Abstract
Study Objectives:
Nocturnal blood pressure (BP) dipping in patients with obstructive sleep apnea (OSA) has not yet been well investigated in Chinese patients, in whom the relationship of OSA and body mass index (BMI) is weaker than that in Caucasians. The aim of this study was to evaluate the BP profile, and the relationships between nocturnal BP and the severity of OSA, in Chinese patients.
Methods:
Consecutive Chinese adult outpatients with suspected OSA had overnight polysomnography (PSG), office BP, and 24-h ambulatory BP monitoring (ABPM). The apnea-hypopnea index (AHI) and nocturnal oxygen saturation level were recorded, and BP patterns were classified based on the ABPM.
Results:
Fifty-six subjects (40 male and 16 female, 48.59 ± 13.27 y) were evaluated. There were 14 patients with mild OSA (25.0%, AHI: 10.56 ± 3.42 events/h), 16 with moderate OSA (28.6%, AHI: 23.536 ± 3.42 events/h) and 26 with severe OSA (46.4%, AHI: 51.52 ± 3.42 events/h). There were 18 dippers (32.1%), 27 non-dippers (48.2%), and 11 reverse dippers (19.6%). As OSA severity increased, non-dipping also increased. A total of 67.9% of the OSA patients showed overall hypertension on ABPM, 57.1% had daytime hypertension only, and 73.2% had nighttime hypertension.
Conclusions:
OSA severity is associated with 24-h BP profiles in a population with only mild increases in BMI. These results can influence clinical practice, OSA management, and hypertension treatment policies.
Citation:
Ma Y, Sun S, Peng CK, Fang Y, Thomas RJ. Ambulatory blood pressure monitoring in Chinese patients with obstructive sleep apnea. J Clin Sleep Med. 2017;13(3):433–439.
Keywords: OSA, ambulatory blood pressure, dipping, non-dipping, reverse dipping, hypertension
INTRODUCTION
Obstructive sleep apnea (OSA) affects approximately 3% to 7% of the general population,1 and is estimated to affect at least 2% of women and 4% of men residing in Western communities.2 Despite the relative paucity of data, compared with large epidemiological investigations3 in Caucasian populations, the overall prevalence of OSA is not necessarily higher in Asians. Rather, sleep-disordered breathing (SDB) may be more prevalent in patients with equivalent body mass indices, because of craniofacial differences.4,5
OSA and hypertension are highly prevalent and coexisting conditions.6,7 The pathophysiologic links between OSA and hypertension are complex processes with an interplay of multifactorial mechanisms.8 Recurrent collapse of the upper airway during sleep leads to physiologic changes including intermittent hypoxemia and hypercapnia, intrathoracic pressure changes, increased sympathetic nervous system activation, and frequent cortical arousals. All these repetitive OSA-induced changes, with consequent increases in catecholamine levels, systemic inflammation, oxidative stress, and endothelial dysfunction, persist into the daytime and contributes to the development of hypertension.9–12
Studies show that 37% to 56% of hypertensive individuals have received a subsequent diagnosis of comorbid OSA,13,14 with levels as high as 71% to 83% among those resistant to hypertension treatment.15,16 In a study involving 2,677 adults, after adjusting for age, body mass index (BMI), and sex, the odds of hypertension increased by 1% for every unit (event/h) increase in the AHI, with the prevalence levels for hypertension being 22.8%, 36.5%, 46%, and 53.6% in subjects with no, mild, moderate, and severe OSA, respectively.17 The frequency of undiagnosed clinical hypertension in apneic patients was reported to be 38.6%.18
BRIEF SUMMARY
Current Knowledge/Study Rationale: Chinese populations have a higher incidence of sleep-disordered breathing (SDB) than Caucasians because of a narrow cranial base and flat midface structure. We aimed to evaluate the BP profile in Chinese patients, in whom the OSA-BMI correlation is weaker.
Study Impact: OSA and its severity are associated with pathological blood pressure patterns, and this association presents even when BMI is at worst mildly elevated. Hypertension pathology in OSA is underestimated unless 24-h BP is measured.
Blood pressure (BP) variability is an important biological phenomenon. As systolic BP (SBP) and diastolic BP (DBP) display significant diurnal variations, the BP varies in a diurnal manner throughout a 24-h period, being higher during the day and lower at night. Healthy individuals have a dipping BP pattern characterized by a nighttime BP that is 10% to 20% lower than their daytime BP (night-day BP ratio > 0.8 and ≤ 0.9). When the nocturnal decrease in BP is less than 10% (night-day BP ratio > 0.9 and ≤ 1.0), it is referred to as a non-dipping pattern. When the nocturnal BP is higher than daytime BP, with the ratio over 1.0, it is referred as a reverse dipping pattern.19
There is evidence that an abnormal BP diurnal rhythm and elevated nocturnal BP is associated with a greater risk of target organ damage and poor cardiovascular prognosis.20,21 A non-dipping pattern is found in 48% to 84% of patients with OSA, and its frequency increases with OSA severity.22,23 However, a high BMI is also associated with BP non-dipping.24,25 Appropriately accounting for the many confounding variables, particularly obesity and age in deciphering the OSA–hypertension connection remains a key challenge.8,26 The aim of this study was to evaluate the BP profile, and the relationships between nocturnal BP and the severity of OSA in Chinese patients, where the correlation of sleep apnea with BMI is weaker than in Caucasian populations.
METHODS
Subjects
This study was approved by the ethical committee of Guang'anmen Hospital. Consecutive Chinese adult outpatients with suspected OSA and undiagnosed hypertension were recruited; written informed consent was obtained. All subjects were assessed with overnight polysomnography (PSG) in the sleep laboratory, and BP assessments including office BP and ambulatory BP monitoring (ABPM). Demographic, anthropometric, and clinical data were obtained at recruitment using specific questionnaires and standard measurements.
Subjects were excluded if any of the following were noted: (1) a disease that potentially affects BP regulation (e.g., Parkinson disease, renal or cardiac transplantation, severe cardiovascular disease); (2) any other sleep disorders or irregular working schedule (e.g., shift workers); (3) substance addiction; (4) previous treatment of OSA by any modality; and (5) treatment with antihypertensive agents.
Measurements
Polysomnography
Attended overnight PSG was performed on each subject in the sleep laboratory for monitoring the electroencephalogram, electrooculogram, chin electromyogram, electrocardiogram, snoring, airflow by nasal pressure, respiratory effort, body position, and oxygen saturation. All sleep studies were manually scored by a professional technician based on the American Academy of Sleep Medicine Scoring Manual version 2.0. Apnea was defined as a cessation in airflow for at least 10 sec, and hypopnea was defined as a decrease in the amplitude of respiratory flow signal of at least 30% for a minimum of 10 sec followed by either a decrease in oxygen saturation of 3% or arousal. All subjects in whom OSA was diagnosed, OSA was classified as mild (AHI ≥ 5 events/h and < 15 events/h), moderate (AHI ≥ 15 events/h and < 30 events/h) and severe (AHI ≥ 30 events/h).
BP Assessment
The office BP was the average of three BPs measured in the sitting position during the visit closest to the ABPM: (1) daytime BP, during 09:00 to 11:00 in the clinic office; (2) night BP, approximately at 21:00 to 22:00 in the sleep laboratory before patients were connected to the recording system; and (3), around 07:00, in the sleep laboratory right after final awakening.
Twenty-four-hour ABPM was performed using validated devices (manufactured by CONTEC, CM506C, Qinhuangdao, China), which were programmed to obtain BP measurement at 30 min intervals during the daytime (06:00 –22:00) and at 60-min intervals during the night (22:00– 06:00). All the subjects were instructed to engage in normal activities but to refrain from strenuous exercise and, at the time of cuff inflation, to stop moving and talking and keep the arm still with the cuff at heart level. They were also asked to provide information including unusual activities or medication changes, in addition to the time of meals, bedtime, and rise time.
Because BP was measured every 30 min during the daytime and every 60 min during the night, all data included in this study had to meet the following inclusion criteria: (1) data collected for no less than 24 h, and (2) no fewer than 8 nighttime recordings of valid BP data, and no fewer than 35 valid recordings in total, and at least 80% of BPs during daytime and nighttime periods, were considered satisfactory. Data included SBP, DBP, mean BP, and heart rate.
Statistical Analysis
SPSS 19.0 (IBM SPSS Statistics, NY, United States) was used for statistical analysis. For power calculation, we assumed α = 0.05, β = 0.10, two-sided significance testing, and 10% study dropout. Under these assumptions, the study would need to enroll at least 48 patients. Standard descriptive statistical calculations were tabulated as mean ± standard deviation of the mean. A Kruskal-Wallis test was used to compare parameters that did not show a normal distribution. Differences in the measurement variables were assessed with analysis of variance, followed by post hoc tests (least significant difference and Dunnett). The chi-square test was used to analyze categorical data. A value of p < 0.05 was considered statistically significant. Partial correlations between variables were analyzed by Pearson test.
RESULTS
Subjects and Demographics
Sixty subjects were referred for further interviews by the sleep specialists in this study. Four subjects were excluded: two subjects did not want to do the overnight sleep study in the laboratory; one subject came to the laboratory late and had to leave early (sleep time was only 4 h); one subject was excluded due to the unqualified signal quality. Therefore, at the end of the study, 56 subjects (40 male and 16 female) were included in the study, with a mean age of 48.59 ± 13.27 y and BMI of 27.53 ± 3.23 kg/m2. All subjects had untreated sleep apnea; the polysomnogram results showed there were 14 with mild sleep apnea (25.0%), age: 49.3 ± 12.47 y, BMI: 25.51 ± 2.00 kg/m2 and AHI: 10.56 ± 3.42 events/h; 16 with moderate sleep apnea (28.6%), age 47.1 ± 14.4 y, BMI: 27.33 ± 3.70 kg/m2 and AHI: 23.53 ± 4.41 events/h and 26 with severe sleep apnea (46.4%), age 49.1 ± 13.4 y, BMI: 28.75 ± 2.96 kg/m2 and AHI = 51.52 ± 11.67 events/h. When the subjects were grouped by the severity of OSA, BMI and AHI showed no significant correlations. In mild OSA (n = 14), r = −0.272, p = 0.348. In moderate OSA (n = 16), r = 0.275, p = 0.302. In severe OSA (n = 26), r = 0.349, p = 0.081.
Ambulatory BP Patterns
Subjects were classified based on BP patterns as shown in Table 1: 18 dippers (32.1%), 27 non-dippers (48.2%), and 11 reverse dippers (19.6%). As shown in Figure 1, BP patterns were classified as dipping (nocturnal BP decrease of 10% to 20% relative to daytime BP, that is night-day BP ratio > 0.8 and ≤ 0.9), non-dipping (nocturnal BP decrease of less than 10%, night-day BP ratio > 0.9 and ≤ 1.0), and reverse dipping (nocturnal BP which is higher than daytime BP, night-day BP ratio > 1.0).
Table 1.
Overall blood pressure patterns.
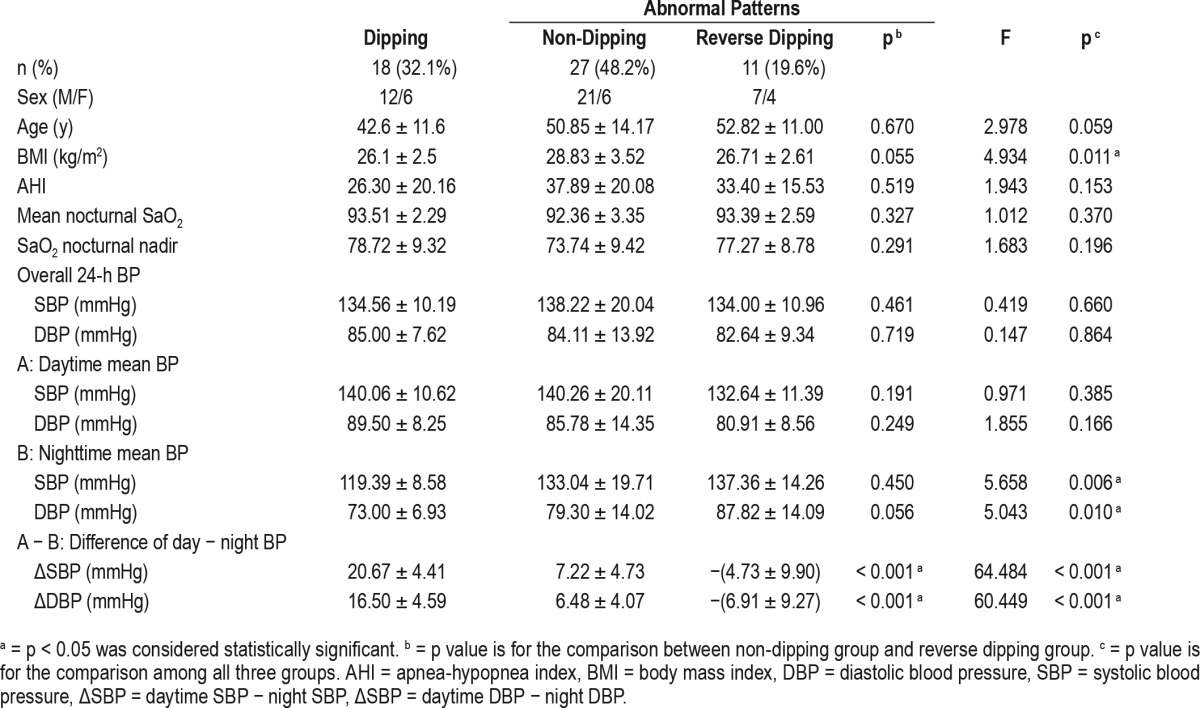
Figure 1. 24-h ambulatory blood pressure patterns.
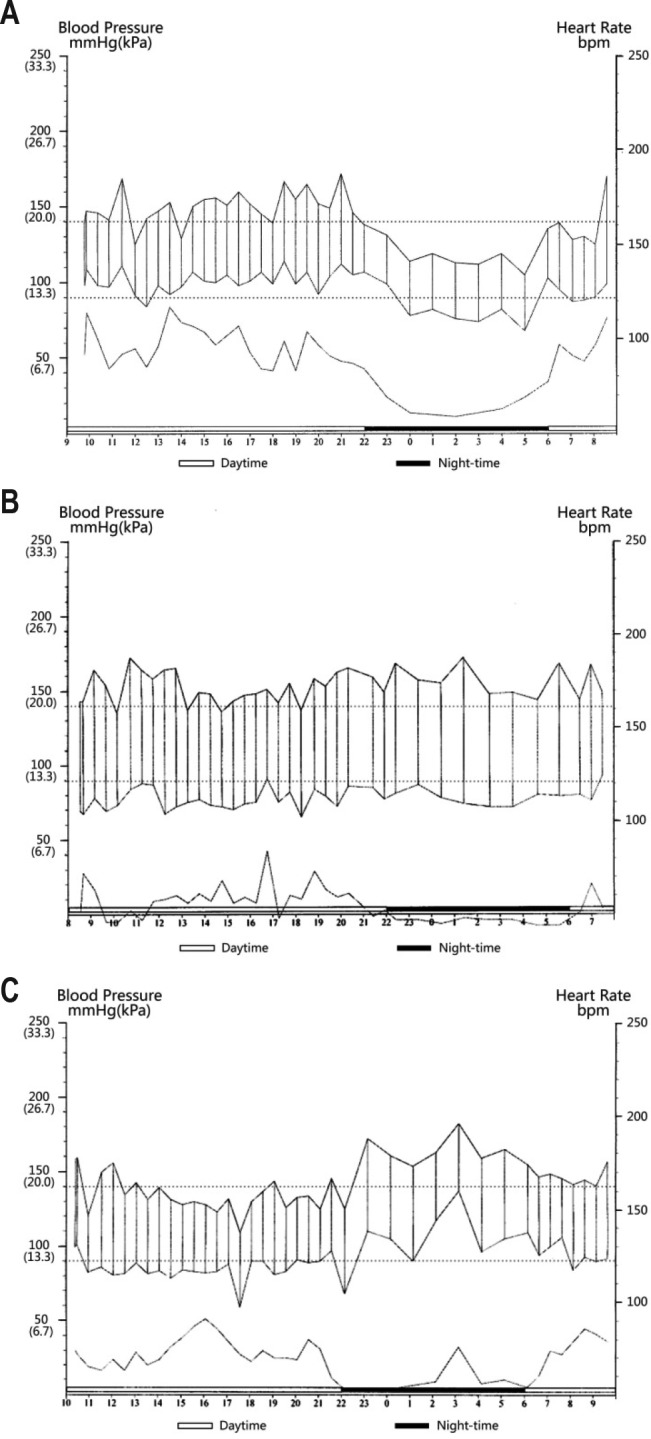
(A) Dipping: blood pressure (BP) normally falls at night. Nocturnal BP decrease is 10% to 20% lower than their daytime BP, a night-day BP ratio between > 0.8 and ≤ 0.9. (B) Non-dipping: nocturnal blood pressure decrease is less than 10%, night-day BP ratio between > 0.9 and ≤ 1.0. (C) Reverse dipping: blood pressure is elevated during sleep and is higher than daytime blood pressure, night-day blood pressure ratio > 1.0.
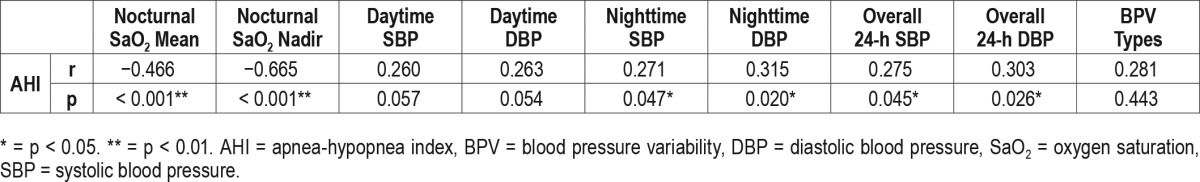
Subjects with normal dipping were relatively younger age than non-dippers and reverse dippers. Non-dippers had significantly higher BMI and higher AHI, as well as lower oxygen saturation (SaO2) nadir, than dippers. The reverse dippers showed a similar BMI and higher AHI, but no significant differences in SaO2 nadir relative to dippers. Compared with dippers, both non-dippers and reverse dippers showed no significant difference in overall BP and daytime BP, but did show significant difference on nighttime SBP and DBP. After adjustment for age and BMI, partial correlation between apnea-hypopnea index, nocturnal saturation of oxygen, and blood pressure was shown in Table 2.
Table 2.
Partial correlation between apnea-hypopnea index, nocturnal saturation of oxygen, and blood pressure (adjusted for age and body mass index).
Sleep Apnea Severity and Dipping
As shown in Figure 2, with increasing sleep apnea severity, dipping is reduced (57.14% in mild, 25.0% in moderate, and 23.08% in severe sleep apnea) and non-dipping increases (35.71% in mild, 43.75% in moderate, and 57.69% in severe sleep apnea). Reverse dipping was higher in moderate (31.25%) than severe (19.23%) sleep apnea. However, the difference among groups was not significant (p = 0.144).
Figure 2. Relative prevalence of dipping, non-dipping, and reverse dipping in relation to sleep apnea severity.
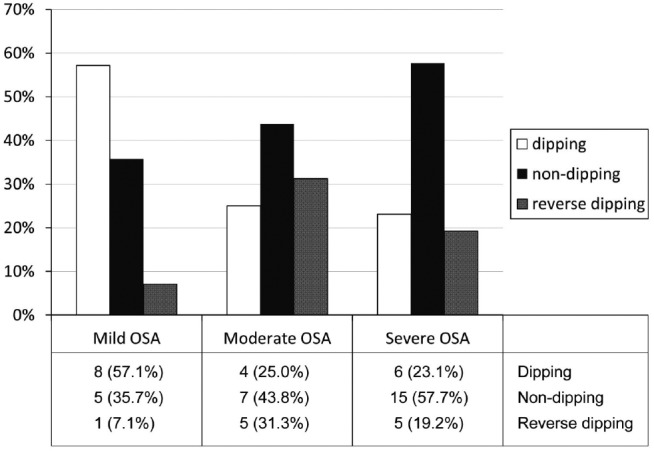
OSA = obstructive sleep apnea.
Hypertension and Ambulatory BP
Based on standard hypertension diagnosis guidelines,27 67.9% of patients with sleep apnea showed overall hypertension, 57.1% had daytime hypertension, and 73.2% had nighttime hypertension. Figure 3 shows the proportions of hypertensives and normotensives in subjects with mild, moderate, and severe sleep apnea. Significant difference can be found in the proportions when daytime (p = 0.030) and nighttime (p = 0.018) BP were analyzed specifically.
Figure 3. Relative proportions of hypertensives and normotensives in individuals with mild, moderate, and severe OSA, with specified systolic and/or diastolic hypertension, using 24-h ambulatory blood pressure measurements.
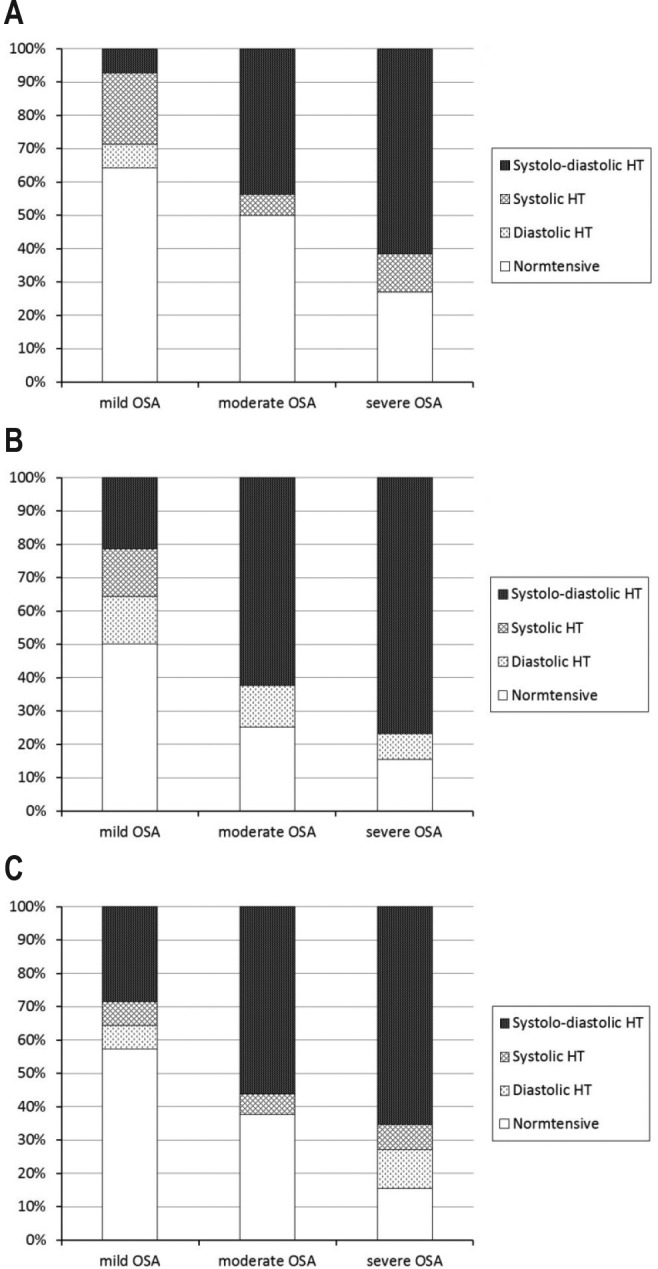
(A) Daytime blood pressure, p = 0.030. (B) Nighttime blood pressure, p = 0.018. (C) 24-h blood pressure, p = 0.155.
DISCUSSION
This study shows that sleep apnea is associated with non-dipping and reverse dipping even in a population where the BMI association of sleep apnea is less than in Western populations. The true degree of BP pathology was evident only on ABPM.
Several cohort studies have consistently shown that OSA is associated with increased cardiovascular mortality, independent of obesity, and cardiovascular risk markers, including sympathetic activation, systemic inflammation, and endothelial dysfunction, are significantly increased in patients with OSA.28 The appropriate accounting for confounding variables, such as BMI and age, in deciphering the OSA–hypertension connection remains a challenge.8,26 Recent clinical and epidemiological studies have reported an independent association between OSA and cardiovascular events.29,30 Regarding ethnic factors, lower BMI is reported as a predictor of OSA in elderly Thai hypertensive patients.31 Severely obese South Asians had significantly greater prevalence and severity of OSA than white Europeans, and OSA may contribute to increased cardiovascular risk in South Asians compared to white Europeans with severe obesity.32 Mechanisms mediating these associations among Chinese or Asian require further investigation.
Our results showed that dipping and non-dipping BP patterns correlated with the severity of OSA. Subjects with an absent nocturnal BP fall showed higher AHI than the dippers. A recent study showed that gradually and progressively deteriorating heart function starts with the extreme dippers, to the dippers and the non-dippers, and finally to the reverse dippers.33 For OSA subjects, some believe that a reverse profile is worse than non-dipping due to the higher nocturnal BP combined with hypoxia and hypercapnia causing a series of abnormal reactions and mechanisms. Others believe that reverse dipping is a transitional phase due to the OSA-elevated BP and characterized by a sympathetic activation of greater magnitude than that seen in the other abnormal nighttime BP patterns. An understanding of the potential factors associated with an altered nighttime BP profile is important because it can help identify persons at risk for abnormal BP patterns and potential target organ damage, and forms the foundation for interventions to prevent/treat alterations in nighttime BP patterns.20 A move to more aggressive targets for diagnosis and management makes it imperative34 that at least when sleep apnea is diagnosed, ABPM should be used to assess BP and determine a diagnosis of hypertension.
In China, where the healthcare system is different and the resources are insufficient, many individuals do not have primary care physicians or general practitioners. Most of the patients who snore and have sleep-related symptoms are not aware of the fact that they may need a medical consultation. Therefore, large numbers of patients with OSA and patients with pathological blood pressure profiles remain untreated. Daytime hypertension is more easily detected by office BP, while hypertension with abnormal blood pressure variability is often missed. There is a need to raise the awareness of the importance of ambulatory BP monitoring in patients with OSA in general. Also, there is a need to research and assess population differences in dipping patterns associated with OSA.
Ambulatory BP could reasonably be considered a standard component of the initial evaluation of patients with sleep apnea, once cardiopulmonary assessments or full PSG has confirmed the diagnosis. The prevalence of hypertension is underdiagnosed in patients with OSA if BP is assessed only by office readings.35 Ambulatory BP monitoring is a better predictor of cardiovascular risk35 and it might be of particular significance in assessment of hypertension in patients with OSA.36 If BP measurements are combined with actigraphy, which is also noninvasive, simple, and cost-effective, the wake-sleep period can be better defined to improve accuracy of computing dipping and non-dipping profiles.
Masked hypertension is more common in patients with sleep apnea.37 Masked hypertension is defined as normal office BP with elevated ambulatory or home BP, and there are several subtypes. Morning hypertension is the most common form, and nighttime hypertension is seen in various conditions that produce non-dipping status, with sleep apnea being one of the major etiologies.38 Regular use of ABPM will “unmask” masked hypertension.
There are several limitations in this study that could have resulted in a biased sample. The main tools used in the outpatient clinics included the STOP-BANG Sleep Apnea Questionnaire, Berlin Questionnaire, and positive pulse oximetry recordings. Only some of the patients underwent a physical examination or imaging by the ear, nose, and throat specialists. Most of them had at least three positive signs, but not everyone was screened by the same tools. Patients were not referred by their physician if a previous diagnosis of hypertension had been made, or were currently using any forms of antihypertensive treatments, or did not have signs of snoring, or were not interested in the study, or other reasons such as travel.
CONCLUSIONS
Sleep apnea and severity of the disorder is associated with pathological BP patterns. This association may present even when BMI is not significantly elevated. Unless 24-h BP is measured, the degree of hypertension pathology in sleep apnea is underestimated.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Peng and Dr. Thomas are co-patent holders for an ECG-based analytic technique for phenotyping sleep and sleep apnea; Beth Israel Deaconess Medical Center, Dr. Peng and Dr. Thomas receive royalties from a license to MyCardio, LLC. Dr. Thomas also is a patent holder for a method to treat central/mixed forms of apnea with adjunctive low concentration carbon dioxide. He has consulted for, and received research grant support from, DeVilbiss-Drive in auto-CPAP algorithm development; he consults for GLG Councils in the general area of sleep disorders. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- ABPM
ambulatory BP monitoring
- AHI
apnea-hypopnea index
- BMI
body mass index
- BP
blood pressure
- DBP
diastolic blood pressure
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SaO2
oxygen saturation
- SBP
systolic blood pressure
- SDB
sleep-disordered breathing
REFERENCES
- 1.Lurie A. Obstructive sleep apnea in adults: epidemiology, clinical presentation, and treatment options. Adv Cardiol. 2011;46:1–42. doi: 10.1159/000327660. [DOI] [PubMed] [Google Scholar]
- 2.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulm Med. 2013;13:10. doi: 10.1186/1471-2466-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang LP, Hsu SC, Lin SW, Ko WS, Chen NH, Tsai YH. Prevalence of snoring and witnessed apnea in Taiwanese adults. Chang Gung Med J. 2008;31(2):175–181. [PubMed] [Google Scholar]
- 5.Lee RW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33(8):1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 7.Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 8.Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: an update. Hypertension. 2014;63(2):203–209. doi: 10.1161/HYPERTENSIONAHA.113.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7(12):677–685. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 10.Freet CS, Stoner JF, Tang X. Baroreflex and chemoreflex controls of sympathetic activity following intermittent hypoxia. Auton Neurosci. 2013;174(1-2):8–14. doi: 10.1016/j.autneu.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Khan A, Patel NK, O'Hearn DJ, Khan S. Resistant hypertension and obstructive sleep apnea. Int J Hypertens. 2013;2013:193010. doi: 10.1155/2013/193010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Testelmans D, Tamisier R, Barone-Rochette G, et al. Profile of circulating cytokines: impact of OSA, obesity and acute cardiovascular events. Cytokine. 2013;62(2):210–216. doi: 10.1016/j.cyto.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Sjostrom C, Lindberg E, Elmasry A, Hagg A, Svardsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax. 2002;57(7):602–607. doi: 10.1136/thorax.57.7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drager LF, Genta PR, Pedrosa RP, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105(8):1135–1139. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest. 2007;132(6):1858–1862. doi: 10.1378/chest.07-1170. [DOI] [PubMed] [Google Scholar]
- 16.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grote L, Hedner J, Peter JH. Mean blood pressure, pulse pressure and grade of hypertension in untreated hypertensive patients with sleep-related breathing disorder. J Hypertens. 2001;19(4):683–690. doi: 10.1097/00004872-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Parati G, Stergiou G, O'Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32(7):1359–1366. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 20.Routledge F, McFetridge-Durdle J. Nondipping blood pressure patterns among individuals with essential hypertension: a review of the literature. Eur J Cardiovasc Nurs. 2007;6(1):9–26. doi: 10.1016/j.ejcnurse.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Izzedine H, Launay-Vacher V, Deray G. Abnormal blood pressure circadian rhythm: a target organ damage? Int J Cardiol. 2006;107(3):343–349. doi: 10.1016/j.ijcard.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, Guilleminault C, Otsuka K, Shiomi T. Blood pressure “dipping” and “non-dipping” in obstructive sleep apnea syndrome patients. Sleep. 1996;19(5):382–387. doi: 10.1093/sleep/19.5.382. [DOI] [PubMed] [Google Scholar]
- 23.Nabe B, Lies A, Pankow W, Kohl FV, Lohmann FW. Determinants of circadian blood pressure rhythm and blood pressure variability in obstructive sleep apnoea. J Sleep Res. 1995;4(S1):97–101. doi: 10.1111/j.1365-2869.1995.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 24.Sherwood A, Routledge FS, Wohlgemuth WK, Hinderliter AL, Kuhn CM, Blumenthal JA. Blood pressure dipping: ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24(9):982–988. doi: 10.1038/ajh.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens. 2002;15(2 Pt 1):111–118. doi: 10.1016/s0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- 26.Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep. 2014;16(1):411. doi: 10.1007/s11906-013-0411-y. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 28.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 31.Sawanyawisuth K, Chindaprasirt J, Senthong V, et al. Lower BMI is a predictor of obstructive sleep apnea in elderly Thai hypertensive patients. Sleep Breath. 2013;17(4):1215–1219. doi: 10.1007/s11325-013-0826-3. [DOI] [PubMed] [Google Scholar]
- 32.Leong WB, Arora T, Jenkinson D, et al. The prevalence and severity of obstructive sleep apnea in severe obesity: the impact of ethnicity. J Clin Sleep Med. 2013;9(9):853–858. doi: 10.5664/jcsm.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanovic BA, Tadic MV, Celic VP. To dip or not to dip? The unique relationship between different blood pressure patterns and cardiac function and structure. J Hum Hypertens. 2013;27(1):62–70. doi: 10.1038/jhh.2011.83. [DOI] [PubMed] [Google Scholar]
- 34.Wright JT, Jr., Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf J, Hering D, Narkiewicz K. Non-dipping pattern of hypertension and obstructive sleep apnea syndrome. Hypertens Res. 2010;33(9):867–871. doi: 10.1038/hr.2010.153. [DOI] [PubMed] [Google Scholar]
- 36.Baguet JP, Hammer L, Lévy P, et al. Night-time and diastolic hypertension are common and underestimated conditions in newly diagnosed apnoeic patients. J Hypertens. 2005;23(3):521–527. doi: 10.1097/01.hjh.0000160207.58781.4e. [DOI] [PubMed] [Google Scholar]
- 37.Kario K. Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens Res. 2009;32(6):428–432. doi: 10.1038/hr.2009.56. [DOI] [PubMed] [Google Scholar]
- 38.Kawano Y, Horio T, Matayoshi T, Kamide K. Masked hypertension: subtypes and target organ damage. Clin Exp Hypertens. 2008;30(3):289–296. doi: 10.1080/10641960802071026. [DOI] [PubMed] [Google Scholar]


