Abstract
Background
Various prognostic indicators have been investigated in neoadjuvant chemotherapy (NAC) treated invasive breast cancer (BC). Our study examines if lymphovascular invasion (LVI) is an independent predictor of survival in women receiving NAC.
Methods
We performed a retrospective analysis in 166 women with operable invasive BC who underwent adriamycin (A) and taxane (T)-based NAC between 2000-2013. Presence of LVI was noted in breast excisions following NAC. Associations between progression-free and overall survival and LVI and other clinicopathologic variables were assessed.
Results
Median follow-up was 31 months (range 1.4-153 months) with a total of 56 events and 24 deaths from any cause. LVI was found in 74 of 166 patients (45%). In univariate analysis, presence of LVI was associated with worse progression-free survival (HR 3.37 95% CI 1.87-6.06, p<0.01) and overall survival (HR 4.35, 95% CI 1.61-11.79, p<0.01). In multivariate models adjusting for breast cancer subtype, LVI was significantly associated with a decrease in progression-free survival (HR 3.76 95% CI 2.07-6.83, p<0.01) and overall survival (HR 5.70 95% CI 2.08-15.64, p<0.01). When stratified by subtype, those with hormone receptor or HER2 positive BCs with no LVI had the most favorable progression-free and overall survival. Those with both LVI and triple negative BC had the worst progression-free and overall survival.
Conclusions
LVI is an important prognostic marker and is associated with worse clinical outcome in breast cancer patients receiving NAC.
Keywords: lymphovascular invasion, neoadjuvant chemotherapy, breast cancer, survival
Introduction
Neoadjuvant chemotherapy (NAC) is a mainstay of treatment for operable and locally advanced breast cancer.[1-4] Several markers have been identified to help predict response to NAC including hormone receptor status, human epidermal growth factor receptor (HER-2) status, histological grade, tumor size, and nodal involvement.[5-10] In addition, response to NAC has been associated with tumor biology, with tumors achieving a pathologic complete response (pCR) being associated with a more favorable clinical outcome compared to those with residual disease.[3,11-15]
Lymphovascular invasion (LVI) is defined as carcinoma cells present within a definite endothelial-lined space (either lymphatic vessels or blood vessels) in the breast.[16,17] While the mechanism of lymphatic metastasis is still largely unknown,[18] the presence of LVI has been extensively studied as a prognostic indicator for progression-free and overall survival in invasive breast cancer. Some studies have shown LVI to be a marker for increased risk of axillary nodal metastases, distant metastases, and death.[19,16,20-22] Yet, others have shown that it is not an independent predictor of overall survival[23] and that its role may be limited to only high-risk groups such as those with positive nodes, tumor size >2cm, high grade, hormone receptor negative tumor, or age <35 years.[24]
The role of LVI as a prognostic marker in NAC treated breast cancer remains unclear. Some studies have shown that LVI is associated with “chemoresistant” cancers[25] and that its absence on core biopsies is associated with a complete pathological response (pCR) and improved survival.[7] However, few studies have examined the role of LVI as an independent predictor of survival with adriamycin (A) and taxane (T)-based NAC regimens.
Our study seeks to evaluate the association of LVI with progression free and overall survival in patients with operable breast cancer treated with NAC. Our hypothesis is that LVI is an independent predictor of survival in NAC treated patients.
Methods
Patient Population
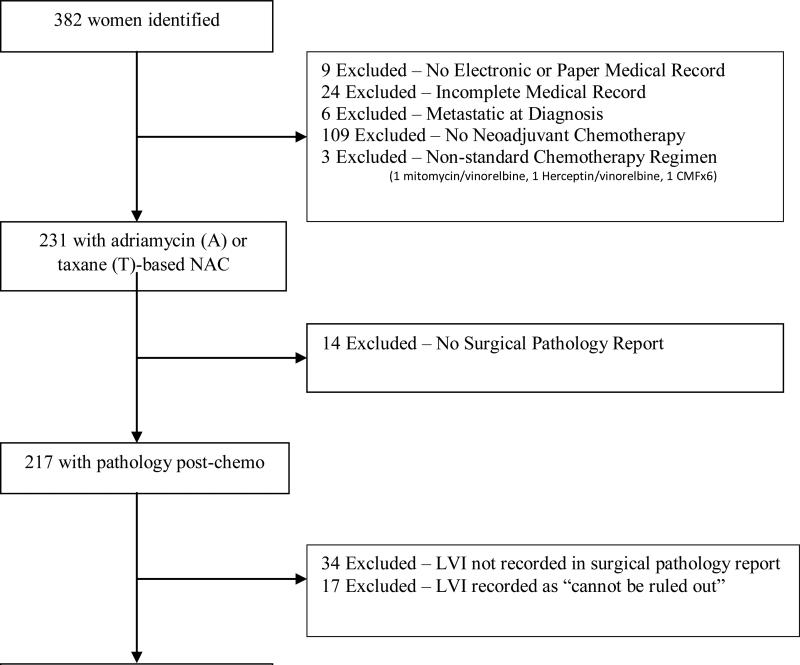
In accordance with Columbia University Medical Center (CUMC) IRB approved protocol (IRB # AAAJ8512), clinical database queries and physician referral were used to identify all women with invasive carcinoma of the breast who received at least part of their care at CUMC and underwent NAC between 2000-2013. Of the 382 patients identified, 33 were excluded for having no electronic/paper chart records (n=9) or incomplete records (n=24) that precluded full data collection. Of the remaining 349, six patients were excluded due to metastatic disease at diagnosis, 109 were excluded as they received no NAC upon further review, and an additional three were excluded as they received non-traditional NAC regimens (1 Mitomycin/Vinorelbine, 1 Herceptin/Vinorelbine, and 1 Cyclophosphamide/Methotrexate/Fluorouracil). Of the remaining 231 women who received adriamycin (A) or taxane (T)-based NAC, 14 were excluded, as they did not have a surgical pathology report performed at CUMC, 34 were excluded, given that none of their pathology reports addressed LVI, and 17 were excluded as the pathology reports could not confer a clear diagnosis of LVI (“cannot be ruled out”). Thus, a total of 166 women were assessed in this analysis. (Figure 1).
Figure 1.
Selection of Patients
Clinical and Pathological Variables
Clinical and pathological data were abstracted from the medical record by two independent researchers. All data were double-verified, and any discrepancies were resolved by oncologists EC and KK. Age was defined in years at pathological diagnosis and was stratified into <50 years of age and ≥50 years of age. Tumor size was defined as the largest dimension on any imaging modality prior to any treatment and was stratified at 0-5cm and >5cm. Grade was defined as the highest grade seen on any biopsy and was defined as low/intermediate grade (grade 1 and 2) and high grade (grade 3). Estrogen receptor (ER) and progesterone receptor (PR) positivity was defined as 10% or greater expression on any biopsy, as the majority of older pathological reports only specified <10% (negative) or ≥10% (positive). However, in accordance with American Society of Clinical Oncology/College of American Pathologist (ASCO/CAP) guidelines from 2010, a separate analysis was also performed where estrogen receptor (ER) and progesterone receptor (PR) positivity was defined as 1% or greater expression on any biopsy.[26] Tumors were considered HER2-positive if they were 3+ by immunohistochemistry (IHC) or demonstrated gene amplification with a ratio of Her-2 /CEP17 ≥2 by in situ hybridization on either the core biopsy or surgical pathology specimen.[27] Based on prior studies, subtype groups were defined as a) hormone receptor positive (ER and/or PR positive) and HER2 negative, b) HER2 positive regardless of hormonal status, and c) triple negative (ER, PR, and HER2 negative).[28] Clinical and pathological staging was determined based on the American Joint Committee on Cancer (AJCC) TNM Staging Manual, 7th edition. Pathological complete response (pCR) was defined as no residual invasive disease in the breast or lymph nodes on surgical pathology specimens (ypT0/Tis ypN0). To assess pathological response and nodal status after NAC, women were divided into three groups: pathological complete response (ypT0/Tis ypN0), those with invasive disease in the breast only (ypT+ ypN0), and those with any invasive disease in lymph nodes (any T and N+), based on prior studies.[29]
LVI was defined based on the CUMC standard pathological definition as presence of carcinoma cells within a definite endothelial-lined space (either lymphatic or blood vessels). This was rarely verified using D2-40 immuno-histochemical stain for lymphatic endothelium and CD31 for endothelium of all vessels. The presence of LVI was evaluated in post-NAC surgical pathology specimens, as well as pre-therapy core biopsies, although the latter less consistently. As only 70 core biopsies addressed the presence or absence of LVI and absence of LVI on core biopsies may represent sampling error, this data element is less reliable. However, there was some agreement (46 out of 70, 66% k=0.4) between the two with 12 out of 70 surgical pathology specimens showing LVI that was not seen on the core biopsy and only 12 out of 70 core biopsies showing LVI that was not seen on surgical pathology specimens. Of the 12 where LVI was seen on the core but not surgical pathology biopsies, three surgical pathology biopsies showed pCR, three showed residual node-negative tumor, and six showed nodal disease only with no residual tumor after NAC. As with prior studies[19], 17 were excluded as the pathologist could not rule out LVI. All pathology specimens were interpreted by trained surgical pathologists.
All women received A-based, T-based, or A/T-based NAC. Women were considered to have received radiation therapy (XRT) if they received any type of whole breast radiation with or without nodal radiation. Hormonal therapy was defined as treatment with any selective estrogen-receptor modulator (SERM) or aromatase inhibitor (AI). Surgery type was stratified into lumpectomy or mastectomy with or without lymph node dissection.
Statistical Analysis
Chi square, Fisher's exact and t-tests were used to compare relevant clinical and pathological variables according to presence or absence of LVI. Progression-free survival was based on the STEEP criteria[30], and events were defined as any local/regional or distant metastasis, contralateral invasive breast cancer (excluding in-situ disease), any secondary, non-breast, invasive cancer, and/or death by any cause. Progression free survival (PFS) and overall survival (OS) were calculated in months from date of definitive surgery to date of first event or death (for OS) or last follow-up in those women without events. Kaplan Meier survival analysis and the log-rank statistic were used to estimate survival differences between groups based on clinically relevant variables. Cox proportional hazard models were used to assess the association of LVI and PFS and OS after adjusting for other covariates, including age, tumor size, grade, subtype, and post-surgical nodal status. Stratified analyses were performed using the a priori determined variable of subtype (triple negative vs. not triple negative). All analyses were performed using SAS 9.4 and STATA 12.0 with significance defined as a two-sided p-value of 0.05.
Results
Demographics
Of the 166 women, 74 had evidence of LVI on pathology (n=59 with invasion into lymphatics and n=15 with invasion into lymphatics and veins), and 92 had no evidence of LVI on post-NAC surgical pathology samples. Of the 166 women, 18 received A-based, 27 received T-based, and 121 received A/T-based NAC. All women completed the entire course of NAC with the exception of four women who had their NAC terminated early due to progression of disease (n=2), long delays in treatment (n=1) and progression as well as toxicity (n=1).
Mean age was 52 in the LVI group and 51 in the no LVI group (Table 1). In both groups, the majority of women self-reported as non-Hispanic White or Hispanic and had invasive ductal carcinomas, tumors between 0-5cm in size, and high-grade breast cancers. Both groups had a similar distribution of subtypes with hormone receptor positive/HER2 negative being the most common (LVI, n=37; no LVI, n=35), followed by HER2 positive (LVI, n=22; no LVI, n= 36), then triple negative breast cancer (TNBC) (LVI, n=15; no LVI, n= 21). One patient with HER2 positive breast cancer did not receive neoadjuvant or adjuvant Herceptin as she was lost to follow-up soon after initial medical oncologist visit. The LVI group had no women who achieved pCR, as expected based on our definition of pCR, and in the no LVI group, n=34 (36%) patients achieved pCR (p<0.001). The LVI group also had significantly higher rates of mastectomy (p<0.001) and post-operative radiation therapy (p-0.006).
Table 1.
Baseline Characteristics by Lymphovascular (LVI) Status
| Variable | LVI (n=74) | No LVI (n=92) | p-value* |
|---|---|---|---|
| Mean Age (SD), years | 52 (1.6) | 51 (1.1) | 0.72 |
| Race | |||
| Non-Hispanic White | 29 (39%) | 30 (33%) | 0.34 |
| Black | 10 (14%) | 24 (26%) | |
| Hispanic | 30 (41%) | 33 (36%) | |
| Other | 1 (1%) | 2 (2%) | |
| Unknown | 4 (5%) | 3 (3%) | |
| Type | |||
| Ductal Carcinoma | 60 (81%) | 77 (86%) | 0.33 |
| Lobular Carcinoma | 5 (7%) | 7 (8%) | |
| Other | 9 (12%) | 5 (6%) | |
| Size | |||
| 0-5cm | 55 (76%) | 74 (80%) | 0.53 |
| >5cm | 17 (24%) | 18 (20%) | |
| Grade | |||
| Low (I & II) | 25 (34%) | 29 (33%) | 0.911 |
| High (III) | 49 (66%) | 59 (67%) | |
| Subtype | |||
| Hormone Receptor+/HER2− | 37 (50%) | 35 (38%) | 0.29 |
| HER2 + | 22 (30%) | 36 (39%) | |
| Triple Negative | 15 (20%) | 21 (23%) | |
| Pathological Staging | |||
| pCR** | 0 (0%) | 34 (36%) | <0.001 |
| T+, N − | 9 (12%) | 29 (32%) | |
| Node + | 65 (88%) | 29 (32%) | |
| Surgery Type | |||
| Lumpectomy | 11 (15%) | 40 (44%) | <0.001 |
| Mastectomy | 63 (85%) | 51 (56%) | |
| Adjuvant Radiation | |||
| Yes | 70 (97%) | 74 (84%) | 0.006 |
| No | 2 (3%) | 14 (16%) | |
| Adjuvant Hormonal Therapy | |||
| Yes | 53 (75%) | 54 (68%) | 0.40 |
| No | 18 (25%) | 25 (32%) | |
| Neoadjuvant/Adjuvant Herceptin | |||
| Yes | 19 (27%) | 36 (52%) | 0.003 |
| No | 51 (73%) | 33 (48%) |
p-values represent t-tests for continuous and chi square or Fisher's exact tests for categorical variables.
pCR – pathological complete response
Survival Analysis
Median follow-up was 31 months (range 1.4-153 months). There were a total of 56 events with 50 of them being recurrence or progression of invasive breast cancer (13 local and 37 distant), 2 being new invasive primary cancers (1 colon and 1 laryngeal) and 4 being death from any cause without evidence of recurrence or progression. Of the 13 local recurrences, six were recurrences in the same breast, three in the lymph nodes, and four in the chest wall. Ten of these women received radiation therapy while two did not (data missing for last woman). There were 24 overall deaths from any cause.
On univariate analysis, presence of LVI was significantly associated with worse PFS (HR 3.37; 95% CI 1.87-6.06; p < 0.01) and OS (HR 4.35; 95% CI 1.61-11.79; p <0.01). Subtype (triple negative as compared to hormone receptor+/HER2- breast cancer) was also significantly associated with worse PFS (HR 2.00; 95% CI 1.06-3.75; p = 0.03) and OS (HR 4.23; 95% CI 1.47-12.17; p < 0.01), (Table 2.1 and 2.2). In addition to presence of LVI and subtype, the presence of lymph node involvement (nodal disease) at the time of definitive surgery (vs. pCR, HR 2.80; 95% CI 1.10-7.11; p = 0.03) was also significantly associated with worse PFS, although post-surgical nodal status itself was not overall significantly associated with either PFS or OS (Table 2.1 and 2.2). Age, size, grade and radiation therapy were not significantly associated with either PFS or OS.
Table 2.1.
Univariate analysis of predictors of progression-free survival (time from definitive surgery)
| No event |
Event |
|||||
|---|---|---|---|---|---|---|
| Characteristics | No. | % | No. | % | HR (95% CI) | P-value |
| Total patients | 110 | 66 | 56 | 34 | ||
| Age (continuous) | 1.00 (0.97, 1.02) | 0.6306 | ||||
| Age group | ||||||
| <50 years | 46 | 61 | 30 | 39 | 1 | - |
| 50+ years | 64 | 71 | 26 | 29 | 0.75 (0.44, 1.28) | 0.2911 |
| LVI | ||||||
| No | 75 | 82 | 17 | 18 | 1 | - |
| Yes | 35 | 47 | 39 | 53 | 3.37 (1.87, 6.06) | <0.0001* |
| Subtype | 0.0352* | |||||
| Hormone positive/HER2 | 51 | 71 | 21 | 29 | 1 | - |
| HER2 positive | 43 | 74 | 15 | 26 | 0.89 (0.45-1.77) | 0.7481 |
| TNBC | 16 | 44 | 20 | 56 | 2.00 (1.06-3.75) | 0.0314* |
| Post-surgical stage and nodal | 0.0610 | |||||
| pCR | 29 | 85 | 5 | 15 | 1 | - |
| T+, N negative | 29 | 76 | 9 | 24 | 1.76 (0.59, 5.26) | 0.3119 |
| N+, regardless of T | 52 | 55 | 42 | 45 | 2.80 (1.10, 7.11) | 0.0303* |
| Size (continuous) | 1.05 (0.92, 1.20) | 0.4745 | ||||
| Size | ||||||
| 0-5 cm | 83 | 64 | 46 | 36 | 1 | - |
| >5 cm | 27 | 77 | 8 | 23 | 0.74 (0.35, 1.57) | 0.4369 |
| Grade | ||||||
| low | 41 | 76 | 13 | 24 | 1 | - |
| High | 66 | 61 | 42 | 39 | 1.79 (0.96, 3.35) | 0.0682 |
Abbreviations: HR, Hazard ratio; CI, confidence interval; LVI: lymphovascular invasion; pCR: pathologic response; TNBC: Triple Negative Breast Cancer
Table 2.2.
Univariate analysis of predictors of overall survival (time from definitive surgery)
| Alive |
Dead |
|||||
|---|---|---|---|---|---|---|
| Characteristics | No. | % | No. | % | HR (95% CI) | P-value |
| Total patients | 142 | 86 | 24 | 14 | ||
| Age (continuous) | 1.02 (0.99, 1.05) | 0.2757 | ||||
| Age group | ||||||
| <50 years | 66 | 87 | 10 | 13 | 1 | - |
| 50+ years | 76 | 84 | 14 | 16 | 1.29 (0.55, 3.01) | 0.5620 |
| LVI | ||||||
| No | 87 | 95 | 5 | 5 | 1 | - |
| Yes | 55 | 74 | 19 | 26 | 4.35 (1.61, 11.79) | 0.0039* |
| Subtype | 0.0898 | |||||
| Hormone positive/HER2 negative | 67 | 93 | 5 | 7 | 1 | - |
| HER2 positive | 51 | 88 | 7 | 12 | 1.52 (0.46-4.97) | 0.4927 |
| TNBC | 24 | 67 | 12 | 33 | 4.23 (1.47-12.17) | 0.0076* |
| Post-surgical stage and nodal status | 0.1434 | |||||
| pCR | 33 | 97 | 1 | 3 | 1 | - |
| T+, N negative | 35 | 92 | 3 | 8 | 2.97 (0.31, 28.59) | 0.3470 |
| N+, regardless of T | 74 | 79 | 20 | 21 | 5.89 (0.79, 44.13) | 0.0844 |
| Size (continuous) | 0.98 (0.79-1.22) | 0.8585 | ||||
| Size | ||||||
| 0-5 cm | 110 | 85 | 19 | 15 | 1 | - |
| >5 cm | 32 | 91 | 3 | 9 | 0.65 (0.19-2.18) | 0.4802 |
| Grade | ||||||
| low | 49 | 91 | 5 | 9 | 1 | - |
| High | 89 | 82 | 19 | 18 | 1.96 (0.72, 5.32) | 0.1854 |
Abbreviations: HR, Hazard ratio; CI, confidence interval; LVI: lymphovascular invasion; pCR: pathologic complete response; TNBC: Triple Negative Breast Cancer
On multivariate analysis, presence of LVI was an independent predictor for a worse PFS survival (HR 3.76, 2.07-6.83, p<0.01) and worse OS (HR 5.70, 2.08-15.64, p<0.01) after adjusting for subtype (Table 3). TNBC was an independent predictor of worse PFS (HR 2.59, 1.37-4.90, p<0.01) and OS (HR 6.06, 2.08-17.68, p<0.01) after adjusting for LVI.
A separate analysis using a cutoff of ≥1% for ER/PR positivity only affected the subtype of 3 women, changing them from TNBC to hormone receptor positive. On univariate analysis using the cutoff of ≥1%, TNBC, as compared to hormone receptor +/HER2- breast cancer, was significantly associated with worse PFS (p=0.05) and a trend towards worse OS (p=0.08). On multivariate analysis both presence of LVI (HR 3.88, 2.13-7.09, p<0.01) and TNBC, as compared to hormone receptor +/HER2- breast cancer, (HR 2.51, 1.31-4.80, p<0.01) were significantly associated with worse PFS. Presence of LVI (HR 5.85, 2.10-16.27, p<0.01) and TNBC, as compared to hormone receptor +/ HER2- breast cancer, (HR 4.41, 1.60-12.21, p<0.01) were also significant predictors of worse OS.
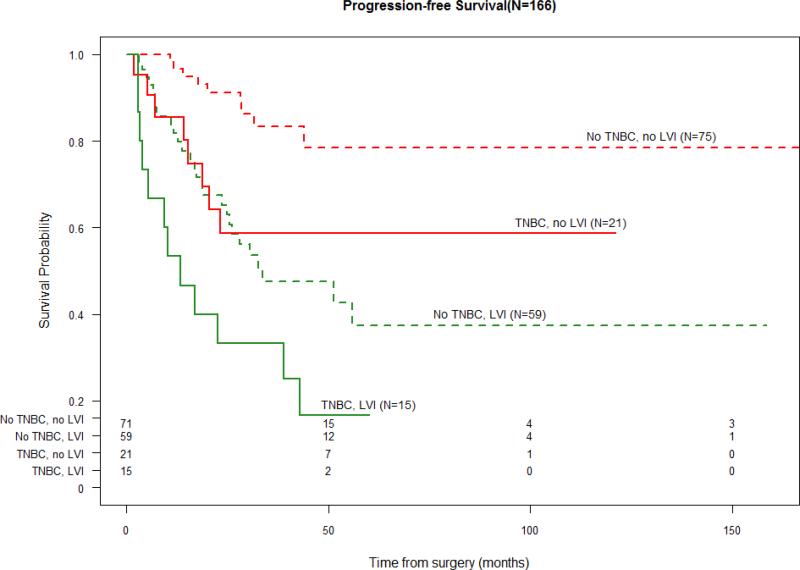
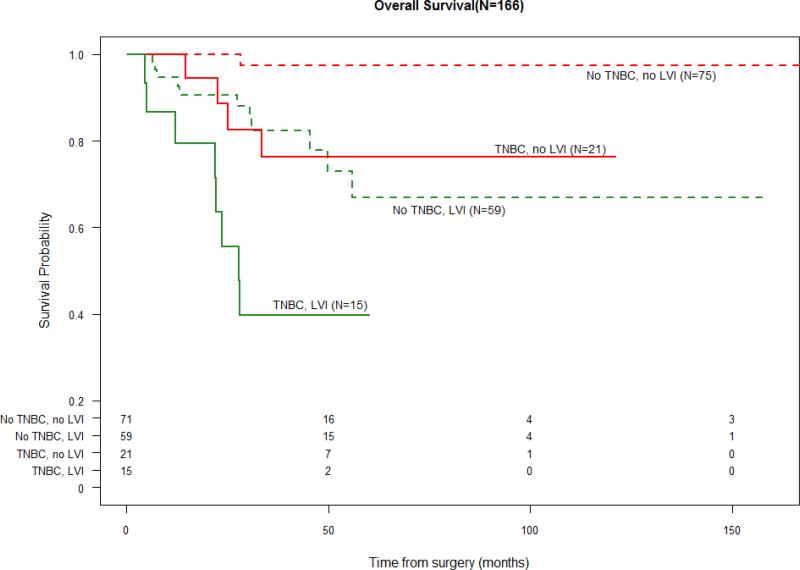
When stratified by triple negative status, those with no TNBC and no LVI had the most favorable PFS and OS. The presence of LVI was associated with a PFS and OS detriment to a similar extent as having TNBC. The presence of both TNBC and LVI was associated with the least favorable survival outcomes (Figure 2.1 and 2.2).
Figure 2.1.
Progression-Free Survival and LVI stratified by “TNBC” vs. “not TNBC”
(Number at Risk displayed in table below survival curves)
Figure 2.2.
Overall Survival and LVI stratified by “TNBC” vs. “not TNBC”
(Number at Risk displayed in table below survival curves)
Discussion
In a population of 166 women receiving NAC for newly diagnosed breast cancer, the presence of LVI was associated with worse PFS and OS. In analysis stratified by triple negative status, LVI appeared to affect survival in a similar manner as having triple negative status, and the presence of both pathologic factors together further worsened PFS and OS.
The results of our study are similar to prior studies of LVI in women receiving NAC. In 115 Japanese women receiving NAC, Tamura et al. found that LVI predicted tumor recurrence and death in multivariate models and could be useful in classifying risk.[5] Others found that LVI was a significant component of clinicopathologic scores that predict response and survival in women receiving NAC.[31] In studies looking at various subtypes of breast cancer, LVI was associated with a worse 5-year recurrence free survival rate.[32] Yet, Huang et al. found that in 542 women treated with NAC and radiation therapy, LVI was only associated with survival on univariate analysis, with other factors, such as skin/nodal involvement, tamoxifen use and subtype, being better predictors of overall survival in multivariate models. However, the NAC regimens used in this previously reported study were older than modern regimens, [33] and few studies have focused on the prognostic implications of LVI in surgical pathology specimens after NAC in such a diverse population.
Our study found that LVI, as seen on post-NAC surgical specimens, was an independent predictor of PFS and OS in women receiving modern anthracycline and/or taxane-containing NAC regimens. This is in agreement with the study by Tamura et al. who found that LVI on post-surgical specimens was a better predictor of survival than LVI on biopsy samples pre-NAC in a small population of Japanese women.[5] Interestingly, we found that LVI was associated with PFS and OS, independent of post-surgical stage/nodal status, suggesting that the presence of LVI represents an independent prognostic marker of poor outcome, outside of its potential association with nodal metastasis. Other studies support this finding and have reported that LVI is an independent predictor of survival in multivariate models with size, grade, age and type in those with node negative disease in patients receiving adjuvant chemotherapy.[19] We also found that LVI affected PFS to a similar extent as having a triple negative subtype, a known poor prognostic factor, and that the two variables in conjunction further decreased PFS. This has been seen in other studies as well, and Sakuma et al. found that in 44 women with triple negative breast cancer, presence of LVI was associated with worse disease free survival.[34]
Although the exact mechanism is unknown, LVI may represent an aggressive tumor or tumor environment that could portend a worse prognosis. Lymphangiogenesis is thought to correlate with lymph node metastasis and may be driven by factors secreted by tumor cells and their environment such as vascular endothelial growth factor C (VEGF-C) and matrix metalloproteinase 9 (MMP-9). [35-37] Studies also show that lymphangiogenesis is affected by histology subtype, with TNBC patients having higher densities of lymphatic microvessels and VEGF-C compared with non-TNBC patients, and subsequently worse OS.[38,20,39] The underlying mechanism for LVI as an independent predictor of poor PFS appears independent from existing nodal invasion, and as it appears to vary with subtype, may reflect not only the aggressiveness of the underlying tumor but also the surrounding tumor micro-environment.
Strengths of this study are that it included a large population of women with breast cancer who received modern regimens of NAC, allowing us to study the prognostic implications of presence of LVI after receiving NAC on survival. Although this was a retrospective study, the data extraction process was double-verified for accuracy. In addition, multiple clinical and pathological covariates were assessed. This is also an ethnically diverse population, which may improve generalizability of results. In addition, we performed our analysis using both the ER/PR positivity cutoff of ≥10% and the newer cutoff of ≥1% with similar results. Limitations include the incomplete assessment of LVI on the core biopsies and the lack of central pathology review. As with all retrospective studies, causality is not possible to assess, and although many potential confounders were accounted for in this study, there is always the possibility of residual confounding. Future research should focus on integrating LVI to delineate this high-risk population, with future studies targeting these patients with new therapeutic approaches.
In conclusion, LVI appears to be an independent predictor of survival in women with invasive breast carcinoma receiving modern NAC regimens. This association also appears to vary by subtype with those with TNBC and LVI having the worst overall prognosis. Further studies in larger cohorts are necessary to determine the prognostic implications of LVI post-NAC in various subtype populations to better inform treatment decisions.
Table 3.1.
Multivariate analysis of predictors of progression-free survival (time from definitive surgery)
| No event |
Event |
|||||
|---|---|---|---|---|---|---|
| Characteristics | No. | % | No. | % | HR (95% CI) | P-value |
| Total patients | 110 | 66 | 56 | 34 | ||
| LVI | ||||||
| No | 75 | 82 | 17 | 18 | 1 | - |
| Yes | 35 | 47 | 39 | 53 | 3.76 (2.07-6.83) | <0.0001* |
| Subtype | 0.0069* | |||||
| Hormone positive/HER2 negative | 51 | 71 | 21 | 29 | 1 | - |
| HER2 positive | 43 | 74 | 15 | 26 | 1.11 (0.56-2.21) | 0.8688 |
| TNBC | 16 | 44 | 20 | 56 | 2.59 (1.37-4.90) | 0.0055* |
Table 3.2.
Multivariate analysis of predictors of overall survival (time from definitive surgery)
| Alive |
Dead |
|||||
|---|---|---|---|---|---|---|
| Characteristics | No. | % | No. | % | HR (95% CI) | P-value |
| Total patients | 142 | 86 | 24 | 14 | ||
| LVI | ||||||
| No | 87 | 95 | 5 | 5 | 1 | - |
| Yes | 55 | 74 | 19 | 26 | 5.70 (2.08-15.64) | 0.0007* |
| Subtype | 0.0020* | |||||
| Hormone positive/HER2 negative | 67 | 93 | 5 | 7 | 1 | - |
| HER2 positive | 51 | 88 | 7 | 12 | 1.89 (0.58-6.22) | 0.2936 |
| TNBC | 24 | 67 | 12 | 33 | 6.06 (2.08-17.68) | 0.0010* |
Abbreviations: HR, Hazard ratio; CI, confidence interval; LVI: lymphovascular invasion; TNBC: Triple Negative Breast
Acknowledgments
Funding: This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number KL2 TR000081. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- LVI
lymphovascular invasion
- NAC
neoadjuvant chemotherapy
- TNBC
triple negative breast cancer
- pCR
pathological complete response
- PFS
progression-free survival
- OS
overall survival
Footnotes
Disclosures: None
Conflict of Interest Statement:
None of the above authors have any conflicts of interest to declare.
References
- 1.Ragaz J. Preoperative (neoadjuvant) chemotherapy for breast cancer: outline of the British Columbia Trial. Recent Results Cancer Res. 1986;103:85–94. doi: 10.1007/978-3-642-82671-9_9. [DOI] [PubMed] [Google Scholar]
- 2.Ragaz J, Baird R, Rebbeck P, Goldie A, Coldman A, Spinelli J. Preoperative adjuvant chemotherapy (neoadjuvant) for carcinoma of the breast: rationale and safety report. Recent Results Cancer Res. 1985;98:99–105. doi: 10.1007/978-3-642-82432-6_11. [DOI] [PubMed] [Google Scholar]
- 3.Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, Winer EP. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26(5):814–819. doi: 10.1200/JCO.2007.15.3510. doi:10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R, Jonat W, Lebeau A, Loibl S, Miller W, Seeber S, Semiglazov V, Smith R, Souchon R, Stearns V, Untch M, von Minckwitz G. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24(12):1940–1949. doi: 10.1200/JCO.2005.02.6187. doi:10.1200/JCO.2005.02.6187. [DOI] [PubMed] [Google Scholar]
- 5.Tamura N, Hasebe T, Okada N, Houjoh T, Akashi-Tanaka S, Shimizu C, Shibata T, Sasajima Y, Iwasaki M, Kinoshita T. Tumor histology in lymph vessels and lymph nodes for the accurate prediction of outcome among breast cancer patients treated with neoadjuvant chemotherapy. Cancer science. 2009;100(10):1823–1833. doi: 10.1111/j.1349-7006.2009.01264.x. doi:10.1111/j.1349-7006.2009.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petit T, Borel C, Ghnassia JP, Rodier JF, Escande A, Mors R, Haegele P. Chemotherapy response of breast cancer depends on HER-2 status and anthracycline dose intensity in the neoadjuvant setting. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7(6):1577–1581. [PubMed] [Google Scholar]
- 7.Keskin S, Muslumanoglu M, Saip P, Karanlik H, Guveli M, Pehlivan E, Aydogan F, Eralp Y, Aydiner A, Yavuz E, Ozmen V, Igci A, Topuz E. Clinical and pathological features of breast cancer associated with the pathological complete response to anthracycline-based neoadjuvant chemotherapy. Oncology. 2011;81(1):30–38. doi: 10.1159/000330766. doi:10.1159/000330766. [DOI] [PubMed] [Google Scholar]
- 8.Chollet P, Amat S, Cure H, de Latour M, Le Bouedec G, Mouret-Reynier MA, Ferriere JP, Achard JL, Dauplat J, Penault-Llorca F. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer. 2002;86(7):1041–1046. doi: 10.1038/sj.bjc.6600210. doi:10.1038/sj.bjc.6600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penault-Llorca F, Vincent-Salomon A. [Roles of the pathologist in neoadjuvant chemotherapy: evaluation of response, prognostic and predictive factors]. Ann Pathol. 2003;23(6):555–563. [PubMed] [Google Scholar]
- 10.Bollet MA, Sigal-Zafrani B, Gambotti L, Extra JM, Meunier M, Nos C, Dendale R, Campana F, Kirova YM, Dieras V, Fourquet A, Institut Curie Breast Cancer Study G Pathological response to preoperative concurrent chemo-radiotherapy for breast cancer: results of a phase II study. Eur J Cancer. 2006;42(14):2286–2295. doi: 10.1016/j.ejca.2006.03.026. doi:10.1016/j.ejca.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Ferriere JP, Assier I, Cure H, Charrier S, Kwiatkowski F, Achard JL, Dauplat J, Chollet P. Primary chemotherapy in breast cancer: correlation between tumor response and patient outcome. Am J Clin Oncol. 1998;21(2):117–120. doi: 10.1097/00000421-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422. doi: 10.1200/JCO.2007.10.6823. doi:10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 13.Rody A, Karn T, Gatje R, Ahr A, Solbach C, Kourtis K, Munnes M, Loibl S, Kissler S, Ruckhaberle E, Holtrich U, von Minckwitz G, Kaufmann M. Gene expression profiling of breast cancer patients treated with docetaxel, doxorubicin, and cyclophosphamide within the GEPARTRIO trial: HER-2, but not topoisomerase II alpha and microtubule-associated protein tau, is highly predictive of tumor response. Breast (Edinburgh, Scotland) 2007;16(1):86–93. doi: 10.1016/j.breast.2006.06.008. doi:10.1016/j.breast.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE, Jr., Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. doi:10.1016/s0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 15.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. doi:10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed RA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. The American journal of surgical pathology. 2007;31(12):1825–1833. doi: 10.1097/PAS.0b013e31806841f6. doi:10.1097/PAS.0b013e31806841f6. [DOI] [PubMed] [Google Scholar]
- 17.Song YJ, Shin SH, Cho JS, Park MH, Yoon JH, Jegal YJ. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. Journal of breast cancer. 2011;14(3):198–203. doi: 10.4048/jbc.2011.14.3.198. doi:10.4048/jbc.2011.14.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124(3):922–928. doi: 10.1172/JCI71606. doi:10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee AH, Pinder SE, Macmillan RD, Mitchell M, Ellis IO, Elston CW, Blamey RW. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer. 2006;42(3):357–362. doi: 10.1016/j.ejca.2005.10.021. doi:10.1016/j.ejca.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed RA, Martin SG, Mahmmod AM, Macmillan RD, Green AR, Paish EC, Ellis IO. Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: findings from a large case series with long-term follow-up. The Journal of pathology. 2011;223(3):358–365. doi: 10.1002/path.2810. doi:10.1002/path.2810. [DOI] [PubMed] [Google Scholar]
- 21.Pinder SE, Ellis IO, Galea M, O'Rouke S, Blamey RW, Elston CW. Pathological prognostic factors in breast cancer. III. Vascular invasion: relationship with recurrence and survival in a large study with long-term follow-up. Histopathology. 1994;24(1):41–47. doi: 10.1111/j.1365-2559.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 22.Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, Macmillan D, Ellis IO. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118(15):3670–3680. doi: 10.1002/cncr.26711. doi:10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 23.Freedman GM, Li T, Polli LV, Anderson PR, Bleicher RJ, Sigurdson E, Swaby R, Dushkin H, Patchefsky A, Goldstein L. Lymphatic space invasion is not an independent predictor of outcomes in early stage breast cancer treated by breast-conserving surgery and radiation. The breast journal. 2012;18(5):415–419. doi: 10.1111/j.1524-4741.2012.01271.x. doi:10.1111/j.1524-47412012.01271.x. [DOI] [PubMed] [Google Scholar]
- 24.Ejlertsen B, Jensen MB, Rank F, Rasmussen BB, Christiansen P, Kroman N, Kvistgaard ME, Overgaard M, Toftdahl DB, Mouridsen HT. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst. 2009;101(10):729–735. doi: 10.1093/jnci/djp090. doi:10.1093/jnci/djp090. [DOI] [PubMed] [Google Scholar]
- 25.Uematsu T, Kasami M, Watanabe J, Takahashi K, Yamasaki S, Tanaka K, Tadokoro Y, Ogiya A. Is lymphovascular invasion degree one of the important factors to predict neoadjuvant chemotherapy efficacy in breast cancer? Breast cancer (Tokyo, Japan) 2011;18(4):309–313. doi: 10.1007/s12282-010-0211-z. doi:10.1007/s12282-010-0211-z. [DOI] [PubMed] [Google Scholar]
- 26.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of oncology practice / American Society of Clinical Oncology. 2010;6(4):195–197. doi: 10.1200/JOP.777003. doi:10.1200/jop.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchrakian N, Flanagan L, Harford J, Gannon JM, Quinn CM. New ASCO/CAP guideline recommendations for HER2 testing increase the proportion of reflex in situ hybridization tests and of HER2 positive breast cancers. Virchows Archiv : an international journal of pathology. 2015 doi: 10.1007/s00428-015-1871-z. doi:10.1007/s00428-015-1871-z. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Wang S, Israel HP, Yan SX, Horowitz DP, Crockford S, Gidea-Addeo D, Clifford Chao KS, Kalinsky K, Connolly EP. Higher locoregional recurrence rate for triple-negative breast cancer following neoadjuvant chemotherapy, surgery and radiotherapy. SpringerPlus. 2015;4:386. doi: 10.1186/s40064-015-1116-2. doi:10.1186/s40064-015-1116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE, Jr., Taghian A, Wickerham DL, Wolmark N. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30(32):3960–3966. doi: 10.1200/JCO.2011.40.8369. doi:10.1200/JCO.2011.40.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman J-AW, Sparano JA, Hunsberger S, Enos RA, Gelber RD. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. Journal of Clinical Oncology. 2007;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Fatah TM, Ball G, Lee AH, Pinder S, MacMilan RD, Cornford E, Moseley PM, Silverman R, Price J, Latham B, Palmer D, Chan A, Ellis IO, Chan SY. Nottingham Clinico-Pathological Response Index (NPRI) after neoadjuvant chemotherapy (Neo-ACT) accurately predicts clinical outcome in locally advanced breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(5):1052–1062. doi: 10.1158/1078-0432.CCR-14-0685. doi:10.1158/1078-0432.ccr-14-0685. [DOI] [PubMed] [Google Scholar]
- 32.Caudle AS, Yu TK, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, Hoffman K, Meric-Bernstam F, Hunt KK, Buchholz TA, Mittendorf EA. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast cancer research : BCR. 2012;14(3):R83. doi: 10.1186/bcr3198. doi:10.1186/bcr3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang EH, Tucker SL, Strom EA, McNeese MD, Kuerer HM, Hortobagyi GN, Buzdar AU, Valero V, Perkins GH, Schechter NR, Hunt KK, Sahin AA, Buchholz TA. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. International journal of radiation oncology, biology, physics. 2005;62(2):351–357. doi: 10.1016/j.ijrobp.2004.09.056. doi:10.1016/j.ijrobp.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 34.Sakuma K, Kurosumi M, Oba H, Kobayashi Y, Takei H, Inoue K, Tabei T, Oyama T. Pathological tumor response to neoadjuvant chemotherapy using anthracycline and taxanes in patients with triple-negative breast cancer. Experimental and therapeutic medicine. 2011;2(2):257–264. doi: 10.3892/etm.2011.212. doi:10.3892/etm.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, Jakesz R, Horvat R, Austrian B, Colorectal Cancer Study G. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240(2):306–312. doi: 10.1097/01.sla.0000133355.48672.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, Bartel G, Krieger S, Kalt R, Hantusch B, Keller T, Nagy-Bojarszky K, Huttary N, Raab I, Lackner K, Krautgasser K, Schachner H, Kaserer K, Rezar S, Madlener S, Vonach C, Davidovits A, Nosaka H, Hammerle M, Viola K, Dolznig H, Schreiber M, Nader A, Mikulits W, Gnant M, Hirakawa S, Detmar M, Alitalo K, Nijman S, Offner F, Maier TJ, Steinhilber D, Krupitza G. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest. 2011;121(5):2000–2012. doi: 10.1172/JCI44751. doi:10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang ZQ, Han YZ, Nian Q, Chen G, Cui SQ, Wang XY. Tumor Invasiveness, Not Lymphangiogenesis, Is Correlated with Lymph Node Metastasis and Unfavorable Prognosis in Young Breast Cancer Patients (</=35 Years). PLoS One. 2015;10(12):e0144376. doi: 10.1371/journal.pone.0144376. doi:10.1371/journal.pone.0144376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohammed RA, Ellis IO, Mahmmod AM, Hawkes EC, Green AR, Rakha EA, Martin SG. Lymphatic and blood vessels in basal and triple-negative breast cancers: characteristics and prognostic significance. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24(6):774–785. doi: 10.1038/modpathol.2011.4. doi:10.1038/modpathol.2011.4. [DOI] [PubMed] [Google Scholar]
- 39.Niemiec J, Adamczyk A, Ambicka A, Mucha-Malecka A, Wysocki W, Mitus J, Rys J. Lymphangiogenesis assessed using three methods is related to tumour grade, breast cancer subtype and expression of basal marker. Pol J Pathol. 2012;63(3):165–171. doi: 10.5114/pjp.2012.31500. [DOI] [PubMed] [Google Scholar]