Abstract
Upon blood vessel injury, platelets are exposed to adhesive proteins in the vascular wall and soluble agonists, which initiate platelet activation, leading to formation of hemostatic thrombi. Pathological activation of platelets can induce occlusive thrombosis, resulting in ischemic events such as heart attack and stroke, which are leading causes of death globally. Platelet activation requires intracellular signal transduction initiated by platelet receptors for adhesion proteins and soluble agonists. Whereas many platelet activation signaling pathways have been established for many years, significant recent progress reveals much more complex and sophisticated signaling and amplification networks. With the discovery of new receptor signaling pathways and regulatory networks, some of the long-standing concepts of platelet signaling have been challenged. This review provides an overview of the new developments and concepts in platelet activation signaling.
Platelet activation signaling plays a critical role in the function of platelets in hemostasis and thrombosis, and has been the subject of intensive investigations for many years. With the explosive increases in our knowledge recently, a much more complex and sophisticated picture of platelet signaling and amplification networks has emerged, and some long-standing concepts of platelet activation are now challenged. Classically, platelet activation is induced by collagen or soluble platelet agonists that bind G-protein-coupled receptors, leading to the activation of platelet adhesion receptors, mainly the integrin αIIbβ3, which mediates platelet adhesion and aggregation. However, various platelet adhesion receptors, which were previously regarded as “passive,” are increasingly recognized to be important in mediating and amplifying platelet activation signals. Increasing numbers of ligand-receptor pairs, which were known to be critical for mediating immune or inflammatory responses in leukocytes, are now shown to play important roles in platelet activation, in the role of platelets in immune or inflammatory responses, and in the coupling between thrombosis and inflammation. Importantly, it is now increasingly evident that a long-established negative regulator of platelet activation, the nitric oxide (NO)-cyclic guanylyl monophosphate (cGMP) pathway, in fact plays biphasic roles in platelet activation and is important in mediating platelet activation induced by several receptor signaling pathways, including recently discovered platelet pattern-recognition receptor signaling pathways. In this review, we will provide an overview and update to the concepts and mechanisms of platelet activation signaling.
With the recent advances and complexity of the platelet signaling network in mind, we divide platelet activation signaling into the following phases: 1) divergent early receptor signaling induced by not only classic soluble platelet agonists but also adhesion receptor ligands and inflammatory stimuli; 2) convergence of early signaling pathways on common intermediates and signal amplification networks; 3) inside-out signaling leading to activation of the main platelet adhesion receptor, the integrin αIIbβ3, which mediates stable platelet adhesion and aggregation; and 4) integrin outside-in signaling, which greatly amplifies platelet activation and thrombus size. Accordingly, we will review each of these phases in the following sections.
Receptor Signaling Pathways Leading to Platelet Activation
Adhesion Receptor-Mediated Platelet Activation Signaling
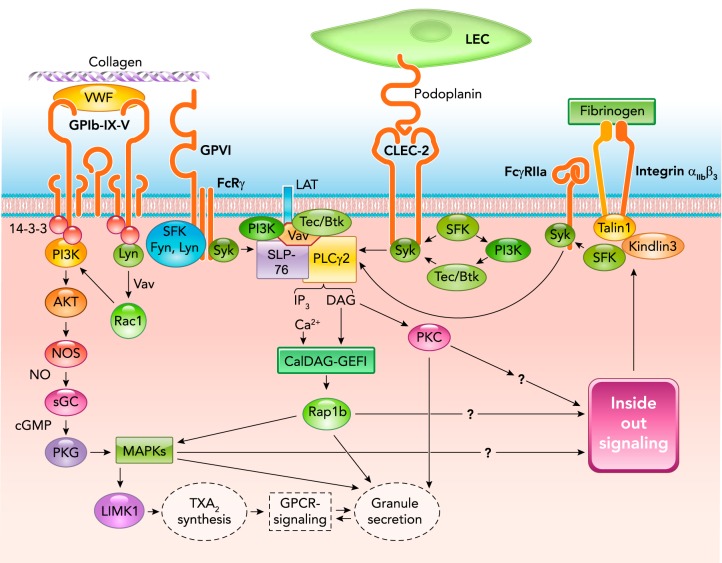
GPIb-IX signaling.
Under flow conditions, particularly at high flow shear rates, the initial adhesion of platelets to the blood vessel wall requires the interaction between immobilized VWF on the surface of endothelium or in the subendothelial matrix with its platelet receptor, the glycoprotein (GP) Ib-IX-V complex. The ligand binding subunit of the GPIb-IX-V receptor complex, GPIbα, contains binding sites for the A1 domain of VWF in its NH2-terminal domain (42, 93, 143, 148, 215). VWF and GPIbα respond to increasing shear force by undergoing conformational changes that increase their affinity for one another (135, 151, 186), forming shear-resistant “catch bonds” or “flex bonds” that allow platelet adhesion under shear stress (93, 108, 135, 232). The cytoplasmic domain of GPIbα is anchored to actin filaments, which underlie the platelet plasma membrane, through interaction with filamin A. This interaction is critical for maintaining membrane structure and platelet shape, and is also an important structural reenforcement for resisting shear force during platelet adhesion (40).
Despite its ability to resist shear, GPIb-IX-mediated platelet adhesion to VWF is transient in nature. Stable adhesion of platelets initiated by VWF/GPIb-IX binding requires the activation of another VWF receptor, integrin αIIbβ3. In fact, it is increasingly evident that VWF binding to GPIb-IX under shear triggers platelet activation signals, leading to integrin activation and integrin-dependent stable platelet adhesion (53, 125, 183). A region within the extracellular juxtamembrane stalk of GPIbα, called the mechanosensitive domain (MSD) (Ala417-Phe483), was shown to unfold and extend when subject to VWF-dependent pulling force (247). Another region in the leucine-rich repeat domain (LRRD) has also been shown to unfold by VWF-mediated pulling force (101). The unfolding of LRRD and MSD plays distinct roles in VWF binding-dependent transduction of pulling force, triggering intracellular signaling as indicated by calcium elevation (100). The mechanical force-signaling coupling appears to require the interaction between GPIb cytoplasmic domain, with an intracellular molecule ζ form of 14-3-3 protein (100).
GPIb-IX also binds thrombin and is important for low dose thrombin-induced platelet activation (78, 95, 242). This function of GPIb-IX requires the thrombin binding sites around three sulfated tyrosine residues (Y276DYY) (27, 48, 55, 143) in the ligand-binding domain of GPIbα, which is distinct from the VWF binding site (27, 47, 48, 55, 143). It is important to note that, under conditions where thrombosis is induced by limited vascular injury (such as the laser-induced arterial injury model), only low concentrations of thrombin are detectable, which, however, are critical for platelet thrombus formation in vivo (57).
GPIb-IX signaling induced by either VWF or thrombin both require the binding of 14-3-3ζ to the cytoplasmic domain of GPIbα (44, 57, 80), a Src family kinase (SFK) (Lyn and possibly Src)-Rac1 signaling pathway (51, 53, 239), and downstream activation of the phosphoinositide 3-kinase (PI3K)-Akt and cGMP-dependent protein kinase (PKG) pathway (127, 128, 239, 240), mitogen-activated protein kinase (MAPK) (ERK1/2 and p38) pathway (69, 126, 128), and LIM kinase 1 (LIMK1) pathway (57, 59). Several other proteins such as Bruton's tyrosine kinase (Btk) (132) and ADAP (102) have also been shown to be important, although it remains to be established whether this is because they play roles in the amplification pathways, such as the ITAM pathway, or the integrin signaling pathway, in which these proteins are known to be important (103, 176). It is interesting that the role of LIMK1 in stimulating VWF and low-dose thrombin-induced platelet activation is specific for the GPIb-IX signaling pathway, because LIMK1 appears to play a negative role in platelet activation induced by the GPIb-IX-independent platelet agonists. LIMK1 promotes VWF-stimulated activation of cytosolic phospholipase A2 (cPLA2) and consequent TXA2 production (59) (FIGURE 1).
FIGURE 1.
Signaling pathways of the major platelet receptors for adhesive ligands leading to integrin αIIbβ3 activation and important roles of ITAM pathway
sGC, soluble guanylyl cyclase; NOS, NO synthase; SFK, src family kinase; LEC, lymphatic endothelial cell; SLP-76, SH2 domain-containing leukocyte phosphoprotein of 76 kDa; Btk, Bruton's tyrosine kinase; Rap1b, RAS-related protein 1b; MAPKs, mitogen-activated protein kinases; TXA2, thromboxane A2.
ITAM signaling in platelet activation triggered during platelet adhesion.
The immunoreceptor tyrosine-based activation motif (ITAM) is a protein sequence motif in the cytoplasmic domain of certain immunoreceptors containing two tyrosine residues within a conserved YxxL/IX6-12 YxxL/I sequence (where x is any amino acid), and it plays an important role in receptor signaling leading to activation of leukocytes (10, 22, 222). ITAM receptors expressed in human platelets include the Fc receptor γ chain (FcRγ), a subunit of certain Ig Fc receptors critically important for their signal transduction, and Fcγ receptor IIa (FcγRIIa), an IgG Fc receptor important for human platelet response to antibody-antigen complexes and aggregated IgG (not expressed in mouse platelets) (180, 225). ITAM receptors are not only critical in signal transduction of immunoreceptors in platelets and leukocytes, they are also important for signal transduction during platelet adhesion (23, 62, 209). FcRγ is reportedly associated with GPVI and GPIb-IX (62), and FcγRIIa with GPIb-IX and integrin αIIbβ3 (202, 248). CLEC2, a platelet podoplanin receptor important for platelet adhesion to lymphatic vessels, has a single ITAM-like YxxL motif known as a hemITAM.
gpvi.
The role of ITAM in platelet adhesion signaling is best exemplified in the collagen receptor GPVI (104, 106, 155), which plays a critical role in robust platelet activation induced by collagen. GPVI forms a noncovalent complex with FcRγ (60, 73, 209) and is reportedly associated with certain SFK, such as Lyn and Fyn, through the intracellular proline-rich domain of GPVI (60, 185). This complex is necessary for transmitting signals induced by collagen binding to the extracellular immunoglobulin-like domains of GPVI (15, 162, 175, 228). Upon collagen-induced clustering of GPVI, ITAM in the associated FcRγ is tyrosine phosphorylated by SFK (60, 177). However, the role of Lyn in GPVI remains controversial, with some studies suggesting that Lyn plays a negative regulatory role in GPVI-mediated ITAM signaling (177), probably via its role in the regulatory immunoreceptor tyrosine-based inhibition motif (ITIM), such as those mediated via PECAM-1 and paired immunoglobulin-like receptor B, which counteracts ITAM signaling (64, 150). Tyrosine phosphorylation of ITAM enables binding of the tyrosine kinase Syk (10, 60, 72, 94, 175, 177) through its dual Src homology 2 (SH2) domains (92), enabling its activation. Interestingly, NOX-dependent reactive oxygen species production has been shown to stimulate GPVI-induced Syk activation, possibly by inhibition of protein tyrosine phosphatases (49). Activated Syk initiates a cascade of events, involving adapter molecules and kinases: SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) (33, 79), linker for activated T-cells (LAT) (171), Grb2 (56), Gads (91), Tec family kinases (Btk and Tec) (12, 176), and PI3K (20, 25, 220), which leads to translocation to the plasma membrane, phosphorylation, and activation of phospholipase C-γ2 (PLCγ2) (20, 175, 205, 218, 221, 222). PLCγ2 hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into 1,2-diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG and IP3 activate protein kinase C and release calcium into the cytosol from intracellular stores, respectively, promoting thromboxane production and granule secretion, inside-out signaling, and integrin activation (120, 221, 222) (FIGURE 1).
clec-2.
The platelet receptor for the transmembrane glycoprotein podoplanin is CLEC-2. In laboratories, the snake venom protein rhodocytin was used to stimulate CLEC-2 signaling (203, 204). The CLEC-2-mediated platelet adhesion to podoplanin-expressing cells (e.g., lymphatic endothelial cells and pericytes) is important for the development and separation of lymphatic vessels from blood vessels (184, 210), in maintaining the integrity of the blood-lymphatic vessel junction, in preventing blood cell flow into lymphatics (86), and possibly also in playing a role in maintaining vascular integrity during inflammation (21, 85). Thus deletion of podoplanin or CLEC-2 in mice causes blood-lymphatic mixing (16, 66, 184, 204, 210). The hemITAM-dependent CLEC-2/PDPN signaling pathway involves many of the same signaling molecules used by full ITAM receptors and appears to require the binding of Syk SH2 domain to the phosphorylated CLEC-2 hemITAM motif (92). However, one report suggests that Syk activation in CLEC-2 signaling involves PI3K and Tec family kinase-mediated Syk phosphorylation, whereas, in GPVI-mediated signaling, PI3K and Tec family kinases are activated downstream of Syk (142). The mechanisms of ITAM or hemITAM motif phosphorylation may also be different. CLEC-2 hemITAM phosphorylation appears to require both Syk and SFK (191). In contrast, GPVI ITAM phosphorylation is mediated by SFK (60, 177) (FIGURE 1).
GPIb-IX and integrin αIIbβ3 are also reportedly associated with ITAM receptors (62, 202, 248). However, it appears that their primary signaling does not require the ITAM signaling pathway (102, 133, 141, 241) but that ITAM signaling plays important roles in the amplification of the signaling initiated by these receptors, leading to significantly greater platelet responses (132, 228, 248). This notion is supported by the data that GPIb-IX-triggered, integrin-dependent stable platelet adhesion to VWF and integrin-mediated platelet spreading are not inhibited by inhibitors of Syk (240), a key enzyme in the ITAM pathway, unlike the ITAM-dependent secondary platelet secretion and aggregation induced via GPIb-IX and integrin signaling pathways. Interestingly, association of Syk with filamin A, which also interacts with GPIbα cytoplasmic domain, reportedly regulates ITAM signaling induced by GPVI (63).
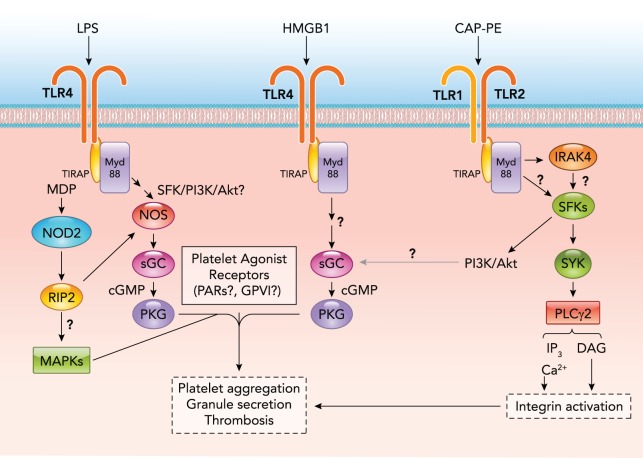
Platelet Activation Signaling Mediated by Pattern Recognition Receptors
Pathogen-associated molecular patterns (PAMPs) are molecules, such as DNA, RNA, glycoproteins, and lipopolysaccharides (LPS), produced by microorganisms. Damage-associated molecular patterns (DAMPs) are molecules [oxidized lipid derivatives, DNA fragments, and proteins, such as high-mobility group box 1 (HMGB1)] released in response to tissue damage. PAMPs and DAMPs are recognized by pattern recognition receptors, such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors, and the receptor for advanced glycation end products (RAGE), and serve as initiating signals for innate immunity and non-infectious inflammatory responses. Members of TLRs and NOD recently have been shown to promote platelet activation. Platelets express several TLRs, including TLR1, TLR2, TLR4, TLR6, and TLR9 (7, 11, 35, 194, 243). [TLR1 and TLR6 have only been reported in human but not in mouse platelets (194).] TLR2 has been shown to be critical for platelet activation induced by a synthetic TLR1-/TLR2-specific agonist, Pam3CSK4 (19), and oxidized lipid derivatives such as carboxyalkylpyrrole-phosphatidylethanolamine (18). TLR9 mediates platelet activation signals induced by carboxy(alkylpyrrole) protein adducts generated during oxidative stress (170). TLR4 is a major platelet receptor for LPS and mediates signals leading to platelet granule secretion in response to LPS (7), and also mediates platelet activation and secretion induced by HMGB1, a DNA-binding protein, which is released by monocytes and macrophages during inflammation (159) and is also stored in platelet granules and released during platelet activation (216). Signaling of TLR2, TLR4, and TLR9 in platelets is dependent on MyD88. Data published by different groups have shown that TLR4 mediates platelet activation signaling by MyD88-dependent activation of the cGMP-PKG pathway (216, 243). The cGMP pathway was not investigated in the TLR2 and TLR9 signaling pathways, although these receptors have been shown to involve SFK and PI3K/AKT, which are known activators of the NO-cGMP pathway, as well as the ITAM pathway (18, 19, 170). Platelets also express NOD2 (but not NOD1), which is a cytosolic receptor recognizing muramyl dipeptide (MDP) found on all bacteria (246). MDP-NOD2 interaction triggers platelet activation via MyD88- and MyD88-dependent activation of MAPK and the NO-cGMP-PKG signaling pathway, which potentiates platelet responses, including aggregation, clot retraction, and thrombosis (246) (FIGURE 2).
FIGURE 2.
Reported signaling pathways of pattern recognition receptors in platelets
Note that TLR receptors all require MyD88 for signaling. LPS, lipopolysaccharide; MDP, muramyl dipeptide; HMGB1, high-mobility group box 1; CAP-PE, carboxyalkylpyrrole-phosphatidylethanolamine; TLR, toll-like receptor; PKG, cGMP-dependent protein kinase; RIP2, receptor-interacting protein-2; TIRAP, toll/interleukin-1 receptor domain-containing adapter protein; Myd88, myeloid differentiation factor 88. The question marks represent unknown or hypothesized pathways requiring further investigation.
Platelet activation induced by pattern recognition receptors is likely to serve as a mechanism for thrombotic response to microbial infection and tissue damage in parallel to innate immunity and inflammation. Conversely, platelet storage of DAMPs, such as HMGB1, and their release during platelet activation is likely to exacerbate inflammation. The importance of platelet activation and thrombosis in inflammation and infection has been suggested in sepsis and during the development of atherosclerosis (21, 39, 131).
In addition to the pattern-recognition receptors, certain oxidized lipid species may stimulate platelets via glycoprotein IV, which involves the MAPK kinase signaling pathway (29). The activation of platelets during immune and inflammatory response may also be stimulated by other thrombotic and inflammatory stimuli, such as antigen-antibody complexes via the FcγRIIA-ITAM pathway, as well as thrombin or platelet-activating factor via G-protein-coupled pathways.
G-Protein-Coupled Receptor-Mediated Platelet Activation Signaling Induced by Soluble Platelet Agonists
Platelets can be activated by many soluble agonists either released from the sites of blood vessel injury and inflammation or from platelets during platelet activation. These agonists activate platelets by binding to their respective receptors on the platelet membrane, most of which are G-protein-coupled receptors (GPCR). GPCRs are a family of membrane proteins with seven transmembrane domains, which upon activation by their ligands elicit intracellular signaling by activating the heterotrimeric guanine nucleotide-binding proteins (G proteins) (166). Heterotrimeric G proteins consist of an α-subunit in complex with the tightly associated β- and γ-subunits. In the inactive state, in which the G-protein complex is receptor-associated, the α-subunit is bound to guanosine diphosphate (GDP) and complexed with βγ-subunits. Agonist binding to GPCRs induces conformational changes that activate the exchange of GDP for guanosine triphosphate (GTP) on the Gα-subunit (113, 214). Consequently, the GTP-bound Gα-subunit dissociates from the βγ-subunits and from the receptor. The GTP-bound α-subunit and the βγ-subunit then interact with their respective downstream effector proteins, propagating signals. The GTP-bound Gα signal propagation is then terminated by GTP hydrolysis, which is mediated by intrinsic GTPase activity of Gα-subunits and greatly accelerated upon binding of a regulator of G-protein signaling (RGS) domain within members of the RGS protein family. Based on primary sequence homology of the Gα-subunit and their effects on downstream targets, heterotrimeric G proteins are grouped into four classes: Gαi/o/z, Gαs, Gαq/11, and Gα12/13 (169).
Gαq.
Upon activation of Gαq-coupled GPCRs, Gαq dissociates from the receptor, and binds and activates PLC β isozymes that catalyze hydrolysis of PIP2 to form second messengers IP3 and DAG. Gαq-null platelets are defective in IP3 release and cannot mobilize calcium in response to GPCR agonist stimulation (168). Gαq is critical for platelet aggregation in response to most platelet GPCR agonists (thrombin, ADP, 5HT, PAF, and TXA2) (166) and is indirectly important in platelet response to adhesion proteins such as collagen (168), which require the amplification signaling induced by TXA2, ADP, etc. for optimal platelet activation (30).
Gα12/13.
A classical pathway of Gα12/13 signaling is the binding of GTP-Gα13 (or Gα12) to the guanine nucleotide exchange factors (GEF) for small GTPase RhoA, including p115RhoGEF, LARG, and GEF-H1, activating RhoA and Rho kinase (ROCK), subsequently promoting phosphorylation of myosin light chain (MLC) and actin-myosin-dependent contraction (74, 110, 152, 153, 167, 206). Platelets express both Gα12 and Gα13; however, only Gα13 is required for low-dose thrombin- or U46619 (a TXA2 analog)-induced platelet aggregation and secretion. Gα13-null platelets also show defects in thrombin and U46619-induced platelet shape change, which is consistent with defects in RhoA activation and MLC phosphorylation, granule secretion, and clot retraction (13, 70, 99, 123, 172). RhoA may not be directly required for integrin activation and platelet aggregation per se, and RhoA-null platelets reportedly displayed enhanced aggregation responses to collagen and CRP(174). Recently, it was shown that GTP-bound Gα13 directly interacts with the cytoplasmic domain of the integrin β3-subunit, and this interaction is critically important in integrin outside-in signaling (77). Interestingly, Gα13 binding to β3 potently inhibits RhoA activation, providing a mechanism for dynamically regulating RhoA and RhoA-dependent contractility (see below) (77).
Gαi and Gαs.
A typical Gαi-coupled receptor is the ADP receptor P2Y12, and the typical Gαs-coupled receptor is the prostaglandin I2 receptor PTGIR (IP receptor). Gαi/o and Gαs both bind to adenylyl cyclase, with Gαi inhibiting but Gαs stimulating the function of adenylyl cyclase to synthesize cAMP (166, 233). cAMP, by stimulating cAMP-dependent protein kinase (PKA), plays a critical role in keeping platelets in a resting state, which is the mechanism by which PGI2 inhibits platelet activation. In contrast, Gαi, by inhibiting synthesis of the potent platelet inhibitory second messenger cAMP, is critically important in platelet activation (2, 14, 17, 179, 195). Platelets express several members of the Gαi family, including Gαi2, Gαi3, and Gαz (166). Defects in one isoform, such as Gαi2, cause only a partial defect in agonist-induced inhibition of cAMP synthesis (52, 96, 233). Gαi stimulates platelet activation not only by regulating cAMP levels but also via cAMP-independent mechanisms (136, 233). In particular, upon Gαi activation, dissociated Gβγ has been shown to interact with and activate PI3Kγ and possibly β-isoforms of PI3K (116, 140, 208, 212), which is important in Gi signaling (25, 37, 88, 105, 130, 187). The Gαi pathway also plays roles in activation of the small GTP-binding RAS-related protein 1b (Rap1b) as well as Rap1b regulator RASA3 (32, 136, 137, 196, 197, 227), and in activating SFKs (230).
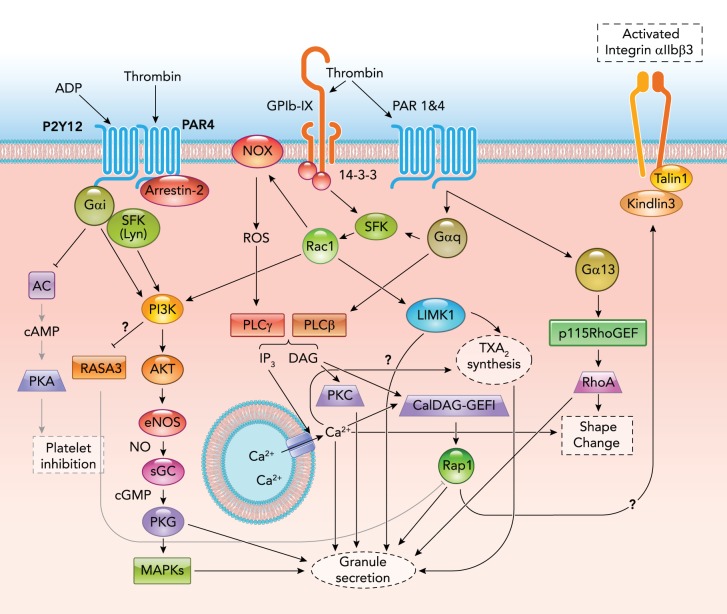
Synergy Between Different Receptor Pathways of Platelet Activation
Several platelet agonists have more than one platelet receptor, and optimal platelet activation induced by these agonists often requires synergy or cooperativity between different receptor pathways. ADP, which is released from platelet-dense granules or damaged cells and tissues (26), requires both Gαq-coupled P2Y1 and Gαi-coupled P2Y12 signaling for platelet activation (166). Platelet activation becomes defective when any of these two receptors or their associated G proteins are blocked or deficient (61, 90, 97). Thus it appears that cooperativity of the Gi and Gq pathway is necessary for ADP-induced platelet activation. Thromboxane A2 (TXA2) has a single receptor (TP) (207), which, however, is coupled to both Gαq and Gα12/13, and both classes of G proteins are important for optimal platelet response (153), particularly at low doses, suggesting synergy between two different pathways. Thrombin-induced platelet activation involves even more complex receptor and signaling pathways. Thrombin has at least three receptors on the platelet surface: PAR1, PAR4, and GPIb-IX in human platelets, and PAR3, PAR4, and GPIb-IX in mouse platelets (38, 45), wherein PAR3 was suggested to be a dock for efficient cleavage and signaling via PAR4 (160). Upon thrombin stimulation, PAR1 has been suggested to form heterodimers with PAR4 that enhance cleavage of PAR4 (8). In addition, PAR4 and P2Y12 have been reported to dimerize, and their interaction promotes PAR signaling, leading to AKT activation (107). PAR1 and PAR4 are both coupled to Gαq, Gα13 and possibly Gαi pathways (controversial, indirect coupling was reported in some studies) (38, 109). Thrombin binds and cleaves these GPCRs to expose a new NH2-terminal sequence, which serves as an internal ligand to interact and activate receptor signaling (217). The role of non-GPCR receptor, GPIb-IX, in thrombin-induced platelet activation has been controversial. Whereas some investigators proposed that GPIb-IX serves as a thrombin dock that promotes thrombin cleavage of PARs (46), other investigators suggested that GPIb-IX transmitted platelet activation signals independent of PARs (3, 178). However, recent work indicates that GPIb-IX is neither a passive thrombin dock nor a PAR-independent thrombin receptor, but that mutual cooperativity between thrombin-induced GPIb-IX signaling and PAR signaling is required for optimal platelet response (57). The synergy of these different receptors greatly enhances platelet sensitivity to low concentrations of thrombin, which is important for arterial thrombosis (57) (FIGURE 3).
FIGURE 3.
Synergy among multiple thrombin receptor signaling pathways
P115 RhoGEF, p115 Rho guanine nucleotide exchange factor; PKA, cAMP-dependent protein kinase; AC, adenylyl cyclase; Rap1b, RAS-related protein 1b; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; ROS, reactive oxygen species; NOX, NADPH oxidase.
Signal Amplification Networks
Whereas receptors for many different platelet agonists and adhesive proteins transmit platelet activation signals, these divergent receptor signaling pathways converge onto common signaling pathways that greatly amplify the initial receptor signals leading to robust platelet responses.
Phospholipase c, Calcium Elevation, and Diacylglycerol
Phospholipase C (PLC) is like a hub upon which numerous platelet-activation signaling pathways converge. Platelets express at least three distinct families of PLC: PLCβ, PLCγ, and PLCδ. In human platelets, PLCγ2, PLCβ2, and PLCβ3 appear to be the predominantly expressed PLC family members (122). PLCβ and PLCγ are activated via different mechanisms (182). The former is activated by binding Gαq and the latter by Syk-mediated protein tyrosine phosphorylation. This difference in regulation is imparted by a COOH-terminal coil-coil domain found in PLCβ that is not present in PLCγ (182). By contrast, PLCγ contains SH2, SH3, and split PH domains that are not found in PLCβ (182). However, recent work suggests that Gαq-coupled agonists, such as thrombin, also induced phosphorylation and activation of PLCγ through a ROS-dependent signaling pathway (50). Conversely, ITAM agonists, such as collagen, induce release of GPCR agonists, such as ADP and TXA2, thus indirectly activating PLCβ via the Gq pathway (161, 198). Among its main functions, PLC catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (182). IP3 binds the IP3 receptor, resulting in Ca2+ release from the dense tubular system into the cytosol, which is a major mechanism of calcium elevation (213). IP3-receptor-mediated Ca2+ release and subsequent depletion of intracellular Ca2+ stores further stimulates store-operated calcium entry (SOCE) (213), causing sustained intracellular Ca2+ elevation. Elevation of intracellular calcium plays a central role in platelet activation induced by all agonists, wherein it is critical to many intracellular signaling events, including integrin inside-out signaling, and activation of many calcium-dependent proteins including PKC, calpain, and calmodulin. Elevation of intracellular calcium is required for almost all platelet functions, including stable platelet adhesion, shape change, aggregation, secretion, exposure of procoagulant activity, and clot retraction. DAG activates conventional (α,β) and novel (δ, θ, η, ε) protein kinase C (PKC) isoforms (83), which are also important for integrin activation and platelet granule secretion. Elevations in intracellular Ca2+ concentrations also lead to activation of the Ca2+- and DAG-regulated guanine nucleotide exchange factor I (CalDAG-GEFI) (41, 198). It is possible that DAG may also be involved in CalDAG-GEFI activation, although CalDAG-GEFI has an atypical low-affinity binding site for DAG (43). CalDAG-GEFI is critical for activation of the small GTPase, RAS-related protein 1b (Rap1b), which is important for granule secretion and for inside-out signaling leading to integrin activation.
Phosphoinositide 3-Kinase (PI3K)-Akt Pathway
The PI3K-Akt signaling pathway is an important signaling pathway inducing platelet granule release and is important for platelet activation and stable platelet adhesion induced by GPIb-IX signaling (127, 128, 239, 240), integrin outside-in signaling, and ITAM signaling (118, 154, 165). This pathway, however, is not required for primary platelet activation induced by GPCR agonists but serves to amplify GPCR signaling. Among different phosphoinositide species, phosphatidylinositol 3,4,5-triphosphate (PIP3) is mainly converted from phosphatidylinositol 4,5-bisphosphate (PIP2) by class I phosphoinositide 3-kinase (PI3K) that phosphorylates the 3 position on the PIP2 inositol ring. Agonist stimulation induces PI3K activation and thus generation of PIP3, which mediates membrane translocation of 3-phosphoinositide-dependent kinase 1 (PDK1) and Akt isoforms via the PIP3-binding PH domains in these proteins, allowing PDK1-mediated phosphorylation and activation of Akt. All three isoforms of Akt are expressed in platelets, and knockout of any one of them reduces low-dose thrombin- and U46619-induced platelet secretion and the secretion-dependent second-wave aggregation (28, 165, 226). Knockout of Akt1 or Akt2 also diminishes VWF-/GPIb-IX-induced platelet activation (240), but only deletion of Akt1 significantly affects collagen-induced platelet secretion and aggregation (28). Akt isoforms, predominantly Akt3, are also important in integrin outside-in signaling promoting platelet spreading. Two Akt effectors have been shown to mediate the PI3K-Akt signaling, leading to granule secretion and platelet activation: nitric oxide (NO) synthase (NOS) and GSK3β. Platelets express endothelial NOS, which is phosphorylated and activated by Akt, and stimulates the cGMP pathway leading to granule secretion and GPIb-IX-dependent platelet activation (see below). GSK3β, however, plays differential roles in different platelet activation pathways: it plays an inhibitory role in thrombin-induced platelet activation and integrin outside-in signaling, which is relieved by Akt-mediated GSK3β phosphorylation and inhibition, but appears to promote collagen-induced platelet activation (165). Thus the role of Akt in collagen-induced platelet activation is unlikely to be mediated by GSK3β, whereas the role of Akt in integrin outside-in signaling is mediated mainly via its phosphorylation and inhibition of GSK3β (118, 154, 164, 165), which has been shown to negatively regulate platelet spreading, clot retraction, and thrombus stability (118, 124). However, the mechanism of GSK3β action is unknown. Recent studies also suggest an important role for class II PI3KC2α in platelet morphology and thrombus stability under flow (158, 211). In addition, class III PI3K vps34 appears to play a role in platelet autophagy (65). Lipid phosphatases, such as PTEN, hydrolyze PIP3 and thus inhibit the PI3K-Akt and cGMP signaling pathway (224).
New Concept in Platelet cGMP Signaling
Cyclic guanosine monophosphate (cGMP) is synthesized by guanylyl cyclases (GC) in many cell types, including platelets, and plays an important role in activating the cGMP-dependent protein kinases (PKG). Despite the early evidence that platelet agonists stimulate cGMP elevation and that exogenous cGMP analogs may have a stimulatory effect on platelets, it has been a textbook concept since the 1980s that the NO-cGMP pathway is a major negative regulator of platelet activation (84). This is mainly due to the finding that soluble guanylyl cyclase (sGC) is activated by NO and that NO donor compounds, which dramatically elevate cGMP levels in platelets, inhibit platelet activation. However, despite development of many compounds that mimic or elevate cGMP since the 1980s, none has emerged as an effective anti-platelet drug. More recently, this concept has been challenged by the discovery of the biphasic roles of the NO-cGMP-PKG signaling pathway in regulating platelet activation: low concentrations of NO and cGMP endogenously synthesized during platelet activation promote platelet activation and significantly increase platelet sensitivity to low concentrations of platelet agonists, including GPIb-IX agonists, ITAM receptor agonists, and GPCR agonists (127, 199, 239, 244). Deficiency in endothelial NOS, inducible NOS, sGC, and PKG, as well as pharmacological inhibitors of the NOS/cGMP/PKG pathway have been shown to inhibit platelet activation in response to low-dose agonists, highlighting the importance of this pathway in stimulating platelet activation (127, 144, 145, 199, 239, 244), Furthermore, the important stimulatory roles of the cGMP-PKG pathway in the platelet activation signaling of pattern recognition receptors have been reported by different groups (216, 243, 246). Only high concentrations of NO (such as that provided by NO donors) and cGMP analogs showed an inhibitory effect on platelet activation, mainly via cGMP-dependent elevation of cAMP and activation of cAMP-dependent protein kinase (244), although PKG may also play a role in the inhibitory phase (147). The biphasic role of the cGMP pathway in platelet activation is likely to be an important mechanism for self-limiting stimulation of platelet response to low-level thrombotic and inflammatory stimuli.
Eicosanoids Pathways
During platelet activation, platelets synthesize biologically active lipid species, called eicosanoids, functioning as second messengers. Eicosanoid synthesis requires calcium-dependent activation of cytosolic phospholipase A2 (PLA2), which hydrolyzes membrane phospholipids to release arachidonic acid (AA). AA is metabolized via the cyclooxygenase and lipoxygenase pathways (5). Cyclooxygenases (COX), which are inhibited by aspirin, initiate the conversion of AA into prostaglandins (PG) (87, 237). Among several PGs synthesized in platelets, thromboxane A2 (TXA2) is a potent platelet agonist and plays an important role in augmenting platelet activation and promoting thrombus formation by binding to its Gq-/G13-coupled platelet receptor. Lipoxygenases initiate the conversion of AA into oxylipins, among which, 12-hydroxyeicosatetraenoic acid (12-HETE) has been reported both to potentiate platelet activation induced by PAR agonists, FcγRIIa cross linking, or GPVI agonists (34, 89, 236, 238), and to inhibit platelet activation induced by collagen (189, 237) and ADP (98). Whereas the reasons for this controversy remain unclear, 12-LOX-null mice reportedly prolongs tail bleeding times (236), and a 12-LOX inhibitor, ML355, inhibits 12-HETE production, platelet aggregation, and secretion induced by FcγRIIa cross linking, as well as by PAR4 agonist peptide-induced platelet activation (138, 238).
Granule Secretion as a Signal Amplification Mechanism
One of the most efficient common mechanisms of signal amplification during platelet activation is secretion of platelet granule contents. Platelets contain three major secretory organelles: dense (δ) granules that contain small molecules (e.g., divalent cations, ADP, ATP, serotonin, and polyphosphates); alpha (α) granules that contain adhesion proteins (e.g., VWF, fibrinogen), growth factors (e.g., platelet-derived growth factor, vascular endothelial growth factors), coagulation factors (e.g., factor V, factor XI), and chemokines (e.g., platelet factor 4, neutrophil-activating peptide-2); and lysosomes that contain degradative enzymes (e.g., cathepsin D, β-hexosaminidase) (76, 114). Secreted granule contents not only enhance platelet activation signaling but also activate and recruit circulating resting platelets, greatly facilitating formation and growth of a thrombus. In addition, granule secretion contributes to physiological vascular remodeling, inflammation, and wound repair, and pathological atherosclerosis and cancer metastasis (54, 71, 75, 117, 131, 146, 188). A critical mechanism for release of granule contents is fusion of granule membranes with plasma membranes on the platelet surface or in the open canalicular system; this fusion enables release of granules into the extracellular milieu. Contraction of platelets then forces out granule cargo. Secretion of granule cargo requires highly regulated and coordinated interactions between N-ethylmaleimide-sensitive fusion protein (NSF), soluble NSF-attachment protein (SNAP), and soluble NSF-attachment protein receptors (SNAREs), which are reviewed elsewhere (76, 114). The granule secretion process may be stimulated by elevations in intracellular Ca2+ and phosphorylation of SNARE proteins by PKC, IKK, and PKG (181). Whereas almost all platelet agonists stimulate platelet granule secretion, it has been long recognized that “strong” agonists such as collagen and thrombin induce granule secretion independent of platelet aggregation. By contrast, the “weak” agonists, such as ADP, or low concentrations of “strong” agonists induce secretion in an aggregation-dependent manner, which requires integrin outside signaling. Several platelet signaling pathways are important in promoting agonist-stimulated platelet granule secretion: 1) calcium elevation (201); 2) PKC signaling; 3) SFK signaling (31, 60, 129, 177, 185, 190, 239); 4) PI3K/AKT and NO/cGMP/PKG signaling pathways (127, 128, 239, 240); 5) MAPK signaling (4, 24, 115, 126); 6) CalDAG-GEFI and Rap1b signaling (41, 197, 227, 245); and 7) small RhoGTPase Rac1 and RhoA signaling (6, 51, 149, 174). The mechanisms by which these pathways promote secretion remain unclear and are the subject of reviews elsewhere (75, 76, 114).
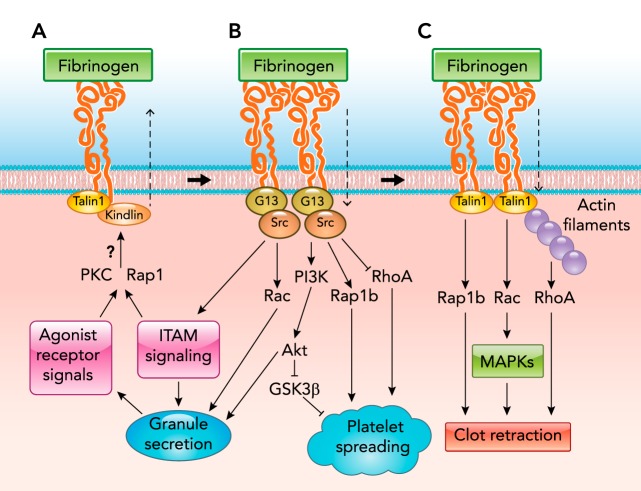
Integrin Inside-Out Signaling
Integrins play key roles in platelet adhesion and aggregation. Platelet integrins include collagen receptor α2β1, fibronectin receptor α5β1, laminin receptor α6β1, and vitronectin receptor αvβ3 (36, 125). The most abundant and important platelet integrin is the integrin αIIbβ3, which serves as a receptor for fibrinogen, VWF, vitronectin, and several other matrix adhesive proteins, and is required for stable platelet adhesion to the vascular wall and platelet aggregation. In circulating platelets, integrin αIIbβ3 is expressed in a “resting” form, wherein it is suggested to be in a bent conformation with a low affinity for ligands. After agonist stimulation, intracellular signals induce the extracellular domain of αIIbβ3 to transform into a suggested extended conformation with a high affinity for ligands (58). This process is known as inside-out signaling. A key step of inside-out signaling is the binding of cytosolic adapter protein talin to a site on the β3 cytoplasmic tail containing an NPXY motif, which is thought to facilitate a secondary interaction with a membrane proximal binding site on β3 (223) (FIGURE 4). These interactions were suggested to disrupt inner and outer membrane clasps held between α- and β-chain cytoplasmic and transmembrane regions triggering αIIbβ3 activation. Deletion of talin1 or mutation of the talin binding site on the β3 cytoplasmic tail impairs agonist-induced integrin activation (163, 173) and platelet aggregation (163). In addition to talin, kindlins are also important in inside-out signaling. Kindlins bind to a β3 COOH-terminal NXXY motif distinct from the talin binding site (157). Several studies suggested that the binding of talin and kindlins cooperates to induce full integrin activation (111, 234). How kindlins induce integrin activation is still a subject of debate. There have been reports suggesting that kindlins facilitate talin binding (139). Others suggest that kindlins, unlike talin, is dispensable for integrin binding to a monovalent ligand (fibronectin tenth type III repeat) but is required for binding to multivalent ligand fibrinogen, possibly by promoting clustering of integrins (156, 234, 235).
The mechanism responsible for inducing talin and kindlin binding to integrins is not totally clear. There have been reports suggesting that the small GTPase Rap1 plays an important role and hypothesizing that Rap1 GTP-interacting adaptor molecule (RIAM) may be the Rap1 effector responsible for inducing talin binding (82, 121, 219). However, although RIAM is important for leukocyte integrin function (112), RIAM deficiency does not affect platelet integrin activation and function (200).
Integrin Outside-In Signaling
Ligand binding to integrins not only mediates platelet adhesion and aggregation, but it also induces intracellular signals. This process is known as outside-in signaling, which, in the early phase, induces stabilization of platelet adhesion, platelet spreading, granule secretion, and amplification of platelet aggregation, which is important for growth of an occlusive intravascular thrombus (58, 125, 192), and, in the later phase, stimulates clot retraction (FIGURE 4). The early phase of outside-in signaling requires the binding of heterotrimeric G protein Gα13 to a conserved EXE motif on the β3 cytoplasmic tail (77, 193). A role for kindlin-3 was also reported for outside-in signaling (157). Integrin ligation induces Gα13 binding and talin dissociation from β3 (193). Gα13 binding is important for integrin-mediated c-Src activation (77). Activation of the β3 COOH-terminal-associated c-Src (9) is required for transmitting outside-in signaling via phosphorylation of β3 cytoplasmic NXXT motifs (1, 119), inhibition of RhoA, activation of the PI3K/Akt pathway, and Rac1 as well as Rap1b, leading to platelet spreading (67, 81, 245) (FIGURE 4). Src is also important in integrin-dependent activation of the ITAM signaling pathway (190, 248). All these events lead to integrin-dependent granule secretion and greatly amplified thrombus growth. In the late phase, calpain cleavage of the COOH-terminal Src binding site in β3 abolishes Src-dependent inhibition of RhoA, resulting in activation of RhoA- and platelet-mediated clot retraction (68, 229). Interestingly, talin becomes re-associated with β3 cytoplasmic domain during the late phase of outside-in signaling and is important for clot retraction (77, 193). Recently, vacuolar protein sorting-associated protein 33B (VPS33B), previously shown to be important in megakaryocyte and platelet α-granule biogenesis (134), was found to bind to the β3 tail. VPS33B-null platelets display defective outside-in signaling and endocytosis of fibrinogen (231).
FIGURE 4.
Integrin signaling pathways
A: integrin inside-out signaling. A key final step of inside-out signaling is the binding of cytosolic adapter protein talin and kindlins. Question marks indicate that the mechanisms remain unclear and require further investigation. B: early phase integrin outside-in signaling, which requires the interaction between Gα13 and Src with the β3 cytoplasmic domain. Talin dissociation occurs during the early phase outside-in signaling. C: late-phase integrin outside-in signaling, which requires talin reassociation and calpain cleavage of Src binding site in the β3 tail.
Conclusions and Perspectives
With significant recent advances in platelet physiology, the intricate platelet activation signaling networks continue to grow in complexity and sophistication. Recent advances also necessitate the revision of some textbook concepts in platelets such as the roles of NO and cGMP in platelet activation. Notably, pattern recognition receptors important for immune response and inflammation utilize the cGMP pathway to transmit platelet activation signals, suggesting a link between inflammation and thrombosis via this self-regulated signal pathway promoting platelet activation and secretion. These advances are largely attributed to widespread use of targeted gene deletion/mutation techniques in mice combined with pharmacological innovations, which not only have generated numerous insights into the mechanisms of platelet activation, but also have created new models and avenues for future research and pharmaceutical development. One important goal of studying platelet activation signaling is to provide targets for developing new drugs that can be used to more precisely treat thrombosis and hemorrhage. In this regard, the discovery of selectively targeting of integrin outside-in signaling without perturbing the ligand binding to integrin αIIbβ3 allows selective inhibition of occlusive thrombosis without apparent effect on hemostasis in mouse models (193), which is in contrast to the adverse effects of hemorrhage associated with current anti-platelet drugs. Clearly, more precise understanding of the complex platelet activation signaling pathways should help us in developing new generations of platelet drugs in treating thrombotic, inflammatory, and hemorrhagic disorders. After all, the precision of our understanding in mechanisms of health and disease determines how precise medicine can be.
Footnotes
This work is supported by National Heart, Lung, and Blood Institute Grants and contracts HL-062350, HL-080264, HL-125356, HHSN268201400007C, and HHSN268201700002C (X.D.) and 5F31 HL-123319 (B.E.).
X.D. holds patents relevant to the subject, and has financial interests in DMT, Inc.
Author contributions: B.E. and X.D. prepared figures; B.E. and X.D. drafted manuscript; B.E. and X.D. edited and revised manuscript; B.E. and X.D. approved final version of manuscript.
References
- 1.Ablooglu AJ, Kang J, Petrich BG, Ginsberg MH, Shattil SJ. Antithrombotic effects of targeting alphaIIbbeta3 signaling in platelets. Blood 113: 3585–3592, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aburima A, Wraith KS, Raslan Z, Law R, Magwenzi S, Naseem KM. cAMP signaling regulates platelet myosin light chain (MLC) phosphorylation and shape change through targeting the RhoA-Rho kinase-MLC phosphatase signaling pathway. Blood 122: 3533–3545, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Adam F, Guillin MC, Jandrot-Perrus M. Glycoprotein Ib-mediated platelet activation. Eur J Biochem 270: 2959–2970, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Adam F, Kauskot A, Nurden P, Sulpice E, Hoylaerts MF, Davis RJ, Rosa JP, Bryckaert M. Platelet JNK1 is involved in secretion and thrombus formation. Blood 115: 4083–4092, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Adler DH, Cogan JD, Phillips JA, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, et al. Inherited human cPLA(2α) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest 118: 2121–2131, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbar H, Kim J, Funk K, Cancelas J, Shang X, Chen L, Johnson J, Williams D, Zheng Y. Genetic and pharmacologic evidence that Rac1 GTPase is involved in regulation of platelet secretion and aggregation. J Thromb Haemost 5: 1747–1755, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood 106: 2417–2423, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Arachiche A, Mumaw MM, De La Fuente M, Nieman MT. Protease-activated receptor 1 (PAR1) and PAR4 heterodimers are required for PAR1-enhanced cleavage of PAR4 by α-thrombin. J Biol Chem 288: 32553–32562, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci USA 100: 13298–13302, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arman M, Krauel K. Human platelet IgG Fc receptor FcgammaRIIA in immunity and thrombosis. J Thromb Haemost 13: 893–908, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 107: 637–641, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood 102: 3592–3599, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Bauer M, Retzer M, Wilde JI, Maschberger P, Essler M, Aepfelbacher M, Watson SP, Siess W. Dichotomous regulation of myosin phosphorylation and shape change by rho-kinase and calcium in intact human platelets. Blood 94: 1665–1672, 1999. [PubMed] [Google Scholar]
- 14.Beck F, Geiger J, Gambaryan S, Veit J, Vaudel M, Nollau P, Kohlbacher O, Martens L, Walter U, Sickmann A. Time-resolved characterization of cAMP/PKA-dependent signaling reveals that platelet inhibition is a concerted process involving multiple signaling pathways. Blood 123: e1–e10, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Berlanga O, Tulasne D, Bori T, Snell DC, Miura Y, Jung S, Moroi M, Frampton J, Watson SP. The Fc receptor gamma-chain is necessary and sufficient to initiate signalling through glycoprotein VI in transfected cells by the snake C-type lectin, convulxin. Eur J Biochem 269: 2951–2960, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu Mm, Zhou D. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 116: 661–670, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Best LC, Martin TJ, Russell RG, Preston FE. Prostacyclin increases cyclic AMP levels and adenylate cyclase activity in platelets. Nature 267: 850–852, 1977. [DOI] [PubMed] [Google Scholar]
- 18.Biswas S, Xin L, Panigrahi S, Zimman A, Wang H, Yakubenko VP, Byzova TV, Salomon RG, Podrez EA. Novel phosphatidylethanolamine derivatives accumulate in circulation in hyperlipidemic ApoE−/− mice and activate platelets via TLR2. Blood 127: 2618–2629, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res 104: 346–354, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobe R, Wilde JI, Maschberger P, Venkateswarlu K, Cullen PJ, Siess W, Watson SP. Phosphatidylinositol 3-kinase-dependent translocation of phospholipase Cγ2 in mouse megakaryocytes is independent of Bruton tyrosine kinase translocation. Blood 97: 678–684, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Boulaftali Y, Hess PR, Getz TM, Cholka A, Stolla M, Mackman N, Owens AP, Ware J, Kahn ML, Bergmeier W. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest 123: 908–916, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulaftali Y, Hess PR, Kahn ML, Bergmeier W. Platelet ITAM signaling and vascular integrity. Circ Res 114: 1174–1184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcγRIIa as the ITAM-bearing receptor mediating αIIbβ3 outside-in integrin signaling in human platelets. Blood 112: 2780–2786, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canobbio I, Reineri S, Sinigaglia F, Balduini C, Torti M. A role for p38 MAP kinase in platelet activation by von willebrand Factor. Thromb Haemost 91: 102–110, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Canobbio I, Stefanini L, Cipolla L, Ciraolo E, Gruppi C, Balduini C, Hirsch E, Torti M. Genetic evidence for a predominant role of PI3Kβ catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood 114: 2193–2196, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Cattaneo M, Gachet C. ADP receptors and clinical bleeding disorders. Arterioscler Thromb Vasc Biol 19: 2281–2285, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Celikel R, McClintock RA, Roberts JR, Mendolicchio GL, Ware J, Varughese KI, Ruggeri ZM. Modulation of alpha thrombin function by distinct interactions with platelet glycoprotein Ib alpha. Science 301: 218–221, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood 104: 1703–1710, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res 102: 1512–1519, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho MJ, Liu J, Pestina TI, Steward SA, Thomas DW, Coffman TM, Wang D, Jackson CW, Gartner TK. The roles of alpha IIb beta3-mediated outside-in signal transduction, thromboxane A2, and adenosine diphosphate in collagen-induced platelet aggregation. Blood 101: 2646–2651, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Cho MJ, Pestina TI, Steward SA, Lowell CA, Jackson CW, Gartner TK. Role of the Src family kinase Lyn in TxA2 production, adenosine diphosphate secretion, Akt phosphorylation, and irreversible aggregation in platelets stimulated with γ-thrombin. Blood 99: 2442–2447, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC II. Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest 115: 680–687, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clements JL, Lee JR, Gross B, Yang B, Olson JD, Sandra A, Watson SP, Lentz SR, Koretzky GA. Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice. J Clin Invest 103: 19–25, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coffey MJ, Jarvis GE, Gibbins JM, Coles B, Barrett NE, Wylie OR, O'Donnell VB. Platelet 12-lipoxygenase activation via glycoprotein VI: involvement of multiple signaling pathways in agonist control of H(P)ETE synthesis. Circ Res 94: 1598–1605, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol 83: 196–198, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood 112: 3011–3025, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cosemans JM, Munnix IC, Wetzker R, Heller R, Jackson SP, Heemskerk JW. Continuous signaling via PI3K isoforms β and γ is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood 108: 3045–3052, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 3: 1800–1814, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J Thromb Haemost 9: 1097–1107, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Cranmer SL, Ashworth KJ, Yao Y, Berndt MC, Ruggeri ZM, Andrews RK, Jackson SP. High shear-dependent loss of membrane integrity and defective platelet adhesion following disruption of the GPIbα-filamin interaction. Blood 117: 2718–2727, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med 10: 982–986, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Cruz MA, Diacovo TG, Emsley J, Liddington R, Handin RI. Mapping the glycoprotein Ib-binding site in the von willebrand factor A1 domain. J Biol Chem 275: 19098–19105, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Czikora A, Lundberg DJ, Abramovitz A, Lewin NE, Kedei N, Peach ML, Zhou X, Merritt RC, Craft EA, Braun DC. Structural basis for the failure of the C1 domain of Ras guanine nucleotide releasing protein 2 (RasGRP2) to bind phorbol ester with high affinity. J Biol Chem 291: 11133–11147, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai K, Bodnar R, Berndt MC, Du X. A critical role for 14-3-3 protein in regulating the VWF binding function of platelet glycoprotein Ib-IX and its therapeutic implications. Blood 106: 1975–1981, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Candia E. Mechanisms of platelet activation by thrombin: a short history. Thromb Res 129: 250–256, 2012. [DOI] [PubMed] [Google Scholar]
- 46.De Candia E, Hall SW, Rutella S, Landolfi R, Andrews RK, De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J Biol Chem 276: 4692–4698, 2001. [DOI] [PubMed] [Google Scholar]
- 47.De Luca M, Facey DA, Favaloro EJ, Hertzberg MS, Whisstock JC, McNally T, Andrews RK, Berndt MC. Structure and function of the von Willebrand factor A1 domain: analysis with monoclonal antibodies reveals distinct binding sites involved in recognition of the platelet membrane glycoprotein Ib-IX-V complex and ristocetin-dependent activation. Blood 95: 164–172, 2000. [PubMed] [Google Scholar]
- 48.De Marco L, Mazzucato M, Masotti A, Ruggeri ZM. Localization and characterization of an alpha-thrombin-binding site on platelet glycoprotein Ib alpha. J Biol Chem 269: 6478–6484, 1994. [PubMed] [Google Scholar]
- 49.Delaney MK, Kim K, Estevez B, Xu Z, Stojanovic-Terpo A, Shen B, Ushio-Fukai M, Cho J, Du X. Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler Thromb Vasc Biol 36: 846–854, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delaney MK, Kim K, Estevez B, Xu Z, Stojanovic-Terpo A, Shen B, Ushio-Fukai M, Cho J, Du X. Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler Thromb Vasc Biol 36: 846–854, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delaney MK, Liu J, Zheng Y, Berndt MC, Du X. A role for Rac1 in glycoprotein Ib-IX-mediated signal transduction and integrin activation. Arterioscler Thromb Vasc Biol 32: 2761–2768, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devanathan V, Hagedorn I, Köhler D, Pexa K, Cherpokova D, Kraft P, Singh M, et al. Platelet Gi protein Gαi2 is an essential mediator of thrombo-inflammatory organ damage in mice. Proc Natl Acad Sci USA 112: 6491–6496, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du X. Signaling and regulation of the platelet glycoprotein Ib-IX-V complex. Curr Opin Hemaotol 14: 262–269, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 121: 1008–1015, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumas JJ, Kumar R, Seehra J, Somers WS, Mosyak L. Crystal structure of the GpIb alpha thrombin complex essential for platelet aggregation. Science 301: 222–226, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Dütting S, Vögtle T, Morowski M, Schiessl S, Schäfer CM, Watson SK, Hughes CE, et al. Growth factor receptor-bound protein 2 contributes to (Hem)immunoreceptor tyrosine-based activation motif-mediated signaling in platelets. Circ Res 114: 444–453, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estevez B, Kim K, Delaney MK, Stojanovic-Terpo A, Shen B, Ruan C, Cho J, Ruggeri ZM, Du X. Signaling-mediated cooperativity between glycoprotein Ib-IX and protease-activated receptors in thrombin-induced platelet activation. Blood 127: 626–636, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estevez B, Shen B, Du X. Targeting integrin and integrin signaling in treating thrombosis. Arterioscler Thromb Vasc Biol 35: 24–29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Estevez B, Stojanovic-Terpo A, Delaney MK, O'Brien KA, Berndt MC, Ruan C, Du X. LIM kinase-1 selectively promotes glycoprotein Ib-IX-mediated TXA2 synthesis, platelet activation, and thrombosis. Blood 121: 4586–4594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ezumi Y, Shindoh K, Tsuji M, Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor gamma chain complex on human platelets. J Exp Med 188: 267–276, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med 5: 1199, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Falati S, Edmead CE, Poole AW. Glycoprotein Ib-V-IX,, a receptor for von Willebrand factor, couples physically and functionally to the Fc receptor γ-chain, Fyn, and Lyn to activate human platelets. Blood 94: 1648–1656, 1999. [PubMed] [Google Scholar]
- 63.Falet H, Pollitt AY, Begonja AJ, Weber SE, Duerschmied D, Wagner DD, Watson SP, Hartwig JH. A novel interaction between FlnA and Syk regulates platelet ITAM-mediated receptor signaling and function. J Exp Med 207: 1967–1979, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan X, Shi P, Dai J, Lu Y, Chen X, Liu X, Zhang K, Wu X, Sun Y, Wang K. Paired immunoglobulin-like receptor B regulates platelet activation. Blood 124: 2421–2430, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng W, Chang C, Luo D, Su H, Yu S, Hua W, Chen Z, Hu H, Liu W. Dissection of autophagy in human platelets. Autophagy 10: 642–651, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finney BA, Schweighoffer E, Navarro-Núñez L, Bénézech C, Barone F, Hughes CE, Langan SA, Lowe KL, Pollitt AY, Mourao-Sa D. CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood 119: 1747–1756, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flevaris P, Li Z, Zhang G, Zheng Y, Liu J, Du X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood 113: 893–901, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flevaris P, Stojanovic A, Gong H, Chishti A, Welch E, Du X. A molecular switch that controls cell spreading and retraction. J Cell Biol 179: 553–565, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia A, Quinton TM, Dorsam RT, Kunapuli SP. Src family kinase A-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets. Blood 106: 3410–3414, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Getz TM, Dangelmaier CA, Jin J, Daniel JL, Kunapuli SP. Differential phosphorylation of myosin light chain (Thr)18 and (Ser)19 and functional implications in platelets. J Thromb Haemost 8: 2283–2293, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghasemzadeh M, Kaplan ZS, Alwis I, Schoenwaelder SM, Ashworth KJ, Westein E, Hosseini E, et al. The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood 121: 4555–4566, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor γ-chain in collagen-stimulated platelets. J Biol Chem 271: 18095–18099, 1996. [DOI] [PubMed] [Google Scholar]
- 73.Gibbins JM, Okuma M, Farndale R, Barnes M, Watson SP. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor gamma-chain. FEBS Lett 413: 255–259, 1997. [DOI] [PubMed] [Google Scholar]
- 74.Goggs R, Williams Christopher M, Mellor H, Poole Alastair W. Platelet Rho GTPases: a focus on novel players, roles and relationships. Biochem J 466: 431–442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev 29: 153–162, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Golebiewska EM, Poole AW. Secrets of platelet exocytosis-what do we really know about platelet secretion mechanisms? Br J Haematol 165: 204–216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong H, Shen B, Flevaris P, Chow C, Lam SC, Voyno-Yasenetskaya TA, Kozasa T, Du X. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science 327: 340–343, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greco NJ, Tandon NN, Jones GD, Kornhauser R, Jackson B, Yamamoto N, Tanoue K, Jamieson GA. Contributions of glycoprotein Ib and the seven transmembrane domain receptor to increases in platelet cytoplasmic [Ca2+] induced by I ± -thrombin. Biochemistry 35: 906–914, 1996. [DOI] [PubMed] [Google Scholar]
- 79.Gross BS, Lee JR, Clements JL, Turner M, Tybulewicz VLJ, Findell PR, Koretzky GA, Watson SP. Tyrosine phosphorylation of SLP-76 is downstream of Syk following stimulation of the collagen receptor in platelets. J Biol Chem 274: 5963–5971, 1999. [DOI] [PubMed] [Google Scholar]
- 80.Gu M, Xi X, Englund GD, Berndt MC, Du X. Analysis of the roles of 14-3-3 in the platelet glycoprotein Ib-IX mediated activation of integrin alphaiib beta3 using a reconstituted mammalian cell expression model. J Cell Biol 147: 1085–1096, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guidetti GF, Torti M. The small GTPase Rap1b: a bidirectional regulator of platelet adhesion receptors. J Signal Trans 2012: 412089, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol 16: 1796–1806, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Harper M, Poole A. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. J Thromb Haemost 8: 454–462, 2010. [DOI] [PubMed] [Google Scholar]
- 84.Haslam RJ, Dickinson NT, Jang EK. Cyclic nucleotides and phosphodiesterases in platelets. Thromb Haemost 82: 412–423, 1999. [PubMed] [Google Scholar]
- 85.Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, Pan Y, Sheng M, Yago T, Silasi-Mansat R. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 502: 105–109, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, Herzog B, Lu M, Nieswandt B, Oliver G. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest 124: 273–284, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirabayashi T, Murayama T, Shimizu T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biol Pharm Bull 27: 1168–1173, 2004. [DOI] [PubMed] [Google Scholar]
- 88.Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, WYMANNM, Montrucchio G. Resistance to thromboembolism in PI3Kγ-deficient mice. FASEB J 15: 2019–2021, 2001. [DOI] [PubMed] [Google Scholar]
- 89.Holinstat M, Boutaud O, Apopa P, Vesci J, Bala M, Oates JA, Hamm HE. Protease-activated receptor signaling in platelets activates cytosolic phospholipase A(2)(α) differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arterioscler Thromb Vasc Biol 31: 435–442, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409: 202–207, 2001. [DOI] [PubMed] [Google Scholar]
- 91.Hughes CE, Auger JM, McGlade J, Eble JA, Pearce AC, Watson SP. Differential roles for the adapters Gads and LAT in platelet activation by GPVI and CLEC-2. J Thromb Haemost 6: 2152–2159, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hughes CE, Finney BA, Koentgen F, Lowe KL, Watson SP. The N-terminal SH2 domain of Syk is required for (hem)ITAM, but not integrin, signaling in mouse platelets. Blood 125: 144–154, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huizinga EG, Tsuji S, Romijn RAP, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Ibα and its complex with von Willebrand Factor A1 domain. Science 297: 1176–1179, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Ichinohe T, Takayama H, Ezumi Y, Arai M, Yamamoto N, Takahashi H, Okuma M. Collagen-stimulated activation of Syk but not c-Src is severely compromised in human platelets lacking membrane glycoprotein VI. J Biol Chem 272: 63–68, 1997. [DOI] [PubMed] [Google Scholar]
- 95.Jamieson GA, Okumura T. Reduced thrombin binding and aggregation in Bernard-Soulier platelets. J Clin Invest 61: 861–864, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jantzen HM, Milstone DS, Gousset L, Conley PB, Mortensen RM. Impaired activation of murine platelets lacking Gαi2. J Clin Invest 108: 477–483, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci USA 95: 8070–8074, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnson EN, Brass LF, Funk CD. Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proc Natl Acad Sci USA 95: 3100–3105, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson GJ, Leis LA, Krumwiede MD, White JG. The critical role of myosin IIA in platelet internal contraction. J Thromb Haemost 5: 1516–1529, 2007. [DOI] [PubMed] [Google Scholar]
- 100.Ju L, Chen Y, Xue L, Du X, Zhu C. Cooperative unfolding of distinctive mechanoreceptor domains transduces force into signals. eLife 5: e15447, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ju L, Lou J, Chen Y, Li Z, Zhu C. Force-induced unfolding of leucine-rich repeats of glycoprotein Ibα strengthens ligand interaction. Biophys J 109: 1781–1784, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, Shattil SJ. Signaling through GP Ib-IX-V activates αIIbβ3 independently of other receptors. Blood 103: 3403–3411, 2004. [DOI] [PubMed] [Google Scholar]
- 103.Kasirer-Friede A, Kang J, Kahner B, Ye F, Ginsberg MH, Shattil SJ. ADAP interactions with talin and kindlin promote platelet integrin αIIbβ3 activation and stable fibrinogen binding. Blood 123: 3156–3165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, Marchese P, Reininger A, Ruggeri ZM, Ware J. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood 102: 1701–1707, 2003. [DOI] [PubMed] [Google Scholar]
- 105.Kauffenstein G, Bergmeier W, Eckly A, Ohlmann P, Leon C, Cazenave J, Nieswandt B, Gachet C. The P2Y12 receptor induces platelet aggregation through weak activation of the αIIbβ3 integrin-a phosphoinositide 3-kinase-dependent mechanism. FEBS Lett 505: 281–290, 2001. [DOI] [PubMed] [Google Scholar]
- 106.Kehrel B, Wierwille S, Clemetson KJ, Anders O, Steiner M, Graham Knight C, Farndale RW, Okuma M, Barnes MJ. Glycoprotein VI is a major collagen receptor for platelet activation: it recognizes the platelet-activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa, and von Willebrand Factor do not. Blood 91: 491–499, 1998. [PubMed] [Google Scholar]
- 107.Khan A, Li D, Ibrahim S, Smyth E, Woulfe DS. The physical association of the P2Y12 receptor with PAR4 regulates arrestin-mediated Akt activation. Mol Pharmacol 86: 1–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim J, Zhang CZ, Zhang X, Springer TA. A mechanically stabilized receptor-ligand flex-bond important in the vasculature. Nature 466: 992–995, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim S, Foster C, Lecchi A, Quinton TM, Prosser DM, Jin J, Cattaneo M, Kunapuli SP. Protease-activated receptors 1 and 4 do not stimulate Gi signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of Gi signaling. Blood 99: 3629–3636, 2002. [DOI] [PubMed] [Google Scholar]
- 110.Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol 144: 745–754, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klapproth S, Moretti FA, Zeiler M, Ruppert R, Breithaupt U, Mueller S, Haas R, Mann M, Sperandio M, Fässler R, Moser M. Minimal amounts of kindlin-3 suffice for basal platelet and leukocyte functions in mice. Blood 126: 2592–2600, 2015. [DOI] [PubMed] [Google Scholar]
- 112.Klapproth S, Sperandio M, Pinheiro EM, Prünster M, Soehnlein O, Gertler FB, Fässler R, Moser M. Loss of the Rap1 effector RIAM results in leukocyte adhesion deficiency due to impaired β2 integrin function in mice. Blood 126: 2704–2712, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci 28: 397–406. [DOI] [PubMed] [Google Scholar]
- 114.Koseoglu S, Flaumenhaft R. Advances in platelet granule biology. Curr Opin Hematol 20: 464–471, 2013. [DOI] [PubMed] [Google Scholar]
- 115.Kramer RM, Roberts EF, Um SL, Börsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA. p38 Mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. J Biol Chem 271: 27723–27729, 1996. [DOI] [PubMed] [Google Scholar]
- 116.Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J Biol Chem 272: 24252–24256, 1997. [DOI] [PubMed] [Google Scholar]
- 117.Labelle M, Begum S, Hynes Richard O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20: 576–590, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laurent PA, Séverin S, Hechler B, Vanhaesebroeck B, Payrastre B, Gratacap MP. Platelet PI3Kβ and GSK3 regulate thrombus stability at a high shear rate. Blood 125: 881–888, 2015. [DOI] [PubMed] [Google Scholar]
- 119.Law DA, DeGuzman FR, Heiser P, Ministri-Madrid K, Killeen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature 401: 808–811, 1999. [DOI] [PubMed] [Google Scholar]
- 120.Lecut C, Schoolmeester A, Kuijpers MJE, Broers JLV, van Zandvoort MAMJ, Vanhoorelbeke K, Deckmyn H, Jandrot-Perrus M, Heemskerk JWM. Principal role of glycoprotein VI in α2β1 and αIIbβ3 activation during collagen-induced thrombus formation. Arterioscler Thromb Vasc Biol 24: 1727–1733, 2004. [DOI] [PubMed] [Google Scholar]
- 121.Lee HS, Lim CJ, Puzon-McLaughlin W, Shattil SJ, Ginsberg MH. RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J Biol Chem 284: 5119–5127, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee SB, Rao AK, Lee KH, Yang X, Bae YS, Rhee SG. Decreased expression of phospholipase C-beta 2 isozyme in human platelets with impaired function. Blood 88: 1684–1691, 1996. [PubMed] [Google Scholar]
- 123.Léon C, Eckly A, Hechler B, Aleil B, Freund M, Ravanat C, Jourdain M, Nonne C, Weber J, Tiedt R, Gratacap MP, Severin S, Cazenave JP, Lanza F, Skoda R, Gachet C. Megakaryocyte-restricted MYH9 inactivation dramatically affects hemostasis while preserving platelet aggregation and secretion. Blood 110: 3183–3191, 2007. [DOI] [PubMed] [Google Scholar]
- 124.Li D, August S, Woulfe DS. GSK3β is a negative regulator of platelet function and thrombosis. Blood 111: 3522–3530, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Z, Delaney MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol 30: 2341–2349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin αIIbβ3. J Biol Chem 276: 42226–42232, 2001. [DOI] [PubMed] [Google Scholar]
- 127.Li Z, Xi X, Gu M, Feil R, Ye RD, Eigenthaler M, Hofmann F, Du X. A stimulatory role for cGMP-dependent protein kinase in platelet activation. Cell 112: 77–86, 2003. [DOI] [PubMed] [Google Scholar]
- 128.Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin αIIbβ3. Blood 107: 965–972, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Z, Zhang G, Liu J, Stojanovic A, Ruan C, Lowell CA, Du X. An important role of the Src family kinase Lyn in stimulating platelet granule secretion. J Biol Chem 285: 12559–12570, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lian L, Wang Y, Draznin J, Eslin D, Bennett JS, Poncz M, Wu D, Abrams CS. The relative role of PLCβ and PI3Kγ in platelet activation. Blood 106: 110–117, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost 106: 827–838, 2011. [DOI] [PubMed] [Google Scholar]
- 132.Liu J, Fitzgerald ME, Berndt MC, Jackson CW, Gartner TK. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood 108: 2596–2603, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J, Pestina TI, Berndt MC, Jackson CW, Gartner TK. Botrocetin/VWF-induced signaling through GPIb-IX-V produces TxA2 in an I ± IIbI23- and aggregation-independent manner. Blood 106: 2750–2756, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lo B, Li L, Gissen P, Christensen H, McKiernan PJ, Ye C, Abdelhaleem M, Hayes JA, Williams MD, Chitayat D, Kahr WHA. Requirement of VPS33B, a member of the Sec1/Munc18 protein family, in megakaryocyte and platelet α-granule biogenesis. Blood 106: 4159–4166, 2005. [DOI] [PubMed] [Google Scholar]
- 135.Lou J, Zhu C. Flow induces loop-to-beta-hairpin transition on the beta-switch of platelet glycoprotein Ib alpha. Proc Natl Acad Sci USA 105: 13847–13852, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lova P, Paganini S, Hirsch E, Barberis L, Wymann M, Sinigaglia F, Balduini C, Torti M. A selective role for phosphatidylinositol 3,4,5-trisphosphate in the Gi-dependent activation of platelet Rap1B. J Biol Chem 278: 131–138, 2003. [DOI] [PubMed] [Google Scholar]
- 137.Lova P, Paganini S, Sinigaglia F, Balduini C, Torti M. A Gi-dependent pathway is required for activation of the small GTPase Rap1B in human platelets. J Biol Chem 277: 12009–12015, 2002. [DOI] [PubMed] [Google Scholar]
- 138.Luci DK, Jameson JB, Yasgar A, Diaz G, Joshi N, Kantz A, Markham K, Perry S, Kuhn N, Yeung J, Kerns EH, Schultz L, Holinstat M, Nadler JL, Taylor-Fishwick DA, Jadhav A, Simeonov A, Holman TR, Maloney DJ. Synthesis and structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl)amino)benzenesulfonamide derivatives as potent and selective inhibitors of 12-lipoxygenase. J Med Chem 57: 495–506, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of β(3) integrins. J Cell Biol 181: 439–446, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maier U, Babich A, Nürnberg B. Roles of non-catalytic subunits in Gβγ-induced activation of class I phosphoinositide 3-kinase isoforms β and γ. J Biol Chem 274: 29311–29317, 1999. [DOI] [PubMed] [Google Scholar]
- 141.Mangin P, Yuan Y, Goncalves I, Eckly A, Freund M, Cazenave JP, Gachet C, Jackson SP, Lanza F. Signaling role for phospholipase Cγ2 in platelet glycoprotein Ibα calcium flux and cytoskeletal reorganization: involvement of a pathway distinct from FcRγ chain and FcγRIIA. J Biol Chem 278: 32880–32891, 2003. [DOI] [PubMed] [Google Scholar]
- 142.Manne BK, Badolia R, Dangelmaier C, Eble JA, Ellmeier W, Kahn M, Kunapuli SP. Distinct pathways regulate Syk protein activation downstream of immune tyrosine activation motif (ITAM) and hemITAM receptors in platelets. J Biol Chem 290: 11557–11568, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marchese P, Murata M, Mazzucato M, Pradella P, De Marco L, Ware J, Ruggeri ZM. Identification of three tyrosine residues of glycoprotein Ib with distinct roles in von Willebrand Factor and -thrombin binding. J Biol Chem 270: 9571–9578, 1995. [DOI] [PubMed] [Google Scholar]
- 144.Marjanovic JA, Stojanovic A, Brovkovych V, Skidgel RA, Du X. Inducible nitric oxide synthase plays a stimulatory role in platelet activation. Blood 106: 1650–1650, 2005. [Google Scholar]
- 145.Marjanovic JA, Stojanovic A, Brovkovych VM, Skidgel RA, Du X. Signaling-mediated functional activation of inducible nitric-oxide synthase and its role in stimulating platelet activation. J Biol Chem 283: 28827–28834, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Massberg S, Konrad I, Schürzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, et al. Platelets secrete stromal cell-derived factor 1α and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med 203: 1221–1233, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Massberg S, Sausbier M, Klatt P, Bauer M, Pfeifer A, Siess W, Fässler R, Ruth P, Krombach F, Hofmann F. Increased adhesion and aggregation of platelets lacking cyclic guanosine 3′,5′-monophosphate kinase I. J Exp Med 189: 1255–1264, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matsushita T, Sadler JE. Identification of amino acid residues essential for von Willebrand factor binding to platelet glycoprotein Ib. Charged-to-alanine scanning mutagenesis of the A1 domain of human von Willebrand factor. J Biol Chem 270: 13406–13414, 1995. [DOI] [PubMed] [Google Scholar]
- 149.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem 280: 39474–39484, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ming Z, Hu Y, Xiang J, Polewski P, Newman PJ, Newman DK. Lyn and PECAM-1 function as interdependent inhibitors of platelet aggregation. Blood 117: 3903–3906, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miura S, Li CQ, Cao Z, Wang H, Wardell MR, Sadler JE. Interaction of von Willebrand factor domain A1 with platelet glycoprotein Ibalpha-(1–289). Slow intrinsic binding kinetics mediate rapid platelet adhesion. J Biol Chem 275: 7539–7546, 2000. [DOI] [PubMed] [Google Scholar]
- 152.Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, Gawaz M, Offermanns S. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med 9: 1418–1422, 2003. [DOI] [PubMed] [Google Scholar]
- 153.Moers A, Wettschureck N, Grüner S, Nieswandt B, Offermanns S. Unresponsiveness of Platelets Lacking Both Gαq and Gα13. Implications For Collagen-Induced Platelet Activation. J Biol Chem 279: 45354–45359, 2004. [DOI] [PubMed] [Google Scholar]
- 154.Moore SF, van den Bosch MT, Hunter RW, Sakamoto K, Poole AW, Hers I. Dual regulation of glycogen synthase kinase 3 (GSK3) α/β by protein kinase C (PKC) α and Akt promotes thrombin-mediated integrin αIIbβ3 activation and granule secretion in platelets. J Biol Chem 288: 3918–3928, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moroi M, Jung SM, Okuma M, Shinmyozu K. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J Clin Invest 84: 1440, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Morse EM, Brahme NN, Calderwood DA. Integrin cytoplasmic tail interactions. Biochemistry 53: 810–820, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med 14: 325–330, 2008. [DOI] [PubMed] [Google Scholar]
- 158.Mountford JK, Petitjean C, Putra HWK, McCafferty JA, Setiabakti NM, Lee H, Tønnesen LL, et al. The class II PI 3-kinase, PI3KC2α, links platelet internal membrane structure to shear-dependent adhesive function. Nat Commun 6: 6535, 2015. [DOI] [PubMed] [Google Scholar]
- 159.Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med 255: 332–343, 2004. [DOI] [PubMed] [Google Scholar]
- 160.Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature 404: 609–613, 2000. [DOI] [PubMed] [Google Scholar]
- 161.Nieswandt B, Bergmeier W, Eckly A, Schulte V, Ohlmann P, Cazenave JP, Zirngibl H, Offermanns S, Gachet C. Evidence for cross-talk between glycoprotein VI and Gi-coupled receptors during collagen-induced platelet aggregation. Blood 97: 3829–3835, 2001. [DOI] [PubMed] [Google Scholar]
- 162.Nieswandt B, Bergmeier W, Schulte V, Rackebrandt K, Gessner JE, Zirngibl H. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRγ chain. J Biol Chem 275: 23998–24002, 2000. [DOI] [PubMed] [Google Scholar]
- 163.Nieswandt B, Moser M, Pleines I, Varga-Szabo D, Monkley S, Critchley D, Fassler R. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med 204: 3113–3118, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.O'Brien KA, Gartner TK, Hay N, Du X. ADP-stimulated activation of Akt during integrin outside-in signaling promotes platelet spreading by inhibiting glycogen synthase kinase-3beta. Arterioscler Thromb Vasc Biol 32: 2232–2240, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.O'Brien KA, Stojanovic-Terpo A, Hay N, Du X. An important role for Akt3 in platelet activation and thrombosis. Blood 118: 4215–4223, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res 99: 1293–1304, 2006. [DOI] [PubMed] [Google Scholar]
- 167.Offermanns S, Laugwitz KL, Spicher K, Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc Natl Acad Sci USA 91: 504–508, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in G alpha(q)-deficient mice. Nature 389: 183–186, 1997. [DOI] [PubMed] [Google Scholar]
- 169.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9: 60–71, 2008. [DOI] [PubMed] [Google Scholar]
- 170.Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ Res 112: 103–112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pasquet JM, Gross B, Quek L, Asazuma N, Zhang W, Sommers CL, Schweighoffer E, et al. LAT is required for tyrosine phosphorylation of phospholipase Cγ2 and platelet activation by the collagen receptor GPVI. Mol Cell Biol 19: 8326–8334, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Paul BZS, Daniel JL, Kunapuli SP. Platelet shape change is mediated by both calcium-dependent and -independent signaling pathways: role of p160 Rho-associated coiled-coil-containing protein kinase in platelet shape change. J Biol Chem 274: 28293–28300, 1999. [DOI] [PubMed] [Google Scholar]
- 173.Petrich BG, Fogelstrand P, Partridge AW, Yousefi N, Ablooglu AJ, Shattil SJ, Ginsberg MH. The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation. J Clin Invest 117: 2250–2259, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, Offermanns S, Krohne G, Kleinschnitz C, Brakebusch C, Nieswandt B. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood 119: 1054–1063, 2012. [DOI] [PubMed] [Google Scholar]
- 175.Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JGJ, Saito T, Tybulewicz VLJ, Watson SP. The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J 16: 2333–2341, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Quek LS, Bolen J, Watson SP. A role for Bruton's tyrosine kinase (Btk) in platelet activation by collagen. Curr Biol 8: 1137–1140, 1998. [DOI] [PubMed] [Google Scholar]
- 177.Quek LS, Pasquet JM, Hers I, Cornall R, Knight G, Barnes M, Hibbs ML, Dunn AR, Lowell CA, Watson SP. Fyn and Lyn phosphorylate the Fc receptor gamma chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood 96: 4246–4253, 2000. [PubMed] [Google Scholar]
- 178.Ramakrishnan V, DeGuzman F, Bao M, Hall SW, Leung LL, Phillips DR. A thrombin receptor function for platelet glycoprotein Ib-IX unmasked by cleavage of glycoprotein V. Proc Natl Acad Sci USA 98: 1823–1828, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Raslan Z, Naseem KM. The control of blood platelets by cAMP signalling. Biochem Soc Trans 42: 289–294, 2014. [DOI] [PubMed] [Google Scholar]
- 180.Reilly MP, Taylor SM, Hartman NK, Arepally GM, Sachais BS, Cines DB, Poncz M, McKenzie SE. Heparin-induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcγRIIA. Blood 98: 2442–2447, 2001. [DOI] [PubMed] [Google Scholar]
- 181.Ren Q, Wimmer C, Chicka MC, Ye S, Ren Y, Hughson FM, Whiteheart SW. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood 116: 869–877, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rhee SG. Regulation of phosphoinositide-specific phospholipase C*. Ann Rev Biochem 70: 281–312, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res 100: 1673–1685, 2007. [DOI] [PubMed] [Google Scholar]
- 184.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, et al. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J 22: 3546–3556, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schmaier AA, Zou Z, Kazlauskas A, Emert-Sedlak L, Fong KP, Neeves KB, Maloney SF, et al. Molecular priming of Lyn by GPVI enables an immune receptor to adopt a hemostatic role. Proc Natl Acad Sci USA 106: 21167–21172, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, Schneider MF. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci USA 104: 7899–7903, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schoenwaelder SM, Ono A, Sturgeon S, Chan SM, Mangin P, Maxwell MJ, Turnbull S, Mulchandani M, Anderson K, Kauffenstein G. Identification of a unique co-operative phosphoinositide 3-kinase signaling mechanism regulating integrin αIIbβ3 adhesive function in platelets. J Biol Chem 282: 28648–28658, 2007. [DOI] [PubMed] [Google Scholar]
- 188.Schumacher D, Strilic B, Sivaraj Kishor K, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24: 130–137, 2013. [DOI] [PubMed] [Google Scholar]
- 189.Sekiya F, Takagi J, Saito Y. Elucidation of a role of plasma albumin during collagen-induced aggregation of bovine platelets. Thromb Res 56: 407–415, 1989. [DOI] [PubMed] [Google Scholar]
- 190.Senis YA, Mazharian A, Mori J. Src family kinases: at the forefront of platelet activation. Blood 124: 2013–2024, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Severin S, Pollitt AY, Navarro-Nunez L, Nash CA, Mourao-Sa D, Eble JA, Senis YA, Watson SP. Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J Biol Chem 286: 4107–4116, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shen B, Delaney MK, Du X. Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr Opin Cell Biol 24: 600–606, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shen B, Zhao X, O'Brien KA, Stojanovic-Terpo A, Delaney MK, Kim K, Cho J, Lam SC, Du X. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature 503: 131–135, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shiraki R, Inoue N, Kawasaki S, Takei A, Kadotani M, Ohnishi Y, Ejiri J, Kobayashi S, Hirata K, Kawashima S, Yokoyama M. Expression of Toll-like receptors on human platelets. Thromb Res 113: 379–385, 2004. [DOI] [PubMed] [Google Scholar]
- 195.Siess W, Lapetina EG. Functional relationship between cyclic AMP-dependent protein phosphorylation and platelet inhibition. Biochem J 271: 815–819, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stefanini L, Bergmeier W. RAP1-GTPase signaling and platelet function. J Mol Med 94: 13–19, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stefanini L, Paul DS, Robledo RF, Chan ER, Getz TM, Campbell RA, Kechele DO, Casari C, Piatt R, Caron KM. RASA3 is a critical inhibitor of RAP1-dependent platelet activation. J Clin Invest 125: 1419, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stefanini L, Roden RC, Bergmeier W. CalDAG-GEFI is at the nexus of calcium-dependent platelet activation. Blood 114: 2506–2514, 2009. [DOI] [PubMed] [Google Scholar]
- 199.Stojanovic A, Marjanovic JA, Brovkovych VM, Peng X, Hay N, Skidgel RA, Du X. A phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. J Biol Chem 281: 16333–16339, 2006. [DOI] [PubMed] [Google Scholar]
- 200.Stritt S, Wolf K, Lorenz V, Vögtle T, Gupta S, Bösl MR, Nieswandt B. Rap1-GTP-interacting adaptor molecule (RIAM) is dispensable for platelet integrin activation and function in mice. Blood 125: 219–222, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science 323: 474–477, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sullam PM, Hyun WC, Szöllösi J, Dong Jf Foss WM, López JA. Physical proximity and functional interplay of the glycoprotein Ib-IX-V complex and the Fc receptor FcγRIIA on the platelet plasma membrane. J Biol Chem 273: 5331–5336, 1998. [DOI] [PubMed] [Google Scholar]
- 203.Suzuki-Inoue K, Fuller GL, García Á, Eble JA, Pöhlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 107: 542–549, 2006. [DOI] [PubMed] [Google Scholar]
- 204.Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K. Essential in Vivo Roles of the C-type Lectin Receptor CLEC-2 embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem 285: 24494–24507, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Suzuki-Inoue K, Inoue O, Frampton J, Watson SP. Murine GPVI stimulates weak integrin activation in PLCgamma2−/− platelets: involvement of PLCgamma1 and PI3-kinase. Blood 102: 1367–1373, 2003. [DOI] [PubMed] [Google Scholar]
- 206.Suzuki Y, Yamamoto M, Wada H, Ito M, Nakano T, Sasaki Y, Narumiya S, Shiku H, Nishikawa M. Agonist-induced regulation of myosin phosphatase activity in human platelets through activation of Rho-kinase. Blood 93: 3408–3417, 1999. [PubMed] [Google Scholar]
- 207.Thomas DW, Mannon RB, Mannon PJ, Latour A, Oliver JA, Hoffman M, Smithies O, Koller BH, Coffman TM. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest 102: 1994–2001, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Thomason PA, James SR, Casey PJ, Downes CP. A G-protein beta gamma-subunit-responsive phosphoinositide 3-kinase activity in human platelet cytosol. J Biol Chem 269: 16525–16528, 1994. [PubMed] [Google Scholar]
- 209.Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor gamma-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J Biol Chem 272: 23528–23531, 1997. [DOI] [PubMed] [Google Scholar]
- 210.Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fässler R. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood 115: 3997–4005, 2010. [DOI] [PubMed] [Google Scholar]
- 211.Valet C, Chicanne G, Severac C, Chaussade C, Whitehead MA, Cabou C, Gratacap MP, Gaits-Iacovoni F, Vanhaesebroeck B, Payrastre B. Essential role of class II PI3K-C2α in platelet membrane morphology. Blood 126: 1128–1137, 2015. [DOI] [PubMed] [Google Scholar]
- 212.Vanhaesebroeck B, Waterfield M. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res 253: 239–254, 1999. [DOI] [PubMed] [Google Scholar]
- 213.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost 7: 1057–1066, 2009. [DOI] [PubMed] [Google Scholar]
- 214.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature 494: 185–194, 2013. [DOI] [PubMed] [Google Scholar]
- 215.Vicente V, Houghten RA, Ruggeri ZM. Identification of a site in the alpha chain of platelet glycoprotein Ib that participates in von Willebrand factor binding. J Biol Chem 265: 274–280, 1990. [PubMed] [Google Scholar]
- 216.Vogel S, Bodenstein R, Chen Q, Feil S, Feil R, Rheinlaender J, Schäffer TE, et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J Clin Invest 125: 4638–4654, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vu TKH, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64: 1057–1068, 1991. [DOI] [PubMed] [Google Scholar]
- 218.Wang D, Feng J, Wen R, Marine JC, Sangster MY, Parganas E, Hoffmeyer A, et al. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity 13: 25–35, 2000. [DOI] [PubMed] [Google Scholar]
- 219.Watanabe N, Bodin L, Pandey M, Krause M, Coughlin S, Boussiotis VA, Ginsberg MH, Shattil SJ. Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin alphaIIbbeta3. J Cell Biol 181: 1211–1222, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Watanabe N, Nakajima H, Suzuki H, Oda A, Matsubara Y, Moroi M, Terauchi Y, et al. Functional phenotype of phosphoinositide 3-kinase p85alpha-null platelets characterized by an impaired response to GP VI stimulation. Blood 102: 541–548, 2003. [DOI] [PubMed] [Google Scholar]
- 221.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost 3: 1752–1762, 2005. [DOI] [PubMed] [Google Scholar]
- 222.Watson SP, Herbert JMJ, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost 8: 1456–1467, 2010. [DOI] [PubMed] [Google Scholar]
- 223.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell 128: 171–182, 2007. [DOI] [PubMed] [Google Scholar]
- 224.Weng Z, Li D, Zhang L, Chen J, Ruan C, Chen G, Gartner TK, Liu J. PTEN regulates collagen-induced platelet activation. Blood 116: 2579–2581, 2010. [DOI] [PubMed] [Google Scholar]
- 225.Worth RG, Chien CD, Chien P, Reilly MP, McKenzie SE, Schreiber AD. Platelet FcgammaRIIA binds and internalizes IgG-containing complexes. Exp Hematol 34: 1490–1495, 2006. [DOI] [PubMed] [Google Scholar]
- 226.Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest 113: 441–450, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by Gi family members in platelets. J Biol Chem 277: 23382–23390, 2002. [DOI] [PubMed] [Google Scholar]
- 228.Wu Y, Suzuki-Inoue K, Satoh K, Asazuma N, Yatomi Y, Berndt MC, Ozaki Y. Role of Fc receptor γ-chain in platelet glycoprotein Ib-mediated signaling. Blood 97: 3836–3845, 2001. [DOI] [PubMed] [Google Scholar]
- 229.Xi X, Flevaris P, Stojanovic A, Chishti A, Phillips DR, Lam SC, Du X. Tyrosine phosphorylation of the integrin beta 3 subunit regulates beta 3 cleavage by calpain. J Biol Chem 281: 29426–29430, 2006. [DOI] [PubMed] [Google Scholar]
- 230.Xiang B, Zhang G, Stefanini L, Bergmeier W, Gartner TK, Whiteheart SW, Li Z. The Src family kinases and protein kinase C synergize to mediate Gq-dependent platelet activation. J Biol Chem 287: 41277–41287, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xiang B, Zhang G, Ye S, Zhang R, Huang C, Liu J, Tao M, et al. Characterization of a novel integrin binding protein, VPS33B, which is important for platelet activation and in vivo thrombosis and hemostasis. Circulation 132: 2334–2344, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, Lopez JA, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest 118: 3195–3207, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yang J, Wu J, Jiang H, Mortensen R, Austin S, Manning DR, Woulfe D, Brass LF. Signaling through Gi family members in platelets: redundancy and specificity in the regulation of adenylyl cyclase and other effectors. J Biol Chem 277: 46035–46042, 2002. [DOI] [PubMed] [Google Scholar]
- 234.Ye F, Petrich Brian G, Anekal P, Lefort Craig T, Kasirer-Friede A, Shattil Sanford J, Ruppert R, Moser M, Fässler R, Ginsberg Mark H. The mechanism of kindlin-mediated activation of integrin αIIbβ3. Curr Biol 23: 2288–2295, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ye F, Snider AK, Ginsberg MH. Talin and kindlin: the one-two punch in integrin activation. Front Med 8: 6–16, 2014. [DOI] [PubMed] [Google Scholar]
- 236.Yeung J, Apopa PL, Vesci J, Stolla M, Rai G, Simeonov A, Jadhav A, et al. 12-Lipoxygenase activity plays an important role in PAR4 and GPVI-mediated platelet reactivity. Thromb Haemost 110: 569–581, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yeung J, Holinstat M. 12-Lipoxygenase: a potential target for novel anti-platelet therapeutics. Cardiovasc Hematol Agents Med Chem 9: 154–164, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yeung J, Tourdot BE, Fernandez-Perez P, Vesci J, Ren J, Smyrniotis CJ, Luci DK, et al. Platelet 12-LOX is essential for FcgammaRIIa-mediated platelet activation. Blood 124: 2271–2279, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yin H, Liu J, Li Z, Berndt MC, Lowell CA, Du X. Src family tyrosine kinase Lyn mediates VWF/GPIb-IX-induced platelet activation via the cGMP signaling pathway. Blood 112: 1139–1146, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yin H, Stojanovic A, Hay N, Du X. The role of AKT in the signaling pathway of the glycoprotein Ib-IX-induced platelet activation. Hemost Thromb Vasc Biol 111: 658–665, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yuan Y, Kulkarni S, Ulsemer P, Cranmer SL, Yap CL, Nesbitt WS, Harper I, et al. The von Willebrand Factor-glycoprotein Ib/V/IX interaction induces actin polymerization and cytoskeletal reorganization in rolling platelets and glycoprotein Ib/V/IX-transfected cells. J Biol Chem 274: 36241–36251, 1999. [DOI] [PubMed] [Google Scholar]
- 242.Zarpellon A, Celikel R, Roberts JR, McClintock RA, Mendolicchio GL, Moore KL, Jing H, Varughese KI, Ruggeri ZM. Binding of thrombin to surface-anchored platelet glycoprotein Ib sulfotyrosines through a two-site mechanism involving exosite I. Proc Natl Acad Sci USA 108: 8628–8633, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang G, Han J, Welch EJ, Richard DY, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol 182: 7997–8004, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang G, Xiang B, Dong A, Skoda RC, Daugherty A, Smyth SS, Du X, Li Z. Biphasic roles for soluble guanylyl cyclase (sGC) in platelet activation. Blood 118: 3670–3679, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang G, Xiang B, Ye S, Chrzanowska-Wodnicka M, Morris AJ, Gartner TK, Whiteheart SW, White GC 2nd, Smyth SS, Li Z. Distinct roles for Rap1b protein in platelet secretion and integrin alphaIIbbeta3 outside-in signaling. J Biol Chem 286: 39466–39477, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang S, Zhang S, Hu L, Zhai L, Xue R, Ye J, Chen L, Cheng G, Mruk J, Kunapuli SP. Nucleotide-binding oligomerization domain 2 receptor is expressed in platelets and enhances platelet activation and thrombosis. Circulation 131: 1160–1170, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang W, Deng W, Zhou L, Xu Y, Yang W, Liang X, Wang Y, Kulman JD, Zhang XF, Li R. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein Ib-IX complex. Blood 125: 562–569, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhi H, Rauova L, Hayes V, Gao C, Boylan B, Newman DK, McKenzie SE, Cooley BC, Poncz M, Newman PJ. Cooperative integrin/ITAM signaling in platelets enhances thrombus formation in vitro and in vivo. Blood 121: 1858–1867, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]