Abstract
Potassium homeostasis has a very high priority because of its importance for membrane potential. Although extracellular K+ is only 2% of total body K+, our physiology was evolutionarily tuned for a high-K+, low-Na+ diet. We review how multiple systems interface to accomplish fine K+ balance and the consequences for health and disease.
“There are known knowns; there are things we know we know. We also know there are known unknowns; that is to say we know there are some things we do not know. But there are also unknown unknowns–the ones we don't know we don't know.” Donald Rumsfeld, U.S. Secretary of Defense, 2001–2006
Organisms are believed to have evolved in a geothermal field environment with a high potassium (K)-to-sodium (Na) ratio, setting the stage for evolution of the internal milieu of modern cells (65). Eukaryotes have high intracellular (ICF) K+, critical for protein synthesis and cell volume regulation maintained by the active uphill pumping of K+ into the cell by the ubiquitous plasma membrane Na-K-ATPase. Na-K-ATPase activity also contributes to low extracellular fluid (ECF) K+, creating a steep transmembrane potassium gradient that is a key determinant of the membrane potential and a source of stored potential energy that is used to drive action potentials, control muscle contractility, and power ion transporters. When ECF [K+] falls or rises, cell membranes hyperpolarize or hypopolarize, respectively, which disrupts normal electrical excitability and can lead to life-threatening cardiac arrhythmias. Thus the ECF [K+] is regulated, i.e., “K+ homeostasis,” within narrow limits by multiple renal and extrarenal mechanisms.
Importantly, the ECF pool of potassium is very small (70 meq) relative to the recommended daily dietary potassium intake (120 meq/day), leading to daily K+ homeostasis challenges (2, 48). That is, ECF K+ must be maintained during absorption of a K+-rich meal as well as during fasting. Normally, daily K+ output equals K+ intake; however, efficient K+ homeostasis requires rapid adaptation. For example, during periods of fasting, ECF K+ is maintained by reducing renal K+ excretion and redistributing muscle ICF K+ to ECF. When the fast is broken by a high-K+ meal (e.g., the hungry fasting lion catches the gazelle), the ingested and absorbed K+ must be rapidly taken up by muscle ICF and/or excreted by the kidney to prevent detrimental fluctuations in ECF [K+]. In contrast, during pregnancy, dietary K+ intake chronically exceeds K+ excretion to facilitate the growth of the fetus; positive K+ balance continues after birth in the growing child until steady-state muscle mass is attained (92).
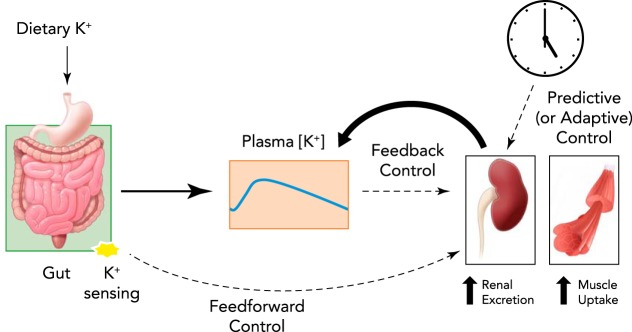
Both feedback and feedforward mechanisms exist to control this vital variable in the face of acute challenges (FIGURE 1). First, ECF [K+] is controlled by feedback mechanisms: increased ECF [K+] stimulates renal K+ excretion by direct effects on renal tubule cells as well as indirect effects mediated by stimulating aldosterone production, both of which stimulate K+ excretion (32, 72, 75). Increased ECF [K+] also promotes cellular K+ uptake, especially by muscle (14). Second, ECF [K+] is controlled by feedforward mechanisms (81, 117): ingested K+ appears to be sensed in the gut, provoking renal K+ excretion and cellular K+ uptake before or without increases in ECF [K+]. Feedforward control, proposed decades ago by Rabinowitz and colleagues (81), was recently shown to operate in humans (79). Third, long-recognized circadian rhythms in renal K+ excretion provide evidence that ECF [K+] may also be modulated by “predictive” control of K+ homeostasis, which enhances K+ excretion during the times when K+ intake is anticipated; recent studies demonstrate that clock genes control these circadian rhythms in K+ excretion (41, 42).
FIGURE 1.
A schematic diagram illustrating three different control mechanisms for ECF K+ homeostasis during dietary K+ intake
Feedback control is driven by a rise of plasma [K+], i.e., perturbation of the system, and feedforward control is driven by the sensing of dietary K+ intake in the gastrointestinal tract, independent of plasma [K+]. Predictive or adaptive control is driven by circadian rhythms.
Feedforward control operates independently of the variable-controlled, activating corrective responses that anticipate changes in the controlled variable (FIGURE 1). An undisputed mechanism of feedforward control is insulin's action to promote K+ shift from ECF to ICF (20, 24). Insulin is secreted during a meal when dietary glucose and K+ are absorbed, and, since a meal can routinely contain as much potassium as is found in the entire ECF, insulin's action to stimulate K+ transport into ICF is critical to buffer the rise in ECF potassium. Insulin secretion from pancreatic β-cells is controlled primarily by plasma glucose and incretin hormones secreted from intestinal cells during food absorption. Thus control of K+ redistribution to ICF by insulin is largely independent of ECF [K+] and thus is considered to be a feedforward control. The feedforward response is also independent of gut sensing of dietary K+ (or dietary K+ intake), since ECF [K+] falls after a K+-deficient meal as a result of insulin action (20, 79).
A number of excellent recent reviews have covered the topic of K+ homeostasis quite comprehensively (31, 32, 42, 72, 75). The goal of this brief analysis is to review new findings (“known knowns”) as well as to identify remaining gaps in our understanding of the mechanisms responsible for potassium homeostasis (“known unknowns”). In addition, we discuss recent findings linking the cardiovascular benefits of a potassium-rich diet to the molecular mechanisms engaged in excreting the potassium.
The Cardiovascular Benefit of a K+-Rich Diet Is a Gift From Our Past
Early terrestrial animals, like hunter-gather groups, consumed diets with high K+ and very low Na+ (71). To maintain extracellular fluid, animals evolved salt-retaining genes, including genes for salty food preference; once salt was commercialized by humans, salt-seeking behaviors drove very high NaCl consumption (10, 86). Consequently, although our human physiology has been fine-tuned to handle high-K+, low-Na+ diets, typical Western diets contain far less K+ than Na+. For example, as people transition from non-industrialized to industrialized societies, their dietary K+-to-Na+ ratio falls from >1 (3, 60) to <0.5 (63). Epidemiological studies repeatedly establish significant inverse relationships between dietary K+-to-Na+ ratio and blood pressure, whether estimated from urinary K+-to-Na+ ratio (45, 50, 85) or diet recall (116). Progression of kidney disease is reported to be blunted with higher K+/Na+ intakes in some (6, 97) but not all (43) studies and warrants careful examination. A study in adolescent girls followed for 10 years suggests it is important to consume a K+-rich diet while young in life: a lower rate of rise in BP was observed in girls consuming a K+-rich diet, yet there was no association with dietary Na+ (13). A recent study of half a million participants in China queried the relationship between fruit consumption (a rich source of diet K+) and cardiovascular disease and discovered lower BP, blood glucose, and rate of cardiovascular deaths in participants that ate fruit frequently vs. rarely (28). Although these studies establish correlations, studies involving dietary K+ recommendations (95) or supplementation also detect similar benefits (1, 12, 114). The highly regarded Dietary Approaches to Stop Hypertension (DASH) investigated the impact of consuming a diet rich in vegetables, fruits, and low-fat dairy products at various levels of Na+ intake and concluded that, although BP was reduced when Na+ was lowered, the effect was amplified by the K+-rich DASH diet, especially at higher Na+ levels, in the elderly, African Americans, and hypertensives; elevating dietary K+ alone also had an effect but not as great as the DASH diet (5, 37, 88). Arguably the most deliberate interventional study was conducted by Chang et al. (17) in a population of elderly male veterans living in retirement communities in Taiwan. K+-enriched (49%) cooking salt was provided to three of five kitchens (condiments like soy sauce not limited); K+ intake increased 70%, and Na intake decreased 25%. Impressively, relative risk of cardiovascular disease was reduced 40% over 3 years in these veterans with a mean age of 75. Likewise, hypertensive Tibetans given 25% KCl table salt exhibit reduced BP after 3 mo (119). Taken together, these studies illustrate the cardiovascular benefits of diets with higher K+-to-Na+ ratios independent of dietary Na+. As summarized in subsequent sections, recent findings demonstrate that the blood pressure-lowering benefit arises from physiological mechanisms recruited to excrete K+ consumed in a K+-rich diet to maintain plasma [K+] in a narrow range.
Overview of Organ System Regulation of K+ Homeostasis: Adaptations to an Acute K+ Load
Multiple organ systems and signaling cascades work in concert to regulate the small pool of ECF [K+]. FIGURE 2 provides an overview of the pathways of K+ compartmental redistribution (solid arrows) and routes of regulatory cross talk (dotted arrows) between organs, both known and potential but unknown, that control the small pool of ECF [K+]. Daily K+ intake is taken up rapidly into the extracellular fluid across the small intestine. Evidence suggests absorption is passive, that is, driven by electrochemical gradients and solvent drag, not unlike reabsorption along the kidney proximal tubule (4). K+ is usually ingested with dietary nutrients that raise plasma glucose, which stimulates insulin secretion. Insulin activates cellular Na-K-ATPase activity, which pumps ECF K+ into the ICF, especially muscle (dotted arrow, a) (59); additionally, a rise in [K+] in muscle T-tubules, evident during exercise, is sufficient to stimulate Na-K-ATPase-driven K+ uptake (25). Exercise training, by increasing muscle mass and the number of sodium pumps in muscle, improves potassium adaptation by increasing the rate of K+ uptake and the size of the buffer pool (21).
FIGURE 2.
An overview of K+ fluxes (solid arrows) and routes of regulatory cross talk (dotted arrows) between organs during dietary K+ intake
See text for explanations of individual K+ fluxes and regulatory cross talk.
ECF K+, via plasma, is continuously filtered into the kidneys, which, ideally, match K+ output to K+ intake to achieve whole body K+ homeostasis. Thus renal K+ excretion is increased or decreased in response to K+-rich or -poor meals, respectively. The molecular mechanisms are beginning to be unraveled. The kidneys regulate the fraction of K+ reabsorbed vs. excreted (bidirectional solid arrows) depending on plasma [K+] (dotted arrow, b), ICF K+ stores (dotted arrow, c), and plasma aldosterone level (dotted arrow, d), which is itself regulated by plasma [K+] (dotted arrow, b). Evidence suggests signals emanating from gut-sensing of [K+] likely stimulate renal K+ excretion (dotted arrow, e). Finally, CNS is implicated from studies of circadian variability in K+ homeostasis (41), but there is a paucity of knowledge supporting specific cross talk between brain and gut, kidney, or muscle (“unknown” dotted arrows, f, g, h, respectively).
These layers of regulation that drive cellular uptake and renal excretion efficiently blunt the rise in ECF during K+ intake. In a recent study (84), we acutely raised plasma [K+] to 5.5 mM in conscious rats via tail vein infusion and calculated its compartmental disposition: at 3 h, only 10% of the infused K+ remained in the ECF, 40% was excreted into the urine, and, by subtraction, 50% of the infused [K+] was redistributed into the ICF. Subsequently, during muscle contraction, K+ redistributes from muscle ICF and reenters ECF pool for excretion (FIGURE 2, solid arrow leaving muscle). Overall, K+ balance studies in people indicate that ∼90% of the ingested K+ is excreted in the urine, and the remaining 10% is excreted in feces (4); this fecal excretion is also regulated by plasma [K+] indirectly via mineralocorticoid regulation of colonic sodium and potassium channels (FIGURE 2, dotted arrow, d) (105) and colonic H-K-ATPase (62, 91).
The Kidneys Match Output to Input: Regions Involved
Feedback control, although very effective, requires an error signal, that is, a deviation from the baseline or set point (46), e.g., a rise in ECF [K+] that drives the compensatory responses such as renal K+ excretion and/or cellular K+ uptake. Studies have verified a rise in ECF [K+] in response to K+ ingestion in rodents (84, 98) and man (79, 80); the K+-rich Paleolithic diet, containing up 400 meq/day, would certainly have provided such an error signal (71, 94). Recent studies have provided some clarity in understanding the molecular mechanisms connecting the increase in plasma K+ to increase in K+ excretion. It has long been appreciated that >80% of the filtered load of K+ is reabsorbed in the proximal tubule and loop of Henle, independent of dietary intake, whereas regulated K+ transport that matches K+ output to intake occurs in the late distal convoluted tubule (DCT) through connecting tubule (CNT) and collecting duct (CD) (72). In fact, more than 50 years ago, Malnic et al. (56) demonstrated, by micropuncture, that in normal and high-K+ diet-fed rats, tubular fluid K+ increases from DCT through cortical CD, evidence for K+ secretion, and, in K+-deficient rats, tubular K+ decreases along the same region, evidence for K+ uptake. The K+ secretion channels were eventually identified as the renal outer medulla K+ channel (ROMK) (44) and tubular flow-sensitive, large-conductance Ca2+-activated K+ (BK) channels (15, 78) and the active K+ reabsorption route as an H-K-ATPase (26).
Molecular Mechanisms Regulating Renal K+ Excretion
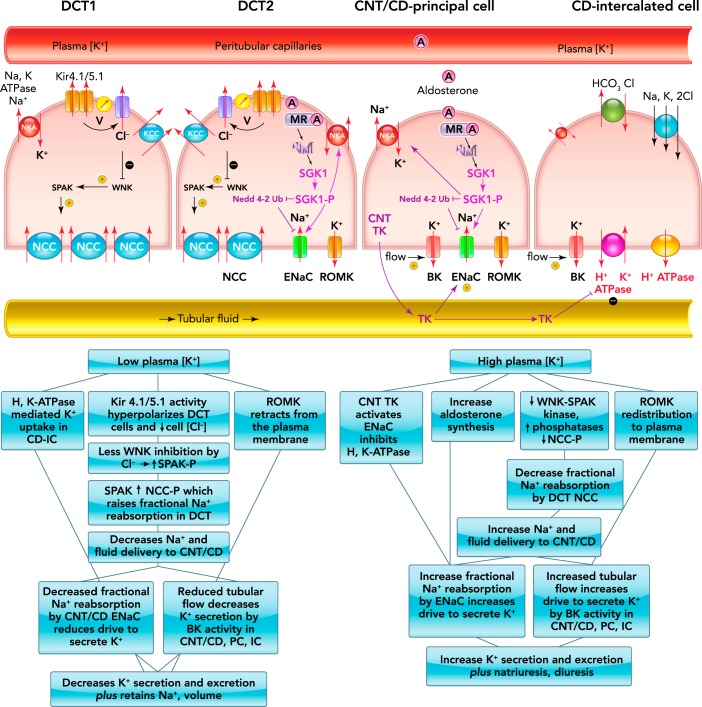
FIGURE 3 (adapted from Ref. 99) illustrates how key cellular and molecular mechanisms, including sensors and effectors, work in concert to regulate K+ output along the distal nephron in the face of variable intake. Remarkably, control of K+ secretion vs. reabsorption is strongly influenced by Na+ handling upstream of these CNT/CD K+ transporters, specifically, by the DCT Na+-Cl− cotransporter (NCC). It was long known that thiazide diuretics, which target and inhibit DCT NCC activity, can provoke urinary K+ loss (83), but there was not a mechanistic connection between NCC and K+ homeostasis until a 2009 study by Vallon et al. (106), which demonstrated that dietary K+ itself regulates NCC phosphorylation (NCC-P) and activation: low K+ increases and high K+ decreases NCC-P abundance. The physiological consequence of DCT NCC activation during low K+ intake is an increase in fractional reabsorption of NaCl in the DCT and, thus, reduced delivery of Na+ and volume to the CNT/CD where less Na+ movement through the apical epithelial Na+ channel (ENaC) reduces the electrochemical driving force for K+ secretion into the tubular fluid via ROMK and flow-sensitive BK channels (7), thus conserving K+ by reducing its excretion; likewise, the consequence of inhibiting NCC during high K+ intake is reduced fractional Na+ reabsorption by NCC and increased Na+ delivery and flow to ENaC, where increased fractional Na+ reabsorption drives K+ secretion and increased flow increases K+ secretion by BK channels, resulting in increased K+ output to match the increased input. The molecular mechanisms involved in the transporters' regulation have begun to be revealed and include regulation of abundance in the membrane (by synthesis and/or trafficking), phosphorylation state (by kinases and phosphatases), and other modifications such as proteolytic cleavage by cellular and tubular proteases.
FIGURE 3.
Schematic diagram and flow charts illustrating molecular events regulating Na+, K+, Cl−, and H+ transport and their transporters
A schematic diagram illustrating molecular events regulating Na+, K+, Cl−, and H+ transport and their transporters in different regions of the distal nephron (top). Also shown are flow charts showing how these individual molecular events work in concert to regulate K+ and Na+ excretion in response to low (bottom left) or high (bottom right) plasma [K+]. BK, high-conductance Ca2+-activated K+ channel; CD, collecting duct; CNT, connecting tubule; DCT, distal convoluted tubule; DCT1, the first portion of the DCT; DCT2, the second portion of the DCT; ENaC, epithelial Na+ channel; IC, intercalated cells; Kir, inwardly rectifying K+ channel; KCC, K+-Cl− cotransporter; MR, mineralocorticoid receptor; NCC, Na+-Cl− cotransporter; NCC-P, NCC phosphorylation; PC, principal cells; ROMK, renal outer medulla K channel; SGK1, serum- and glucocorticoid-inducible kinase 1; SGK1-P, SGK1 phosphorylation; SPAK, Ste20p-related proline and alanine-rich kinase; SPAK-P, SPAK phosphorylation; TK, tissue kallikrein; V, voltage or membrane potential; WNK, with-no-lysine kinase.
The first portion of the DCT (DCT1) expresses high levels of apical NCC and basolateral Na-K-ATPase, which together effect transepithelial NaCl reabsorption in this region. The basolateral heteromeric K+ channel (Kir 4.1/5.1) not only recycles K+ pumped in by the Na-K-ATPase across this membrane but its activity is responsive to changes in extracellular [K+] in a manner that can set off a signaling cascade that regulates apical NCC activity (118). Said another way, Kir 4.1/5.1 is a plasma [K+] sensor that plays a role in feedback control to normalize plasma K+ (99, 102). Although some details remain to be confirmed in vivo, when ECF [K+] is reduced, as during K+-deficient intake, K+ exit through Kir 4.1/5.1 increases, which hyperpolarizes the membrane potential and increases basolateral Cl− conductance and exit, which lowers cell [Cl−] in cultured cells (101) and in native DCT (76). Lowering cell [Cl−] relieves its inhibition of the with-no-lysine kinase (WNK) and Ste20p-related proline and alanine-rich kinase (SPAK) cascade, and promotes SPAK phosphorylation and its activation of NCC (11). This feedback system works in a physiological range in vivo: pooled and modeled results suggest that decreasing plasma [K+] from 4.5 to 3 mM lowers cell [Cl−] by 5 mM and raises NCC-P three- to fourfold (102).
Reciprocally, an acute rise in plasma [K+] dephosphorylates NCC within minutes (98) and in a physiological range typical of that measured after a meal (84). Whether raising plasma K+ reduces the gradient for K+ exit via the Kir4.1/5.1 enough to raise cell [Cl−] and inhibit the WNK-SPAK cascade has been hard to determine in native kidney tissue (76). Our group measured a >50% decrease in NCC-P associated with raising plasma [K+] to 5.5 mM in rats, and although there was an accompanying 35% decrease in SPAK-P, it did not reach significance, suggesting that the decrease in NCC-P may not require a decrease in SPAK-P. A recent study using kidney tissue studied ex vivo confirmed the cascade from low plasma [K+] to increased WNK-SPAK activity and higher NCC-P, but this same study failed to demonstrate a connection between decreased cell [Cl−] conductance and NCC dephosphorylation (76). These authors discussed evidence that NCC-P is controlled by not only kinases but also by protein phosphatases (PP) (39, 51, 77) yet showed that the decrease in NCC-P during exposure to high extracellular [K+] persists during PP1, PP2A, and PP3 inhibition (76). They concluded that potassium regulation of NCC activity involves both Cl−-dependent and -independent pathways, including WNK-SPAK and unidentified signaling mechanisms. Since trafficking of NCC out of the apical membrane can play a role in lowering NCC activity (35, 89, 90), this mechanism should also be added to the “known unknown” category for further investigation. The bigger question of how high plasma K+ dephosphorylates NCC is another unknown that warrants further investigation given the prevalence of hyperkalemia in hospitalized populations and in chronic kidney diseases.
The second portion of the DCT (DCT2) expresses all the components expressed in the DCT1 (Kir 4.1/5.1, NCC, Na-K-ATPase, WNK-SPAK cascade) as well as components expressed in the CNT and CD principal cells (CD-PC) including ENaC, ROMK, mineralocorticoid receptor (MR), and 11β-hydroxysteroid dehydrogenase type 2 (36), which degrades glucocorticoids, thus conferring aldosterone specificity to the MR. A more complete cataloging of proteins expressed along the DCT is reviewed elsewhere (7, 58), and aldosterone regulation of K+ homeostasis is discussed in the next section. The DCT2 is notable for co-expression of NCC and the ENaC. As discussed, the inhibition of NCC in DCT1 during hyperkalemia increases delivery of both salt and water to the CNT/CD-PC, where it increases ENaC-mediated fractional reabsorption of Na+. But what is the effect of NCC inhibition on ENaC located in the same cell? We established, using native gels, that apical membrane NCC is localized to 700-kDa multimers in rat kidneys (52). Using this strategy, the Hoover lab recently showed that ENaC subunits are also localized to the 700-kDa multimer in a cultured DCT cell line and validated the NCC-ENaC association by co-immunoprecipitation, electronmicroscopy, and FRET (64); the study also demonstrated that thiazide diuretic inhibition of NCC reduced ENaC open probability 50% in these cultured cells (64). Although tantalizing, it remains to be established whether indirect regulation of ENaC via NCC-ENaC association and thiazide inhibition is also evident and physiologically relevant in the native DCT2 (a known unknown).
The next regions downstream are the CNT, the last portion of the nephron's distal tubule, followed by the CD, composed of principal cells (CD-PC) and intercalated cells (CD-IC). Relevant to K+ handling along this region, the CNT shares transport characteristics with the CD-PC, and these will be discussed together as CNT/CD-PC; it is worth noting that DCT2 and CNT reabsorb and secrete a much higher fraction of the filtered loads of Na+ and K+ than CD-PC, which probably plays a relatively minor role unless a very high K+/low Na+ diet is consumed (61, 73). Neither CNT nor CD-PC express NCC or its kinase SPAK. Kir 4.1 is expressed in this region but plays only a minor role in regulating membrane potential, probably because there are at least two other unidentified K+ channels expressed in the basolateral membranes of CNT/CD (99). If and how this region senses plasma [K+], independent of aldosterone, are not known. The transporters in the CNT/CD-PC include the basolateral Na-K-ATPase, which pumps K+ from the blood side into the cell and the apical ROMK and BK channels that can secrete the cellular K+ into the tubule lumen if a favorable driving force is provided. Na-K-ATPase couples K+ uptake and Na+ extrusion from the cell into the blood, which maintains low cellular [Na+], and this low Na+ is the driving force for Na+ to move from tubular fluid through apical ENaC into the cell, creating the electrochemical gradient for K+ to be secreted into the tubular fluid via ROMK. There is tight synchronizing of Na-K-ATPase and ENaC transport activities, mediated by hormones and kinases, to prevent fluctuations in cell volume when Na+ reabsorption is increased or decreased in this region (33).
In response to altered dietary K+, both ENaC and ROMK traffic between apical membranes and intracellular compartments and are modified by protease and kinase cascades, thus impacting the number of active channels in the membrane (35, 53, 61, 112, 113). In general, high K+ intake rapidly increases apical expression of both ENaC subunits and ROMK (35) to drive K+ secretion, and the increased tubular flow that results from inhibition of DCT Na+ reabsorption stimulates activity of BK channels. [Signaling mechanisms for BK discussed in a recent review (15).] In parallel, elevated plasma [K+] stimulates aldosterone production by adrenals, which, via MR receptors, stimulate serum- and glucocorticoid-inducible kinase 1 (SGK1) transcription and activation, which both activate Na-K-ATPase and promote ENaC activity. By inactivating the ubiquitin ligase Nedd 4-2, which promotes ENaC degradation, SGK1 indirectly promotes apical accumulation of ENaC by reducing endocytosis (57). Interestingly, the serine protease tissue kallikrein (TK), synthesized in renal CNT cells, amplifies the excretion of a K+ load by both proteolytic activation of ENaC (74) and inhibition of CD-IC H-K-ATPase (16), identifying TK functionally as a kaliuretic factor. TK acts rapidly in an aldosterone- and kinin-independent manner to facilitate excretion of a K+ load; how CNT TK is regulated by dietary K+ intake or plasma [K+] and how it inhibits H-K-ATPase activity are unknowns remaining to be determined (29). In contrast, K+-deficient intake reduces ENaC (30, 66), even during Na+ restriction (34), and provokes ROMK retraction from the apical membrane mediated by the effects of cSrc family protein tyrosine kinase on ROMK (53) and on SGK1-WNK4 inhibition of ROMK (54), effected via clathrin-mediated endocytic machinery (112, 113). Since low-K+ diets stimulate DCT Na+ reabsorption, flow to the CNT/CD is reduced, minimizing BK-mediated K+ secretion. CD-IC apical colonic H-K-ATPase activity is increased during K+ depletion, which can further reduce K+ excretion (22, 29).
Update on Role of Aldosterone in K+ Homeostasis
Aldosterone synthesis is stimulated by elevated plasma [K+] and acts on the kidney to increase urinary K+ excretion, a classic feedback mechanism that acts to normalize plasma [K+]. The DCT NCC has been viewed as an aldosterone target, yet this is not consistent with the suppression of NCC by elevated plasma K+, which is viewed as important for shifting Na+ reabsorption downstream from NCC to ENaC to drive K+ secretion and kaliuresis. However, a direct test of this notion, by treating mice with a thiazide to inhibit NCC, measured natriuresis but not kaliuresis, indicating that simply increasing Na+ and volume delivery to the CD is not sufficient to activate ENaC and ROMK channel activity (47). Elevated aldosterone production, stimulated directly by hyperkalemia, likely contributes to K+ secretion by triggering SGK1 transcription, which, via well described signaling cascades, initiates redistribution and/or activation of ENaC and ROMK channels (35, 38, 40, 115). Recent studies in mice lacking aldosterone synthase (AS−/−) (105) or lacking renal tubular MR−/− (23, 100) provide more answers and new questions. Complete aldosterone deficiency (AS−/−) lowers abundance of renal NCC and NCC-P relative to that in AS+/+ mice; 2% K+ diet further depresses NCC-P in both genotypes, which raises flow and Na+ delivery downstream. Abundance of both activated α-ENaC and ROMK is higher in AS−/− mice, likely driven by compensatory elevated angiotensin II levels, consistent with aldosterone-independent K+ adaptation. In contrast, in the colon of AS−/− mice, the responses of Na+ and K+ channels to K+ loading are absent, indicating dependency of colonic K+ adaptation on aldosterone (105). In a study to directly visualize whether aldosterone regulates NCC as well as ENaC, the Loffing lab generated mice with MR selectively knocked out of ∼20% of renal tubule cells to view cells with and without MR in the same image (23). NCC and NCC-P abundance and their responses to low-salt diet (which elevates plasma aldosterone) were unchanged in MR−/− vs. MR+/+ cells; further down the nephron in the collecting system, α-ENaC and Na-K-ATPase were significantly reduced in MR−/− cells and did not respond to low-salt diet. In a parallel study, the Ellison group generated renal tubule-specific MR−/− mice that developed salt wasting and hyperkalemia, both likely due to deficient ENaC expression; NCC and NCC-P abundance were also very low in these mice but could be elevated by K+ restriction, indicating that the suppression of NCC in the MR−/− was secondary to hyperkalemia rather than the lack of MR. Together, these studies support the idea that aldosterone and MR directly regulate ENaC yet indirectly regulate NCC abundance and phosphorylation secondary to altering ENaC, K+ secretion, and ECF K+ disruptions.
Molecular Mechanisms of Feedforward Control
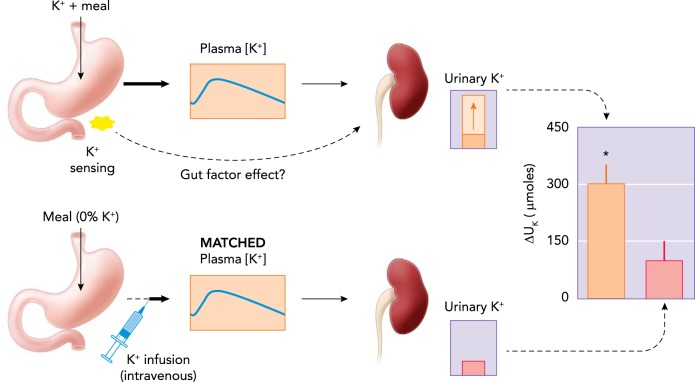
Is there anticipatory K+ excretion independent of a rise in plasma K+ at the DCT? When the long-fasting seal (108) that has been conserving K+ by maximizing renal reabsorption and shifting K+ from ICF to ECF returns to the sea and eats a tuna, it would be very helpful to preemptively convey the message from the K+-rich gut to the kidneys to prepare to secrete K+ and to the muscles to take it up. Gut-mediated feedforward control of plasma K+ homeostasis was proposed almost three decades ago by Rabinowitz and colleagues (81) based on their finding that plasma [K+] did not measurably increase in rats during their active period when K+ intake and K+ excretion were both elevated. They proposed that dietary K+ was sensed in the splanchnic bed and signaled to the kidney to increase K+ excretion to prevent a rise in plasma [K+]. Supporting this idea, Oh and Youn (69) demonstrated that, after ingesting K+ with a meal, feedforward control plays a major role in stimulating renal K+ excretion (FIGURE 4). A recent study by Preston et al. (79) showed that feedforward control of K+ excretion also operates in humans: when oral K+ is provided with a K+-deficient meal, serum [K+] did not increase but K+ excretion increased sharply. The molecular mechanisms underlying the feedforward control of K+ excretion (or muscle uptake) are not known and remain to be unraveled. There are numerous possibilities for sensing of dietary K+ in the gut. Dietary K+ causes huge variations in intestinal [K+]. A simple idea is that intestinal cells sense the rise in luminal [K+] and secrete peptide hormones to regulate renal K+ excretion; we have tried unsuccessfully to identify gut peptides whose secretion is affected by dietary K+ intake (69) or pituitary peptides that might play a role in K+ homeostasis (69, 70). Another possibility that remains to be explored is a role of the gut microbiota. Recent studies have provided ample evidence that the gut microbiota play a crucial role in host physiology and pathology (87). K+ transport plays a critical role in bacterial growth and survival and regulates protein (or toxin) secretion or interaction with the host (55, 68). It is conceivable that, in response to dietary K+, gut bacteria secrete substances that act as signals to affect K+ homeostasis of the host. Although the mechanisms by which dietary K+ is sensed in the gut and the signals transmitted are unknown, we postulate that kidneys possess sensors to respond to the signals (independent of plasma [K+]) to alter K+ excretion. A key unknown is whether the renal mechanism affecting feedforward regulation are the same as feedback control mechanisms via the DCT effectors (Kir 4.1/5.1, cell Cl−, WNK-SPAK and NCC-phosphorylation state).
FIGURE 4.
A demonstration of the operation of a feedforward control during normal dietary K+ intake in rats
Top: a normal, K+-containing meal may increase renal K+ excretion by increasing plasma [K+] and/or activating a feedforward control in response to gut sensing of dietary K+ intake. Bottom: when the same but K+-deficient meal was given to rats (no gut sensing of dietary K+ and thus feedforward control) and plasma [K+] was matched by intravenous K+ infusion, renal excretion was significantly smaller (69), indicating that a predominant portion of the increase in renal K+ excretion during normal dietary K+ intake is due to a feedforward control.
Chronic K+ Adaptation Independent of Plasma [K+]
Choi et al. (19) found that high fat feeding in rats decreased insulin stimulation of both glucose and K+ uptake. Whereas decreased glucose uptake arose from high dietary fat, decreased K+ uptake (and renal K+ excretion) occurred because rats ate less of the calorie-dense, high-fat diet and, consequently, less K+, since the reduced K+ uptake was normalized by K+ supplementation. More recently, Nguyen and Moe confirmed that insulin-stimulated cellular K+ uptake was preserved in humans with Type 2 diabetes, whereas cellular glucose uptake was reduced (67). Both studies illustrate that molecular mechanisms of insulin-stimulated K+ uptake are distinct from insulin-stimulated glucose uptake. Thus the reduced cellular K+ uptake and renal K+ excretion observed in high-fat-fed rats represent adaptive responses to reduced K+ intake (reduced to one-third of controls fed normal diet) (19). Interestingly, plasma [K+] was not altered in the high-fat-feeding study, indicating that renal or extrarenal K+ adaptation can occur without changes in plasma [K+]. To directly support this, a subsequent study by Chen et al. (18) confirmed (in rats fed normal fat chow) that chronically reducing K+ intake to one-third of normal resulted in robust downregulation of renal K+ excretion as well as cellular K+ uptake without changes in plasma [K+] or aldosterone concentration. Oh et al. (70) further determined that these K+ adaptations occur very rapidly, following overnight feeding with reduced K+ intake. Taken together, these studies demonstrate that both renal and extrarenal K+ adaptation can occur very rapidly (overnight) and without measurable changes in plasma [K+]. This K+ adaptation without changes in plasma [K+] identifies an intriguing gap: we hypothesize there are signals generated from altered K+ intake to which renal and extrarenal tissues respond, even in the absence of changes in aldosterone and plasma K+ concentrations, such as brief transients in plasma [K+], or gut factors. Additionally, there is evidence that the brain is involved in the regulation of electrolyte balance (82). Impaired potassium homeostasis is evident in hypophysectomized rats (70); in particular, both K+ excretion and K+ tolerance are reduced, despite normal K+ intake, similar to rats maintained on low-potassium diets, suggesting that hypophysectomized rats do not sense the dietary K+ intake and are, rather, geared to K+ conservation. One idea is that the pituitary releases a humoral factor subsequent to gut sensing of K+ intake that participates in both acute control of K+ homeostasis during absorptive periods and also exerts postabsorptive K+ handling independent of plasma [K+] (117). This unknown remains to be identified. Likewise, the possibility that NCC phosphorylation is also regulated by a mechanism independent of plasma [K+] warrants consideration, i.e., that NCC phosphorylation is increased when K+ intake is modestly reduced and renal K+ excretion is decreased without changes in plasma [K+] as in the Chen et al. study (18).
Wang and colleagues (9, 110) have shown that K+ depletion increases protein tyrosine kinase (i.e., cSrc)-mediated phosphorylation of ROMK, which reduces K+ secretion and excretion by translocation of the K+ channels out of the apical membrane, effects mediated by NADPH oxidase activation and superoxide anion production. A more recent study by this group further reported that these changes occurred in the absence of changes in plasma [K+] in rodents maintained on a low (0.1%) vs. normal (1%) K+ diet (111). Chen et al., with the collaboration of Wang and colleagues (18), also showed that modest K+ deprivation increased cSrc expression and ROMK phosphorylation without changes in plasma K+ or aldosterone levels. Taken together, these findings suggest that NADPH oxidase in the kidney may be involved in plasma [K+]-independent regulation of renal K+ excretion, an unknown meriting further exploration.
Is there cross talk between muscle and kidney to maintain ECF [K+]? This question is in the category “unknown unknowns” but should be discussed. In the postabsorptive state (when no dietary K+ is absorbed), K+ removed from the ECF by excretion must be replenished by K+ donated by ICF stores, mainly muscles, to maintain constant plasma [K+]. This takes on importance during fasting and disease states when K+ output is chronically greater than K+ intake. How do muscles sense that renal K+ excretion is altered or how much K+ to donate in the absence of changes in ECF [K+]? More than 10 years ago, we determined that, during dietary K+ deprivation and falling plasma [K+], muscle Na-K-ATPase α2 catalytic isoform and active K+ uptake are reduced, favoring redistribution of K+ from ICF to ECF to buffer the fall in ECF [K+] (20, 103, 104). That finding suggests that the link between renal K+ excretion and muscle K+ redistribution could simply be plasma [K+]. Subsequently, discussed above, we showed that when dietary K+ was reduced to one-third of control, both renal K+ excretion and active K+ uptake were reduced before any change in plasma [K+] or muscle Na-K-ATPase α2 (18), suggesting some cross talk. Also supporting cross talk, a recent study by Veiras et al. (107) demonstrates that angiotensin II provokes a mild hypokalemia in rats, attributed to ENaC activation driving K+ secretion and excretion. When angiotensin II-infused rats were fed a single K+-rich meal, plasma [K+] rose from 3.6 to 4.7 mM, but K+ excretion did not increase, suggesting that the ingested K+ is preferentially routed to replenish intracellular K+ stores when whole body K+ is depleted. This would be a useful adaptation when a long-fasting lioness catches a gazelle. Exercise is another scenario that may illuminate whether muscle-kidney cross talk exists: during intense exercise, the rate of passive K+ leakage from working muscles exceeds the rate of active K+ reuptake, resulting in a rise in plasma [K+], e.g., to 6 mM [K+] during intense rowing (8). Whether the signaling cascade reducing NCC dephosphorylation is activated (leading to negative K+ balance) or suppressed via cross talk is a discoverable unknown that could identify novel mechanisms of K+ adaptation.
Elucidation of the Cardiovascular Benefits of a Potassium-Rich Diet
A discussion of the physiology of K+ homeostasis would be incomplete without considering the negative impact of the rise in dietary Na-to-K ratio as humans transitioned from a natural diet to a Western diet. The homeostatic mechanisms developed to maintain K+ homeostasis, discussed above, evolved in animals consuming a diet (plants and meat) very rich in K+ and low in Na+; the homeostatic responses are arguably unnecessary when the high-Na+/low-K+ “Western diet” is consumed. Significantly, the recent literature reveals (FIGURE 3) that the mechanisms evolved to excrete a K+-rich diet require the excretion of Na+ (reduced NCC activity) and thereby exert a cardiovascular benefit, analogous to a diuretic (without the diuretic's negative side effects). In fact, a number of investigations have come to the conclusion that the body places a higher priority on maintaining plasma [K+] than on maintaining the effective circulating volume. As a consequence, responses to maintain K+ homeostasis while consuming a low-K+/high-Na+ Western diet can actually raise blood pressure: Vitzhum et al. (109) reported that dietary K+ depletion drives higher SPAK and NCC activity and provokes renal Na+ retention and higher blood pressure (even when ENaC or MR were antagonized) to minimizing Na+ delivered to the CNT/CD-PC and K+ secretion; these findings were confirmed by Terker et al. (102). Jensen et al. (49) report that high K+ intake provokes rapid NCC dephosphorylation and urinary Na+ loss even under Na+-depleted conditions. Frindt and Palmer measured ENaC channel activity during Na+ depletion and discovered that ENaC activity was undetectable during low K+ intake, despite Na+ depletion, presumably to minimize ENaC-driven K+ secretion and urinary K+ loss (34). These studies and others support the conclusion that K+ homeostasis is prioritized over maintenance of extracellular Na+ and volume, a reality that likely contributes to elevated blood pressure in populations with low K+ intake.
Conclusions
Recent studies reviewed herein have changed unknowns to knowns and revealed new unknowns. Regarding feedback regulation, the functional identification of the Kir 4.1/5.1 channel as a K+ sensor in the DCT unmasked a feedback loop between low plasma [K+], NCC phosphorylation in DCT, and reduced K+ secretion downstream in the CCD. Important unknowns remain concerning feedback during high plasma [K+], including mechanisms of rapid NCC dephosphorylation and H-K-ATPase activation, and how/whether plasma [K+] is sensed in the CNT/CCD. These warrant further investigation given the prevalence of hyperkalemia in the hospitalized population and in patients taking renin angiotensin system inhibitors. On a related point, the effects of gastric K+-binding resins (93) on K+ homeostasis mechanisms in hyperkalemic patients are largely unknown.
Feedforward control of K+ homeostasis is likely unnecessary in populations consuming high-Na+/low-K+ diets, yet takes on prominence in the setting of high-K+/low-Na+ diet given the small pool of ECF potassium. For example, a K+-rich meal (lion catches the gazelle) is generally well tolerated because of our evolutionary adaptation, but intravenous K+ infusion of a similar load can be deadly; a lack of feedforward control (via insulin or gut factor) driving K+ clearance from the ECF by cell uptake and renal excretion distinguishes intravenous K+ infusion from oral K+ intake. This example underscores the importance of feedforward control in K+ homeostasis. Significant unknowns remain, including cross talk between gut (intake) and muscle (ICF storage pools) and brain (central control). For example, when the long-fasting seal eats a tuna, are there signals between these organs that “decide” to replenish muscle stores rather than excrete the ingested [K+]? Do feedback and feedforward regulation use the same intrarenal mechanisms? When plasma [K+] is elevated by exercise, is renal K+ excretion blunted to facilitate muscle reuptake?
The recently elucidated molecular mechanisms of K+ homeostasis indicate that elevating dietary K+ should contribute to a lowering of the global burden of hypertension. Silver and Farley (96) editorialize that manufacturers should reduce Na+ added during food processing and process food in a way that maintains natural cation composition. They recommend public policies that promote increased K+ intake, lower the cost of fruits and vegetables, a known barrier to consumption (27), and display K+ content on nutritional facts panels. The recently described “known knowns” should drive high-priority strategies to accomplish these global public health goals.
Footnotes
The authors are supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-083785 (to A. A. McDonough) and American Diabetes Association Basic Science Award 1-16-IBS-332 (to J. H. Youn).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: A.A.M. and J.H.Y. interpreted results of experiments; A.A.M. and J.H.Y. prepared figures; A.A.M. and J.H.Y. drafted manuscript; A.A.M. and J.H.Y. edited and revised manuscript; A.A.M. and J.H.Y. approved final version of manuscript.
References
- 1.Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc 88: 987–995, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adrogue HJ, Madias NE. The impact of sodium and potassium on hypertension risk. Semin Nephrol 34: 257–272, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 356: 1966–1978, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 107: 548–571, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 47: 296–308, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Araki S, Haneda M, Koya D, Kondo K, Tanaka S, Arima H, Kume S, Nakazawa J, Chin-Kanasaki M, Ugi S, Kawai H, Araki H, Uzu T, Maegawa H. Urinary potassium excretion and renal and cardiovascular complications in patients with Type 2 Diabetes and normal renal function. Clin J Am Soc Nephrol 10: 2152–2158, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology 26: 115–123, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Atanasovska T, Petersen AC, Rouffet DM, Billaut F, Ng I, McKenna MJ. Plasma K+ dynamics and implications during and following intense rowing exercise. J Appl Physiol 117: 60–68, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH. Role of gp91phox -containing NADPH oxidase in mediating the effect of K restriction on ROMK channels and renal K excretion. J Am Soc Nephrol 18: 2037–2045, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batuman V. Salt and hypertension: why is there still a debate? Kidney Int Suppl 3: 316–320, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazua-Valenti S, Gamba G. Revisiting the NaCl cotransporter regulation by with-no-lysine kinases. Am J Physiol Cell Physiol 308: C779–C791, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: a meta-analysis of randomized controlled trials. J Hypertens 33: 1509–1520, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Buendia JR, Bradlee ML, Daniels SR, Singer MR, Moore LL. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatr 169: 560–568, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Bundgaard H, Schmidt TA, Larsen JS, Kjeldsen K. K+ supplementation increases muscle [Na+-K+-ATPase] and improves extrarenal K+ homeostasis in rats. J Appl Physiol 82: 1136–1144, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Carrisoza-Gaytan R, Carattino MD, Kleyman TR, Satlin LM. An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron. Am J Physiol Cell Physiol 310: C243–C259, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambrey R, Picard N. Role of tissue kallikrein in regulation of tubule function. Curr Opin Nephrol Hypertens 20: 523–528, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, Tsai SY, Pan WH. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr 83: 1289–1296, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Chen P, Guzman JP, Leong PK, Yang LE, Perianayagam A, Babilonia E, Ho JS, Youn JH, Wang WH, McDonough AA. Modest dietary K+ restriction provokes insulin resistance of cellular K+ uptake and phosphorylation of renal outer medulla K+ channel without fall in plasma K+ concentration. Am J Physiol Cell Physiol 290: C1355–C1363, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Choi CS, Lee FN, McDonough AA, Youn JH. Independent regulation of in vivo insulin action on glucose versus K+ uptake by dietary fat and K+ content. Diabetes 51: 915–920, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Choi CS, Thompson CB, Leong PK, McDonough AA, Youn JH. Short-term K+ deprivation provokes insulin resistance of cellular K+ uptake revealed with the K+ clamp. Am J Physiol Renal Physiol 280: F95–F102, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Clausen T. Quantification of Na+,K+ pumps and their transport rate in skeletal muscle: functional significance. J Gen Physiol 142: 327–345, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crambert G. H-K-ATPase type 2: relevance for renal physiology and beyond. Am J Physiol Renal Physiol 306: F693–F700, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Czogalla J, Vohra T, Penton D, Kirschmann M, Craigie E, Loffing J. The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflügers Arch 468: 849–858, 2016. [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Felig P, Ferrannini E, Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol Endocrinol Metab 238: E421–E427, 1980. [DOI] [PubMed] [Google Scholar]
- 25.DiFranco M, Hakimjavadi H, Lingrel JB, Heiny JA. Na,K-ATPase alpha2 activity in mammalian skeletal muscle T-tubules is acutely stimulated by extracellular K+. J Gen Physiol 146: 281–294, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doucet A, Marsy S. Characterization of K-ATPase activity in distal nephron: stimulation by potassium depletion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F418–F423, 1987. [DOI] [PubMed] [Google Scholar]
- 27.Drewnowski A, Rehm CD, Maillot M, Monsivais P. The relation of potassium and sodium intakes to diet cost among U.S. adults. J Hum Hypertens 29: 14–21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du H, Li L, Bennett D, Guo Y, Key TJ, Bian Z, Sherliker P, Gao H, Chen Y, Yang L, Chen J, Wang S, Du R, Su H, Collins R, Peto R, Chen Z, Kadoorie Biobank S. China fresh fruit consumption and major cardiovascular disease in China. N Engl J Med 374: 1332–1343, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74: 325–349, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elkjaer ML, Kwon TH, Wang W, Nielsen J, Knepper MA, Frokiaer J, Nielsen S. Altered expression of renal NHE3, TSC, BSC-1, and ENaC subunits in potassium-depleted rats. Am J Physiol Renal Physiol 283: F1376–F1388, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Ellison DH, Terker AS. Why your mother was right: how potassium intake reduces blood pressure. Trans Am Clin Climatol Assn 126: 46–55, 2015. [PMC free article] [PubMed] [Google Scholar]
- 32.Ellison DH, Terker AS, Gamba G. Potassium and its discontents: new insight, new treatments. J Am Soc Nephrol 27: 981–989, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feraille E, Dizin E. Coordinated control of ENaC and Na+,K+-ATPase in renal collecting duct. J Am Soc Nephrol 27: 2554–2563, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frindt G, Houde V, Palmer LG. Conservation of Na+ vs. K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol 301: F14–F20, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funder JW. Apparent mineralocorticoid excess. J Steroid Biochem Mol Biol 165: 151–153, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension 67: 733–739, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Gleason CE, Frindt G, Cheng CJ, Ng M, Kidwai A, Rashmi P, Lang F, Baum M, Palmer LG, Pearce D. mTORC2 regulates renal tubule sodium uptake by promoting ENaC activity. J Clin Invest 125: 117–128, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glover M, Mercier Zuber A, Figg N, O'Shaughnessy KM. The activity of the thiazide-sensitive Na(+)-Cl cotransporter is regulated by protein phosphatase PP4. Can J Physiol Pharmacol 88: 986–995, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Grahammer F, Nesterov V, Ahmed A, Steinhardt F, Sandner L, Arnold F, Cordts T, Negrea S, Bertog M, Ruegg MA, Hall MN, Walz G, Korbmacher C, Artunc F, Huber TB. mTORC2 critically regulates renal potassium handling. J Clin Invest 126: 1773–1782, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gumz ML, Rabinowitz L. Role of circadian rhythms in potassium homeostasis. Semin Nephrol 33: 229–236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gumz ML, Rabinowitz L, Wingo CS. An integrated view of potassium homeostasis. N Engl J Med 373: 60–72, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He J, Mills KT, Appel LJ, Yang W, Chen J, Lee BT, Rosas SE, Porter A, Makos G, Weir MR, Hamm LL, Kusek JW, Chronic Renal Insufficiency Cohort Study I. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 27: 1202–1212, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hedayati SS, Minhajuddin AT, Ijaz A, Moe OW, Elsayed EF, Reilly RF, Huang CL. Association of urinary sodium/potassium ratio with blood pressure: sex and racial differences. Clin J Am Soc Nephrol 7: 315–322, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houk JC. Control strategies in physiological systems. FASEB J 2: 97–107, 1988. [DOI] [PubMed] [Google Scholar]
- 47.Hunter RW, Craigie E, Homer NZ, Mullins JJ, Bailey MA. Acute inhibition of NCC does not activate distal electrogenic Na+ reabsorption or kaliuresis. Am J Physiol Renal Physiol 306: F457–F467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: National Academies Press, 2004. [Google Scholar]
- 49.Jensen IS, Larsen CK, Leipziger J, Sorensen MV. Na+-dependence of K+-induced natriuresis, kaliuresis and NCC dephosphorylation. Acta Physiol (Oxf) 218: 49–61, 2016. [DOI] [PubMed] [Google Scholar]
- 50.Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ, Joosten MM. Urinary potassium excretion and risk of developing hypertension: the prevention of renal and vascular end-stage disease study. Hypertension 64: 769–776, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Lazelle RA, McCully BH, Terker AS, Himmerkus N, Blankenstein KI, Mutig K, Bleich M, Bachmann S, Yang CL, Ellison DH. Renal deletion of 12 kDa FK506-binding protein attenuates tacrolimus-induced hypertension. J Am Soc Nephrol 27: 1456–1464, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee DH, Maunsbach AB, Riquier-Brison AD, Nguyen MT, Fenton RA, Bachmann S, Yu AS, McDonough AA. Effects of ACE inhibition and ANG II stimulation on renal Na-Cl cotransporter distribution, phosphorylation, and membrane complex properties. Am J Physiol Cell Physiol 304: C147–C163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin DH, Sterling H, Wang WH. The protein tyrosine kinase-dependent pathway mediates the effect of K intake on renal K secretion. Physiology 20: 140–146, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Lin DH, Yue P, Yarborough O, 3rd Scholl UI, Giebisch G, Lifton RP, Rinehart J, Wang WH. Src-family protein tyrosine kinase phosphorylates WNK4 and modulates its inhibitory effect on KCNJ1 (ROMK). Proc Natl Acad Sci USA 112: 4495–4500, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Ho KK, Su J, Gong H, Chang AC, Lu S. Potassium transport of Salmonella is important for type III secretion and pathogenesis. Microbiology 159: 1705–1719, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malnic G, Klose RM, Giebisch G. Micropuncture study of renal potassium excretion in the rat. Am J Physiol 206: 674–686, 1964. [DOI] [PubMed] [Google Scholar]
- 57.McCormick JA, Bhalla V, Pao AC, Pearce D. SGK1: a rapid aldosterone-induced regulator of renal sodium reabsorption. Physiology 20: 134–139, 2005. [DOI] [PubMed] [Google Scholar]
- 58.McCormick JA, Ellison DH. Distal convoluted tubule. Compr Physiol 5: 45–98, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McKenna MJ, Gissel H, Clausen T. Effects of electrical stimulation and insulin on Na+-K+-ATPase ([3H]ouabain binding) in rat skeletal muscle. J Physiol 547: 567–580, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287: F593–F601, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Meneton P, Schultheis PJ, Greeb J, Nieman ML, Liu LH, Clarke LL, Duffy JJ, Doetschman T, Lorenz JN, Shull GE. Increased sensitivity to K+ deprivation in colonic H,K-ATPase-deficient mice. J Clin Invest 101: 536–542, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mente A, O'Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S, Investigators P. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 371: 601–611, 2014. [DOI] [PubMed] [Google Scholar]
- 64.Mistry AC, Wynne BM, Yu L, Tomilin V, Yue Q, Zhou Y, Al-Khalili O, Mallick R, Cai H, Alli AA, Ko B, Mattheyses A, Bao HF, Pochynyuk O, Theilig F, Eaton DC, Hoover RS. The sodium chloride cotransporter (NCC) and epithelial sodium channel (ENaC) associate. Biochem J 473: 3237–3252, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mulkidjanian AY, Bychkov AY, Dibrova DV, Galperin MY, Koonin EV. Origin of first cells at terrestrial, anoxic geothermal fields. Proc Natl Acad Sci USA 109: 821–830, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen MT, Yang LE, Fletcher NK, Lee DH, Kocinsky H, Bachmann S, Delpire E, McDonough AA. Effects of K+-deficient diets with and without NaCl supplementation on Na+, K+, and H2O transporters' abundance along the nephron. Am J Physiol Renal Physiol 303: F92–F104, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen TQ, Maalouf NM, Sakhaee K, Moe OW. Comparison of insulin action on glucose versus potassium uptake in humans. Clin J Am Soc Nephrol 6: 1533–1539, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ochrombel I, Ott L, Kramer R, Burkovski A, Marin K. Impact of improved potassium accumulation on pH homeostasis, membrane potential adjustment and survival of Corynebacterium glutamicum. Biochim Biophys Acta 1807: 444–450, 2011. [DOI] [PubMed] [Google Scholar]
- 69.Oh KS, Oh YT, Kim SW, Kita T, Kang I, Youn JH. Gut sensing of dietary K(+) intake increases renal K(+)excretion. Am J Physiol Regul Integr Comp Physiol 301: R421–R429, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh YT, Kim J, Youn JH. Role of pituitary in K+ homeostasis: impaired renal responses to altered K+ intake in hypophysectomized rats. Am J Physiol Regul Integr Comp Physiol 304: R1166–R1174, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation 52: 146–151, 1975. [DOI] [PubMed] [Google Scholar]
- 72.Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 10: 1050–1060, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palmer LG, Frindt G. Na+ and K+ transport by the renal connecting tubule. Curr Opin Nephrol Hypertens 16: 477–483, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Patel AB, Chao J, Palmer LG. Tissue kallikrein activation of the epithelial Na channel. Am J Physiol Renal Physiol 303: F540–F550, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Penton D, Czogalla J, Loffing J. Dietary potassium and the renal control of salt balance and blood pressure. Pflügers Arch 467: 513–530, 2015. [DOI] [PubMed] [Google Scholar]
- 76.Penton D, Czogalla J, Wengi A, Himmerkus N, Loffing-Cueni D, Carrel M, Rajaram RD, Staub O, Bleich M, Schweda F, Loffing J. Extracellular K+ rapidly controls NCC phosphorylation in native DCT by Cl−-dependent and -independent mechanisms. J Physiol 596: 6319–6331, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Picard N, Trompf K, Yang CL, Miller RL, Carrel M, Loffing-Cueni D, Fenton RA, Ellison DH, Loffing J. Protein phosphatase 1 inhibitor-1 deficiency reduces phosphorylation of renal NaCl cotransporter and causes arterial hypotension. J Am Soc Nephrol 25: 511–522, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pluznick JL, Sansom SC. BK channels in the kidney: role in K(+) secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Preston RA, Afshartous D, Rodco R, Alonso AB, Garg D. Evidence for a gastrointestinal-renal kaliuretic signaling axis in humans. Kidney Int 88: 1383–1391, 2015. [DOI] [PubMed] [Google Scholar]
- 80.Rabelink TJ, Koomans HA, Hene RJ, Dorhout Mees EJ. Early and late adjustment to potassium loading in humans. Kidney Int 38: 942–947, 1990. [DOI] [PubMed] [Google Scholar]
- 81.Rabinowitz L. Homeostatic regulation of potassium excretion. J Hypertens 7: 433–442, 1989. [DOI] [PubMed] [Google Scholar]
- 82.Rabinowitz L, Aizman RI. The central nervous system in potassium homeostasis. Front Neuroendocrinol 14: 1–26, 1993. [DOI] [PubMed] [Google Scholar]
- 83.Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev 80: 277–313, 2000. [DOI] [PubMed] [Google Scholar]
- 84.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA. Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodrigues SL, Baldo MP, Machado RC, Forechi L, Molina Mdel C, Mill JG. High potassium intake blunts the effect of elevated sodium intake on blood pressure levels. J Am Soc Hypertens 8: 232–238, 2014. [DOI] [PubMed] [Google Scholar]
- 86.Rossier BC, Baker ME, Studer RA. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev 95: 297–340, 2015. [DOI] [PubMed] [Google Scholar]
- 87.Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology 31: 283–293, 2016. [DOI] [PubMed] [Google Scholar]
- 88.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG 3rd, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 344: 3–10, 2001. [DOI] [PubMed] [Google Scholar]
- 89.Sandberg MB, Maunsbach AB, McDonough AA. Redistribution of distal tubule Na+-Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol 291: F503–F508, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl− cotransporter to apical membrane. Am J Physiol Renal Physiol 293: F662–F669, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Sangan P, Rajendran VM, Mann AS, Kashgarian M, Binder HJ. Regulation of colonic H-K-ATPase in large intestine and kidney by dietary Na depletion and dietary K depletion. Am J Physiol Cell Physiol 272: C685–C696, 1997. [DOI] [PubMed] [Google Scholar]
- 92.Satlin LM. Developmental regulation of expression of renal potassium secretory channels. Curr Opin Nephrol Hypertens 13: 445–450, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Schaefer JA, Gales MA. Potassium-binding agents to facilitate renin-angiotensin-aldosterone system inhibitor therapy. Ann Pharmacother 50: 502–510, 2016. [DOI] [PubMed] [Google Scholar]
- 94.Sebastian A, Frassetto LA, Sellmeyer DE, Morris RC Jr. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin Nephrol 26: 447–453, 2006. [DOI] [PubMed] [Google Scholar]
- 95.Siani A, Strazzullo P, Giacco A, Pacioni D, Celentano E, Mancini M. Increasing the dietary potassium intake reduces the need for antihypertensive medication. Ann Int Med 115: 753–759, 1991. [DOI] [PubMed] [Google Scholar]
- 96.Silver LD, Farley TA. Sodium and potassium intake: mortality effects and policy implications: comment on “Sodium and potassium intake and mortality among US adults”. Arch Intern Med 171: 1191–1192, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O'Donnell MJ, Mann JF, Clase CM, ONTARGET and TRANSCEND. Investigators. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int 86: 1205–1212, 2014. [DOI] [PubMed] [Google Scholar]
- 98.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. [DOI] [PubMed] [Google Scholar]
- 99.Su XT, Wang WH. The expression, regulation, and function of Kir4.1 (Kcnj10) in the mammalian kidney. Am J Physiol Renal Physiol 311: F12–F15, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang CL, Ellison DH. Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thompson CB, Choi C, Youn JH, McDonough AA. Temporal responses of oxidative vs. glycolytic skeletal muscles to K+ deprivation: Na+ pumps and cell cations. Am J Physiol Cell Physiol 276: C1411–C1419, 1999. [DOI] [PubMed] [Google Scholar]
- 104.Thompson CB, McDonough AA. Skeletal muscle Na,K-ATPase alpha and beta subunit protein levels respond to hypokalemic challenge with isoform and muscle type specificity. J Biol Chem 271: 32653–32658, 1996. [DOI] [PubMed] [Google Scholar]
- 105.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J. Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26: 425–438, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Veiras LC, Han J, Ralph DL, McDonough AA. Potassium supplementation prevents sodium chloride cotransporter stimulation during angiotensin II hypertension. Hypertension 68: 904–912, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Viscarra JA, Ortiz RM. Cellular mechanisms regulating fuel metabolism in mammals: role of adipose tissue and lipids during prolonged food deprivation. Metabolism 62: 889–897, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vitzthum H, Seniuk A, Schulte LH, Muller ML, Hetz H, Ehmke H. Functional coupling of renal K+ and Na+ handling causes high blood pressure in Na+ replete mice. J Physiol 592: 1139–1157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol 66: 547–569, 2004. [DOI] [PubMed] [Google Scholar]
- 111.Wang ZJ, Sun P, Xing W, Pan C, Lin DH, Wang WH. Decrease in dietary K intake stimulates the generation of superoxide anions in the kidney and inhibits K secretory channels in the CCD. Am J Physiol Renal Physiol 298: F1515–F1522, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Welling PA. Regulation of renal potassium secretion: molecular mechanisms. Semin Nephrol 33: 215–228, 2013. [DOI] [PubMed] [Google Scholar]
- 113.Welling PA. Roles and regulation of renal K channels. Annu Rev Physiol 78: 415–435, 2016. [DOI] [PubMed] [Google Scholar]
- 114.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 277: 1624–1632, 1997. [DOI] [PubMed] [Google Scholar]
- 115.Yang L, Frindt G, Lang F, Kuhl D, Vallon V, Palmer LG. SGK1-dependent ENaC processing and trafficking in mice with high dietary K intake and elevated aldosterone. Am J Physiol Renal Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ, Hu FB. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 171: 1183–1191, 2011. [DOI] [PubMed] [Google Scholar]
- 117.Youn JH. Gut sensing of potassium intake and its role in potassium homeostasis. Semin Nephrol 33: 248–256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci USA 111: 11864–11869, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao X, Yin X, Li X, Yan LL, Lam CT, Li S, He F, Xie W, Sang B, Luobu G, Ke L, Wu Y. Using a low-sodium, high-potassium salt substitute to reduce blood pressure among Tibetans with high blood pressure: a patient-blinded randomized controlled trial. PLos One 9: e110131, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]