Abstract
Study Objectives:
Describe common symptoms, comorbidities, functional limitations, and treatment responsiveness among patients with narcolepsy. Investigate the effect of pediatric onset of narcolepsy symptoms on time to diagnosis of narcolepsy and presence of comorbid depression.
Methods:
Cross-sectional survey of 1,699 people in the United States with self-reported diagnosis of narcolepsy. We utilized mixed-methods data analyses to report study findings.
Results:
Most participants reported receiving a diagnosis of narcolepsy more than 1 y after symptom onset. We found that the strongest predictor of this delayed diagnosis was pediatric onset of symptoms (odds ratio = 2.4, p < 0.0005). Depression was the most common comorbidity but we detected no association with pediatric onset of narcolepsy symptoms. Overall, participants reported that fatigue and cognitive difficulties were their most burdensome symptoms in addition to sleepiness and cataplexy. The majority of participants reported residual daytime fatigue and/or sleepiness despite treatment. Most participants reported they could not perform at work or school as well as they would like because of narcolepsy symptoms.
Conclusions:
This study provides unique insight into the narcolepsy disease experience. The study quantifies the problem of diagnostic delay for narcolepsy patients in the United States and highlights that symptoms are more likely to be missed if they develop before 18 y of age. These results suggest that narcolepsy awareness efforts should be aimed at parents, pediatric health care providers, school professionals, and children/adolescents themselves. Disease burden is high because of problems with fatigue, cognition, and persistence of residual symptoms despite treatment.
Citation:
Maski K, Steinhart E, Williams D, Scammell T, Flygare J, McCleary K, Gow M. Listening to the patient voice in narcolepsy: diagnostic delay, disease burden and treatment efficacy. J Clin Sleep Med. 2017;13(3):419–425.
Keywords: cataplexy, children, delayed diagnosis, narcolepsy, symptoms, treatment
INTRODUCTION
Narcolepsy is a chronic neurological disorder characterized by excessive daytime sleepiness and other core symptoms including hypnagogic/hypnopompic hallucinations, sleep paralysis, and disrupted nocturnal sleep. Approximately 50% to 60% of patients with narcolepsy have episodes of cataplexy,1,2 an abrupt loss of muscle tone induced by a strong emotion (e.g., laughter, anticipation, anger). The presence of cataplexy distinguishes the two subtypes of narcolepsy: narcolepsy with cataplexy (narcolepsy type 1) and narcolepsy without cataplexy (narcolepsy type 2).3 Of note, delayed diagnosis among narcolepsy patients is a well-described clinical problem as patients often receive the diagnosis years after symptom onset.4,5 More than 50% of patients report symptom onset before 18 y of age1,6, but it is unknown if pediatric onset of symptoms contributes to delayed diagnosis. Children/adolescents with narcolepsy may not be able to articulate disease symptoms as well as adults and are often reliant on external sources, such as parents and teachers, to note problematic sleepiness. Furthermore, pediatric patients with narcolepsy can have atypical forms of cataplexy, including cataplectic facies and positive motor movements similar to dyskinesias,7,8 making diagnosis more challenging.
BRIEF SUMMARY
Current Knowledge/Study Rationale: It is unclear what patient factors contribute to the problem of diagnostic delays for narcolepsy. Furthermore, health care providers and researchers tend to focus on assessments of core narcolepsy symptoms to determine treatment efficacy but it is not clear if these are the symptoms of most importance to patients for daily functioning.
Study Impact: This phenomenological study provides novel data on narcolepsy disease experience that informs these gaps in the literature. Specifically, symptom onset before 18 y of age contributes to delayed diagnosis but not to reports of comorbid depression. Study results are informative for health care providers and narcolepsy patient advocates to improve diagnostic delays and care of patients with narcolepsy. Results may also inform the development of future patient-reported outcome tools.
There are few longitudinal studies on narcolepsy that inform on symptom stability, comorbidities, functional limitations, and treatment efficacy.9 More recently, impaired cognition, inattention, and a high prevalence of mood disorders have been reported in cross-sectional studies of patients with narcolepsy, suggesting a broad range of comorbidities that affect quality of life and daytime functionality.10,11 To gain better understanding of the narcolepsy disease experience for future drug development, the United States Food and Drug Administration (FDA) held a public meeting entitled “Narcolepsy Patient-Focused Drug Development” on September 24, 2014 with narcolepsy advocacy groups and patients with narcolepsy.12 The purpose of this meeting was to systematically gather patients' perspectives on their condition and available therapies to treat their symptoms.13 A consortium of narcolepsy advocacy groups (Wake Up Narcolepsy, Inc., Project Sleep, and FasterCures, a Center of the Milken Institute, Inc.) worked together under the heading of “Unite Narcolepsy” to develop and disseminate a survey on narcolepsy disease experience to patients with narcolepsy in the United States to provide data for this meeting. Although this survey aimed to provide information for the FDA meeting, it also gives unique insight into patients' symptom burden, comorbidities, and treatment goals that can be useful for clinical care.
In this study, we provide a descriptive analysis of the Unite Narcolepsy survey data. This analysis focused on the patient perspective and highlights four main domains: (1) diagnostic delay, (2) symptom burden, (3) comorbidities associated with narcolepsy, and (4) general treatment efficacy. Given that the literature suggests duration of narcolepsy symptoms affects disease burden,4,14 we also assessed the effect of pediatric onset (symptoms before 18 years of age) on time to diagnosis and presence of self-reported depression. We hypothesized that pediatric onset of symptoms would be associated with a diagnostic delay of greater than 1 year given the unique presentation of cataplexy symptoms children can experience and challenges in obtaining information on symptoms from children. We further hypothesized that pediatric onset of symptoms and delayed diagnosis would be related to current reporting of depression because of the presence of untreated symptoms during a critical developmental time when identity and independence are being formed.15
METHODS
Survey Instrument
Wake Up Narcolepsy, Inc., a nonprofit narcolepsy patient advocacy group, sponsored the development and dissemination of the Unite Narcolepsy survey. The survey was developed for the purpose of providing patient perspectives on narcolepsy symptoms and treatment for a public meeting on narcolepsy sponsored by the FDA. The FDA held this meeting on September 24, 2014 as part of their Patient-Focused Drug Development Initiative and a transcript of the meeting12 and a summary report13 are accessible online. The survey questions were based on questions posed by the FDA in the Federal Register announcement about the meeting,16 and peer-reviewed literature and patient experience was used to develop multiple-choice answers. Five key opinion leaders reviewed the survey to establish content validity. Cognitive validity was checked with a pilot test of the questionnaire with 15 patients with narcolepsy. This pilot test also helped gauge which questions required free text responses. The survey was accessible to patients from August 26, 2013 to November 15, 2013 on a Unite Narcolepsy website18 and promoted through patient advocacy websites, social media, web postings, and web advertisements. Interim analysis of the data was presented at the FDA public meeting on September 24, 2014 and available on the Unite Narcolepsy website.17
The survey was composed of 29 questions with partially categorized responses; participants could provide open text comments for each question. Questions queried respondent concepts of disease burden (e.g., effect of symptoms on daily activities, changes made in life due to narcolepsy symptoms, most significant symptoms), diagnostic delay (time from symptom development to diagnosis), treatment response (e.g., frequency of daytime sleepiness with and without treatment), disease stability (change in condition over time), and downsides of current treatment. Categorical responses for survey questions varied from yes/no, frequency of events (e.g., frequency of cataplexy), and statements that best describe participants' feelings about symptoms. On six questions, participants were asked to check all statements that apply to them (e.g., Are there specific activities that are important to you but you cannot do at all or as fully as you would like because of your condition? Please check all that apply: Sleep through the night, perform as I'd like at work or school…). The survey included open-ended questions about other conditions participants had in addition to narcolepsy (Question 6), what treatments helped symptoms (Question 17), and “substantial downsides” of current therapies taken for narcolepsy (Question 25). We reviewed free-text responses and created a coding dictionary. ES coded all responses and KM independently coded a subset of 100 responses for each question to ensure validity; these coders achieved 100% agreement in coding terms. The full survey questionnaire is available in supplemental material.
Participants
Patients with narcolepsy, “hypersomnia conditions,” or their caretakers were invited to complete the survey, and data collection was anonymous. If respondents took the survey more than once, we counted only their most recent responses. In total, we collected data from 2,017 self-selected, unique participants and analyzed responses only from participants who reported a diagnosis of narcolepsy made by a health care provider (n = 1,699). The survey instrument had limited demographic data but included age (categorical responses).
Data/Statistical Analysis
Boston Children's Hospital provided Institutional Review Board approval to access survey data from Unite Narcolepsy and perform data analysis. Given the number of websites and other informal methods that promoted the survey, it is not possible to estimate a response rate. We performed mixed methods, qualitative and quantitative analyses on survey responses at Boston Children's Hospital using IBM SPSS Statistics software (version 23, IBM Corporation, Armonk, NY) and SAS (version 9.4, SAS Institute, Cary, NC). We used chi-square tests for bivariate analysis of categorical responses within a single question. We used logistic regression analysis with pairwise deletion to examine factors associated with delayed diagnosis [> 1 y vs. ≤ 1 y (reference)]. Factors in this model included (1) pediatric onset of symptoms (onset of symptoms < 18 y age vs. ≥ 18 y age), (2) cataplexy (no cataplexy vs. has cataplexy symptoms) and (3) current age (< 35 y vs. ≥ 35 y). We performed content analysis to extract themes from free-text responses to open-ended questions until thematic saturation was achieved. We then used these themes to tabulate and analyze the top five responses. We again used logistic regression to study factors associated with depression reporting. We extracted rates of self-reported depression from free-text responses in Question 6 (“Are there other conditions you have to manage”). For analysis of self-reported depression, we included the aforementioned three factors and presence of diagnostic delay (> 5 years vs. ≤ 5 years) in our logistic regression model. We included factors of current age at time of survey and presence of cataplexy in our logistic regression models because of prior data suggesting they may be alternative predictors or confounders of our outcomes of interest.4 Values of p < 0.05 were considered significant.
Missing data for each question ranged from 1% to 35%. We analyzed characteristics of respondents and non-respondents for key questions regarding time to diagnosis (Q9) and symptom stability (Q15). Respondents to both questions reported cataplexy more frequently than nonrespondents (p < 0.0001) but current age was similar between these groups (p > 0.5).
RESULTS
Respondents
We present data on available respondent characteristics in Table 1. Approximately 92% of respondents (1,552/1,697) were age 18 y or older at the time of survey completion. Approximately, two-thirds of the respondents (997/1,475) reported having cataplexy whereas 22.3% (329/1,475) reported no cataplexy, and 10.1% (149/1,475) were not sure if they had cataplexy. Sixty-two percent of participants (843/1,365) reported that their symptoms began before age 18 y. The majority of respondents reported taking medications prescribed by a health care provider for narcolepsy symptoms (81.6%, 1,047/1,283). Participants reported the following current medication usage: 20.9% (231/1,103) armodafinil, 32.2% (360/1,118) amphetamine, 29% (348/1,199) modafinil, 35.2% (422/1,199) sodium oxybate. It is not possible to report on combinations of medications taken based on survey design.
Table 1.
Respondent information.

Time to Diagnosis
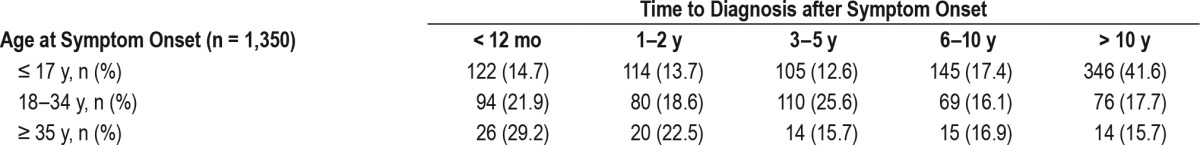
Approximately 82% of respondents (1,193/1,451) reported that they received a diagnosis of narcolepsy 1 y or more from symptom onset (Figure 1). Table 2 highlights the duration of diagnostic delays according to participants' ages at time of diagnosis. Logistic regression was used to assess the association between diagnostic delay and pediatric onset of symptoms, presence of cataplexy, and current age. This model was statistically significant (p < 0.0005), explained 7.9% of the variance in 1 y or more of diagnostic delay (Nagelkerke R squared), and correctly classified 81.9% of the cases. All factors were significant (p ≤ 0.003; Table S1 in the supplemental material). The absence of cataplexy (odds ratio [OR] = 1.79, 95% confidence interval [CI] 1.22–2.62) and pediatric onset of symptoms (OR = 2.41, 95% CI 1.75–3.33) increased odds of delayed diagnosis but current respondent age younger than 35 y was protective of delayed diagnosis (OR = 0.35, 95% CI 0.25–0.49). We did not find a significant interaction between pediatric onset of symptoms and cataplexy (p = 0.47).
Figure 1. Time from symptom onset to diagnosis of narcolepsy (n = 1,451).
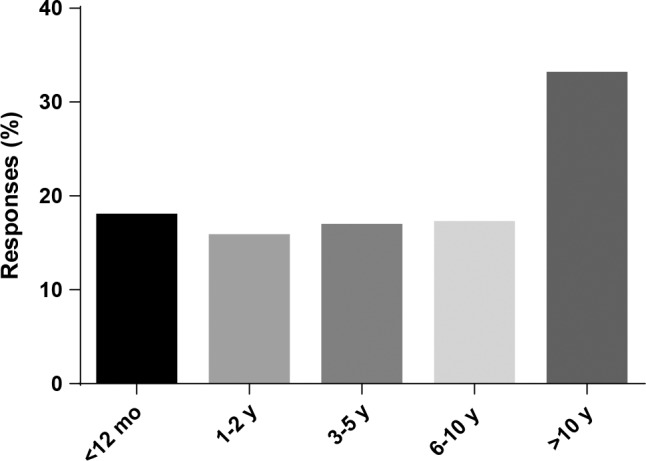
Table 2.
Time to diagnosis after symptom onset by age category.
Symptom Control/Comorbid Conditions
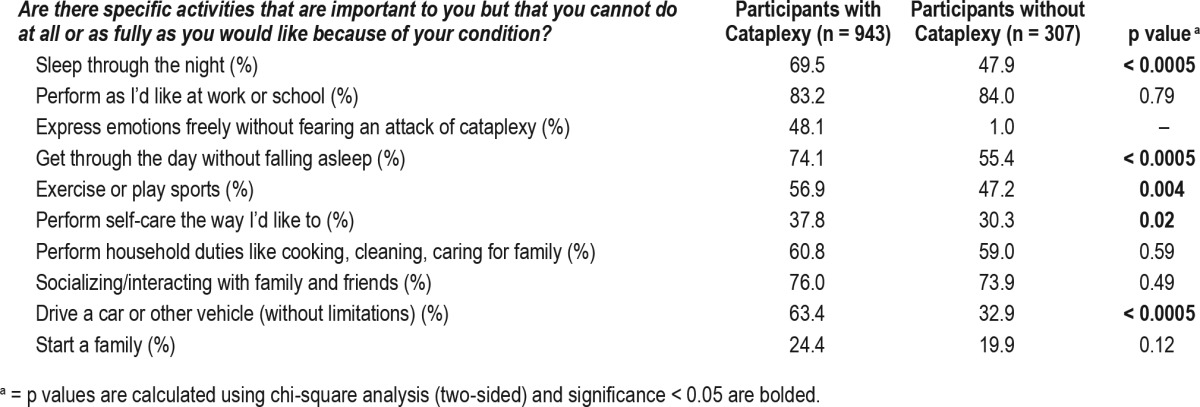
The survey asked participants to check the top three most significant symptoms that affect their lives and frequencies of these responses are presented in Table 3. Among 943 respondents with cataplexy, excessive daytime sleepiness (75.3%), cognitive difficulties (difficulty thinking, remembering, concentrating, or paying attention; 46.9%), and cataplexy (41.1%) were the most frequently reported symptoms. Similarly, among the 313 respondents without cataplexy, excessive daytime sleepiness (83.3%), general fatigue (54%), and cognitive difficulties (53%) were most commonly reported. Respondents without cataplexy reported mental fog, mood problems, general fatigue, and feeling unrefreshed in the morning more frequently than participants with cataplexy (Table 3). To further assess disease burden, participants were asked if there are any specific activities that are important to them that they cannot do at all or as fully as they would like because of their condition. Most commonly, respondents reported that they could not perform at work or school as well as they would like (84.2%, 1,171/1,391). Table 4 lists other activities respondents reported as problematic. Overall, respondents with cataplexy tended to report higher frequencies of lifestyle limitations compared to those without cataplexy in domains of expressing emotion freely, driving, sleeping through the night, exercising/playing sports, and performing self-care (p's < 0.02). Sixty-seven percent of respondents (973/1,450) reported having other diagnoses they had to manage in addition to narcolepsy. The most common diagnoses reported in the 991 free text responses were depression (24.8%), anxiety (17.7%), obstructive sleep apnea (9%), fibromyalgia (7.4%), and celiac disease (1.4%). Using logistic regression, we found no main effects for pediatric onset of narcolepsy or delayed diagnosis of more than 5 y on self-reported depression (p's > 0.18; Table S2 in the supplemental material).
Table 3.
Most significant symptoms reported by participants that impact life.
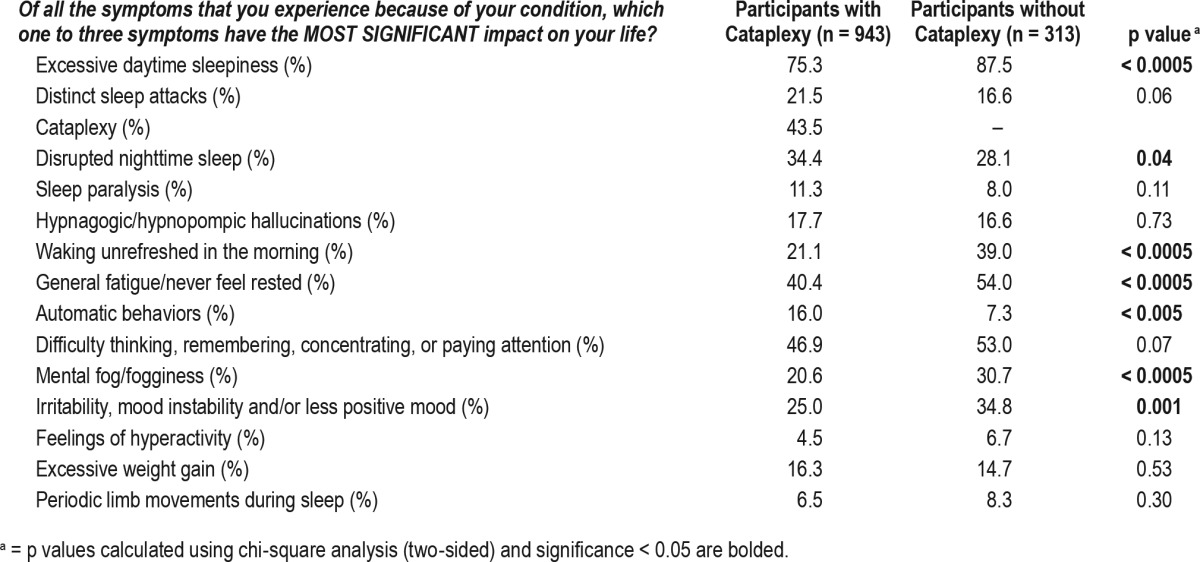
Table 4.
Specific activity limitations reported by participants with narcolepsy.
Additionally, participants were asked how their condition changed over time. Almost 68% of respondents (930/1,373) indicated that their condition was about the same since diagnosis. In contrast, 22.1% (304/1,373) responded that symptoms were worse and more unpredictable since diagnosis, 5.9% (81/1,373) felt more overwhelmed and less prepared to manage narcolepsy symptoms, and 4.3% (59/1,373) were unsure if symptoms changed.
Treatments Effects
Participants were asked what makes their symptoms better. Nonpharmacologic interventions reported were sleep hygiene (e.g., naps, regular schedule; 36%, 401/1,114), dietary changes (17.9%, 199/1,114), exercise (14.5%,161/1,114), supplements/alternative medicine (10.4%, 116/1,114), and environmental changes (sunlight, fresh air, keeping room cool; 4.3%, 48/1,114). Most respondents reported that prescribed medications provided substantial improvement (43.6%, 559/1,283) or some improvement (45.1%, 578/1,283) in their abilities to perform specific activities important in their daily lives. However, with treatment, only 3.9% (52/1,338) reported no daytime sleepiness (broadly defined as sleepiness, sleep attacks fatigue and/or not feeling rested), whereas 57.1% (764/1,338) reported experiencing daytime sleepiness and/or fatigue at least three times per day. Among respondents with cataplexy taking anti-cataplexy treatments, 53.6% (690/1,287) reported no cataplexy, 34.3% (441/1,287) reported one to two episodes of cataplexy per day, and 12.1% (156/1,287) reported three or more episodes of cataplexy per day. Respondents reported substantial problems with current therapies including side effects (52.7%, 581/1,103), lifestyle effect (41.3%, 455/1,102), inadequate efficacy (23.6%, 260/1,103), insurance problems (22.8%, 252/1,103), and cost (16.9%, 186/1,103). The survey did not ask participants to specify which treatment/ medication caused reported effects or problems for more granular analyses.
DISCUSSION
The results of this survey provide a unique body of information about disease burden from the perspective of patients with narcolepsy. Most participants (82%) reported more than 1 y delay in obtaining a diagnosis of narcolepsy after symptom onset. The odds of this diagnostic delay among patients with pediatric onset of symptoms were more than double the odds of those with adult onset of symptoms. Results of the survey also showed a broader range of symptoms associated with narcolepsy. Although clinical and research outcome measures focus on assessments of excess daytime sleepiness and cataplexy,18 participants reported their most troublesome symptoms included general fatigue and subjective cognitive difficulties (difficulty thinking, remembering, concentrating, or paying attention). Depression was the most common comorbidity with narcolepsy reported by 25% of participants. Counter to our hypothesis, we did not find an association of depression with pediatric onset of symptoms or delayed diagnosis. Most participants reported some improvement in narcolepsy symptoms with treatment, but about half still had daily cataplexy with anti-cataplexy treatment and most participants (57%) experienced daytime sleepiness and/or fatigue at least three times per day. Such findings suggest a high symptom burden even with treatment. More importantly, most participants reported functional difficulties with performing at school/work, interacting with family and friends, and performing household activities because of narcolepsy symptoms.
Diagnostic delay in narcolepsy is an important clinical problem because it can result in misdiagnosis, inappropriate medication exposure, and/or delayed treatment and can result in impaired productivity, poor academic performance, and safety concerns such as drowsy driving.5 Furthermore, early diagnosis close to symptom onset may become critical as more is discovered about possible autoimmune etiologies of narcolepsy19,20 and immunomodulating treatments that can alter disease trajectory.21,22 Morrish et al. conducted a cross-sectional survey of 215 narcolepsy patients in the United Kingdom and reported that narcolepsy was diagnosed in 66% of patients within 5 y of symptom onset.4 In multivariate regression modeling, the study authors found that absence of cataplexy and longer symptom duration were present, resulting in delayed diagnosis. In our survey with a larger cohort of patients, we found that a diagnosis was made in 50% of patients within 5 y of symptom onset and only 18% received a diagnosis within 1 y of symptom onset.
We found that the strongest predictor of delayed diagnosis was pediatric onset of symptoms (OR = 2.4, p < 0.0005). Consistent with the literature, we found that absence of cataplexy was also associated with diagnostic delays5,23 but we found no interaction between pediatric onset of symptoms and presence/ absence of cataplexy to suggest that narcolepsy type 1 or 2 is missed more frequently in children/adolescents. Our results show that early recognition of pediatric narcolepsy is problematic in the United States. However, participants who were younger than 35 y reported less delayed diagnosis than older participants in our model, perhaps suggesting overall improvement of awareness of narcolepsy among health care providers.
Diagnosis of pediatric narcolepsy may be particularly problematic because children/adolescents may not be able to clearly articulate manifestations of episodic core symptoms nor have the insight to recognize problematic excessive daytime sleepiness. Notably, symptoms of narcolepsy may present differently among children. Excessive daytime sleepiness in children can manifest as attentional problems, externalizing behaviors (such as hyperactivity), and/or emotional lability,24,25 leading to possible misdiagnosis of primary attention deficit hyperactivity disorder or other psychopathology. Children with narcolepsy type 1 can also present with atypical cataplexy with static appearance of hypotonia, ptosis, and/or jaw slackening and dyskinetic movements7 that may be mistaken for other neurological disorders. Last, given the epidemic of insufficient sleep among otherwise healthy adolescents,26,27 health care providers may incorrectly ascribe daytime sleepiness to insufficient sleep syndrome and not consider central nervous system etiologies.
This survey also highlights the daily effect of symptoms that extend beyond the core features of narcolepsy.3 Subjective cognitive impairments such as mental fog and difficulty thinking, remembering, concentrating, or paying attention were among the most significant symptoms affecting daily life. Such symptoms may contribute to high reporting of participants (83%) feeling impaired in school or at work, yet cognitive difficulties are often unaddressed in the clinical management of narcolepsy. The cause of this cognitive impairment is unclear, but sleepiness and general fatigue can impair attention and executive functioning in patients with narcolepsy,10,28,29 and co-morbid depression can reduce subjective ratings of attention.29 These studies suggest that clinical assessment and management of sleepiness is important, but additional clinical tools to assess depressive symptoms and cognition are needed to optimize patient functioning. In addition, participants commonly reported symptoms including irritability, mood changes, general fatigue, and feeling unrefreshed after nocturnal sleep. Although these symptoms are less specific, they may be useful in increasing the sensitivity of future screening questionnaires for narcolepsy types 1 and 2. Furthermore, the common occurrence of these comorbidities may suggest novel neuroinflammatory and/or neurochemical mechanisms inherent to narcolepsy that could be explored in future research.
We also examined a range of comorbid conditions and disease symptom stability in this study. Rates of self-reported depression (24.8%) and anxiety (17.7%) in our cohort were comparable to other cross-sectional studies of patients with narcolepsy.11,30–32 Youth with chronic illness have been shown to have increased rates of depressive symptoms in adulthood compared to peers without chronic illness,33 but we did not find an association of depression with pediatric onset of narcolepsy. Given data showing the economic and social burdens of delayed diagnosis of narcolepsy,34,35 we were surprised to find no association between a greater than 5-y delay in diagnosis and depression in our cohort. Possibly, the duration of narcolepsy symptoms is a more important factor to the development of depression rather than when in the lifespan symptoms developed.
Very little is known about how the symptoms of narcolepsy vary across life. Clinical experience suggests that symptoms are fairly stable, though cataplexy has been reported to develop36 and fluctuate years after the onset of sleepiness.8 Most of our participants reported stable symptoms since disease onset, but over one-fourth reported worsening. Admittedly, it is not clear from the data provided (Q15: How has your condition changed over time?) whether the condition actually changes or if ability to cope with symptoms varies across time. However, we report these findings to prompt future longitudinal research on narcolepsy disease trajectory. Fluctuations in symptom stability could be caused by a host of factors, including environmental changes, stressors, comorbidities, tolerance to medications, and changes in routine, but it is possible that narcolepsy worsens with progressive loss of hypocretin neurons or is modified by chronic alterations of compensating monoaminergic neural networks.37
Though this survey does not enable comparisons between treatment types, it provides clinically useful information regarding the efficacy of medications and behavioral interventions. Most participants in this study (81%) were on typical medications for narcolepsy. Just over one-half of the participants taking anti-cataplexy medications reported fewer than one episode of cataplexy each day, suggesting relatively good efficacy. Still, it is unknown if this residual cataplexy is mild and tolerable and thus no further adjustments in treatments were needed, or if the cataplexy is treatment resistant. In contrast, most participants reported persistent daytime sleepiness and/or fatigue despite treatment, and most reported problematic side effects with their current medications. Sample bias is possible as participants completed the survey for the FDA and thus may represent a population with more treatment difficulties. Still, the results suggest the need for comparative effectiveness studies as well as continued drug development. Of note, 36% of participants reported benefit with adhering to good sleep hygiene and 14% to 20% of participants reported benefit with dietary changes and exercise, suggesting an integrated approach of pharmacologic and lifestyle changes can optimize symptom control.
This study has some limitations. Demographic details of participants such as gender, socioeconomic factors, and education were not included in this survey and could be important confounders in our regression analyses. The diagnosis of narcolepsy was based on self-report, and the health records of participants were not available to confirm the diagnosis of narcolepsy nor listed comorbidities. However, two-thirds of the participants reporting a narcolepsy diagnosis also reported having cataplexy, a symptom unique to narcolepsy type 1. Furthermore, reported rates of cataplexy, symptom onset before age 18 y and comorbid depression are consistent with prior studies based on data collected from medical records.1,2,38 For reasons previously mentioned, self-selection bias could influence study results. Last, missing data ranged from 1% to 35% per question. Missing data analyses for two questions on time to diagnosis and symptom stability showed that respondents more frequently reported cataplexy than nonrespondents. This and higher reporting of cataplexy symptoms among respondents may suggest that patients with narcolepsy type 2 may be underrepresented in the reported survey results.
CONCLUSIONS
These survey results provide novel insight into factors contributing to delays in the diagnosis of narcolepsy and patients' disease experience. Narcolepsy typically begins in children and adolescents, yet these patients are most likely to experience delays in diagnosis. Thus, this study highlights the importance of directing advocacy and educational efforts for narcolepsy awareness to parents/caretakers, clinical providers, schools, and children/adolescents themselves. In addition, this study reports the range of symptoms that extend beyond the “core” diagnostic symptoms of narcolepsy specified in International Classification of Sleep Disorders.3 The core symptoms are essential for diagnosis, but daily life with narcolepsy is also affected by cognition, mood, and nighttime sleep. Such findings underscore the need for better narcolepsy clinical tools using patient-reported outcomes to more effectively treat patients. Next, the high rates of depression and anxiety in this patient population indicate that psychological and social support services could play a critical role in improving patients' well-being. Last, these results emphasize the need to develop more effective pharmacologic and non-pharmacologic approaches to improve narcolepsy disease control. Overall, this study suggests that much work is needed in the realms of patient advocacy and clinical care to improve diagnosis, monitor health status, and treat narcolepsy symptoms. Additionally, we hope this study will prompt future research directions in understanding the longitudinal trajectory of narcolepsy symptoms and etiology of reported comorbidities including mood problems, fatigue, and cognitive difficulties.
DISCLOSURE STATEMENT
This study was funded by a grant from Wake Up Narcolepsy. Dr. Maski received grant support from Wake Up Narcolepsy. for this research study and receives research support from Jazz Pharmaceuticals. Dr. Steinhart has recieved research support from Jazz Pharmaceuticals. Dr. Scammell receives research support from Eisai Pharmaceuticals and Jazz Pharmaceuticals, and is a consultant for Jazz Pharmaceuticals, Reset Therapeutics, Merck, Purdue Pharma, Ono Pharmaceuticals, and Janssen. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- CI
confidence interval
- FDA
United States Food and Drug Administration
- OR
odds ratio
REFERENCES
- 1.Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25(2):197–202. doi: 10.1093/sleep/25.2.197. [DOI] [PubMed] [Google Scholar]
- 2.Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20(11):1012–1020. [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 4.Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004;5(1):37–41. doi: 10.1016/j.sleep.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15(5):502–507. doi: 10.1016/j.sleep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30(1):13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 7.Serra L, Montagna P, Mignot E, Lugaresi E, Plazzi G. Cataplexy features in childhood narcolepsy. Mov Disord. 2008;23(6):858–865. doi: 10.1002/mds.21965. [DOI] [PubMed] [Google Scholar]
- 8.Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain. 2013;136(Pt 12):3787–3795. doi: 10.1093/brain/awt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruck D, Costa A. The natural history of health and symptoms in narcolepsy: a 10 year longitudinal study. Aus J Primary Health. 2003;9(1):59–67. [Google Scholar]
- 10.Lecendreux M, Lavault S, Lopez R, et al. Attention-deficit/hyperactivity disorder (ADHD) symptoms in pediatric narcolepsy: a cross-sectional study. Sleep. 2015;38(8):1285–1295. doi: 10.5665/sleep.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores NM, Villa KF, Black J, Chervin RD, Witt EA. The humanistic and economic burden of narcolepsy. J Clin Sleep Med. 2016;12(3):401–407. doi: 10.5664/jcsm.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Public Meeting on Narcolepsy Patient-Focused Drug Development. United States Food and Drug Administration Web site. [Accessed March 3, 2016]. http://www.fda.gov/ForIndustry/UserFees/PrescriptionDrugUserFee/ucm359018.htm. Updated December 10, 2014.
- 13.The Voice of the Patient. Report date June 2014. United States Food and Drug Administration Web site. [Accessed September 29, 2016]. http://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM402907.pdf.
- 14.Ingravallo F, Gnucci V, Pizza F, et al. The burden of narcolepsy with cataplexy: how disease history and clinical features influence socio-economic outcomes. Sleep Med. 2012;13(10):1293–1300. doi: 10.1016/j.sleep.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Erikson EH. Identity Youth and Crisis. New York, NY: W W Norton; 1968. [Google Scholar]
- 16.Narcolepsy Public Meeting on Patient-Focused Drug Development. United States Federal Register Web site. [Accessed September 26, 2016]. https://www.gpo.gov/fdsys/pkg/FR-2013-07-19/xml/FR-2013-07-19.xml#seqnum43209.
- 17.Unite Narcolepsy Interim Survey Results. Unite Narcolepsy Web site. [Accessed September 29, 2016]. http://www.unitenarcolepsy.org/survey-results. Published 2013.
- 18.Krahn LE, Hershner S, Loeding LD, et al. Quality measures for the care of patients with narcolepsy. J Clin Sleep Med. 2015;11(3):335–355. doi: 10.5664/jcsm.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed SS, Volkmuth W, Duca J, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med. 2015;7(294):294ra105. doi: 10.1126/scitranslmed.aab2354. [DOI] [PubMed] [Google Scholar]
- 20.Partinen M, Kornum BR, Plazzi G, Jennum P, Julkunen I, Vaarala O. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol. 2014;13(6):600–613. doi: 10.1016/S1474-4422(14)70075-4. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen S, Biering-Sorensen B, Kornum BR, et al. Early IVIg treatment has no effect on post-H1N1 narcolepsy phenotype or hypocretin deficiency. Neurology. 2012;79(1):102–103. doi: 10.1212/WNL.0b013e31825dce03. [DOI] [PubMed] [Google Scholar]
- 22.Coelho FM, Pradella-Hallinan M, Alves GR, Bittencourt LR, Tufik S. Report of two narcoleptic patients with remission of hypersomnolence following use of prednisone. Arq Neuropsiquiatr. 2007;65(2A):336–337. doi: 10.1590/s0004-282x2007000200028. [DOI] [PubMed] [Google Scholar]
- 23.Baumann CR, Mignot E, Lammers GJ, et al. Challenges in diagnosing narcolepsy without cataplexy: a consensus statement. Sleep. 2014;37(6):1035–1042. doi: 10.5665/sleep.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown TE, McMullen WJ., Jr Attention deficit disorders and sleep/arousal disturbance. Ann N Y Acad Sci. 2001;931:271–286. doi: 10.1111/j.1749-6632.2001.tb05784.x. [DOI] [PubMed] [Google Scholar]
- 25.Geissler J, Romanos M, Hegerl U, Hensch T. Hyperactivity and sensation seeking as autoregulatory attempts to stabilize brain arousal in ADHD and mania? Atten Defic Hyperact Disord. 2014;6(3):159–173. doi: 10.1007/s12402-014-0144-z. [DOI] [PubMed] [Google Scholar]
- 26.2006 Teens and Sleep. National Sleep Foundation Web site. [Accessed September 29, 2016]. http://www.sleepfoundation.org/article/sleep-america-polls/2006-teens-and-sleep.
- 27.Owens J Adolescent Sleep Working G, Committee on Adolescence. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134:e921–e932. doi: 10.1542/peds.2014-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayard S, Croisier Langenier M, Cochen De Cock V, Scholz S, Dauvilliers Y. Executive control of attention in narcolepsy. PloS One. 2012;7(4):e33525. doi: 10.1371/journal.pone.0033525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamarian L, Hogl B, Delazer M, et al. Subjective deficits of attention, cognition and depression in patients with narcolepsy. Sleep Med. 2015;16(1):45–51. doi: 10.1016/j.sleep.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Inocente CO, Gustin MP, Lavault S, et al. Depressive feelings in children with narcolepsy. Sleep Med. 2014;15(3):309–314. doi: 10.1016/j.sleep.2013.08.798. [DOI] [PubMed] [Google Scholar]
- 31.Overeem S, Mignot E, van Dijk JG, Lammers GJ. Narcolepsy: clinical features, new pathophysiologic insights, and future perspectives. J Clin Neurophysiol. 2001;18(2):78–105. doi: 10.1097/00004691-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14:488–492. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Ferro MA, Gorter JW, Boyle MH. Trajectories of depressive symptoms during the transition to young adulthood: the role of chronic illness. J Affect Dis. 2015;174:594–601. doi: 10.1016/j.jad.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Jennum P, Ibsen R, Petersen ER, Knudsen S, Kjellberg J. Health, social, and economic consequences of narcolepsy: a controlled national study evaluating the societal effect on patients and their partners. Sleep Med. 2012;13(8):1086–1093. doi: 10.1016/j.sleep.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Taddei RN, Werth E, Poryazova R, Baumann CR, Valko PO. Diagnostic delay in narcolepsy type 1: combining the patients' and the doctors' perspectives. J Sleep Res. 2016 May 6; doi: 10.1111/jsr.12420. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Andlauer O, Moore HT, Hong SC, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35(9):1247–1255. doi: 10.5665/sleep.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsujino N, Tsunematsu T, Uchigashima M, et al. Chronic alterations in monoaminergic cells in the locus coeruleus in orexin neuron-ablated narcoleptic mice. PloS One. 2013;8(7):e70012. doi: 10.1371/journal.pone.0070012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fortuyn HA, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry. 2010;32(1):49–56. doi: 10.1016/j.genhosppsych.2009.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


