Abstract
Herpes simplex encephalitis (HSE) can be complicated by adverse events in the acute phase. We herein present the case of a 71-year-old woman with HSE complicated by cerebral hemorrhage. She presented with acute deterioration of consciousness and fever and was diagnosed with HSE based on the detection of herpes simplex virus-1 in the cerebrospinal fluid by a polymerase chain reaction. The cerebral hemorrhage developed during acyclovir therapy; however, its diagnosis was delayed for 2 days. After the conservative treatment of the cerebral hemorrhage, the patient made a near-complete recovery. Cerebral hemorrhage should be considered as an acute-phase complication of HSE.
Keywords: cerebral hemorrhage, herpes simplex encephalitis
Introduction
Without appropriate management, Herpes simplex encephalitis (HSE) is a potentially fatal disease (1). The mortality and morbidity rates remain high, even when an early diagnosis is made based on the detection of the herpes simplex virus (HSV) in the cerebrospinal fluid (CSF) by a polymerase chain reaction (PCR), and despite the advent of acyclovir therapy (2). In particular, the acute-phase mortality and long-term outcome in patients with severe HSE remain major concerns (3). Although the severity and poor outcomes of HSE are mostly associated with acute-phase complications (2,3), these important adverse events are not well understood because of the low frequency of the condition and the paucity of reported cases. This lack of understanding may contribute to a lack of awareness by clinicians and, consequently, to the poor outcomes of patients with HSE. We herein report the case of a patient with HSE that was complicated by cerebral hemorrhage (the diagnosis of which was delayed), which developed at the end of 2 weeks of acyclovir therapy.
Case Report
A 71-year-old woman was admitted to our hospital in a state of unconsciousness following fever and headache. She experienced epigastric discomfort and dizziness 8 days before admission and gradually lost her appetite. Two days later, she developed a fever of 38.2°C, headache, and nausea. She was evaluated twice by the outpatient service of our hospital for fever and was prescribed acetaminophen both times. However, in addition to the persistence of fever and headache, there was an acute worsening of her state of consciousness on the day of admission, and she was transported to our emergency department by ambulance. The patient was placed under observation for 4 years at another hospital due to the presence of an unruptured aneurysm of the right middle cerebral artery. In addition, she had undergone bilateral cataract surgery 3 years previously. She had no medical history of immunodeficiency. There was no family history of sudden death or intracranial hemorrhage. She was not taking any regular medications. The patient was a non-smoker and did not regularly consume alcohol.
On physical examination, her Glasgow coma scale (GCS) score was E1V1M4, her body temperature was 38.8°C, her blood pressure was 116/66 mmHg, her pulse rate was 80/min, and her respiratory rate was 16/min with an O2 saturation of 99% (with a flow rate of 3 L/min). Her pupils were round and equally dilated (size, 3 mm). A head and neck examination revealed no anemia, icterus, or other abnormalities. The results of a cardiovascular examination were normal, and auscultation revealed normal lung sounds. An abdominal examination was unremarkable with no obvious hepatosplenomegaly. A neurological examination revealed no neck stiffness and normal deep tendon reflexes; however, slightly rigid muscle tone and bilateral pathological Babinski reflexes were observed.
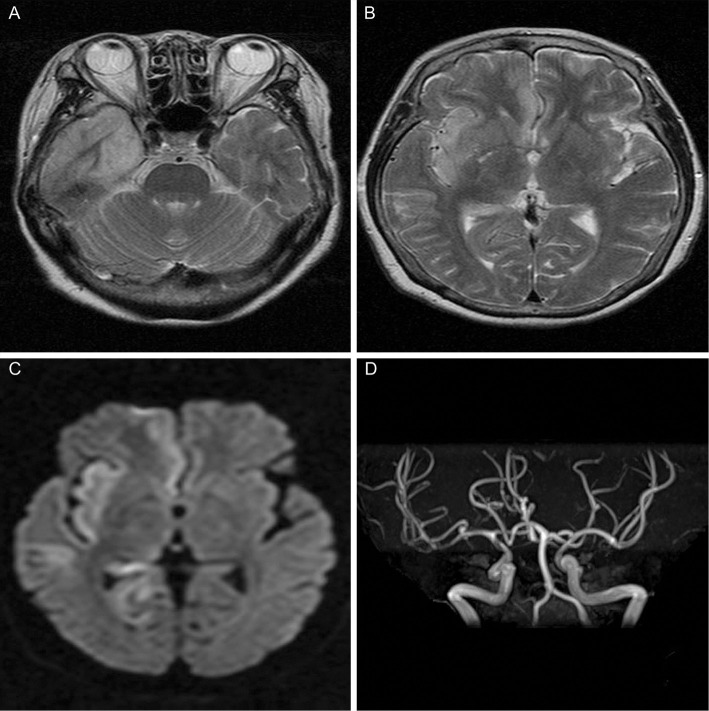
Laboratory investigations revealed a normal white blood cell count (7,350/μL), a slightly elevated C-reactive protein (0.36 mg/dL) level, elevated lactate dehydrogenase (313 IU/L) and creatine kinase (939 IU/L) levels, hyponatremia (128 mEq/L), hypokalemia (2.9 mEq/L), hypochloremia (88 mEq/L), and a normal blood glucose level (126 mg/dL). A urinalysis revealed no abnormal findings. A chest X-ray and electrocardiogram revealed no abnormalities. The right anterior medial temporal lobe and insular cortex displayed high signal intensity on T2-weighted and diffusion-weighted brain magnetic resonance imaging (Fig. 1A-C), and the patient's aneurysm, which was already known to exist, was observed at the bifurcation of the distal M1 segment of the middle cerebral artery using magnetic resonance angiography (Fig. 1D). A CSF analysis detected moderate lymphocytic leukocytosis (170/μL), a moderately increased protein level (77 mg/dL), and a slightly decreased glucose level (53 mg/dL). Gram staining of the patient's CSF was negative, and blood and CSF cultures showed no bacterial growth. A PCR of the CSF to detect HSV-1 and HSV-2 revealed the presence of HSV-1 DNA. She was therefore diagnosed with HSE.
Figure 1.
Magnetic resonance imaging on the day of admission. Axial T2-weighted images (A, B) and an axial diffusion-weighted image (C) show an area of high signal intensity in the right anterior medial temporal lobe and insular cortex. Magnetic resonance angiography (D) shows an aneurysm at the bifurcation of the distal M1 segment of the right cerebral artery.
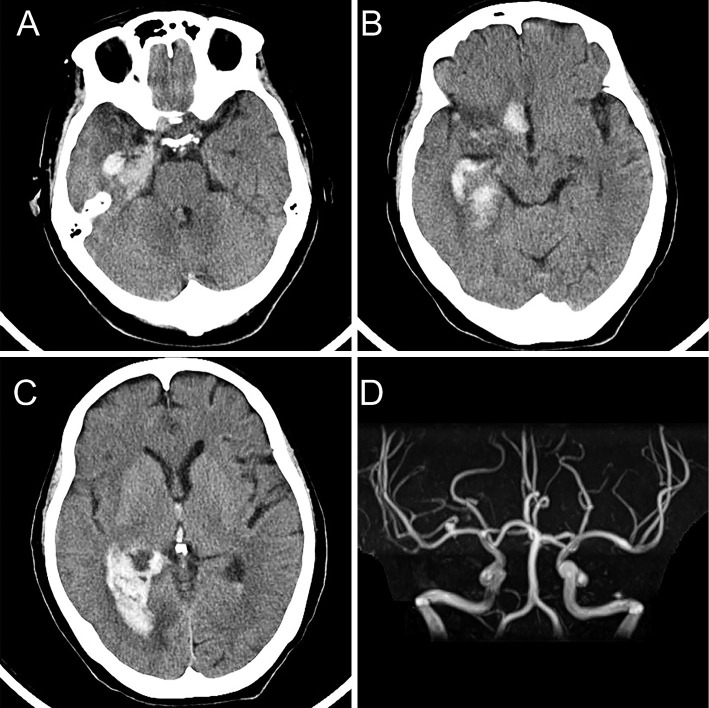
The patient was treated with acyclovir (10 mg/kg, intravenously, three times per day [ideal body weight]), glycerol (200 mL, intravenously, twice a day), and additional supportive therapies. Although the patient vomited on the day of admission, her symptoms disappeared within the first 2 days. Her consciousness gradually recovered to GCS E4V4M6, and her temperature returned to 37.3°C on day 11 after admission. However, she vomited during the night on day 12, and her GCS slightly worsened to E3V4M6, and she displayed a slightly elevated body temperature of 37.6°C on day 13. An exacerbation of HSE was considered, and a CSF analysis including a PCR for HSV-1 was performed, which revealed mild bloody fluid with moderate lymphocytic leukocytosis (178/μL) and a markedly increased protein level (350 mg/dL). Brain computed tomography imaging performed on day 14 revealed cerebral hemorrhage in the right medial temporal lobe and right basal frontal area, with a right lateral ventricular rupture (Fig. 2A-C). Although magnetic resonance angiography revealed an increase in the size of right cerebral arterial aneurysm (Fig. 2D), no evidence of subarachnoid hemorrhage was observed by computed tomography or magnetic resonance imaging. She was transported to the neurosurgery department of another hospital for possible surgical intervention. However, the patient was successfully treated with conventional therapy without surgery. Acyclovir therapy was terminated based on the results of the second PCR for HSV, which was negative. After 2 months of rehabilitation, the patient made a near-complete recovery. There was no recurrence of encephalitis or intracranial hemorrhage.
Figure 2.
Computed tomography and magnetic resonance imaging at the time of cerebral hemorrhage. Axial computed tomography shows acute cerebral hemorrhage in the right medial temporal lobe (A) and the right basal frontal area (B). A right lateral ventricular rupture is also observed (C). Magnetic resonance angiography (D) shows an increase in the size of the right cerebral arterial aneurysm at the bifurcation of the distal M1 segment of the right cerebral artery.
Discussion
We herein report a case of cerebral hemorrhage that developed in a patient who was undergoing acyclovir treatment for HSE. Although the patient made a near-complete recovery, the diagnosis of the cerebral hemorrhage was delayed. Initially, an acute exacerbation of HSE was considered, as intracranial vascular disease was not included in the differential diagnosis. This case suggests that acute-phase complications of HSE need to be better understood to avoid missing the onset of adverse events that can be managed successfully if they are diagnosed early.
The patient's cerebral hemorrhage appears to have been associated with HSE due to the following reasons. First, the site of hemorrhage was consistent with the region in which HSE-associated inflammation was observed. Second, the timing of the onset of hemorrhage was typical for HSE-related cerebral hemorrhage (4). Although the precise cause of cerebral hemorrhage in HSE patients remains unclear, based on the pathological findings in similar cases, we hypothesize that the rupture of the small vasculitic vessels of the brain due to HSV-induced inflammation was a possible mechanism of our patient's bleeding (5,6).
In addition to cerebral hemorrhage (4), seizures (2,3), increased intracranial pressure (3,7-10), stroke (11,12), subarachnoid hemorrhage (13), and central diabetes insipidus (14) have been reported as complications during the acute phase of HSE (this includes the period of acyclovir therapy). The list of reported complications is shown in Table. Although the prevalence of these complications is not well known, seizures and increased intracranial pressure, which have been reported in one in three and one in five HSE cases, appear to be among the most common (2,3). The onset of these complications is early. Cerebral hemorrhage and increased intracranial pressure have mostly been observed during acyclovir therapy, within 2 weeks after admission (3,4,7-10); the condition of patients may decline in this period due to HSE and not because of complications, which can pose a challenge for the differential diagnosis. Furthermore, the symptoms of these two complications, which include deteriorated consciousness, headache, hemiparesis, hemiplegia, and third nerve palsy, are similar to those of HSE (3,4,7-10). Thus, the possibility of a diagnostic delay may be higher in patients with cerebral hemorrhage and increased intracranial hypertension than in those with other complications. However, these two complications frequently require surgical intervention (3,4,7-10). Because both complications can be detected by computed tomography (as in our case), clinicians should consider repeat computed tomography to allow for the early detection of these potential complications in patients who display a worsening clinical condition despite the administration of acyclovir treatment.
Table.
Acute-phase Complications in Patients with Herpes Encephalitis.
| Complication | Prevalence |
Duration from admission to complication |
Complication associated symptoms |
Surgical intervention |
Reference |
|---|---|---|---|---|---|
| Central diabetes insipidus | Unknown | 3 weeks | Polydipsia and polyuria | No | (14) |
| Cerebral hemorrhage | Unknown | 0-16 days | Deterioration of consciousness, hemiparesis, hemiplegia, no symptom, and third nerve palsy | Yes (50%) | (4) |
| Increased intracranial pressure | 21%* | 0-12 days | Deterioration of consciousness, headache, and third nerve palsy | Yes | (3, 7-10) |
| Seizure | 33% | Unknown | seizure | No | (2) |
| Stroke | Unknown | Present on admission | Hemiparesis and impaired vision | No | (11, 12) |
| Subarachnoid hemorrhage | Unknown | 26 days | Deterioration of consciousness | No | (13) |
*In intensive care unit patients
In conclusion, the diagnosis of cerebral hemorrhage during the early phase of HSE may be delayed because of the similarity of its symptoms to the primary disease and due to the worsening of encephalitis. Clinicians should consider cerebral hemorrhage and other acute-phase complications and re-perform imaging studies in HSE patients who do not show any improvement during acyclovir treatment.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Whitley RJ, Soong SJ, Dolin R, Galasso GJ, Ch'ien LT, Alford CA. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious Diseases collaborative antiviral study. N Engl J Med 297: 289-294, 1977. [DOI] [PubMed] [Google Scholar]
- 2. Raschilas F, Wolff M, Delatour F, et al. . Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis 35: 254-260, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Jouan Y, Grammatico-Guillon L, Espitalier F, Cazals X, François P, Guillon A. Long-term outcome of severe herpes simplex encephalitis: a population-based observational study. Crit Care 19: 345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodríguez-Sainz A, Escalza-Cortina I, Guio-Carrión L, et al. . Intracerebral hematoma complicating herpes simplex encephalitis. Clin Neurol Neurosurg 115: 2041-2045, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Fukushima Y, Tsuchimochi H, Hashimoto M, et al. . A case of herpetic meningoencephalitis associated with massive intracerebral hemorrhage during acyclovir treatment: a rare complication. No Shinkei Geka 38: 171-176, 2010. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 6. Lo WB, Wilcock DJ, Carey M, Albanese E. Neurological picture. Herpes encephalitis complicated by cerebral hemorrhage. J Neurol Neurosurg Psychiatry 84: 1404-1406, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Safain MG, Roguski M, Kryzanski JT, Weller SJ. A review of the combined medical and surgical management in patients with herpes simplex encephalitis. Clin Neurol Neurosurg 128: 10-16, 2015. [DOI] [PubMed] [Google Scholar]
- 8. González Rabelino GA, Fons C, Rey A, Roussos I, Campistol J. Craniectomy in herpetic encephalitis. Pediatr Neurol 39: 201-203, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Yan HJ. Herpes simplex encephalitis: the role of surgical decompression. Surg Neurol 57: 20-24, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Counsell CE, Taylor R, Whittle IR. Focal necrotising herpes simplex encephalitis: a report of two cases with good clinical and neuropsychological outcomes. J Neurol Neurosurg Psychiatry 57: 1115-1117, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terlizzi V, Improta F, Di Fraia T, et al. . Primary herpes virus infection and ischemic stroke in childhood: a new association? J Clin Neurosci 21: 1656-1658, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Sas AM, Niks EH, Lequin MH, Catsman-Berrevoets CE, de Wit MC. Herpes simplex virus type-1 encephalitis and occipital ischemic stroke. Pediatr Neurol 41: 294-296, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Tonomura Y, Kataoka H, Yata N, Kawahara M, Okuchi K, Ueno S. A successfully treated case of herpes simplex encephalitis complicated by subarachnoid bleeding: a case report. J Med Case Rep 4: 310, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Canton A, Simo R, Mesa J, Molina C, Rovira A, Montalban X. Central diabetes insipidus: a complication of herpes simplex encephalitis. J Neurol Neurosurg Psychiatry 61: 325-326, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]