Abstract
Study Objectives:
Restless legs syndrome, also known as Willis-Ekbom disease (RLS/WED), is a frequent condition, though its pathophysiology is not completely understood. The diagnosis of RLS/WED relies on clinical criteria, and the only instrumental tool, the suggested immobilization test, may lead to equivocal results. Recently, neurophysiological parameters related to F-wave duration have been proposed as a diagnostic aid. The aim of this study is to assess and compare the diagnostic values of these parameters in diagnosis of RLS/WED.
Methods:
Fifteen women affected by primary RLS/WED and 17 age- and sex- matched healthy subjects. A complete electroneurographic evaluation, including nerve conduction studies (NCS), cutaneous silent period (CSP), and F-wave parameters, namely amplitude, F-wave duration (FWD), and the ratio between FWD and duration of the corresponding compound muscle action potential (FWD/CMAPD).
Results:
No subject showed alterations of the NCS. However, FWD and FWD/CMAPD of both upper and lower limbs were significantly longer in patients than controls. Tibial FWD/CMAPD best discriminated RLS/WED patients from controls. A cutoff of 2.06 yielded a sensitivity of 69.2%, a specificity of 94.1%, a positive predictive power of 90%, and a negative predictive power of 80% (area under the curve = 0.817; 95% confidence interval = 0.674–0.959). The combination of ulnar or tibial FWD/CMAPD increases the sensitivity (85.7%) while slightly decreasing the specificity (87.5%, positive predictive value: 85.7%, negative predictive value: 87.5%).
Conclusions:
Lower limb FWD/CMAPD ratio may represent a supportive diagnostic tool, especially in cases of evening lower leg discomfort of unclear interpretation.
Citation:
Congiu P, Fantini ML, Milioli G, Tacconi P, Figorilli M, Gioi G, Pereira B, Marrosu F, Parrino L, Puligheddu M. F-wave duration as a specific and sensitive tool for the diagnosis of restless legs syndrome/Willis-Ekbom disease. J Clin Sleep Med. 2017;13(3):369–375.
Keywords: diagnostic tool, F waves, restless legs syndrome/Willis-Ekbom disease
INTRODUCTION
Restless legs syndrome (RLS), also known as Willis-Ekbom disease (WED), is a sensorimotor disorder characterized by the need to move the legs, generally associated with unpleasant sensations. Its symptoms typically appear during rest and show a circadian rhythm, occurring or worsening in the evening.1–5 The diagnosis of RLS/WED is based on the criteria set in 1995, revised in 2003,4 and recently updated in order to improve the sensitivity in the diagnosis.3 The suggested immobilization test (SIT)6 has been developed to assess RLS/WED symptoms, but to date no instrumental examination allows a clear-cut diagnosis of RLS/WED. The pathophysiology of RLS/WED has not been fully elucidated; an alteration of the spinal hypothalamic dopaminergic tract that modulates the excitability of various neuronal population is suggested.7–13
Different studies carried out on spinal excitability assessing reflexes in patients affected by RLS-PLMD has led to contrasting results, with some showing an increase in spinal excitability,14,15 while others showed no differences between patients and controls.16–20
BRIEF SUMMARY
Current Knowledge/Study Rationale: Currently, the diagnosis of RLS/WED is based on clinical criteria and exclusion of secondary causes and mimicking conditions; there are no instrumental tools able to distinguish between subjects affected by RLS/WED and other conditions characterized by leg discomfort, especially at night. The aim of this study is to evaluate the sensibility and the specificity of some F-wave parameters, easily obtainable during routine nerve conduction study, in the diagnosis of primary RLS/WED.
Study Impact: The introduction of these parameters may represent a new diagnostic instrumental tool that is easy to perform and that may contribute to a correct diagnosis of primary RLS/WED and therefore to the most appropriate therapy.
Recently, Isak et al.21 suggested that the exploration of some parameters, namely the F-wave duration (FWD), the ratio between FWD and the duration of related compound muscle action potential (CMAPD), FWD/CMAPD, and the silent period ratio (SPR) would be helpful in the diagnosis of primary RLS/WED. CMAP is the early response due to activation of muscle fibers by electrical stimulation of a peripheral motor or mixed nerve. These measures are believed to evaluate spinal segmental motor neurons excitability,22,23 although some recent reports suggested that F waves offer a flawed measure of motor neuron excitability.24,25
In the current study, to further explore the role of these parameters in the diagnosis of RLS/WED, we aimed to assess the accuracy and compare the diagnostic values of each of these measures in a group of patients affected by primary RLS/ WED.
METHODS
Among 86 patients affected by RLS referred to the outpatient service of Sleep Disorders Center – University of Cagliari between 2008 and 2012, 15 female subjects affected by primary RLS were consecutively enrolled in the study (Table 1 shows demographic and clinical data of patients). The clinical diagnosis of RLS/WED was made according to standard diagnostic criteria at the time of the study.4 All RLS patients were affected by chronic-persistent RLS/WED. They did not have any disorders that could affect dopaminergic transmission or spinal excitability, they were drug free, and the experimental design was performed before starting a treatment. The control subjects were 17 volunteers, matched for sex and age and acquired in similar circadian conditions to RLS/WED patients, previously recruited to a set normative database among medical, technical, and administrative staff members of our hospital or their relatives (mean age 52.5 ± 8.7 y, range 42–65). None was complaining of RLS/WED symptoms. The Ethical Committee approved the study, and informed consent was obtained by participants.
Table 1.
Demographic and clinical data of patients with restless legs syndrome/Willis-Ekbom disease.

Exclusion criteria in both patients and controls were: history of radiculopathies and peripheral neuropathies, rheumatoid arthritis or other autoimmune diseases, diabetes, and renal failure. To rule out these conditions, all subjects underwent a complete hematologic evaluation including hematocytometric test, iron and glycometabolic state, and hepatic and renal function, as well as neurophysiological examination of the peripheral nervous system through the study of nerve conduction velocity.
Clinical and Instrumental Evaluation
All subjects underwent a clinical examination by a physician expert in sleep medicine at our sleep disorder center, and they subsequently underwent nerve conduction studies (NCS) at the electromyography (EMG) laboratory. NCS included: (1) motor nerve conduction velocity and F responses of the left median nerve, right ulnar nerve, left peroneal nerve, and right tibial nerve; (2) sensory nerve conduction velocity of the left superficial radial nerve and right sural nerve; and (3) bilateral soleus H reflex.
The neurophysiological study was carried out with a Nicolet-Viking II electromyograph through conventional cup surface electrodes. Data from nerve conduction velocity were compared with the normative data of our laboratory. All examinations were performed between 15:00 and 18:00 (for organizational reasons); room temperature was maintained constant (22°C); and skin temperature varied between 32°C and 34°C. For all nerves, two sessions were carried out to verify the reproducibility of the responses. After assessing nerve conduction parameters (namely latencies, amplitudes of motor and sensory potentials, and conduction velocities), we assessed F responses and the cutaneous silent period (CSP).
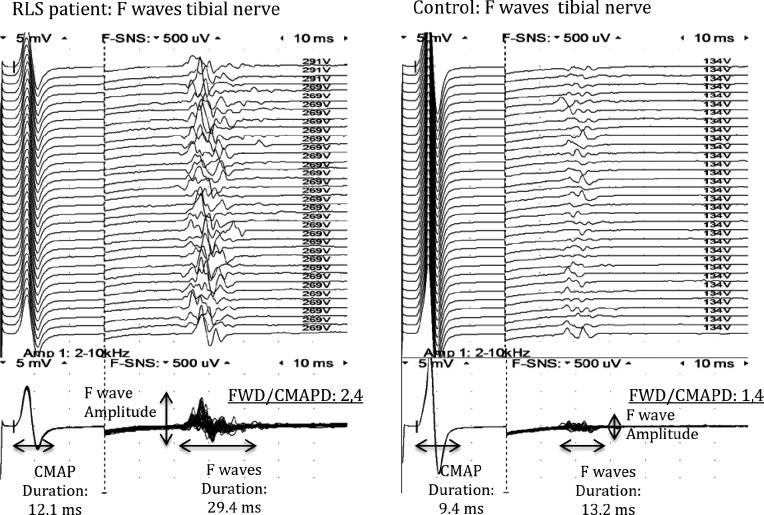
The F wave26 parameters evaluated were: amplitude (measured from peak to peak); minimum latency (measured as the shortest latency to the onset of the first deflection in the 32 consecutives traces); maximum latency (measured as the longest latency to the onset of the first deflection for the 32 traces); mean latency (mean F-waves latency of all traces); FWD (measured from the onset of the deflection to the final return to the baseline), and persistence (percentage of stimuli that evoke a F response)27–29 (Figure 1). For each parameter, we have first measured the value for each single F response, and then we have calculated the mean value. F waves were recorded in upper limbs by stimulating the right ulnar nerve and recording from the abductor digiti minimi, and in lower limbs by stimulating the right tibial nerve and recording from the abductor hallucis. In our laboratory the mean persistence of F-waves for ulnar and tibial nerves was 85% and 98%, respectively, in contrast to 50% in median and peroneal nerves. For that reason, only the parameters coming from the first two nerves were considered. Stimulation has been carried out with a bar stimulator with supramaximal intensity at 1 Hz frequency. Sensitivity was 5 mV/ division for M response and 0.5 μV/division for F responses. The filter setting was 2 Hz–10 KHz, and 32 sweeps were recorded for each nerve. F parameters correlated to spinal excitability were FWD, the ratio between FWD and the CMAPD (measured from the onset of the deflection to the final return to the baseline), and the amplitude of F waves (FWA) (Figure 1).
Figure 1. F waves of tibial nerve in a patient with restless legs syndrome (RLS) and in a control subject.
CMAP = compound muscle action potential, CMAPD = compound muscle action potential duration, FWD = F-wave duration.
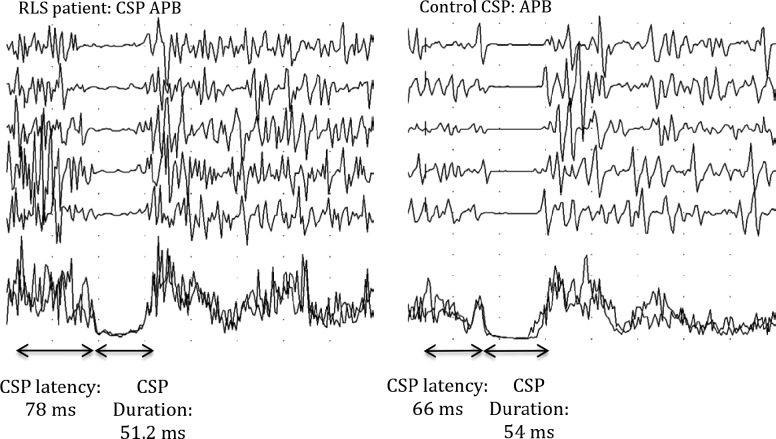
For all subjects, the CSP30 has been recorded in the lower limbs from the right anterior tibialis muscle during constant maximal contraction, by stimulating the dorsolateral branch of the homolateral sural nerve with 50 mA intensity. We assessed the following CSP parameters: initial latency, terminal latency, and duration (time interval between the beginning and end of CSP) (Figure 2). In the upper limbs, CSP has been recorded from the left abductor pollicis brevis muscle by stimulating the digital branch of the median nerve at the second finger homolaterally with 25 mA intensity. The filter setting was 20 Hz–10 KHz. We evaluated the initial and final latency of the CSP, its duration, and the ratio between duration in upper and lower limbs, named the SPR (Figure 2).
Figure 2. Cutaneous silent period (CSP) of the anterior tibialis muscle (AT) in a patient with restless legs syndrome (RLS) and in a control subject.
Statistical Analysis
Sample size has been estimated according to recommendations by Cohen, who has defined effect size (ES, difference between the means divided by standard deviation) bounds as: small (ES: 0.2), medium (ES: 0.5), and large (ES: 0.8, “grossly perceptible and therefore large”). We calculated that 17 subjects per group would be necessary to highlight an ES equal to 1 for a type I error α = 0.05 (two-tailed) and statistical power of 80%, which corresponds to a minimal difference for tibial FWD/CMAPD value equals 0.3 points (for a standard deviation at 0.3, according to Isak et al.21). In order to assess the gaussian distribution of FWD data, the FWD/CMAPD and CSP ratios of both groups, the D'Agostino and Pearson normality test was performed. We compared data between the two groups with the unpaired Student t-test, in case of gaussian distribution, and with the Mann-Whitney U test in case of nonparametric distribution, with significance level at α = 0.05. The frequency of abnormal ulnar and tibial responses was compared in RLS/WED patients and controls by means of the two-sided Fisher exact test. Finally, the relationships between both ulnar and tibial FWD as well as FWD/CMAPD responses and age and RLS/ WED duration were assessed by the Pearson product-moment correlation test or by the Spearman rank correlation test, for variables that were not normally distributed. In order to evaluate the ability of these parameters to discriminate between RLS/WED patients and control subjects, a receiver operating characteristic (ROC) analysis was performed, and thresholds were determined according to estimation of usual indexes, namely Liu, Yuden, and statistical distribution. Area under the curve (AUC), sensitivity, specificity, and positive and negative predictive values were expressed with 95% confidence interval (CI). Sensitivity analysis was performed in order to verify if the missing data would affect results: (1) analysis on the whole data (per protocol), (2) imputation of missing data for the parameters whose data were missing, namely ulnar and tibial FWD. As proposed by some statisticians, we chose to report all the individual p values without any mathematical correction.31 A particular focus was given to the magnitude of differences and to the clinical relevance.32
RESULTS
Age and sex composition of the two groups of subjects were not significantly different. All nerve conduction velocity data (latencies, amplitudes of motor and sensory potentials, and conduction velocities normalized per age and height) were within the normal limits in both patients and controls, as shown in Table S1 in the supplemental material. Ulnar F-wave parameters were available in 14 of 15 RLS patients and in 16 of 17 healthy controls, whereas tibial F-wave parameters were collected in 13 of 15 RLS patients and in 17 of 17 healthy controls. One patient did not tolerate the procedure and F-wave parameters were not assessed; tibial or ulnar F-wave both duration and amplitude (but not its latency) was not clearly identified in one patient and one control, respectively, due to noise artifact. There were no differences between patients and controls in the mean amplitude of F-wave as well as in minimum latency of F-wave in both tibial and ulnar nerve, indicating the absence of alterations in the distal and proximal conduction.
Table 2 illustrates CSP and F-wave parameters in RLS/ WED patients and controls. No significant differences were found in either CSP parameters or F-wave amplitude between patients and controls, whereas a significant increase of the F-wave duration was observed in RLS/WED patients. Indeed, mean FWD and mean FWD/CMAPD ratio in both ulnar and tibial nerves were significantly higher in patients compared to controls.
Table 2.
Cutaneous silent period and F-waves variables in patients with restless legs syndrome/Willis-Ekbom disease and in control subjects.
After ROC curve analysis, the following thresholds were determined for the FWD-related parameters: ulnar FWD = 15.5 msec, ulnar FWD/CMAPD = 1.30, tibial FWD = 23.3 msec, tibial FWD/CMAPD = 2.06. According to these cutoff values, the frequency of abnormal ulnar FWD was higher in RLS/WED than controls (n = 7 of 14 (50.0%) versus n = 1 of 16 (6.25%), Fisher exact p = 0.01), as well as that of the abnormal ulnar FWD/CMAPD ratio (n = 6 of 14 (42.86%) versus n = 1 of 16 (6.25%) between patients and controls (p = 0.031). Likewise, the frequency of abnormal both FWD and FWD/CMAPD ratio in tibial nerve were higher in patients than controls (n = 9 of 13 (69.2%) versus n = 2/17 (11.8%), and n = 9/13 (69.2%) versus n = 1/17 (5.9%), respectively) (p = 0.002 and p < 0.001).
Figure 3 illustrates the ROC curves for ulnar and tibial nerves FWD-related parameters. Tibial FWD/CMAPD ratio best discriminated RLS/WED patients from controls (AUC: 0.87 [95% CI: 0.74; 1.00]).
Figure 3. Receiver operating characteristic curves for tibial and ulnar nerve F-wave duration parameters.
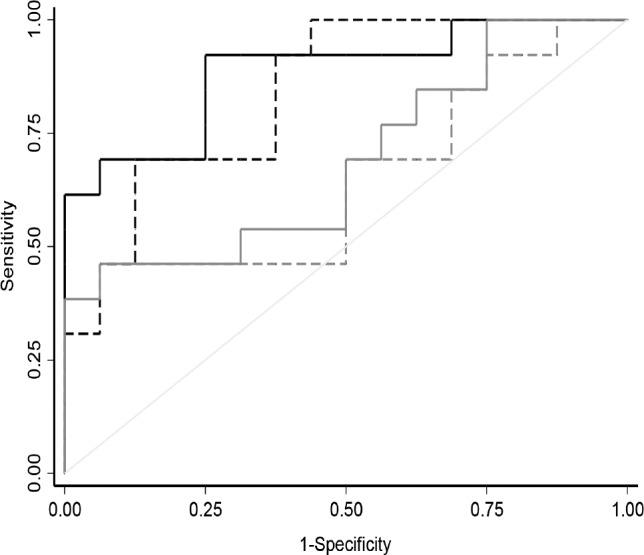
Continuous black line = tibial FWD/CMAPD, dotted black line = tibial FWD, continuous gray line = ulnar FWD/CMAPD, dotted gray line = ulnar FWD. CMAPD = compound muscle action potential duration, FWD = F-wave duration.
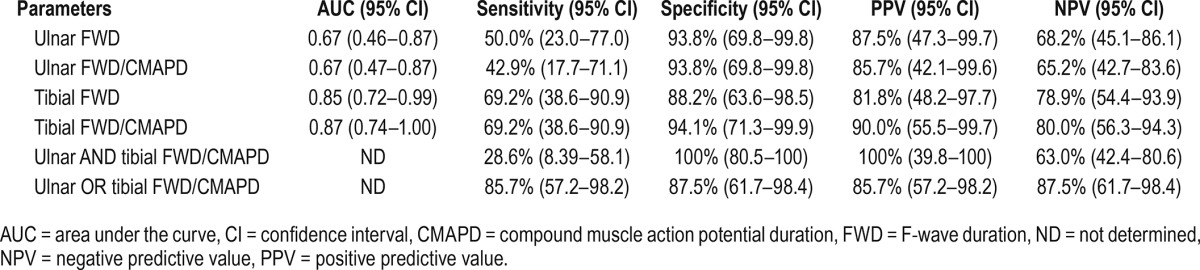
Table 3 shows AUC, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each FWD-related parameter, as well as in combination (ulnar and tibial FWD/CMAPD and ulnar or tibial FWD/CMAPD). Specificity of each FWD-related parameter was always greater than 88% in both ulnar and tibial nerves. Tibial FWD/CMAPD ratio showed a sensitivity of 69.2%, a specificity of 94.1%, a PPV of 90%, and a NPV of 80%. The combination of either ulnar or tibial FWD/CMAPD yielded to a sensitivity of 85.7%, a specificity of 87.5%, a PPV of 85.7%, and a NPV of 87.5%. After performing sensitivity analysis per protocol and imputation of missing data, we found that results were analogous and their interpretation was not modified by the weight of the missing data. Finally, in RLS/WED patients, no significant correlation was found between both ulnar and tibial FWD or the FWD/ CMAPD ratio, and age or duration of RLS.
Table 3.
Sensitivity, specificity, positive predictive value, and negative predictive value for the F-wave duration parameters.
DISCUSSION
RLS/WED is a very frequent condition, but currently, there are no instrumental investigations that can establish or support the diagnosis of primary RLS/WED. The SIT6 was developed to assess RLS/WED symptoms, but it has some limitations, leading to false-negative results in patients with RLS who happen to experience fewer or no symptoms during the test.33,34
Moreover, several conditions may mimic RLS symptoms, such as myalgia, neuropathic pain, venous stasis, leg cramps, positional discomfort, and akathisia, and some patients with RLS/WED may also have one or more of these comorbidities. According to the International RLS Study group, “this situation requires focus on the differentiating characteristics of each condition for both diagnosis and assessment of effect. Then, the clinician can use this information to reach appropriate treatment decisions.”5 Omitting the co-occurring diagnosis of RLS/WED in these patients may have a significant effect on treatment choices.
In view of this particular need, a diagnostic tool with a high specificity, together with a good sensitivity, would be required. In a 2011 study, Isak et al.21 proposed the use of some parameters to help the diagnosis of primary RLS/WED, namely FWD, FWD/CMAPD ratio, and SPR. Results of the present study confirm these important findings, showing that the first two parameters are very useful for the diagnosis of RLS/WED. Indeed, FWD and FWD/CMAPD values, which are easily inferred from the F responses carried out during the clinical routine EMG examination, were significantly increased in patients compared to controls. More specifically, in our study, tibial FWD/CMAPD ratio showed a very high specificity (94%), a good sensitivity (69%), as well as a very high PPV (90%) and NPV (80%), so that a positive result in these parameters appears to signal with a great degree of certainty that RLS/WED is present; a negative result highly predicts the absence of RLS/WED. In addition, tibial and ulnar FWD/CMAPD together (the presence of at least one altered FWD/CMAPD ratio in ulnar or tibial nerve) yielded to a very higher sensitivity, a slightly lower specificity and PPV, and a higher NPP. Based on the current results, the exploration of ulnar nerve in addition to the tibial nerve may improve the sensitivity while slightly reducing the specificity, although the main limitation of the procedure is that in some individuals, F waves cannot be evoked, especially when CMAP amplitude is small. Tibial FWD/CMAPD ratio assessment alone (compared to the extensive ulnar and tibial assessment) maintains the best specificity, and has the advantage of requiring less time and having higher patient acceptance.
Concerning tibial FWD/CMAPD parameter, despite the relative moderate sample size, the statistical power seems satisfactory with a high ES of 1.47 (95% CI [0.64; 2.27]). With 17 controls and 13 patients, the statistical power was greater than 95% to show the difference 1.68 ± 0.40 versus 2.23 ± 0.31.
Moreover, consistent with previous observations,21 this study confirms that RLS/WED patients have a prolongation of FWD and of the FWD/CMAPD ratio, suggesting an increased motoneuronal excitability. FWD is primarily a function of the number of motoneurons recruited by means of an antidromic pathway, and its prolongation suggests that, at normal persistence (i.e., the ratio between the number of recorded F-waves and the number of delivered stimuli), the recruitment of motoneurons is altered, mainly due to an alteration of the recruiting pattern.21 Effectively, the substantial increase in FWD and in the FWD/CMAPD ratio may suggest an altered modulation of spinal motoneurons. In fact, the substantial increase in FWD and in the FWD/ CMAP ratio may suggest an altered modulation of spinal motoneurons, particularly those of smaller size and lower velocity, which would be no longer inhibited by the small interneurons located in the anterior spinal cord.21
Moreover, similar to what has been observed by Isak et al.,21 an alteration of these aforementioned parameters was present in both lower and upper limbs, even though the patients under examination complained of symptoms almost exclusively in the lower limbs. Furthermore, in the current study, no correlation was observed between either the ulnar or tibial FWD or FWD/CMAPD, and age and duration of RLS, showing that in primary RLS the spinal hyperexcit-ability is unrelated to demographic variables or duration of the disorder.
In the current study, patients with RLS/WED did not show significant abnormalities in the CSP, perhaps because of the relatively narrow number of subjects or the method of stimulation. To date, there have been few studies about the CSP in RLS/WED reporting conflicting results that may be due to small sample size or small differences in methodology. These findings may concern the intensity of the painful stimuli (standard fixed versus based on individual sensory threshold) or the condition of painful stimulus administration (e.g., during 100% versus 50% of voluntary contraction).18,21,35,36
CONCLUSIONS
Our data bring new evidence that these parameters, namely tibial FWD/CMAPD ratio, can be used as instrumental support in the diagnosis because they have high specificity and sensitivity and accuracy in discriminating patients affected by RLS/WED from controls. The possibility of easily obtaining such parameters in routine NCS, together with their high spec-ificity (more than 88% for all parameters and 94% for tibial FWD/CMAPD ratio) support the utility of this procedure in the differential diagnosis of those forms of evening discomfort in the lower limbs presenting with diagnostic doubts.
Further studies including more subjects are warranted to corroborate the current findings, so that the FWD and FWD/ CMAPD ratio may become an instrumental diagnostic tool. Moreover, future studies assessing spinal excitability before and after treatment with dopamine agonists in these patients might help to shed light into the pathophysiology of RLS/WED.
DISCLOSURE STATEMENT
The work for this study was performed at Sleep Disorders Center, University of Cagliari, Italy. This was not an industry sponsored study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author roles
*,^Patrizia Congiu: research project: conception, organization, execution; manuscript preparation: writing of the first draft, review and critique.
*Maria Livia Fantini: statistical analysis: design, execution, review and critique; manuscript preparation: review and critique.
*Giulia Milioli: manuscript preparation: review and critique.
^Paolo Tacconi: research project: organization, execution; manuscript preparation: review and critique.
*,^Michela Figorilli: research project: execution.
*Gioia Gioi: research project: organization, execution.
Bruno Pereira: statistical analysis: design, execution.
°Francesco Marrosu: manuscript preparation: review and critique.
*Liborio Parrino: manuscript preparation: review and critique, revision of the language (native English speaker).
*Monica Puligheddu: research project: conception, organization; manuscript preparation: review and critique; statistical analysis: review and critique.
*certified sleep medicine expert, ^certified EMG\ENG PNS expert, °full professor.
ABBREVIATIONS
- APB
abductor pollicis brevis muscle
- AT
anterior tibilis muscle
- AUC
area under the curve
- CI
confidence interval
- CMAP
compound muscle action potential
- CMAPD
compound muscle action potential duration
- CSP
cutaneous silent period
- CSP ratio
ratio of lower extremity to upper extremity of CSP duration
- CV
conduction velocity
- EMG
electromyography
- ES
effect size
- FWA
F-waves amplitude
- FWD
F-waves duration
- FWD/CMAPD
the ratio between FWD and the duration of the corresponding CMAP
- IRLSSS
international restless legs syndrome severity scale
- NCS
nerve conduction studies
- ND
not determined
- NPV
negative predictive value
- PLMD
periodic limb movement disorder
- PPV
positive predictive value
- RLS
Restless legs syndrome
- ROC
receiver operating characteristic
- SAP
sensitive action potential
- SIT
suggested immobilization test
- WED
Willis-Ekbom disease
REFERENCES
- 1.Walters AS. Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Mov Disord. 1995;10(5):634–642. doi: 10.1002/mds.870100517. [DOI] [PubMed] [Google Scholar]
- 2.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespérance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12(1):61–65. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 3.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/ Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria - history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Borreguero D, Williams AM. An update on restless legs syndrome (Willis-Ekbom disease): clinical features, pathogenesis and treatment. Curr Opin Neurol. 2014;27(4):493–501. doi: 10.1097/WCO.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 6.Montplaisir J, Boucher S, Nicolas A, et al. Immobilization tests and periodic leg movements in sleep for the diagnosis of restless leg syndrome. Mov Disord. 1998;13(2):324–329. doi: 10.1002/mds.870130220. [DOI] [PubMed] [Google Scholar]
- 7.Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67(1):125–130. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 8.Manconi M, Ferri R, Zucconi M, et al. Preferential D2 or preferential D3 dopamine agonists in restless legs syndrome. Neurology. 2011;77(2):110–117. doi: 10.1212/WNL.0b013e3182242d91. [DOI] [PubMed] [Google Scholar]
- 9.Skagerberg G, Björklund A, Lindvall O, Schmidt RH. Origin and termination of the diencephalo-spinal dopamine system in the rat. Brain Res Bull. 1982;9(1-6):237–244. doi: 10.1016/0361-9230(82)90136-8. [DOI] [PubMed] [Google Scholar]
- 10.Qu S, Ondo WG, Zhang X, Xie WJ, Pan TH, Le WD. Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res. 2006;168(1-2):152–156. doi: 10.1007/s00221-005-0075-1. [DOI] [PubMed] [Google Scholar]
- 11.Skagerberg G, Lindvall O. Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res. 1985;342(2):340–351. doi: 10.1016/0006-8993(85)91134-5. [DOI] [PubMed] [Google Scholar]
- 12.Paci D, Lanuzza B, Cosentino FII, et al. Subclinical abnormal EMG activation of the gastrocnemii during gait analysis in restless legs syndrome: a preliminary report in 13 patients. Sleep Med. 2009;10(3):312–316. doi: 10.1016/j.sleep.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Rijsman RM, Stam CJ, de Weerd AW. Abnormal H-reflexes in periodic limb movement disorder; impact on understanding the pathophysiology of the disorder. Clin Neurophysiol. 2005;116(1):204–210. doi: 10.1016/j.clinph.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology. 2000;54(8):1609–1616. doi: 10.1212/wnl.54.8.1609. [DOI] [PubMed] [Google Scholar]
- 15.Wechsler LR, Stakes JW, Shahani BT, Busis NA. Periodic leg movements of sleep (nocturnal myoclonus): an electrophysiological study. Ann Neurol. 1986;19(2):168–173. doi: 10.1002/ana.410190210. [DOI] [PubMed] [Google Scholar]
- 16.Bucher SF, Trenkwalder C, Oertel WH. Reflex studies and MRI in the restless legs syndrome. Acta Neurol Scand. 1996;94(2):145–150. doi: 10.1111/j.1600-0404.1996.tb07045.x. [DOI] [PubMed] [Google Scholar]
- 17.Quatrale R, Manconi M, Gastaldo E, et al. Neurophysiological study of corticomotor pathways in restless legs syndrome. Clin Neurophysiol. 2003;114(9):1638–1645. doi: 10.1016/s1388-2457(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 18.Entezari-Taher M, Singleton JR, Jones CR, Meekins G, Petajan JH, Smith AG. Changes in excitability of motor cortical circuitry in primary restless legs syndrome. Neurology. 1999;53(6):1201–1205. doi: 10.1212/wnl.53.6.1201. [DOI] [PubMed] [Google Scholar]
- 19.Gorsler A, Liepert J. Influence of cabergoline on motor excitability in patients with restless legs syndrome. J Clin Neurophysiol. 2007;24(6):456–460. doi: 10.1097/WNP.0b013e31815a0038. [DOI] [PubMed] [Google Scholar]
- 20.Nardone R, Ausserer H, Bratti A, et al. Cabergoline reverses cortical hyperexcitability in patients with restless legs syndrome. Acta Neurol Scand. 2006;114(4):244–249. doi: 10.1111/j.1600-0404.2006.00669.x. [DOI] [PubMed] [Google Scholar]
- 21.Isak B, Uluc K, Salcini C, Agan K, Tanridag T, Us O. A neurophysiological approach to the complex organisation of the spine: F-wave duration and the cutaneous silent period in restless legs syndrome. Clin Neurophysiol. 2011;122(2):383–390. doi: 10.1016/j.clinph.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Milanov IG. A comparison of methods to assess the excitability of lower motoneurones. Can J Neurol Sci. 1992;19(1):64–68. [PubMed] [Google Scholar]
- 23.Fang J, Cui L-Y, Liu MS, et al. F wave study in amyotrophic lateral sclerosis: assessment of segmental motoneuronal dysfunction. Chin Med J (Engl) 2015;128(13):1738–1742. doi: 10.4103/0366-6999.159346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke D. Inability of F waves to control for changes in the excitability of the motoneurone pool in motor control studies. Clin Neurophysiol. 2014;125(2):221–222. doi: 10.1016/j.clinph.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 25.McNeil CJ, Butler JE, Taylor JL, Gandevia SC. Testing the excitability of human motoneurons. Front Hum Neurosci. 2013;7:152. doi: 10.3389/fnhum.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher MA. F-waves--physiology and clinical uses. ScientificWorldJournal. 2007;7:144–160. doi: 10.1100/tsw.2007.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raudino F. F-wave: sample size and normative values. Electromyogr Clin Neurophysiol. 1997;37(2):107–109. [PubMed] [Google Scholar]
- 28.Nobrega JAM, Pinheiro DS, Manzano GM, Kimura J. Various aspects of F-wave values in a healthy population. Clin Neurophysiol. 2004;115(10):2336–2342. doi: 10.1016/j.clinph.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Mesrati F, Vecchierini MF. F-waves: neurophysiology and clinical value. Neurophysiol Clin. 2004;34(5):217–243. doi: 10.1016/j.neucli.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Floeter MK. Cutaneous silent periods. Muscle Nerve. 2003;28(4):391–401. doi: 10.1002/mus.10447. [DOI] [PubMed] [Google Scholar]
- 31.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 33.Michaud M, Paquet J, Lavigne G, Desautels A, Montplaisir J. Sleep laboratory diagnosis of restless legs syndrome. Eur Neurol. 2002;48(2):108–113. doi: 10.1159/000062996. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Borreguero D, Kohnen R, Boothby L, Tzonova D, Larrosa O, Dunkl E. Validation of the multiple suggested immobilization test: a test for the assessment of severity of restless legs syndrome (Willis-Ekbom Disease) Sleep. 2013;36(7):1101–1109. doi: 10.5665/sleep.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han J-K, Oh K, Kim B-J, et al. Cutaneous silent period in patients with restless leg syndrome. Clin Neurophysiol. 2007;118(8):1705–1710. doi: 10.1016/j.clinph.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 36.Öz O, Erdoğan Ç, Yücel M, et al. Effect of pramipexole on cutaneous-silent-period parameters in patients with restless legs syndrome. Clin Neurophysiol. 2012;123(1):154–159. doi: 10.1016/j.clinph.2011.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.