Abstract
We report a case of a 68-year-old female who was a known case of diabetes mellitus and chronic liver disease and presented with complaints of dry cough and other constitutional symptoms since one month. During initial investigations, the patient was found to have peripheral blood eosinophilia. Upon investigating further,the patient was found to have mediastinal lymphadenopathy and fine-needle aspiration of mediastinal lymph nodes showed features of tuberculosis. The patient was started on anti-tubercular treatment and her eosinophil counts returned to normal levels. Correlation between eosinophilia and tuberculosis has not been established in classical literature. This case highlights the same association and raises awareness on this crucial finding. Coexistence of eosinophilia and tuberculosis in our patient is suggested since peripheral blood eosinophilia improved with anti-tubercular treatment. The exact pathogenesis of coexistence of tuberculosis and peripheral blood eosinophilia yet remains to be deciphered, but tissue pathology is mainly associated with the discharge of toxic eosinophil products.
Keywords: Eosinophilia, Tuberculosis, Lymph nodes
What’s Known
To the best of our knowledge, an association between tuberculosis and peripheral eosinophilia has not been reported in the past many years.
Reported cases are scarce.
What’s New
We reported myelolipoma in a patient with 17 alpha-hydroxylase deficiency.
Our patient, with 46XY karyotype and female phenotype, had no gonads and we concluded that his testes had undergone severe atrophy early in embryonic life.
Introduction
Peripheral blood eosinophilia can be caused by many allergic, infectious, and neoplastic disorders, which necessitate an array of different investigations and subsequent treatment for these conditions. A key objective of the early assessment is to recognize a disorder that requires definite treatment. The peripheral blood eosinophil count does not exactly estimate the risk of organ damage in each patient, even though complications associated with eosinophilia are more predominant in patients with greater eosinophil counts(>1500 eosinophils/microL). A patient with mild peripheral blood eosinophilia might also have noteworthy organ involvement by eosinophils.1 Tuberculosis is an infectious bacterial disease caused by Mycobacterium tuberculosis, which most commonly affects the lungs. The classic symptoms of active TB infection are cough, sputum with or without haemoptysis, and fever along with constitutional symptoms. In pulmonary tuberculosis patients rise in haemoglobin levels are used as markers for reflecting a good response to management. In addition, a decrease in platelet count, white cell count, and erythrocyte sedimentation rate (ESR) were regarded as good indicators for disease control in a study done by Omar et al.2 However, an association between eosinophilia and tuberculosis has not been established in classical literature.
Case Report
A 68-year-old female, a resident of Karnal (Haryana, North India), a known case of diabetes mellitus (for 5 years on diet control), and chronic liver disease (for 3 years) presented with complaints of cough, generalized weakness, and significant weight loss since one month. The cough was insidious in onset, dry in nature, and not associated with any aggravating or relieving factor. She was diagnosed with chronic liver disease 3 years ago and an evaluation was done earlier for the same and was found to be cryptogenic in origin. There were no similar complaints among her family members. On examination, she was thin built and pale. The patient was afebrile and haemodynamically stable. There were no palpable lymph nodes. Systemic examination was unremarkable.
Initial blood investigations revealed anaemia (haemoglobin 10.2 g/dl), total leucocyte count 12x109 cells/l, platelet count 150x109 cells/l, erythrocyte sedimentation rate 59 mm/hour and differential count was neutrophil 41%, lymphoctes 27% and eosinophils 32%. Mean corpuscular volume and mean corpuscular haemoglobin concentration were 84 fL and 32 g/dl, respectively. Peripheral blood smear showed normocytic normochromic anaemia with eosinophilia (figure 1). Absolute eosinophil count was 4x109 cells/l. Hepatic functions were normal except for hypoalbuminemia (2.78 g/dl). Renal function tests were normal. ELISA for HIV-1 and HIV-2, anti-hepatitis C antibody, hepatitis B surface antigen were negative. Reaction to the tubercular skin test was less than 10 mm. Chest radiograph showed increased bronchovascular markings with right hilar prominence (figure 2). Stool samples tested two times but did not reveal any ova or parasite. Ultrasound abdomen showed coarse liver echotexture. During the course of hospital stay, the patient continued to have a dry cough and generalized weakness. The patient was given deworming measures (albendazole and diethylcarbamazine). Repeat blood counts revealed persistent eosinophilia. Test for echinococcus serology was negative. Anti-filarial antibodies were negative. Tests for anti-nuclear and anti-neutrophil cytoplasmic antibodies were negative. Echocardiography was normal with no evidence of vegetation. A contrast-enhanced CT of the thorax and abdomen was done, which revealed enlarged non-necrotic mediastinal lymph nodes with features suggestive of chronic liver disease. Microscopic examination of needle-aspirated bone marrow and biopsy examination revealed increased eosinophilic precursors with no abnormal cells. Endoscopic bronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes was consistent with tubercular inflammation with acid-fast stain positive for bacilli. Acid-fast bacilli culture grew Mycobacterium tuberculosis over a period of 3 weeks in the BACTEC medium. The patient was started on anti-tubercular treatment (isoniazid, rifampicin, ethambutol, and pyrazinamide) and her eosinophil counts gradually returned to normal levels with in 1 week.
Figure 1.
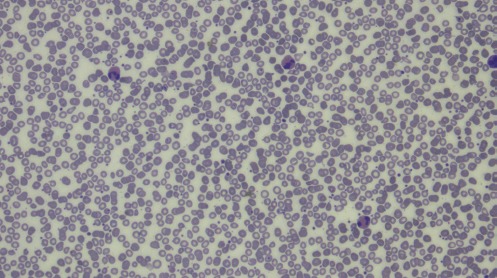
Peripheral smear showing marked eosinophilia.
Figure 2.
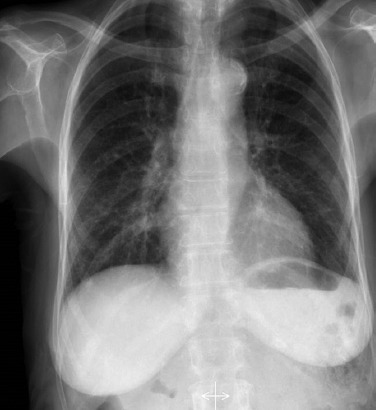
Chest radiograph showing increased bronchovascular markings with right hilar prominence.
Discussion
The common causes for raised eosinophil count are parasitic conditions, allergy/atopy, eczema, urticaria, allergic rhinitis, angioneurotic oedema, reactive eosinophilia subsequent to T-cell lymphoma, B-cell lymphoma, acute lymphoblastic leukemia, eosinophilic leukemia, idiopathic hypereosinophilic syndrome, allergic drug reactions and collagen vascular diseases such as rheumatoid arthritis, eosinophilic fasciitis or allergic angiitis.
The medical literature does not mention tuberculosis as a well-known cause of eosinophilia, but the same was recognized in our patient. To the best of our knowledge, this has not been reported forthe past many years. Ray et al. suggested the possibility of early hypersensitivity reaction to the mycobacterium antigen triggering florid tropical pulmonary eosinophilia state in susceptible patients.3 IL-5 has been found to be the most important cytokine providing the expansion of peripheral eosinophilia in patients with pulmonary tuberculosis.4 Tissue pathology is mainly associated with the discharge of toxic eosinophil products. These products include eosinophil cationic protein, major basic protein,and eosinophil-derived neurotoxin. Tissue injury might also be caused by the discharge of reactive oxygen species.
Gill et al. reported a case of peripheral eosinophilia in abdominal tuberculosis. He diagnosed the patients a case of abdominal tuberculosis on the basis of histopathological examination of peritoneal tissue.5 Similarly, Flores et al. reported a case of peripheral blood eosinophilia with tuberculosis. The patient presented with weakness, fatigue, weight loss, and lymphadenopathy. Biopsy of lymph node revealed granulomatous lesion.6
Hsu et al. reported mild peripheral blood and peritoneal fluid eosinophilia in a patient undergoing peritoneal dialysis. Eosinophilia persisted despite stopping the dialysis, but it disappeared after starting antitubercular treatment.7 Uraemic patients are immunocompromised and have a larger threat of developing tuberculosis from reactivation of silent disease. The finding of tuberculosis in uraemic patients is frequently made in the first year after the beginning of dialysis treatment as the immunity of such patients is significantly diminished with renal failure. A comparatively high rate of extrapulmonary involvement of tuberculosis, especially in lymph nodes has been reported from patients with renal failure.
Tropical pulmonary eosinophiliais widespread in the tropical and subtropical regions of the world; including India.8 It is an occult form of filariasis and results from a blown-up immune response to filarial parasites (Wuchereria bancrofti and Brugiamalayi). The pathogenesis of tropical pulmonary eosinophilia is rampant Type I, III and IV hypersensitivity reactions to filarial antigens. The treatment with steroids shows beneficial effect in treating this condition.9
There is another important cause for peripheral blood eosinophilia, which is eosinophilic pneumonia. The term chronic eosinophilic pneumonia was coined by Carrington and colleagues in 1968 to explain a situation characterized by the presence of blood eosinophilia and pulmonary eosinophilic infiltration for which no reason was found. Chronic eosinophilic pneumonia frequently occurs in middle-aged women, but can also occur at any age in both sexes. Cough with mucoid or no sputum, wheezing weight loss, malaise, dyspnoea, and night sweats are the main symptoms. Asthma is present in nearly half of such cases and tends to be of new onset. It has been described with radiotherapy treatment given for breast cancer.10
All well-known causes of eosinophilia (drug allergy, leukaemia, lymphoma, parasitic infection, autoimmune disorder, vasculitis, HIV, tropical pulmonary eosinophilia) were excluded by detailed history, clinical examination, and laboratory investigations. There have been very few case reports of tubercular association with eosinophilia in literature. Besides, the clinical response shown by the patient and normalization of eosinophil count after starting anti-tubercular treatment (isoniazid 5 mg/kg/day, rifampicin 10 mg/kg/day, ethambutol 15 mg/kg/day, and pyrazinamide 25 mg/kg/day) proves the association of tuberculosis with peripheral eosinophilia.
History taking and physical examination are the most important steps for diagnosis. History of travel, associated collagen vascular disease, altered immune status, drug intake, and airway obstruction are essential components to consider in history taking. The final diagnosis until the end of time rests with the response to treatment, even with infectious syndromes.
We therefore conclude that further research is needed to understand the pathophysiology of marked eosinophilia in tuberculosis and to establish it as a cause.
Conflict of Interest: None declared.
References
- 1.Tefferi A. Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment. Mayo Clin Proc. 2005;80:75–83. doi: 10.1016/S0025-6196(11)62962-5. [DOI] [PubMed] [Google Scholar]
- 2.Al-Omar I, Al-Ashban R, Shah A. Hematological abnormalities in Saudis suffering from pulmonary tuberculosis and their response to the treatment. Research Journal of pharmacology. 2009;3:78–85. [Google Scholar]
- 3.Ray D, Abel R. Hypereosinophilia in association with pulmonary tuberculosis in a rural population in south India. Indian J Med Res. 1994;100:219–22. [PubMed] [Google Scholar]
- 4.Kolobovnikova Iu V, Urazova OI, Novitskii VV, Mikheeva KO, Goncharov MD. [Molecular mechanisms of formation of blood eosinophilia under pulmonary tuberculosis] Vestn Ross Akad Med Nauk. 2012:58–62. [PubMed] [Google Scholar]
- 5.Gill AM. Eosinophilia in Tuberculosis. Br Med J. 1940;2:220–1. doi: 10.1136/bmj.2.4154.220. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flores M, Merino-Angulo J, Tanago JG, Aquirre C. Late generalized tuberculosis and eosinophilia. Arch Intern Med. 1983;143:182. [PubMed] [Google Scholar]
- 7.Hsu SC, Lan RR, Tseng CC, Lai CT, Huang JJ. Extrapulmonary tuberculous infection manifested as peritoneal fluid eosinophilia in a continuous ambulatory peritoneal dialysis patient. Nephrol Dial Transplant. 2000;15:284–5. doi: 10.1093/ndt/15.2.284. [DOI] [PubMed] [Google Scholar]
- 8.Mullerpattan JB, Udwadia ZF, Udwadia FE. Tropical pulmonary eosinophilia--a review. Indian J Med Res. 2013;138:295–302. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray S, Kundu S, Goswami M, Maitra S. Tropical pulmonary eosinophilia misdiagnosed as miliary tuberculosis: a case report and literature review. Parasitol Int. 2012;61:381–4. doi: 10.1016/j.parint.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Cottin V, Frognier R, Monnot H, Levy A, DeVuyst P, Cordier JF, et al. Chronic eosinophilic pneumonia after radiation therapy for breast cancer. Eur Respir J. 2004;23:9–13. doi: 10.1183/09031936.03.00071303. [DOI] [PubMed] [Google Scholar]


