Abstract
Background:
Given the potential role of the 5-hydroxytryptamine-3 receptor in the pathogenesis of schizophrenia, this study was performed to determine whether ondansetron plus risperidone could reduce the negative and depressive symptoms in patients with treatment-resistant schizophrenia.
Methods:
In a double-blinded, placebo-controlled, randomized trial (IRCT registration # 201112125280N7), in 2012–2013 in Mashhad, Iran, 38 patients with treatment-resistant schizophrenia received risperidone either combined with a fixed dose (4–8 mg/d) of ondansetron (n=18) or with a placebo (n=20) for 12 weeks. The patients were evaluated using the Positive and Negative Syndrome Scale (PANSS), Wechsler’s Adult Intelligence Scale-Revised (WAIS-R), and Hamilton’s Rating Scale for Depression (HRSD) at baseline and 12 weeks later. Changes in the inventories were used to evaluate the efficacy of the treatment. The t test, Chi-square test, and SPSS (version 16) were used to analyze the data. The statistical significance was set atP<0.05.
Results:
Ondansetron plus risperidone was associated with a significantly larger improvement in the PANSS overall scale and subscales for negative symptoms and cognition than was risperidone plus placebo (P<0.001). The WAIS-R scale results indicated significant differences between the 2 groups before and after administrating the medicine and the placebo. The administration of ondansetron significantly improved visual memory based on the subtests of the WAIS (P<0.05). Ondansetron had no positive effects on depressive symptoms (effect size=0.13).
Conclusion:
This study confirmed that ondansetron, as an adjunct treatment, reduces negative symptoms in patients with schizophrenia and can be used as a potential adjunctive strategy particularly for negative symptoms and cognitive impairments. Trial Registration Number: IRCT201112125280N7
Keywords: Schizophrenia, Risperidone, Ondansetron, Depression, Negative symptoms
What’s Known
5-hydroxytryptamine3 receptor has a potential role in the pathogenesis of schizophrenia.
What’s New
As an adjunct treatment, ondansetron significantly improved negative symptoms in schizophrenia. •Ondansetron increased intelligence scores in some of subscales of the WAIS test. •Ondansetron had no positive effect on depression symptoms.
Introduction
Schizophrenia is a mental disorder characterized by the separation of thought processes and poor emotional awareness. Schizophrenia comprises negative and positive symptoms. It is arguably one of the most debilitating disorders.1 Schizophrenia is not identical in clinical forms, prognosis, and response to treatment. There are hypotheses suggesting that the dysfunction of neurotransmitters has a unique role in the onset and progress of the disease.2 Although positive symptoms (hallucinations, delusions, disorganized speech, and thinking) are common, the negative symptoms of schizophrenia such as blunted affect, emotional withdrawal, poor rapport, difficulty in abstract thinking, lack of spontaneity, and flow of conversation are often more prominent.3
Cognitive impairment and negative symptoms are very important in impairing occupation and social function.4 Negative syndromes are diagnosed using interview-based measures, the Positive and Negative Syndrome Scale (PANSS), and a scale for the assessment of negative symptoms (SANS). The negative symptoms are classified in 5 subscales: anhedonia, avolition, affective blunting, social isolation, and alogia.5 Primary negative symptoms are often referred to as the deficit syndrome. Individuals with the deficit syndrome have been found to have more cognitive deficits and poorer outcomes than patients without it.6 Despite all the improvements in the treatment of schizophrenia, in the past decade, increasingly, more researchers have focused on studying the negative symptoms of schizophrenia.7 It should be borne in mind that aside from negative symptoms, depressive symptoms are prevalent in patients with schizophrenia.8
The neurotransmitters found to be involved in schizophrenia symptoms are dopamine, gamma-amino butyric acid, glutamate, acetylcholine, serotonin, and histamine. Serotonin or 5-hydroxytryptamine was the first neurotransmitter studied in schizophrenia. Antipsychotic medicines act on serotonin usually by inhibiting its re-uptake (mode of action). Two central 5-HT receptors (5-HT2A and 2C) are candidates for the pharmacogenetic analysis of the effects of medicines in the treatment of schizophrenia. It is known that serotonin 5-hydroxytryptamine-3 (5-HT3) receptors play a role in the pathogenesis of cognitive dysfunction in patients with schizophrenia.9
Ondansetron, an antagonist of the serotonin 5-HT3 receptor, has been used for the treatment of schizophrenia.10 Ondansetron is often used in conjunction with other medications to prevent nausea and vomiting due to cancer chemo-radiotherapy, surgery, anxiety, depression, and migraine headaches.11 Ondansetron can be an option for the treatment of negative symptoms considering the etiological hypotheses related to neurotransmitters.10-13 As ondansetron affects the serotonergic system via the 5-HT3 receptors and in animal models and alcoholic people, independent of its effect on drinking behavior, has reduced depressive symptoms,14,15 it may potentially have antidepressant activities in schizophrenic patients who have depressive symptoms, too. Findings from experimental studies on the effects of ondansetron on the negative symptoms in schizophrenia are scarce. We could not find any study evaluating the antidepressant effects of ondansetron in schizophrenia.11 Hence, obsessive compulsive disorder (OCD) and schizophrenia are clinically similar to some extent and are frequently comorbid.16,17 On the other hand, there are some evidence on the effect of ondansetron on OCD.18 Accordingly, the purpose of the present study was to evaluate the effects of ondansetron on the negative and depressive symptoms of patients with schizophrenia in a control-experimental design.
Patients and Methods
Study Design and Procedures
The present study was a double-blinded, randomized, controlled trial of parallel groups of patients with treatment-resistant schizophrenia (IRCT registration #IRCT201112125280N7), which was performed in 2012–2013 in Mashhad, Iran.
The sample size was determined using the analysis of covariance power formula, n=2[zα + zβ]2s2 (1 − R2)/d2, with zα=2.24, zβ=0.842 (corresponding to power=0.80), R=the correlation between baseline and the end-of-study measures of the primary outcome, d=the difference between the groups, and s=the standard deviation of the primary outcome. We planned to enroll 30 participants per group, which was expected to enable us to detect an effect size of 0.73 with power of 0.80. The actual recruitment was only about 20 participants per group, given the observed R, we had 0.9 power to detect an effect size of 0.49. Fifty patients were recruited from the inpatient and outpatient services of Hejazi Hospital and all the inpatients enrolled in the study were in the hospital because of the lack of social and familial support. The sampling method of this study was purposeful and nonprobable. The inclusion criteria consisted of a DSM-IV-TR diagnosis of schizophrenia regardless of the type of schizophrenia and being in a stable phase of the illness, which means no psychotic episode during a 2-month period preceding study participation. The diagnosis was confirmed by at least 2 senior psychiatrists. All the subjects had been receiving a similar dosage of an atypical antipsychotic medicine (risperidone 4–6 mg/d) for at least 2 months prior to enrollment. The cutoff point for the negative PANSS was >15.13 Clinical examination, medical history, and medicine dosage adjustment were used to diagnose negative symptoms secondary to antipsychotic medicines. General and neurological physical examinations were performed, and electroencephalogram and electrocardiogram were obtained before starting the medication. Fasting blood sugar, complete blood count, serum creatinine, total bilirubin, aspartate aminotransferase, and alanine aminotransferase were evaluated for all the patients. We excluded cardiac disease and comorbid neurological disorders. (The minimal cognitive ability was evaluated using the Mini-Mental State Examination [MMSE]).19 Patients with scores under 20 were excluded from the study. The other exclusion criteria were drug or alcohol abuse or dependency (except smoking); use of medicines affecting cognitive abilities such as antidepressants, mood stabilizers, or a second antipsychotic (plus risperidone); pregnancy; breast feeding; or use of uncertain contraceptive methods.
Process
Since we aimed to evaluate the effects of ondansetron on the negative symptoms of schizophrenia, the first step of the study was to differentiate the secondary negative symptoms from the primary symptoms. The patients were clinically evaluated using the PANSS before the commencement of the treatment. This scale was used to assess clinical psychopathology. The PANSS test was repeated after 1month. Patients whose PANSS test did not change >20% within 4 weeks were selected for this study. The patients were evaluated regarding the secondary negative symptoms, including depression, mental retardation, and complications of antipsychotic medicines. Hamilton’s Rating Scale for Depression (HRSD)20 was used to exclude patients with major depression and to evaluate the efficiency of ondansetron on the depressive syndrome during the study period. Wechsler’s Adult Intelligence Scale-Revised (WAIS-R) was drawn upon to assess the patients’ cognitive ability and the effect of ondansetron on the patients’ cognition.21
Following the initial assessment, the subjects were randomly (simple random sampling using a random number table) assigned to 1 of the 2 groups: placebo and intervention. All the subjects received risperidone tablets (Sobhan Pharmaceutical Group, Iran) and ondansetron (Tehran Shimi Co., Iran). The placebo pills were made in the same shape as ondansetron by the Faculty of Pharmacy, Mashhad University of Medical Sciences, from inert and inactive ingredients (e.g., saline, forms of sugar, etc.).
Thirty-eight inpatients and outpatients with chronic schizophrenia received ondansetron (4–8 mg/d/PO) or a placebo combined with risperidone (8 mg/d/PO) for 12 weeks. The daily dose of ondansetron was 4 mg in the first week, and an extra 4 mg was added in the second week. Neither the study population nor the staff administrating the medication knew whether they were using the drug or the placebo (double blinding). The PANSS, WAIS-R, and HRSD tests were administered at the beginning and at the end of the study, and their results were considered final results. Consequently, the PANSS scores, cognitive performance, and HRSD scores were the outcome measures. The study design is depicted in figure 1.
Figure 1.
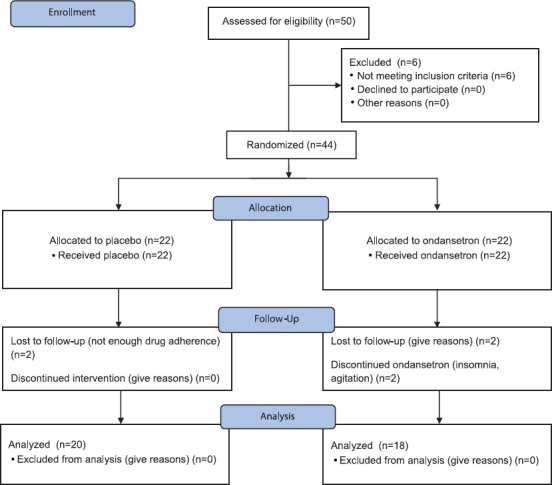
CONSORT flow diagram shows the sampling procedure.
Instruments
Positive and Negative Syndrome Scale for Schizophrenia (PANSS): The PANSS is a psychological scale used to evaluate the severity of symptoms in patients with schizophrenia. It is widely utilized for the assessment of the efficiency of antipsychotic therapy.13 The PANSS scale consists of 30 items, scoring from 1 (not present) to 7 (extremely severe). The PANSS refers to 2 types of symptoms in schizophrenia: positive symptoms, which are an excess or distortion of normal functions, and negative symptoms, which are a reduction or loss of normal functions.22 The validity and reliability of the Farsi version of the PANSS have been confirmed previously (r=0.99).23,24
Hamilton Rating Scale for Depression (HRSD): The HRSD is a multiple-item questionnaire used to provide an indication of depression and is considered a guide to evaluate recovery.20 A score of 0–7 is considered normal. Scores ≥20 indicate moderate, severe, or very severe depression. The test has a good reliability coefficient (0.89)25 and acceptable validity in Farsi (0.65).26
Wechsler’s Adult Intelligence Scale-Revised (WAIS-R): The WAIS-R is a test designed to measure intelligence in adults and older adolescents.20 A study in Iran indicated reliability of 0.69–0.87 on test-retest stability, with internal consistency of 0.77–0.88.27
Statistical Analysis
Both parametric and nonparametric methods were used to evaluate the data. The t test and the Chi-square test were used to analyze the data. Repeated measure design was utilized to assess the effect of the variables. The effect of ondansetron was evaluated with the t test between the placebo and medicine groups. The independent t test was employed to examine the relation between ondansetron and reduction in the negative syndrome. The Chi-square test was drawn upon to assess the effect of admission type on the results of this study. All the statistical analyses were performed with SPSS, version 16, and statistical significance was set atP <0.05.
Ethical Considerations
In the present study, ethical principles were observed in accordance with the Declaration of Helsinki. The research protocol was approved by the Ethics Committee of Mashhad University of Medical Sciences. The patients or their legal custodians provided informed consent in accordance with the procedures outlined by the institutional review board, and they were informed that they could withdraw from the experiment at any time.
Results
From the 44 participants, 38 patients completed the study (n=20 placebo group and n=18 experimental group). Nearly 76% (38/50) completed the 12-week treatment course. The mean age of the subjects in the control and experimental groups was 40.35 years (CI: 37.43 to 43.26) and 36.61 years (CI: 34.02 to 39.20), respectively. The demographic variables are summarized in table 1.
Table 1.
Demographic variables in the experimental and placebo groups
| Variables | Experimental group | Placebo group | P value | ||
|---|---|---|---|---|---|
| Frequency | Percentage | Frequency | Percentage | ||
| Age | 12 | 66.7 | 10 | 50 | 0.071 |
| 20–40 | 6 | 33.3 | 10 | 50 | |
| 40–60 | |||||
| Sex | 16 | 88.9 | 19 | 95 | 0.595 |
| Male | 2 | 11.1 | 1 | 5 | |
| Female | |||||
| Education | 18 | 100 | 20 | 100 | - |
| Under diploma | |||||
| Marital Status | 9 | 50 | 6 | 30 | 0.438 |
| Single | 5 | 27.8 | 7 | 35 | |
| Married | 4 | 22.2 | 7 | 35 | |
| Divorce | |||||
PANSS Score
To determine whether the patients using ondansetron had significant improvement in their negative symptoms compared with the placebo group, we compared the scores of the PANSS at 2 consecutive points and found significant differences between them in the experimental group. Also, the differences in the first and the second points of the negative PANSS were significant between the 2 groups (P<0.001). The efficacy of ondansetron on the negative and positive PANSS was not similar in the experimental group in that it was more effective on the negative PANSS. The result of the paired t test confirmed the significant difference between the positive and negative PANSS improvement in the experimental group (P<0.001) (table 2). We also assessed the effect of ondansetron on different kinds of negative symptoms before and after the treatment. The result of the Wilcoxon test suggested significant differences in the negative symptoms except for “spontaneity and flow of conversation” (N6). Our results showed that the greatest changes in the subscore of the PANSS belonged to passive/apathetic social withdrawal. The data are summarized in table 3. The repeated measures analysis showed significant differences between the 2 groups in terms of the changes in the negative PANSS scores (P<0.01), while the differences between the study groups did not constitute statistical significance concerning the changes in the positive PANSS scores (P=0.614).
Table 2.
Comparison of the negative and positive PANSS scores between the medicine and placebo groups
| Groups | P value | Effect size | ||
|---|---|---|---|---|
| Mean±SD (95%CI) | ||||
| Placebo | Medicine | |||
| Negative PANSS1 | ||||
| NP12 | 30.2±3.07 (28.8-31.5) | 28±4.11 (26.1-30.0) | 0.068 | 1.79 |
| NP23 | 30.2±3.38 (28.3-31.7) | 23.33±3.77 (21.6-25.1) | 0.001 | |
| DNP4 | 0±0.85 (-0.37-0.37) | 4.66±1.32 (4.04-5.27) | 0.001 | |
| Positive PANSS5 | ||||
| PP16 | 18.15±1.92 (17.30-18.99) | 19.22±2.26 (18.1-80.26) | 0.239 | 0.7 |
| PP27 | 18.45±2.32 (17.43-19.46) | 18.11±2.9 (16.77-9.45) | 0.124 | |
| dPP8 | -0.3±1.08 (-0.77-0.17) | 1.11±2.16 (0.11-2.11) | 0.25 | |
1: Positive and negative syndrome scale for schizophrenia; 2: Negative PANSS1- baseline; 3: Negative PANSS2- week 12; 4: Difference negative PANSS=NP1-NP2; 5: Positive and negative syndrome scale for schizophrenia; 6: Positive PANSS1; 7: Positive PANSS2; 8:Difference positive PANSS=PP1-PP2
Table 3.
Comparison of the effects of ondansetron on different kinds of negative syndromes before and after the treatment in the experimental group by the Wilcoxon tes
| Negative syndromes | Mean±SD Confidence Interval | Mean and difference | P value | |
|---|---|---|---|---|
| Before | After | |||
| N1: Blunted affect | 4.11±0.75 (3.76-4.46) | 3±7.66 (-0.54-6.54) | 1.11 | <0.001 |
| N2: Emotional withdrawal | 4.22±0.54 (3.97-4.47) | 2.83±0.51 (2.59-3.07) | 1.39 | <0.001 |
| N3: Poor rapport | 4.11±0.58 (3.84-4.38) | 2.83±0.61 (2.55-3.11) | 1.28 | <0.001 |
| N4: Passive/apathetic social withdrawal | 3.94±0.72 (3.61-4.27) | 2.5±0.61 (2.22-2.78) | 1.44 | <0.001 |
| N5: Difficulty in abstract thinking | 4.11±0.9 (3.69-4.53) | 3.61±0.84 (3.22-3.99) | 0.5 | 0.003 |
| N6: Lack of spontaneity and flow of conversation | 3.61±0.91 (3.19-4.03) | 3.38±0.97 (2.93-3.83) | 0.23 | 0.279* |
| N7: Stereotyped thinking | 3.83±0.85 (3.44-4.22) | 3.44±0.78 (3.08-3.80) | 0.39 | <0.001 |
There were significant differences between the 2 groups in all the fields of negative syndrome, except in N6
Depressive Symptoms
The HRSD scores were evaluated to assess depression between the medicine and placebo groups. Ondansetron had no positive effects on depression syndromes (table 4). The repeated measures analysis showed no significant differences between the 2 groups apropos the changes in the HRSD scores (P=0.693).
Table 4.
Comparison of the effects of ondansetron and placebo on the HRSD before and after the treatment between the 2 groups
| Groups | P value | Effect size | |
|---|---|---|---|
| MeanSD | |||
| Confidence Interval | |||
| Experimental group | Placebo group | ||
| 17.27±3.61 (15.60-18.94) | 17.65±3.71 (16.02-19.28) | 0.740 | 0.13 |
| 16.77±2.92 (15.42-18.11) | 17.33±0.67 (17.04-17.62) | 0.515 | |
| 1.04±0.5 (0.81-1.27) | 1.13±0.35 (0.98-1.28) | 0.593 | |
HRSD: Hamilton’s rating scale for depression; Ham1: Hamilton’s rating scale for depression at the beginning of the study; Hame2: Hamilton’s rating scale for depression at the end of the study; dHam: Ham1–Ham2=difference Hamilton
Cognition
The results obtained from the WAIS-R, in addition to the score of the independent t test for a complete picture and information subscales, showed no significant differences between the 2 groups before and after administrating the medicine and the placebo. However, the results of object assembly and comprehension test subscales showed significant differences between the 2 groups, before and after the treatment. The results of the subscales of the WAIS-R are summarized in table 5.
Table 5.
Comparison of the WAIS-R subscales between the medicine and placebo groups
| Subscales | Baseline | Week12 | Effect size | ||||
|---|---|---|---|---|---|---|---|
| Mean±SD | P value | Mean±SD | P value | ||||
| Confidence Interval | Confidence Interval | ||||||
| Placebo | Medicine | Placebo | Medicine | ||||
| Complete picture | 7.55±2.16 (7.46-7.64) | 8.61±1.57 (7.88-9.34) | 0.096 | -0.15±0.74 (-0.47-0.17) | -0.22±0.64 (-0.52-0.08) | 0.75 | 0.1 |
| Object assembly | 25.65±2.9 (24.38-26.92) | 28.66±1.32 (28.25-29.47) | <0.001 | 0.45±0.6 (0.19-0.71) | -3.05±1.1 (-3.56-2.54) | <0.001 | 1.77 |
| Information | 7.05±3.10 (6.14-8.86) | 8.94±2.66 (7.71-10.17) | 0.052 | 0.05±0.825 (-0.31-0.41) | -0.11±0.75 (-0.46-0.24) | 0.873 | 0.2 |
| Comprehension | 6.55±1.99 (5.68-7.23) | 9.00±1.78 (8.18-9.82) | <0.001 | 0.05±0.82 (-0.31-0.41) | -0.11±0.75 (-0.46-0.24) | <0.001 | 1.48 |
Complications of Ondansetron
Ondansetron was administrated for 18 patients. Eleven (61.11%) patients had some kind of complications. Among the 7 (38.89%) patients who had complications, 2 (11.11%) patients suffered from insomnia and restlessness and 2 (11.11%) patients complained from constipation; the rest of the patients reported confusion, nausea, and exhaustion.
Discussion
In our study, after 12 weeks’ treatment, ondansetron (4–8 mg/d) augmented with risperidone (4–6 mg/d) resulted in greater improvement in the PANSS overall scale and subscales for negative symptoms and cognition, compared to the placebo plus risperidone. Also, we assessed the effects of ondansetron on verbal and performance intelligence using the WAIS-R test. In the comprehension and object assembly subscales, we found significant differences between the 2 groups. In this study, ondansetron had no positive effects on depression symptoms (effect size=0.13).
Previous studies have shown that serotonin (5-HT)-receptor antagonists possess therapeutic potential for schizophrenia.12,28 An article review about the effect of ondansetron on the treatment of schizophrenia in 2010 suggested that ondansetron might be effective in the treatment of schizophrenia, especially negative symptoms. This review proposed that further randomized, double-blinded, active-controlled studies would be helpful in determining the role of ondansetron in the treatment of schizophrenia.10 Just one clinical trial about the augmentation of risperidone with ondansetron was in this review and similar to our findings showed positive effects of ondansetron on the negative symptoms as assessed with the PANSS.12
Brishink and Cutis29 used ondansetron to augment the effects of clozapine in the treatment of schizophrenia, and their findings suggested a significant difference between the 2 study groups. Zhang et al.30 evaluated the effects of the augmentation of haloperidol with ondansetron in patients with treatment -resistant schizophrenia and reported that the ondansetron-treated group had a significantly higher proportion of subjects with a 30% improvement rate from baseline in the PANSS total and negative scores. The authors’ results also showed positive effects on cognition too, which chimes in with our results. One meta-analysis based on limited data concluded that ondansetron added to therapy had significant effects on the PANSS total and negative scores. However, in that review, ondansetron was not superior to the placebo in the PANSS positive scores, as we found out in our study.31 In another study conducted in 2014, the augmentation of the usual treatment of schizophrenia with ondansetron had positive, albeit not significant, effects on the PANSS total score.32
Levkoritzet al.33 showed that a short treatment period with ondansetron led to improvement in visual memory in schizophrenia. In their crossover study, the authors aimed to assess the effects of ondansetron on a variety of memory tasks in patients with schizophrenia. The beneficial effects of ondansetron on memory and intelligence could be due to the exclusion of serotonin from presynaptic areas.
Also, Akhundzadeet al.12 found that combining ondansetron with antipsychotic (risperidone) significantly improved visual memory, negative symptoms, and cognitive disorders in chronic, treatment-resistant schizophrenia. Deakinaet al.34 indicated the improving effects of ondansetron alone or in combination with simvastatin on verbal and visual learning related to the new learning. Mohammadiet al.35 demonstrated positive significant effects of ondansetron on visual memory regarding the WAIS-R data analysis. Another study mentioned that the 5-HT3 receptor regulation was able to improve cognitive deficits and extrapyramidal side effects.36 Likewise, Adleret al.37 performed a study to evaluate the efficacy of ondansetron in improving p50 auditory gatingin patients with schizophrenia under treatment.
Ondansetron had no positive effects on depression symptoms in our study. In contrast, one study in 2014 demonstrated the therapeutic effect of ondansetron on depression in mice.14 Another research claimed that ondansetron in alcoholic individuals was able to improve depression, probably because of its serotonergic effects.15 Also, Picheet al.38 reported significant therapeutic effects of ondansetron on depression. Another study also emphasized the antidepressant effect of ondansetron on schizophrenia and other psychiatric disorders.39 The different methods of the evaluation of depression may be the reason for the inconsistency in the results.
Most studies have shown that ondansetron is well tolerated without any acute complications.11,31 In a study by Bennett,10 only 2 patients abandoned the treatment because of aggression and insomnia. Some patients had minor complications such as insomnia, constipation, confusion, nausea, and fatigue. In our study, 2 subjects left the study because of ondansetron complications (insomnia and agitation).
In schizophrenia, some factors may be correlated with a worse prognosis. Empirical findings have confirmed that negative symptoms are linked with treatment prognosis.7 Nevertheless, findings from experimental studies regarding the effects of ondansetron on the negative symptoms in schizophrenia are not clearly explained. Furthermore, the degree of the influence of negative symptoms on treatment prognosis has yet to be fully elucidated. It is also likely that factors that have effects on negative symptoms are not well understood. The serotonergic system is involved in schizophrenia and OCD. A review of literature demonstrated some therapeutic effects of ondansetron augmentation on the usual drug regimen of treatment-resistant OCD,18 as is the case in treatment-resistant schizophrenia.10 Future studies may be able to find a common pathway in the pathogenesis and treatment of schizophrenia and OCD insofar as sometimes schizophrenia prodrome is similar to OCD-like symptoms and there are similarities between the clinical manifestations of OCD and schizophrenia.16,17 Some cognitive and negative components in schizophrenia may be the result of comorbid OCD.17 Indeed, OCD may lead to or aggravate stereotyped thinking and social and emotional withdrawal in terminal stages. Comprehension and object assembly and other neurocognitive tasks may be impaired by OCD.16,17
Although a standard treatment for schizophrenia has emerged, many unanswered questions still remain. One important question is whether ondansetron is sufficient to decrease negative symptoms in schizophrenia.
The present study also underscores the importance of experimental studies in identifying brain mechanisms involved in the cognition and neuropathology of schizophrenia. Knowledge of negative symptoms in schizophrenia is growing, and the number of studies in this field has increased with a view to understanding the nature of schizophrenia as well as the possibilities of its treatment.
There are a number of limitations in the current study, first and foremost among which is its relatively small sample size, precluding the generalization of its results to other populations without further studies. Another limitation was incomplete adherence to the CONSORT standard of reporting clinical trials.40
Conclusion
The results of the current study showed that the administration of ondansetron significantly improved negative symptoms and cognitive disorders. Moreover, it increased the intelligence scores in some of the subscales of the WAIS-R test. It means that ondansetron can improve cognitive function. These preliminary results suggest that ondansetron may have a role in improving the symptoms of individuals with schizophrenia. The study results affirmed that ondansetron, as an adjunct treatment to conventional therapy, is useful particularly in reducing negative symptoms.
Acknowledgment
We appreciate the Vice Chancellorship of Mashhad University of Medical Sciences and Dr. Masoud Ahmadzad Asl. This research was performed as a dissertation after approval by the Ethics Committee of Mashhad University of Medical Sciences.
Conflict of Interest: None declared.
References
- 1.Martin E. Concise Medical Dictionary. 8th ed. New York: Oxford University Press; 2010. [Google Scholar]
- 2.Wong DF. Molecular Brain Imaging in Schizophrenia. In: Sadock BJ, Sadock VA, Ruiz P, Kaplan HI, editors. Kaplan & Sadock’s comprehensive textbook of psychiatry. 9th ed. Phiadilphia: Lippocott Williams and willkins; 2009. p. 1527. [Google Scholar]
- 3.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 4.Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr Clin North Am. 2005;28:613–33. doi: 10.1016/j.psc.2005.05.004. 26. [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick B, Fischer B. Subdomains within the negative symptoms of schizophrenia: commentary. Schizophr Bull. 2006;32:246–9. doi: 10.1093/schbul/sbj054. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephen NL. Phenomenology of schizophrenia. In: Sadock BJ, Sadock VA, Ruiz P, Kaplan HI, editors. Kaplan & Sadock’s comprehensive textbook of psychiatry. Vol. 2. Phiadilphia: Lippocott Williams and willkins; 2009. pp. 1433–46. [Google Scholar]
- 7.Makinen J, Miettunen J, Isohanni M, Koponen H. Negative symptoms in schizophrenia: a review. Nord J Psychiatry. 2008;62:334–41. doi: 10.1080/08039480801959307. [DOI] [PubMed] [Google Scholar]
- 8.Castle DJ, Slott Jensen JK. Management of depressive symptoms in schizophrenia. Clin Schizophr Relat Psychoses. 2015;9:13–20. doi: 10.3371/CSRP.CAJE.103114. [DOI] [PubMed] [Google Scholar]
- 9.Dean B. Understanding the pathology of schizophrenia: recent advances from the study of the molecular architecture of postmortem CNS tissue. Postgrad Med J. 2002;78:142–8. doi: 10.1136/pmj.78.917.142. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett AC, Vila TM. The role of ondansetron in the treatment of schizophrenia. Ann Pharmacother. 2010;44:1301–6. doi: 10.1345/aph.1P008. [DOI] [PubMed] [Google Scholar]
- 11.Miyata K, Honda K. [Serotonin (5-HT) 3 receptors: antagonists and their pharmacological profiles] Nihon Yakurigaku Zasshi. 1994;104:143–52. doi: 10.1254/fpj.104.143. [DOI] [PubMed] [Google Scholar]
- 12.Akhondzadeh S, Mohammadi N, Noroozian M, Karamghadiri N, Ghoreishi A, Jamshidi AH, et al. Added ondansetron for stable schizophrenia: a double blind, placebo controlled trial. Schizophr Res. 2009;107:206–12. doi: 10.1016/j.schres.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Lindenmayer JP, Bossie CA, Kujawa M, Zhu Y, Canuso CM. Dimensions of psychosis in patients with bipolar mania as measured by the positive and negative syndrome scale. Psychopathology. 2008;41:264–70. doi: 10.1159/000128325. [DOI] [PubMed] [Google Scholar]
- 14.Gupta D, Radhakrishnan M, Kurhe Y. Ondansetron, a 5HT3 receptor antagonist reverses depression and anxiety-like behavior in streptozotocin-induced diabetic mice: possible implication of serotonergic system. Eur J Pharmacol. 2014;744:59–66. doi: 10.1016/j.ejphar.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BA, Ait-Daoud N, Ma JZ, Wang Y. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27:1773–9. doi: 10.1097/01.ALC.0000095635.46911.5D. [DOI] [PubMed] [Google Scholar]
- 16.Bottas A, Cooke RG, Richter MA. Comorbidity and pathophysiology of obsessive-compulsive disorder in schizophrenia: is there evidence for a schizo-obsessive subtype of schizophrenia? J Psychiatry Neurosci. 2005;30:187–93. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 17.Zink M. Comorbid Obsessive-Compulsive Symptoms in Schizophrenia: Insight into Pathomechanisms Facilitates Treatment. Adv Med. 2014;2014:317980. doi: 10.1155/2014/317980. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375–91. doi: 10.1016/j.psc.2014.05.006. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s synopsis of psychiatry: behavioral science/clinical psychiatry. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 20.Hedlund JL, Vieweg BW. The Hamilton rating scale for depression: a comprehensive review. Journal of Operational Psychiatry. 1979;10:149–65. [Google Scholar]
- 21.Alan S Kaufman, Elizabeth O Lichtenberger. Assessing adolescent and adult intelligence. 3rd ed. Hoboken: Wiley; 2006. [Google Scholar]
- 22.Baker RW, Kinon BJ, Maguire GA, Liu H, Hill AL. Effectiveness of rapid initial dose escalation of up to forty milligrams per day of oral olanzapine in acute agitation. J Clin Psychopharmacol. 2003;23:342–8. doi: 10.1097/01.jcp.0000085406.08426.a8. [DOI] [PubMed] [Google Scholar]
- 23.Omranifard V, Karahmadi M, Jannesary Z, Maracy M. Efficacy of modified compliance therapy for schizophrenia patients. J Res Med Sci. 2012;17:S258–63. [Google Scholar]
- 24.Bigdeli I, Farzin A, Talepasand S. Prospective memory impairments in schizophrenic patients. Iran J Psychiatry Behav Sci. 2014;8:57–63. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 25.Gharaei B. Review some of the cognitive pattern in patients with comorbid anxiety and depression [dissertation] [Tehran]: Iran University of Medical Sciences; 1993. p. 102. [Google Scholar]
- 26.Tozandeh Jani H. Comparison of Efficacy of anxiety control, drug and combiation of them in treatment of patients with anxiety disorders [dissertation] [Tehran]: Iran University of Medical Sciences; 1993. p. 114. [Google Scholar]
- 27.Abedi MR. Standardization of Wechsler Adult Intelligence Scale-R (WAIS-R) [dissertation] [Tehran]: Iran University of Medical Sciences; 1994. p. 83. [Google Scholar]
- 28.Costall B, Naylor RJ. 5-HT3 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:27–37. doi: 10.2174/1568007043482624. [DOI] [PubMed] [Google Scholar]
- 29.Briskin JK, Curtis JL. Augmentation of clozapine therapy with ondansetron. Am J Psychiatry. 1997;154:1171. doi: 10.1176/ajp.154.8.1171a. [DOI] [PubMed] [Google Scholar]
- 30.Zhang ZJ, Kang WH, Li Q, Wang XY, Yao SM, Ma AQ. Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomized, placebo-controlled study. Schizophr Res. 2006;88:102–10. doi: 10.1016/j.schres2006.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Kishi T, Mukai T, Matsuda Y, Iwata N. Selective serotonin 3 receptor antagonist treatment for schizophrenia: meta-analysis and systematic review. Neuromolecular Med. 2014;16:61–9. doi: 10.1007/s12017-013-8251-0. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhry IB, Husain N, Drake R, Dunn G, Husain MO, Kazmi A, et al. Add-on clinical effects of simvastatin and ondansetron in patients with schizophrenia stabilized on antipsychotic treatment: pilot study. Ther Adv Psychopharmacol. 2014;4:110–6. doi: 10.1177/2045125313511487. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levkovitz Y, Arnest G, Mendlovic S, Treves I, Fennig S. The effect of Ondansetron on memory in schizophrenic patients. Brain Res Bull. 2005;65:291–5. doi: 10.1016/j.brainresbull2003.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Deakin J, Chaudhry I, Parker A, Dunn G, Kazmi A, Drake R, et al. Therapeutic Trials of Minocycline, Ondansetron and Simvastatin in Schizophrenia. European Psychiatry. 2015;30:71. [Google Scholar]
- 35.Mohammadi N, Noroozian M, Karamghadiri N, Akhondzadeh S. 5-HT3 antagonist for cognition improvement in schizophrenia: a double blind, placebo-controlled trial. Basic Clin Neurosci. 2010;1:10–4. [Google Scholar]
- 36.Shimizu S, Mizuguchi Y, Ohno Y. Improving the treatment of schizophrenia: role of 5-HT receptors in modulating cognitive and extrapyramidal motor functions. CNS Neurol Disord Drug Targets. 2013;12:861–9. doi: 10.2174/18715273113129990088. [DOI] [PubMed] [Google Scholar]
- 37.Adler LE, Cawthra EM, Donovan KA, Harris JG, Nagamoto HT, Olincy A, et al. Improved p50 auditory gating with ondansetron in medicated schizophrenia patients. Am J Psychiatry. 2005;162:386–8. doi: 10.1176/appi.ajp.162.2.386. [DOI] [PubMed] [Google Scholar]
- 38.Piche T, Vanbiervliet G, Cherikh F, Antoun Z, Huet PM, Gelsi E, et al. Effect of ondansetron, a 5-HT3 receptor antagonist, on fatigue in chronic hepatitis C: a randomised, double blind, placebo controlled study. Gut. 2005;54:1169–73. doi: 10.1136/gut.2004.055251. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bétry C, Etiévant A, Oosterhof C, Ebert B, Sanchez C, Haddjeri N. Role of 5-HT (3) Receptors in the Antidepressant Response. Pharmaceuticals. 2011;4:603–29. doi: 10.3390/ph4040603. [ PMC Free Article] [DOI] [Google Scholar]
- 40.Suvarna V. ‘Consort 2010: a standard for reporting clinical trials revised anew? Perspect Clin Res. 2010;1:87–9. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]


