Abstract
Background:
Despite the large number of papers published on the efficiency of different exogenous gonadotropins, no confirmed protocol exists. Therefore, the aim of the present study was to compare the efficacy of 4 exogenous gonadotropins in IVF/ICSI cycles.
Methods:
This study, performed from January 2014 to May 2014, recruited 160 women referred to Ghadir Mother and Child Hospital and Dena Hospital, Shiraz, Iran. The patients underwent standard downregulation and were randomly divided into 4 groups of A, B, C, and D and were administered hMG, hFSH, rFSH, and combined sequential hFSH/rFSH, respectively. Then, the duration of stimulation, number of oocytes and embryos as well as their quality, implantation rate, biochemical and clinical pregnancy rate, and live birth rate in each group were evaluated.
Results:
Group D patients required significantly fewer ampoules of FSH than did the women in groups A, B, and C (P=0.004). The duration of stimulation was significantly longer in group C than in groups A and D (P=0.030). The serum estradiol level was significantly higher in group D than in groups B and C (P=0.005). A significantly higher number of large-sized follicles was observed in group D than in group B (P=0.036).
Conclusion:
Our data revealed no statistically significant differences in the mean oocyte number, embryo quality, clinical pregnancy rate, or live birth rate between the hMG, hFSH, rFSH, and sequential hFSH/rFSH protocols. However, several differences in the duration of stimulation, serum estradiol levels, and number of large-sized follicles were detected between the groups. Trial Registration Number: IRCT201408116541N7
Keywords: Gonadotropins, Oocytes, Pregnancy
What’s Known
Many studies have compared different exogenous gonadotropins for controlled ovarian stimulation to show which kind is more suitable and leads to greater IVF success.
Which gonadotropin has more efficacy for controlled ovarian stimulation is not confirmed and it remains a controversial issue.
What’s New
There are no significant differences in the mean oocyte number, embryo quality, clinical pregnancy rate, or live birth rate between hMG, hFSH, rFSH and sequential hFSH/rFSH.
Several differences in the duration of stimulation, serum estradiol levels, and the number of large size were detected among the groups.
Introduction
Today, assisted reproductive technology (ART) has become a well-established and highly efficient therapy for infertility. In ART, it is well understood that the most important factors for maximizing the success rate of in vitro fertilization (IVF) are retrieving greater numbers of high-quality oocytes using controlled ovarian hyperstimulation (COH) and establishing a receptive endometrium.1-4 Therefore, COH plays a principal role in achieving a high ART success rate.
Nowadays, the use of long protocols using the gonadotropin-releasing hormone (GnRH) analog plus gonadotropins for COH has gained widespread popularity.5-7 Various gonadotropin preparations are commercially available and used for COH such as human menopausal gonadotropin (hMG), human-derived follicle-stimulating hormone (hFSH), and recombinant FSH (rFSH). hMG contains FSH and luteinizing hormone activity, while rFSH comprises only FSH, and in comparison to hFSH, rFSH includes a high proportion of fewer acidic isoforms with high purity and high in vitro bioactivity.8 There are many controversies surrounding which kind of exogenous gonadotropin is more suitable and leads to greater IVF success.
Some studies have demonstrated that a better outcome in terms of oocyte and embryo quality, subsequent pregnancy rates, and live birth rate is obtained when hMG is used for ovarian stimulation, as compared with rFSH.9-11 However, other studies have shown that rFSH is as effective as urinary FSH or hMG in terms of the number of oocytes and embryos obtained and the total gonadotropin dose needed.7,9,12
Studies that compared hFSH with rFSH noted increased ovarian recruitment of follicles in the rFSH group.13,14 Daya12 showed that rFSH was better than hFSH in terms of the pregnancy rate, while van Wely et al. illustrated a borderline significant difference of 5% higher clinical pregnancy rate in women stimulated with hFSH compared with rFSH.7 Selman et al.8 demonstrated that the combination of hFSH/rFSH for ovarian stimulation had a positive effect on follicular development, oocyte quality, embryo development, and clinical outcome in patients with repeated IVF failures.
Therefore, despite the large number of papers published on COH protocols comparing the efficiency of different exogenous gonadotropins, no confirmed protocol exists, and it is not quite clear which is superior to the others. Thus, the objective of the current study was to compare the efficacy of 4 different ovarian stimulation protocols, comprising hFSH, rFSH, hMG, and sequential use of hFSH and rFSH, on oocyte and embryo quality and IVF treatment outcome in patients undergoing IVF or intracytoplasmic sperm injection (ICSI).
Patients and Methods
Patients
This double-blinded, randomized, clinical trial study was registered in the Iranian Registry of Clinical Trials (code: IRCT201408116541N7) and approved by the Institutional Review Board and the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (code: CT-P-92-7249). The study was performed from January 2014 to May 2014. Written informed consent was obtained from each participant. A flow chart of the study design is depicted in figure 1. The CONSORT flow diagram is depicted in figure 2. The sample size used in this study was determined based on the criteria established by Kutner et al.15 using the following formula: type I error (α) =0.05, power of analysis (1-β=0.95), Effect Size 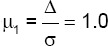 and number of groups=4.
and number of groups=4.
Figure 1.
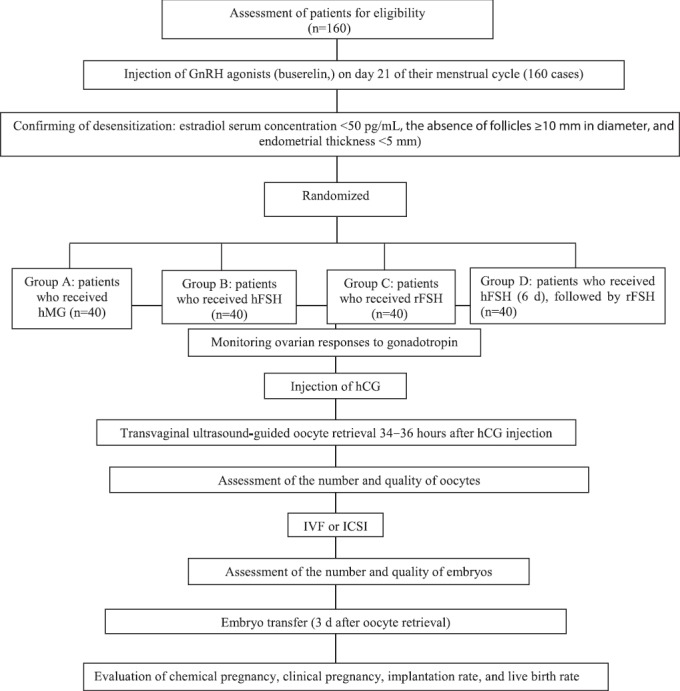
Study flow chart of the evaluation of the efficacy of different ovarian stimulation protocols, consisting of hFSH, rFSH, hMG, and sequential use of hFSH and rFSH, on oocyte and embryo quality and IVF treatment outcome in patients undergoing IVF or ICSI.
Figure 2.
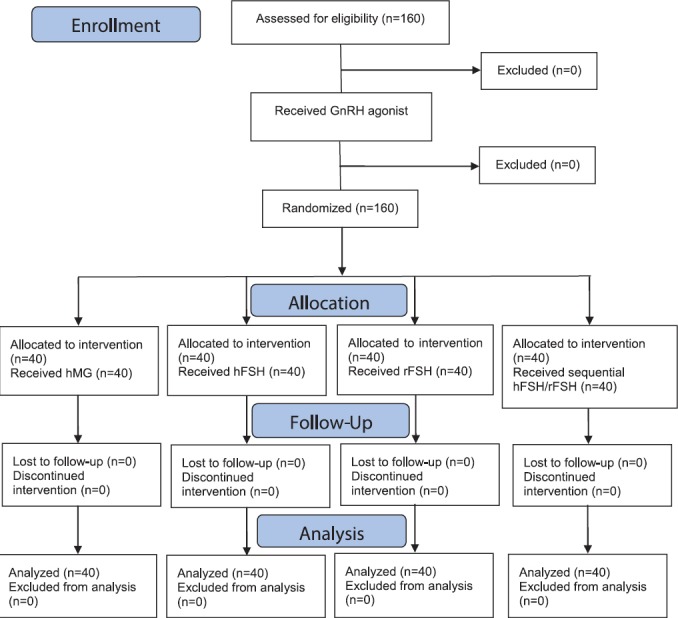
CONSORT flow diagram shows the sampling procedure.
The study group consisted of 160 women referred to 2 hospital-affiliated IVF centers for infertility treatment in Shiraz, Iran (Ghadir Mother and Child Hospital and Dena Hospital). Patients with unexplained or male factor infertility were included in the study if they met the following criteria: 1) age between 20 and 38 years; 2) body mass index (body weight divided by the square of body height) between 19 and 29 kg/m2; 3) history of regular menstrual cycles, ranging from 25–35 days; 4) no relevant systemic disease, severe endometriosis, or uterine or ovarian abnormalities; 5) no more than 3 previous IVF cycles; and 6) no previous IVF cycle with a poor response or the ovarian hyperstimulation syndrome. Additionally, patients with FSH >10 IU/mL, with <5 follicles in antral follicle count, and anti-Müllerian hormone <1 ng/mL were excluded from the study.
Ovarian Stimulation
After the patients were assessed for eligibility according to the mentioned criteria, a standard downregulation protocol was performed for all of them via a subcutaneous injection of GnRH agonists (0.5 mg of buserelin, Suprefact, Serono), on day 21 of their menstrual cycle (1 wk before the expected menses). Subsequently, on day 2 of the next menstrual cycle, after confirming desensitization (estradiol serum concentration <50 pg/mL, the absence of follicles ≥10 mm in diameter, and endometrial thickness <5 mm), the patients were randomized by a person independent of the research team using a computer-generated random-number list. Thereafter, ovarian stimulation was commenced for the study population as follows: group A: 40 patients who received hMG (Menogon®, Ferring Pharmaceuticals A/S, Copenhagen, Denmark); group B: 40 patients who received hFSH (Fostimon®, IBSA Institut Biochimique SA, Geneva, Switzerland); group C: 40 patients who received rFSH (Gonal-F®, Merck, Serono, Rome, Italy); and group D: 40 patients who received hFSH (FostimonX®, IBSA Institut Biochimique SA, Geneva, Switzerland) for the first 6 days, followed by rFSH (Gonal-F®, Merck, Serono, Rome, Italy). In all the 4 groups, the gonadotropin administration was continued up to the day of human chorionic gonadotropin injection (hCG) (Gonasi® HP, IBSA Italia, Rome, Italy). It should be mentioned that both the subjects of the study and the investigators performing the study were blind to the type of the gonadotropin each patient received for ovarian stimulation.
The monitoring of ovarian responses to gonadotropin stimulation during the treatment cycle began from day 6, using transvaginal sonography and the measurement of the plasma E2 level every 3 days. Each change in the gonadotropin dose was performed according to the follicle size and the plasma E2 level. The treatment was continued until the observation of at least 2 follicles having reached 17–18 mm in diameter (leading follicles) and some other follicles 14–16 mm in diameter. When the leading follicle was 18–20 mm, and there were at least 3 follicles of 16–17 mm, gonadotropin administration was stopped, and an intramuscular injection of 10,000 IU of hCG was administered for final oocyte maturation. Finally, 34–36 hours after hCG injection, transvaginal ultrasound-guided oocyte retrieval was performed.
IVF, ICSI, and Assessment of Oocyte and Embryo Quality
Oocyte maturity was tested according to the presence or absence of a germinal vesicle and first polar body and was graded as GV, MI, or MII according to the criteria established by Veeck et al.16,17 Subsequently, IVF or ICSI, based on indications, was performed. ICSI was used in the cases with male factor infertility. After fertilization, embryo scoring was carried out on the day of embryo transfer (3 d after oocyte retrieval).16,17 The embryos were graded as I, II, or III, where I indicates the best-quality embryo and III indicates the lowest-quality embryo. The luteal phase was supported by an intramuscular injection of 2 vials of progesterone (50 mg, Iran Hormone, Tehran, Iran) daily, from the day of oocyte retrieval for 3 days and continued with intravaginal progesterone (400 mg, Cyclogest®, Actavis UK Ltd., Barnstaple, UK) twice per day.
Assessment of Pregnancy, Implantation Rate, and Pregnancy Outcome
Two weeks after embryo transfer, the chemical pregnancy test was carried out by evaluation of serum β-hCG. In addition, clinical pregnancy was evaluated by observing the pregnancy sac 6 weeks after embryo transfer, and the implantation rate was determined by the number of gestational sacs divided by the number of embryos transferred.
End Points and Outcome Measures
The primary end points were oocyte and embryo quality and pregnancy outcomes. The secondary endpoints were the duration of stimulation, plasma E2 level on the day of hCG administration, number of used ampoules or vials of gonadotropin, number of large-sized follicles, total number of collected oocytes and transferred embryos, and implantation and miscarriage rates.
Statistical Analysis
Statistical analysis was performed using SPSS, version 16 (IBM, Armonk, USA). For the analysis of the data, the one-w ay ANOVA test was used followed by the Tukey test to compare the means. A P value <0.05 was considered statistically significant.
Results
According to table 1, age, body mass index, duration of infertility, and endometrial thickness at baseline were similar in all the groups.
Table 1.
Demographic characteristics of the women receiving hMG, hFSH, rFSH, and sequential hFSH/rFSH
| Characteristics | Group A hMG | Group B hFSH | Group C rFSH | Group D Sequential hFSH/rFSH | P value |
|---|---|---|---|---|---|
| Age | 31.90±5.0 | 31.32±5.01 | 30.25±3.45 | 32.35±4.53 | 0.382 |
| Body mass index (kg/m2) | 23.98±2.70 | 24.4±2.91 | 24.69±2.26 | 24.69±2.26 | 0.923 |
| Duration of infertility (y) | 6.60±3.68 | 6.50±3.62 | 7.60±6.43 | 5.40±3.71 | 0.757 |
| Endometrial thickness at first day (mm±SD) | 2.90±1.44 | 3.30±1.33 | 3.10±0.99 | 2.70±1.41 | 0.763 |
| Endometrial thickness on hCG day (mm±SD) | 7.60±1.42 | 7.70±1.41 | 7.90±1.96 | 8.30±1.70 | 0.792 |
| Number of ampoules or vials of gonadotropin | 18.8±6.52 | 18.7±5.91 | 19.8±6.09 | 15.8±3.85▲,■,• | 0.004 |
| Duration of stimulation (day±SD) | 8.90±2.90 | 9.1±2.1 | 10±2.4٭,• | 8.80±1.71 | 0.030 |
| 17ß-estradiol level on the day of triggering | 1940.0±479.1 | 1925.9±675.3 | 2223.6±1026.1 | 2531.07±1087▲,■ | 0.005 |
The significance was considered at P≤0.05.
: Statistically significant differences between groups A and D;
: Statistically significant differences between groups B and D;
: Statistically significant differences between groups C and D;
: Statistically significant differences between groups A and C
The number of ampoules or vials of gonadotropin administered was lower in group D than in the other groups; this difference was statistically significant compared to groups A, B, and C.
The duration of stimulation was longer in group C than in the other 3 groups, and the difference in group C in comparison to groups A and D was statistically significant.
Endometrial thickness and the estradiol level on the day of hCG administration were higher in group D than in the other groups. Apropos the estradiol level, this difference was significant in group D in comparison to groups A and B.
As is shown in table 2, the number of large-sized follicles was high in group D and then in group C, compared to groups A and B. This difference between groups B and D was statistically significant.
Table 2.
Ovarian response and oocyte maturity in the patients receiving hMG, hFSH, rFSH, or sequential hFSH/rFSH
| Characteristics | Group A hMG | Group B hFSH | Group C rFSH | Group D Sequential hFSH/rFSH | P value |
|---|---|---|---|---|---|
| Number of large follicles | 9.9±4.7 | 8.9±4.8 | 10.8±7.0 | 12.45±5.4■ | 0.036 |
| Number of all retrieved oocytes | 388 | 328 | 448 | 433 | 0.068 |
| Number of retrieved oocytes/patient | 9.5±4.83 | 8.2±4.7 | 11.2±6. 7 | 10.8±5.5 | 0.067 |
| Number of degenerated oocytes (%) | 8 (2.1) | 8 (2.44) | 12 (2.7) | 20 (4.6) | 0.178 |
| Number of GV oocytes (%) | 24 (6.3) | 21 (6.40) | 22 (5.0) | 18 (4.2) | 0.906 |
| Number of MI oocytes (%) | 32 (8.4) | 30 (9.15) | 35 (7.9) | 27 (6.2) | 0.923 |
| Number of MII oocytes (%) | 316 (83.2) | 269 (82.01) | 375 (84.5) | 368 (85.0) | 0.069 |
Significance was considered at P≤0.05.
: Statistically significant differences between groups B and D; NS: Nonsignificant
The number of retrieved oocytes was higher in groups C and D than in groups A and B, but the difference did not constitute statistical significance. The number of degenerated oocytes was higher in group D than in groups A, B, and C; the difference, however, was not statistically significant. No statistically significant differences were observed in the number of GV and MI oocytes between the studied groups, but the number of mature oocytes (MII) was higher in group C and then in group D than in groups A and B; nevertheless, the difference was not statistically significant. The lowest number of MII oocytes was observed in group B.
According to table 3, the number of transferred embryos was not different between the groups. The highest proportion of grade-I embryos and the lowest proportion of grade-II and grade-III embryos were in group D, followed by groups C, B, and A.
Table 3.
Embryo score of the patients after treatment with hMG, hFSH, rFSH, or sequential hFSH/rFSH
| Characteristics | Group A hMG | Group B hFSH | Group C rFSH | Group D Sequential hFSH/rFSH | P value |
|---|---|---|---|---|---|
| Number of embryos transferred/patient (mean±SD) | 2.9±0.7 | 2.6±0.9 | 2.8±0.7 | 2.8±0.7 | 0.530 |
| Grade-I embryos (%) | 33 (28.9) | 41 (39.42) | 55 (50.0) | 65 (59.09) | 0.054 |
| Grade-II embryos (%) | 52 (45.6) | 37 (35.9) | 36 (32.7) | 35 (31.83) | 0.688 |
| Grade-III embryos (%) | 29 (25.4) | 26 (25) | 19 (17.3) | 10 (9.09) | 0.106 |
Significance was considered at P≤0.05
As is shown in table 4, the chemical and clinical pregnancy rate, implantation rate, and live birth rate were high in group D, followed by group C, in comparison to the other groups; nonetheless, the difference was not statistically significant. In addition, the abortion rate was highest in group D.
Table 4.
Clinical outcome of the patients after treatment with hMG, hFSH, rFSH, or sequential hFSH/rFSH
| Characteristics | Group A hMG | Group B hFSH | Group C rFSH | Group D SequentialhFSH/rFSH | P value |
|---|---|---|---|---|---|
| Biochemical pregnancy rate (%) | 22 (55) | 22 (55) | 22 (55) | 28 (70) | 0.432 |
| Clinical pregnancy rate (%) (n) | 18 (45) | 15 (37.5) | 20 (50) | 23 (57.5) | 0.296 |
| Implantation rate per embryo transferred (%) | 15.6±17.9 | 15.55±25.69 | 22.7±27.6 | 25.2±24.6 | 0.176 |
| Live birth rate per clinical pregnancy (n) (%) | 11 (61.11) | 9 (60) | 16 (80) | 19 (82) | 0.614 |
| Abortion rate per clinical pregnancy (%) | 2 (11.11) | 2 (13.13) | 3 (15) | 8 (34.87) | 0.862 |
Significance was considered at P≤0.05
Discussion
Among the different protocols for COH, the use of the GnRH analog plus gonadotropins (long protocol or standard protocol) is popular, owing to its more favorable results. The literature abounds with studies comparing exogenous gonadotropins for COH, but the issue still remains controversial.
Exogenous ovarian stimulation increases oocyte yield but may compromise the developmental competence of the oocytes in stimulated cycles.18 In this study, we evaluated the efficacy of 4 different ovarian stimulation protocols using different gonadotropins in women undergoing IVF or ICSI programs.
According to our results, the number of ampoules used was significantly lower in the sequential protocol than that in the other 3 protocols and the duration of stimulation in the rFSH-alone protocol was significantly longer than that in the hFSH and hMG protocols. Gerli et al.19 demonstrated that stimulation with the sequential protocol, compared with the rFSH protocol, necessitated a low gonadotropin dose and short duration of stimulation for the stimulation of ovaries. Other studies have shown no significant differences between the use of rFSH and hFSH or the sequential protocol in the duration of stimulation and the dose of gonadotropin used.6,13,14,20,21 These contradictory results may have originated from diversity not only among the products of pharmaceutical companies but also among patients’ race and physiological status.
The effect of serum estradiol level on the day of hCG on ART outcome is controversial. It is said that although the estradiol level increases endometrial proliferation, uterine perfusion, oocyte development and maturation, number of embryos transferred, implantation, delivery, and pregnancy rate, the supraphysiological level of estradiol may not only cause endometrial damage and disrupt the implantation but also exert negative effects on IVF-ICSI outcome. Nevertheless, this hypothesis has yet to be confirmed.22,23
COH leads to the development of groups of follicles of differing sizes. Gonadotropin stimulation changes in the steroid profile result in modifying the microenvironment of the developing follicle and its oocyte. Precise evaluation of follicle size is highly important, and it has been shown that larger follicles at the time of retrieval have consistently mature oocytes with a higher rate of fertilization.24
We observed that the level of estradiol was significantly higher in the sequential protocol than in the hFSH and hMG protocols, resulting in more large-sized follicles, retrieved oocytes, and MII oocytes in this protocol. Nonetheless, it did not lead to a clear increase in the endometrial thickness of these groups compared to the other 2 groups.
The sequential use of hFSH/rFSH is the same as the natural physiologic cycle, where more acidic isoforms of FSH are produced in the follicular phase, when the estradiol level is low, and fewer acidic isoforms are produced in the late follicular and periovulatory phase, when estradiol is high.21 The significantly high number of large-sized follicles in the sequential protocol in comparison to the rFSH protocol may be related to the combined used of acidic (hFSH) and less acidic isoforms (rFSH) of FSH, which mimics the physiology of the normal menstrual cycle and is an important mechanism for the regulation of the final stages of follicle and oocyte maturation.25
Furthermore, in the rFSH protocol, the level of estradiol was nonsignificantly higher than that with the hFSH and hMG protocols. Gholami et al.13 showed a significantly high level of estradiol in the rFSH protocol compared with the hFSH protocol. Other studies have shown no differences in the estradiol level and endometrial thickness between the sequential, rFSH, hFSH, and hMG protocols.8,19,21,26,27
Although not significant, the number of retrieved oocytes and MII oocytes was high in the sequential and rFSH protocols compared with the hFSH and hMG protocols. However, the number of degenerated oocytes was nonsignificantly high in the sequential protocol compared to the other 3 groups. In other studies, no significant differences in the number of retrieved oocytes have been seen between the different protocols.8,19,27,28 We observed no significant differences in the number of retrieved oocytes and mature oocytes between the hFSH and rFSH patients, chiming in with other studies.6,21 Gerli et al.19 observed that the number of MII oocytes was significantly higher in the patients who received the sequential protocol than in the patients who received rFSH alone.
Therefore, using a sequential protocol, our patients reached higher estradiol levels and sufficient numbers of suitable follicles with fewer ampoules and lower durations of stimulation. Furthermore, in the rFSH protocol, despite the need for more ampoules and a longer duration of stimulation than in the other groups, more retrieved oocytes and higher numbers of MI and MII oocytes were produced than with the hMG and hFSH protocols, although these differences were not significant.
We observed that the use of the sequential and rFSH protocols, by comparison with the hMG or hFSH protocol, nonsignificantly led to more good-quality (grade I) embryos. In addition, the number of low-quality embryos was lowest in the sequential protocol, followed by the rFSH, hFSH, and hMG protocols. Selman et al.8 and Gerli et al.19 showed that the number of good-quality embryos was significantly high in the sequential protocol in comparison to the hFSH and rFSH protocols. In other studies, the number of good-quality embryos is similar in the rFSH, hMG, and hFSH protocols.13,24,27
Although the total number of transferred embryos was not different between the groups, the implantation and pregnancy and live birth rates were higher in the sequential protocol. This may be related to the higher number of good-quality embryos produced in the patients who received the sequential protocol. Selman et al.8,21 and Gerli et al.19 showed that the implantation rate and pregnancy and delivery rates were significantly high using the sequential protocol in comparison to the hFSH and rFSH protocols. In the rFSH protocol, these parameters were slightly higher than in the hFSH and hMG protocols. Gholami et al.13 and Selman et al.26 reported that the implantation rate and pregnancy rate were similar between the rFSH and hFSH protocols. In contrast, Daya12 demonstrated that rFSH was better than hFSH in terms of the pregnancy rate, while van Wely et al.7 showed a significantly high clinical pregnancy rate with the hFSH protocol compared with rFSH. Ludwig et al.27 and Turhan et al.5 showed that the pregnancy and live birth rates were similar between the rFSH and hMG protocols. These differences may be due to the heterogeneity of patients in the analysis, their age, type of GnRH analog suppressions, gonadotropin doses, etc. Nonetheless, the results of the study by Selman et al.8 and our results showed that the sequential protocol was better than the other protocols in terms of clinical pregnancy and the live birth rate. Our results regarding the superiority of rFSH over hFSH differed from their results. In our study, the pregnancy rate was higher in the sequential and rFSH protocols, although the abortion rate was higher in these protocols as well; however, the overall outcome (the live birth rate) stood higher in these 2 groups (not significantly). Accordingly, we concluded that the rFSH and sequential hFSH/rFSH protocols yielded more mature oocytes, but the sequential protocol was more valuable in terms of embryo quality, as was seen in implantation, pregnancies, and live birth rate. Still, there was no clear difference between the hMG and hFSH protocols.
The sequential use of hFSH/rFSH is the same as the natural physiologic cycle, where more acidic isoforms of FSH are produced in the follicular phase, when the estradiol level is low, and fewer acidic isoforms are produced in the late follicular and periovulatory phase, when estradiol is high. This may be an important mechanism for the regulation of the final stages of follicle and oocyte maturation.21,25 Therefore, the difference and distribution of exogenously applied gonadotropins should be determined and used for ovarian stimulation.
Obviously, these differences in the effect of FSH isoforms on follicular development patterns strongly suggest that oocyte development is also likely to be influenced, that normal follicle development and ultimately normal oocyte function depend on an appropriate balance of sequential differentiation, and that this balance is strongly influenced by FSH isoform distribution.28
Conclusion
In conclusion, the sequential protocol was able to improve the success rate of ART and could, as such, be deemed a valuable protocol in IVF programs. Further large randomized trials are needed to yield a precise estimation of any difference between the above-mentioned protocols.
Acknowledgement
This research was partially extracted from a thesis written by “Solmaz Rezaee”, MD, supported by Shiraz University of Medical Sciences with the grant number of 7249.
Conflict of Interest: None declared.
References
- 1.Kamel RM. Assisted reproductive technology after the birth of louise brown. J Reprod Infertil. 2013;14:96–109. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurunath S, Pandian Z, Anderson RA, Bhattacharya S. Defining infertility--a systematic review of prevalence studies. Hum Reprod Update. 2011;17:575–88. doi: 10.1093/humupd/dmr015. [DOI] [PubMed] [Google Scholar]
- 3.Alaee S, Novin MG, Noroozian M, Yeganeh F, Pakravesh J, Heidari M, et al. Evaluation of progesterone receptor, FKBP51 and FKBP52, associated with uterine receptivity, in endometrial tissue of women with repeated implantation failure. Acta Endocrinologica (Buc) 2014;10:329–39. doi: 10.4183/aeb.2014.329. [DOI] [Google Scholar]
- 4.Lai Q, Zhang H, Zhu G, Li Y, Jin L, He L, et al. Comparison of the GnRH agonist and antagonist protocol on the same patients in assisted reproduction during controlled ovarian stimulation cycles. Int J Clin Exp Pathol. 2013;6:1903–10. doi: 10.1016/j.fertnstert.2005.02.053. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turhan N, Pekel A, Ayrim A, Kasap B, Bayrak Ö. Effectiveness of HP-hMG versus r-FSH in patients undergoing IVF/ICSI cycles with moderate male-factor infertility. Turk J Med Sci. 2013;43:144–9. [Google Scholar]
- 6.Mohamed MA, Sbracia M, Pacchiarotti A, Micara G, Linari A, Tranquilli D, et al. Urinary follicle-stimulating hormone (FSH) is more effective than recombinant FSH in older women in a controlled randomized study. Fertil Steril. 2006;85:1398–403. doi: 10.1016/j.fertnstert.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 7.van Wely M, Bayram N, van der Veen F. Recombinant FSH in alternative doses or versus urinary gonadotrophins for ovulation induction in subfertility associated with polycystic ovary syndrome: a systematic review based on a Cochrane review. Hum Reprod. 2003;18:1143–9. doi: 10.1093/humrep/deg229. [DOI] [PubMed] [Google Scholar]
- 8.Selman H, Pacchiarotti A, Rinaldi L, Crescenzi F, Lanzilotti G, Lofino S, et al. Simultaneous administration of human acidic and recombinant less acidic follicle-stimulating hormone for ovarian stimulation improves oocyte and embryo quality, and clinical outcome in patients with repeated IVF failures. Eur Rev Med Pharmacol Sci. 2013;17:1814–9. [PubMed] [Google Scholar]
- 9.Al-Inany H, Aboulghar M, Mansour R, Serour G. Meta-analysis of recombinant versus urinary-derived FSH: an update. Hum Reprod. 2003;18:305–13. doi: 10.1093/humrep/deg088. [DOI] [PubMed] [Google Scholar]
- 10.Al-Inany H, Aboulghar MA, Mansour RT, Serour GI. Ovulation induction in the new millennium: recombinant follicle-stimulating hormone versus human menopausal gonadotropin. Gynecol Endocrinol. 2005;20:161–9. doi: 10.1080/09513590400027232. [DOI] [PubMed] [Google Scholar]
- 11.Al-Inany HG, Abou-Setta AM, Aboulghar MA, Mansour RT, Serour GI. Efficacy and safety of human menopausal gonadotrophins versus recombinant FSH: a meta-analysis. Reprod Biomed Online. 2008;16:81–8. doi: 10.1016/s1472-6483(10)60559-7. [DOI] [PubMed] [Google Scholar]
- 12.Daya S. Updated meta-analysis of recombinant follicle-stimulating hormone (FSH) versus urinary FSH for ovarian stimulation in assisted reproduction. Fertil Steril. 2002;77:711–4. doi: 10.1016/s0015-0282(01)03246-0. [DOI] [PubMed] [Google Scholar]
- 13.Gholami H, Vicari E, Molis M, La Vignera S, Papaleo E, Cappiello F. Pregnancy outcome following in vitro fertilization-embryo transfer (IVF-ET) in women aged <37, undergoing ovulation induction with human FSH compared with recombinant FSH: a randomised controlled study. Eur Rev Med Pharmacol Sci. 2010;14:97–102. [PubMed] [Google Scholar]
- 14.Baker VL, Fujimoto VY, Kettel LM, Adamson GD, Hoehler F, Jones CE, et al. Clinical efficacy of highly purified urinary FSH versus recombinant FSH in volunteers undergoing controlled ovarian stimulation for in vitro fertilization: a randomized, multicenter, investigator-blind trial. Fertil Steril. 2009;91:1005–11. doi: 10.1016/j.fertnstert.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 15.Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied linear statistical models. New York: McGraw-Hill Irwin; 2005. p. 1408. [Google Scholar]
- 16.Veeck LL. Oocyte assessment and biological performance. Ann N Y Acad Sci. 1988;541:259–74. doi: 10.1111/j.1749-6632.1988.tb22263.x. [DOI] [PubMed] [Google Scholar]
- 17.Veeck L. An atlas of human gametes and conception. London: Parthenon; 1999. [Google Scholar]
- 18.Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- 19.Gerli S, Di Renzo GC. Establishing a combined stimulation protocol hFSH followed by rFSH might represent a breakthrough in the IVF practice. Eur Rev Med Pharmacol Sci. 2013;17:2091–6. [PubMed] [Google Scholar]
- 20.Ye H, Huang G, Pei L, Zeng P, Luo X. Efficacy of sequential treatment protocol with highly purified urinary FSH and recombinant FSH for controlled ovarian stimulation. Fertility and Sterility. 2011;96:S254. doi: 10.1016/j.fertnstert.2011.07.975. [DOI] [Google Scholar]
- 21.Selman H, Pacchiarotti A, El-Danasouri I. Ovarian stimulation protocols based on follicle-stimulating hormone glycosylation pattern: impact on oocyte quality and clinical outcome. Fertil Steril. 2010;94:1782–6. doi: 10.1016/j.fertnstert.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. 2010;93:442–6. doi: 10.1016/j.fertnstert.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 23.Kara M, Kutlu T, Sofuoglu K, Devranoglu B, Cetinkaya T. Association between serum estradiol level on the hCG administration day and IVF-ICSI outcome. Iran J Reprod Med. 2012;10:53–8. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehri S, Levi Setti PE, Greco K, Sakkas D, Martinez G, Patrizio P. Correlation between follicular diameters and flushing versus no flushing on oocyte maturity, fertilization rate and embryo quality. J Assist Reprod Genet. 2014;31:73–7. doi: 10.1007/s10815-013-0124-9. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurgan T, Montjean D, Demirol A, Menezo YJ. Sequential (hFSH +recFSH) vs homogenous (hFSH or recFSH alone) stimulation: clinical and biochemical (cumulus cell gene expression) aspects. J Assist Reprod Genet. 2014;31:657–65. doi: 10.1007/s10815-014-0208-1. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selman HA, De Santo M, Sterzik K, Coccia E, El-Danasouri I. Effect of highly purified urinary follicle-stimulating hormone on oocyte and embryo quality. Fertil Steril. 2002;78:1061–7. doi: 10.1016/s0015-0282(02)04202-4. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig M, Rabe T, Bühler K, Diedrich K, Felberbaum R. Efficacy of recombinant human FSH in comparison to urinary hMG following a long down-regulation protocol–an analysis of 24,764 ART-cycles in Germany. Journal für Reproduktionsmedizin und Endokrinologie. 2004;1:82–90. [Google Scholar]
- 28.Esteves SC, Schertz JC, Verza S, Jr, Schneider DT, Zabaglia SF. A comparison of menotropin, highly-purified menotropin and follitropin alfa in cycles of intracytoplasmic sperm injection. Reprod Biol Endocrinol. 2009;7:111. doi: 10.1186/1477-7827-7-111. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


