pneumococcal challenge.
Conclusion
These results suggest that TACI A181E heterozygosity results in TACI haploinsufficiency with increased susceptibility to pneumococcal infection. This has important implications for asymptomatic TACI A181E carriers.
Keywords: TACI, CVID, Natural antibodies, pneumococcal susceptibility, B cells
INTRODUCTION
The transmembrane activator and calcium modulator ligand (CAML) interactor (TACI) is a tumor necrosis factor receptor (TNFR) superfamily member expressed on B cells 1. The TNF family members, B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL), are ligands for TACI 2–4. The extracellular (EC) region of TACI contains two cysteine rich domains (CRDs). Ligand binding causes clustering of the intracellular (IC) domains of TACI, recruitment of signaling molecules that include CAML and TNFR associated factors (TRAF) proteins, and activation of the transcription factors NFAT and NFκB 1, 5–7. TACI is important in immunoglobulin (Ig) class switching, Ig production, regulation of B cell homeostasis and the antibody response to type II T-independent (TI) antigens, which include natural antibodies to bacterial antigens and the pneumococcal polysaccharide component phosphocholine (PC) 5, 8–10.
TNFRSF13B, which encodes TACI, is mutated in up to 10% of patients with common variable immunodeficiency (CVID) 8, 11, 12. Most (90%) of these patients are heterozygous for the TACI C104R or A181E mutations. The C104R mutation, located in the second CRD, abolishes ligand binding 8, 11. The A181E mutation, located in the transmembrane domain, does not affect ligand binding, but severely impairs ligand-induced signaling 13. B cells from CVID patients with heterozygous C104R and A181E mutations in TACI have impaired response to TACI ligation in vitro 8, 11.
About 1 in 200 of reportedly healthy subjects are heterozygous for the A181E mutation (ExAC database, http://exac.broadinstitute.org/variant/17-16843729-G-T). Thus, the A181E mutation is not a cause of CVID but a risk factor and modifier for the disease. The impact of TACI A181E heterozygosity on B cell function in these subjects is not known. We herein show that the levels of natural antibodies to PC in apparently healthy adults subjects never immunized with pneumococcal antigens are significantly lower in A181E heterozygotes compared to TACI sufficient controls. Because humans have complex heterogeneous genetic backgrounds, we investigated the impact of an isolated A144E mutation in mice, which corresponds to the A181E mutation in humans, on the immune response to T-independent (TI) type II antigens in order to determine the mechanistic basis of the impaired anti-PC response in apparently healthy A181E carriers with no CVID. We demonstrate that heterozygosity for mTACI A144E causes TACI haploinsufficiency and increased susceptibility to pneumococcal infection.
RESULTS
Asymptomatic human carriers of the TACI A181E mutation have impaired natural antibody responses
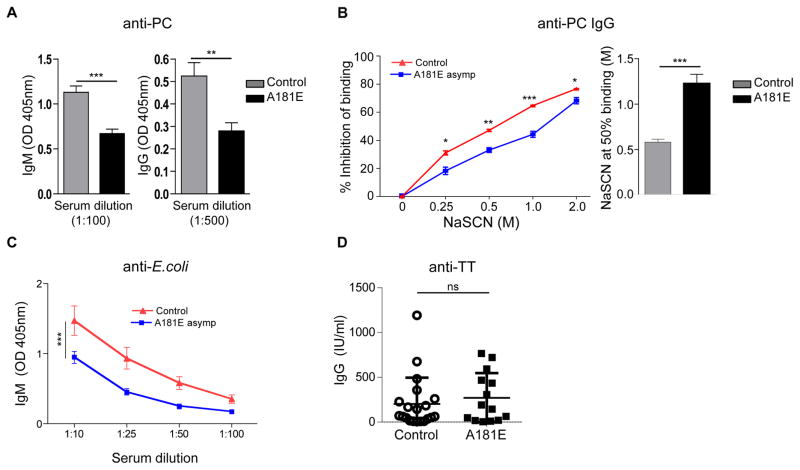
Antibodies to PC, which is present on all S. pneumoniae strains as part of the cell wall polysaccharide 17, 18, are thought to protect against invasive pneumococcal disease in humans 19. PC antibodies were measured in sera from 14 A181E heterozygotes and 20 TACI sufficient Swedish blood donors who had never been vaccinated with pneumococcal antigens, as pneumococcal vaccination was not performed in Sweden for their age group. Serum levels of natural IgM and IgG antibodies to PC were significantly lower in the A181E heterozygotes compared to controls (Fig 1,A). However, the affinity of anti-PC IgG antibodies was significantly higher in A181E heterozygotes compared with controls (Fig 1,B). Serum levels of natural IgM antibodies to E. coli were also significantly lower in the A181E heterozygotes compared to controls (Fig 1,C). Serum levels of IgM, IgG and IgA of antibodies to the T dependent (TD) antigen tetanus toxoid were not significantly different between the two groups (Fig 1,D and data not shown). These results indicate that the heterozygous A181E mutation impairs the natural antibody response to TI bacterial antigens in reportedly healthy human subjects.
Figure 1. Blood donor carriers of the TACI A181E mutation have impaired natural antibody responses.
A, Phosphocholine (PC) specific IgM and IgG antibody, B. Inhibition of anti-PC IgG binding to PC by increasing concentrations of NaSCN (left panel) and NaSCN molarity resulting in 50% inhibition of the O.D. (right panel). C, E. coli specific IgM and D, TT specific IgG in sera from Swedish asymptomatic subjects carrying a heterozygous TACI A181E mutation (n=14) and healthy controls (n=20). Data are expressed as OD at 405 nm or IU/ml. Sera were used at 1:100 dilution in A (for IgM), B, and D, and 1:500 dilution in A (IgG). Columns or symbols and bars in A–D represent means ± SEM. * p<0.5, ** p<0.01, *** p<0.001 by Student’s t-test.
Phenotypic analysis of lymphocytes in TACI A144E knock-in mice
TNF family ligands transduce signals though inducing clustering of the IC domain of their receptors, and most TNFRs also have some degree of pre-ligand association, which is thought to facilitate signaling 20, 21. We examined whether the hTACI A181E and mTACI A144E could assemble with their WT counterparts. Flag-tagged mutants and their Myc-tagged WT counterparts were co-transfected in 293T cells with Flag-tagged and Myc-Tagged WT constructs used as positive controls. The A181E hTACI and A144E mTACI mutants assembled with their WT counterparts, albeit less efficiently than WT TACI with itself (Fig E2,A and B). The assembly of the mutants with WT TACI is not unexpected, given that the extracellular CRD1 domain, which is critical for the pre-association of WT TACI, is intact in these mutants.
We previously showed that the hTACI A181E and mTACI A144E mutations severely impair ligand-induced activation of NFκB in 293T cell transfectants 13. Additional studies demonstrated that both A181E hTACI and A144E mTACI mutants strongly inhibited the ability of co-transfected WT TACI to activate NFκB in 293 T cells (Fig E2,C and D). Furthermore, TACI driven B cell responses of TACI+/− mice that carry a mTACI A144E expressing transgene were intermediate between those of TACI−/− mice and TACI+/− mice 13. These results indicate that the mutants, when overexpressed, may act as dominant negatives. However, overexpression systems are not physiologic. To definitively address the effect of the heterozygous A144E mutation on B cell function, we generated gene targeted knock-in mice that carry a Tnfrsf13b allele that encodes the TACI A144E mutant in the endogenous locus, mimicking humans heterozygous for the A181E TACI mutation (Fig E1).
Homozygous TACIA144E/A144E mice, heterozygous TACI+/A144E mice and WT TACI+/+ littermates, as well as TACI+/− and TACI−/− mice, were bred for more than 10 generations on the C57BL/6 background. None of the mutants differed in growth, weight, or health from their WT littermates, and all had normal lymphocyte cellularity in the thymus, bone marrow (BM), and spleen (data not shown). B cell development in the BM, T and B cell distribution in the spleen, and B cell subsets in the spleen and peritoneal cavity were comparable in all five strains, with the exception, as previously reported 5, 9, 10, of a significant increase in the percentage of B cells with a concomitant decrease in the percentage of T cells in the spleen of TACI−/− mice (Fig E3).
The A144E mutation severely impairs TACI expression in B cells
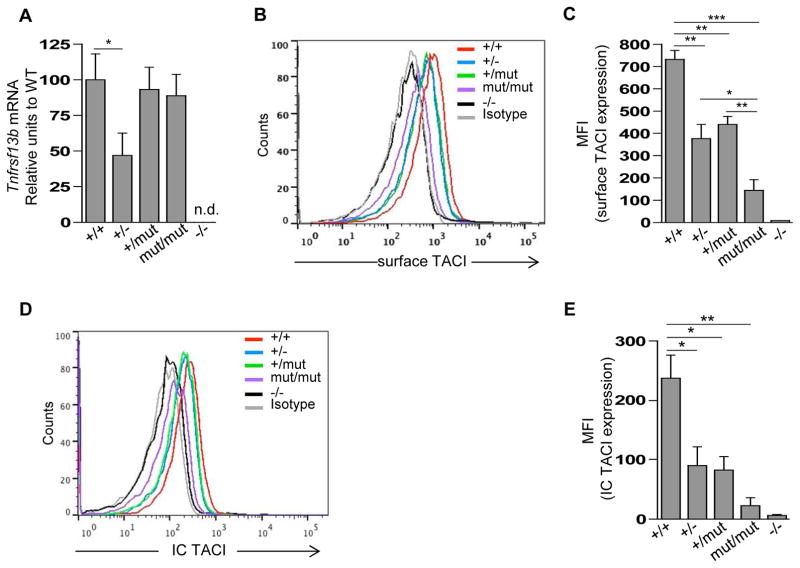
B cells from TACIA144E/A144E and TACI+/A144E mice expressed comparable levels of Tnfrsf13b mRNA as WT B cells, whereas B cells from TACI+/− mice expressed approximately half the WT level of Tnfrsf13b mRNA (Fig 2,A). FACS analysis revealed that the intensity of TACI expression on B cells was markedly diminished in TACIA144E/A144E mice and was approximately half normal in TACI+/A144E and TACI+/− mice (Fig 2,B and C). Intracellular FACS analysis revealed approximately half normal TACI expression in B cells from TACI+/A144E and TACI+/− mice, and virtually no TACI expression in B cells from TACIA144E/A144E mice (Fig 2,D and E). These results indicate that the mutant TACI protein is poorly expressed, and demonstrate that the heterozygous A144E mutation results in haploinsufficiency.
Figure 2. The A144E mutation impairs TACI surface expression, but not mRNA expression, in mouse B cells.
A, qRT-PCR analysis of Tnfrsf13b mRNA levels in purified B220+ splenic B cells from TACI+/+ (+/+), TACI+/− (+/−), TACI+/A144E (+/mut), TACIA144E/A144E (mut/mut), and TACI−/− (−/−) mice. The mRNA expression of Tnfrsf13b compared to that of the housekeeping gene Gapdh is shown as a percentage of the ratio in B cells from TACI+/+ WT controls. B and C, Representative FACS analysis (B) and pooled data (C) of the mean fluorescence intensity (MFI) of TACI surface expression on gated B220+ splenic B cells. D and E, Representative FACS analysis (D) and pooled data (E) of intracellular TACI expression in gated B220+ peripheral B cells. MFI data presented are the net value of TACI expression (MFI of TACI minus MFI of isotype control). Columns and bars in A, C and E represent mean ± SEM (n=3–5 mice per group). * p<0.05, ** p<0.01, *** p<0.001 by Student’s t-test n.d.= not detected.
TACI+/A144E mice exhibit functional TACI haploinsufficiency in vitro
B cell proliferation to APRIL and IgG1 secretion in response to APRIL+IL-4 were significantly and comparably reduced in TACI+/A144E and TACI+/− mice relative to WT controls (Fig 3,A and B). B cells from TACIA144E/A144E and TACI−/− mice failed to respond to APRIL stimulation. There was no evidence of increased apoptosis or cell death in APRIL+IL4 stimulated cultures of B cells from TACI mutant mice compared to WT controls, as determined by Annexin V and propidium iodide staining (data not shown). B cells from all five strains of mice proliferated and secreted IgG1 comparably in response to anti-CD40+IL-4 (Fig 3,A and B). These results indicate that the heterozygous TACI A144E mutation impairs TACI function in B cells in vitro.
Figure 3. Proliferation and in vitro immunoglobulin production in response to APRIL are impaired in B cells from A144E TACI mutant mice.
A and B, Proliferation in response to APRIL (A) and IgG1 secretion in response to APRIL+IL-4 (B) by splenic B cells from TACI+/+ (+/+) TACI+/− (+/−), TACI+/A144E (+/mut), TACIA144E/A144E (mut/mut), and TACI−/− (−/−) mice. Stimulation with anti-CD40+IL-4 was used as a control. Columns and bars represent means ± SEM (n= 5–7 mice per group). * p<0.05, ** p<0.01 by Student’s t-test.
TACI+/A144E mice have impaired antibody response to TNP-Ficoll, but normal antibody response to TD antigen
TACI−/− mice have decreased serum immunoglobulin levels9, 13, 22. Serum IgM, IgG and IgA levels were comparable in TACI+/A144E mice, TACI+/− mice and TACI+/+ controls (Fig 4,A). Serum IgM, IgG and IgA levels were significantly lower in TACIA144E/A144E mice compared to TACI+/+ controls and not significantly different than those of TACI−/− mice.
Figure 4. Serum immunoglobulin levels and antibody responses to TNP-Ficoll in A144E TACI mutant mice.
A, Serum IgM, IgG and IgA levels from non-immunized 8–12 week-old TACI+/+ (+/+), TACI+/− (+/−), TACI+/A144E (+/mut), TACIA144E/A144E (mut/mut) and TACI−/− (−/−) mice. Each symbol represents an individual mouse and the horizontal lines indicate the mean (n=11–15 mice per group). B, IgM and IgG anti-TNP responses 14 days after immunization with TNP-Ficoll. C. IgG anti-NP response after immunization with NP-OVA. Data in B and C are presented as the optical density (OD) readings at 405 nm. Symbols and bars represent mean ± SEM (n= 5–11 mice per group). * p<0.05, ** p<0.01, *** p<0.001, ns= not significant. Student’s t-test was used in A, and two-way ANOVA was used in B.
TACI is essential for the antibody response to the type II TI antigen TNP-Ficoll in mice, as TACI−/− mice fail to mount IgM and IgG anti-TNP responses to immunization with TNP-Ficoll 9 (Fig 4,B). TACI+/A144E mice, like TACI+/− mice, had significantly and comparably lower IgM and IgG anti-TNP responses to TNP-Ficoll than TACI+/+ controls (Fig 4,B). The antibody responses to TNP-Ficoll of TACIA144E/A144E mice were significantly lower than those of TACI+/A144E and TACI+/− mice, and although they were higher than those of TACI−/− mice, the difference was not significant. These results indicate that the heterozygous TACI A144E mutation impairs the B cell response to type II TI antigen in vivo.
The anti-TNP antibody response to TNP-KLH in TACI+/A144E mice, TACIA144E/A144E TACI+/− mice and TACI−/− mice was comparable to that of WT controls when measured on day 28 post-immunization (data not shown), consistent with the previously reported normal response of TACI−/− mice to TNP-KLH22. It was recently reported that TACI−/− mice fail to sustain the anti-NP IgG antibody response to the TD antigen NP-OVA 14. We observed no significant decrease in the levels of IgG anti-NP antibodies on days 35 and 56 post-immunization with OVA-NP in any of the mutant strains compared to WT controls (Fig 4,C). The normal response of TACI+/A144E mice to TD antigens indicates that the mutant does not exert non-specific effects on B cell differentiation into antibody producing cells.
TACI+/A144E mice have decreased levels of natural antibodies to PC and increased susceptibility to pneumococcal inhalation challenge
Antibodies to PC protect unimmunized mice from death following pneumococcal challenge 23. Serum levels of natural IgM and IgG antibodies to PC were readily detected in TACI+/+ mice but were virtually absent in TACI−/− mice, indicating that this response is largely TACI dependent (Fig 5,A). Serum levels of natural IgM and IgG antibodies to PC were significantly lower in TACI+/A144E mice, and TACI+/− mice compared to TACI+/+ controls (Fig 5,A). These results demonstrate that the heterozygous A144E mutation impairs the natural antibody response to PC.
Figure 5. Decreased levels of natural antibodies to phosphocholine and increased susceptibility to pneumococcal inhalation challenge in A144E TACI mutant mice.
A, IgM (left) and IgG (right) PC specific antibody levels in sera from non-immunized 8–12 week-old TACI+/+ (+/+) TACI+/− (+/−), TACI+/A144E (+/mut), and TACI−/− (−/−) mice expressed as OD at 405 nm. n=7 each group. B, Kaplan-Meier survival curves following intranasal challenge of 8–12 week-old unimmunized naïve TACI+/+ (+/+), TACI+/− (+/−), TACI+/A144E (+/mut) and TACI−/− (−/−) mice with a S. pneumoniae serotype 3 strain (n= 12–23 per group). Symbols and bars in A indicate mean ± SEM and symbols in B represent the percent of surviving mice. * p<0.05, ** p<0.01, *** p<0.001. Two-way ANOVA was used in A and Log-rank (Mantel-Cox) test was used in B.
We next examined the impact of the heterozygous A144E mutation on the response of unimmunized mice to pneumococcus. Analysis of Kaplan-Meier survival curves following intranasal challenge of unimmunized, pneumococcus-naive mice with S. pneumoniae serotype 3 revealed that 78% (18 of 23) of TACI+/+ mice survived 10 days post-challenge (Fig 5,B). In contrast none of 12 TACI−/− mice survived the challenge, indicating that protection of naïve mice against death from intranasal pneumococcal challenge is largely TACI dependent. The survival rates of TACI+/A144E heterozygous mice (33%, 4 of 12 mice) and TACI+/− mice (38%, 5 of 13 mice) on day 10 post-challenge were significantly lower than that of TACI+/+ controls, but did not significantly differ from each other (Fig 5,B). These results demonstrate that the heterozygous A144E mutation increases the susceptibility of naïve mice to death following intranasal pneumococcal challenge.
DISCUSSION
We show that the levels of natural antibodies to PC, which are implicated in resistance to pneumococcal infection, are significantly lower in reportedly healthy TACI A181E heterozygous adults and that mice heterozygous for TACI A144E, the murine counterpart to A181E, demonstrate increased susceptibility to pneumococcal infection. These results suggest that heterozygosity for the TACI A181E mutation may confer an increased risk for pneumococcal infections.
We identified reportedly healthy adult Swedish blood donors who were heterozygous for the TACI A181E mutation, consistent with the observation that relatives of CVID patients who also carry the A181E mutation can be clinically healthy 24. Blood donors heterozygous for the TACI A181E mutation had significantly lower levels of natural IgM and IgG antibodies to PC compared to TACI sufficient controls. However, the affinity of IgG antibodies to PC was significantly increased in the A181E carriers compared to controls. It was previously reported that the affinity of IgG antibodies to T-dependent antigens is higher in TACI−/− mice compared to WT controls 14. Ligation of high affinity B cell receptors (BCR) by antigen delivers an apoptotic signal 25. TACI has also been reported to deliver an apoptotic signal to B cells while BAFF-R which shares common ligands with TACI delivers a survival signal 5, 26–28. Decreased TACI signaling and/or increased BAFF-R signaling may allow better survival of B cells that express high affinity BCRs resulting in the production of higher affinity antibodies. It would be of interest to study the post-vaccination antibody response to pneumococcal polysaccharides in these apparently healthy carriers of the A181E mutation. However, this was not possible, since all subjects were de-identified. TACI A181E carriers also had significantly lower levels of natural IgM antibodies to E. coli antigens, raising the possibility that they may be susceptible to infections with organisms other than pneumococcus. The ~0.7% incidence of A181E heterozygosity in the cohort of Swedish blood donors we studied is more than 100 fold higher than the 1 in 25,000 estimated incidence of CVID in Sweden. Thus, the majority of the A181E heterozygotes studied are unlikely to be closely related to patients with CVID, suggesting that their impaired natural antibody response is not caused by genes or factors associated with CVID.
Our studies with TACI A144E knock-in mice clearly indicate that the TACI A144E mutant protein is poorly expressed in B cells. This is consistent with the location of this mutation at a predicted stability center of the protein 29. Poor expression of the A144E mutant protein explains the observation that the level of TACI surface and intracellular protein in B cells of TACI+/A144E heterozygous mice was about half that of WT B cells, comparable to that of B cells from TACI+/− mice. Thus, the heterozygous A144E mutation results in haploinsufficiency of surface TACI expression in mouse B cells. TACI surface expression is reduced in B cells from CVID patients and their asymptomatic relatives heterozygous for the A181E TACI mutation compared to normal controls 24.
Mice heterozygous for the A144E TACI mutation displayed functional TACI haploinsufficiency. B cell proliferation in response to APRIL stimulation, IgG1 secretion in response to stimulation with APRIL+IL-4 and, more importantly, antibody responses to the type II TI antigen TNP-Ficoll, but not to the TD antigens NP-OVA and TNP-KLH were significantly reduced in heterozygous TACI+/A144E mice and similar to those of haploinsufficient TACI+/− mice. Consistent with their poor expression of TACI, TACIA144E/A144E homozygous mice, like TACI−/− mice, had markedly diminished TACI expression on B cells and a virtually absent response to APRIL in vitro. Unlike TACI−/− mice, TACIA144E/A144E mice had residual anti-TNP-Ficoll antibody responses and no expansion of splenic B cells, possibly, suggesting the residual presence of some functionally relevant receptors in these mice
We showed that TACI is critical for the production of natural IgM and IgG antibodies to PC in mice, as evidenced by the virtual absence of these antibodies in the serum of TACI−/− mice. While we did not examine the opsonic activity of sera from the mice for pneumococcal bacteria in vitro, we demonstrated that TACI is critical for the survival of unimmunized mice following pneumococcal intranasal challenge, as all TACI−/− mice succumbed after challenge compared to 22% of WT controls. Both the levels of IgM and IgG antibodies to PC, and the fraction of mice that survived pneumococcal challenge, were significantly and similarly reduced in TACI+/A144E mice and TACI+/− mice compared to WT controls. Thus TACI haploinsufficiency caused by the heterozygous A144E TACI mutation, or loss of a TACI allele, significantly increases the susceptibility of mice to pneumococcal disease.
According to the Center for Disease Control, pneumococcal disease is an important health problem accounting for 400,000 hospitalizations per year in U.S. and with a case fatality rate of 5–7% in the elderly (http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pneumo.pdf). Our findings in TACI+/− mice suggest that lack of expression of TACI encoded by a mutant TNFRSF13B allele in humans may impair their antibody response to pneumococcus, and increase susceptibility to pneumococcal infection. Consistent with this hypothesis, patients with the Smith-Magenis syndrome, who have a chromosome 17p11.2 microdeletion that includes TNFRSF13B, have decreased TACI surface expression, a poor response to pneumococcus vaccination and recurrent respiratory tract infections 30. While one needs to draw a cautious conclusion regarding human susceptibility to a given microorganism based on murine data, the A181E carriers, like the A144E knock-in mice, had significantly lower levels of natural antibodies to PC. Antibodies to PC, which is present on all S. pneumoniae strains as part of the cell wall polysaccharide, are thought to protect against invasive pneumococcal disease in humans 19. Thus, it is not far-fetched to propose that the increased susceptibility of the A144E mice to S. pneumoniae challenge is relevant to A181E carriers. It would be of interest to study over time whether healthy adults who carry the A181E mutation, including those related to CVID patients with this mutation, are at increased susceptibility to pneumococcal infections and to examine healthy individuals who have had a pneumococcal infection for A181E heterozygosity
Our results suggest that TACI A181E carriers, like TACI+/A144E mice, may be more susceptible to infection with pneumococcus and possibly other polysaccharide encapsulated bacteria. Since TACI function is also disrupted in mice heterozygous for TACI C76R, the counterpart of the human TACI C104R mutation 31, this mutation, like the A181E mutation, may also increase the risk of infection. This would have important implications for the ~1% of individuals who carry one of these TACI mutations.
METHODS
TACI expression and co-immunoprecipitation in transfectants
293T cells were transfected with Myc-tagged WT hTACI or mTACI, and either Flag-tagged WT hTACI, A181E hTACI, WT mTACI or A144E mTACI, using FuGENE 6 (Roche). Forty-eight hours later, lysates were examined for expression and co-immunoprecipitation of Myc-tagged and Flag-tagged proteins as previously described 7.
NFκB reporter assay
293T cells were transfected with 100 ng WT or mutant TACI expression plasmid alone or in combination, along with 100 ng NFκB-luciferase reporter plasmid and 10 ng control pRL-TK plasmid (Promega). Four hours later, trimeric ZZ-APRIL (50 ng/mL a gift from ZymoGenetics) was added. Reporter gene activity was determined 20 hours later using a dual-luciferase reporter assay system (Promega) as previously reported 22.
Supplementary Material
Key messages.
#x02022; TACI A181E heterozygosity in asymptomatic subjects is associated with decreased levels of natural antibodies to the pneumococcal polysaccharide phosphocholine and E. coli antigens.
Heterozygosity for mTACI A144E, which corresponds to hTACI A181E, results in TACI haploinsufficiency with impaired production of natural antibodies to phosphocholine, and increased susceptibility to pneumococcal infection.
Acknowledgments
The authors thank Drs. Michel Massaad and Ulrich Salzer for useful discussions. The work was supported by National Institutes of Health (NIH) grants AI076210 (RSG), K08AI114968 (EJ), AI100114 (RM), the Translational Research Program at Boston Children’s Hospital (RM), and the NIAMS Intramural Research Program.
Abbreviations
- APRIL
a proliferation-inducing ligand
- BM
bone marrow
- CAML
calcium modulator ligand
- CFP
cyan fluorescent protein
- CRD
cysteine rich domain
- CVID
Common variable immunodeficiency
- EC
Extracellular
- FRET
Fluorescence resonance energy transfer
- IC
Intracellular
- NFκB
Nuclear factor kappaB
- NFAT
nuclear factor of activated T cells
- NP-OVA
4-hydroxy-3-nitrophenyl acetyl-ovalbumin
- PC
Phosphocholine
- TACI
Transmembrane activator and calcium modulator ligand interactor
- TI
T independent
- TD
T dependent
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- TNP-KLH
trinitrophenyl-keyhole limpet hemocyanin
- WT
wild type
- YFP
yellow fluorescent protein
Footnotes
The authors have no conflicting financial interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.von Bulow GU, Bram RJ. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–41. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- 2.Xia XZ, Treanor J, Senaldi G, Khare SD, Boone T, Kelley M, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–43. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan M, Marsters SA, Grewal IS, Wang H, Ashkenazi A, Dixit VM. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat Immunol. 2000;1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 4.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: A Tutorial on B Cell Survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 5.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–88. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 6.Marsters SA, Yan M, Pitti MR, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF Homologues BLys and APRIL with the TNF receptor homologues BCMA and TACI. Current Biology. 2000;10:785–8. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 7.Garibyan L, Lobito AA, Siegel RM, Call ME, Wucherpfennig KW, Geha RS. Dominant-negative effect of the heterozygous C104R TACI mutation in common variable immunodeficiency (CVID) J Clin Invest. 2007;117:1550–7. doi: 10.1172/JCI31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. TACI is mutated in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–34. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 9.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 10.Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. 2001;2:638–43. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- 11.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Pomar N, Detkova D, Arostegui JI, Alvarez A, Soler-Palacin P, Vidaller A, et al. Role of TNFRSF13B variants in patients with common variable immunodeficiency. Blood. 2009;114:2846–8. doi: 10.1182/blood-2009-05-213025. [DOI] [PubMed] [Google Scholar]
- 13.Lee JJ, Rauter I, Garibyan L, Ozcan E, Sannikova T, Dillon SR, et al. The murine equivalent of the A181E TACI mutation associated with common variable immunodeficiency severely impairs B-cell function. Blood. 2009;114:2254–62. doi: 10.1182/blood-2008-11-189720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuji S, Stein L, Kamada N, Nunez G, Bram R, Vallance BA, et al. TACI deficiency enhances antibody avidity and clearance of an intestinal pathogen. J Clin Invest. 2014;124:4857–66. doi: 10.1172/JCI74428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. The Journal of clinical investigation. 1998;101:1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer W. Phosphocholine of pneumococcal teichoic acids: role in bacterial physiology and pneumococcal infection. Research in microbiology. 2000;151:421–7. doi: 10.1016/s0923-2508(00)00174-1. [DOI] [PubMed] [Google Scholar]
- 18.Gang TB, Hammond DJ, Jr, Singh SK, Ferguson DA, Jr, Mishra VK, Agrawal A. The phosphocholine-binding pocket on C-reactive protein is necessary for initial protection of mice against pneumococcal infection. The Journal of biological chemistry. 2012;287:43116–25. doi: 10.1074/jbc.M112.427310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenberg HB, McCool TL, Weiser JN. Cross-reactivity of human immunoglobulin G2 recognizing phosphorylcholine and evidence for protection against major bacterial pathogens of the human respiratory tract. J Infect Dis. 2004;190:1254–63. doi: 10.1086/424517. [DOI] [PubMed] [Google Scholar]
- 20.Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–4. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 21.Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, et al. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–7. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 22.Lee JJ, Jabara HH, Garibyan L, Rauter I, Sannikova T, Dillon SR, et al. The C104R mutant impairs the function of transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) through haploinsufficiency. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yother J, Forman C, Gray BM, Briles DE. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infection and immunity. 1982;36:184–8. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Gallo M, Radigan L, Almejun MB, Martinez-Pomar N, Matamoros N, Cunningham-Rundles C. TACI mutations and impaired B-cell function in subjects with CVID and healthy heterozygotes. The Journal of allergy and clinical immunology. 2013;131:468–76. doi: 10.1016/j.jaci.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eeva J, Pelkonen J. Mechanisms of B cell receptor induced apoptosis. Apoptosis. 2004;9:525–31. doi: 10.1023/B:APPT.0000038032.22343.de. [DOI] [PubMed] [Google Scholar]
- 26.Goenka R, Matthews AH, Zhang B, O’Neill PJ, Scholz JL, Migone TS, et al. Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med. 2014;211:45–56. doi: 10.1084/jem.20130505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–97. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanno Y, Sakurai D, Hase H, Kojima H, Kobata T. TACI induces cIAP1-mediated ubiquitination of NIK by TRAF2 and TANK to limit non-canonical NF-kappaB signaling. J Recept Signal Transduct Res. 2010;30:121–32. doi: 10.3109/10799891003634509. [DOI] [PubMed] [Google Scholar]
- 29.Salzer U, Bacchelli C, Buckridge S, Pan-Hammarstrom Q, Jennings S, Lougaris V, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113:1967–76. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinen J, Martinez-Gallo M, Gu W, Cols M, Cerutti A, Radigan L, et al. Transmembrane activator and CAML interactor (TACI) haploinsufficiency results in B-cell dysfunction in patients with Smith-Magenis syndrome. The Journal of allergy and clinical immunology. 2011;127:1579–86. doi: 10.1016/j.jaci.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacchelli C, Buckland KF, Buckridge S, Salzer U, Schneider P, Thrasher AJ, et al. The C76R transmembrane activator and calcium modulator cyclophilin ligand interactor mutation disrupts antibody production and B-cell homeostasis in heterozygous and homozygous mice. J Allergy Clin Immunol. 2011;127:1253–9. e13. doi: 10.1016/j.jaci.2011.02.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.