Abstract
By using a feedback system control scheme, the best combination of formononetin, ononin, calycosin, and calycosin‐7‐O‐β‐d‐glucoside derived from Astragali Radix was shown to activate a hypoxia response element, a regulator for erythropoietin (EPO) transcription, in kidney fibroblast. In cyclophosphamide‐induced anemic rats, the treatment of combined flavonoids, or EPO, improved the levels of red blood cells, white blood cells, hemoglobin, and hematocrit. In addition, the altered levels of antioxidant capacity, super oxidase dismutase, and malondialdehyde, triggered in anemic rats, were restored to control levels by the treatment of flavonoids. Here, we proposed a possible therapy by using the common flavonoids in treating anemia.
Keywords: feedback system control, flavonoids combination, hematological parameters
Abbreviations
- CF
combined flavonoids
- CYP
cyclophosphamide
- DE
differential evolution
- EPO
erythropoietin
- ESA
erythropoiesis‐stimulating agents
- FSC
feedback system control
- HIF
hypoxia‐induced factor
- HRE
hypoxia responsive element
- MDA
malondialdehyde
- SOD
super oxidase dismutase
- T‐AOC
total antioxidant capacity
- TCM
traditional Chinese medicine
Anemia is a blood disorder associated with a series of health problems, for example, chronic kidney disease, congestive heart failure, chronic renal failure, diabetic, and nondiabetic renal disease 1. In addition, the treatment of cancer in combining radiotherapy, chemotherapy, and noncardiac or nonvascular surgery could lead to anemia 2, 3. Usually, blood transfusion is required for the anemic patients before therapy and surgery of these illnesses 4. Nevertheless, blood transfusion should be avoided wherever possible because of high risk of infection, mortality, and deaths 5, 6.
Erythropoiesis‐stimulating agents (ESA), that is, recombinant human epoein‐α and darbepoetin‐α, have been utilized for over two decades for the treatment of anemia associated with various diseases, in particular, cancer therapy‐induced anemia 7, 8. In clinical studies, the usage of ESAs in patients could cause a high risk of mortality, thromboembolic event, tumor progression, myocardial infarction, stroke, and hypertension 9, 10. Cyclophosphamide (CYP), a nitrogen mustard alkylating agent, is used in the treatment of cancer chemotherapy and autoimmune disorders 11, 12. In clinical application, CYP exhibits many side effects, including bone marrow suppression and cytotoxicity leading to progressive anemia, oxidative stress, and immunological suppression 13, 14.
Due to its low toxicity, traditional Chinese medicine (TCM) has a long history of utilization as complementary health food supplements 15. Historically, Astragali Radix (roots of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao or A. membranaceus (Fisch.) Bge.) is considered as major herb in stimulating ‘Qi’. Pharmacological study of Astragali Radix has shown that it could enhance biological functions, such as hepatoprotective effects, neuroprotective functions, hematopoietic, antioxidative, immunological properties, and antiaging activities 16, 17. The major ingredients within Astragali Radix, that is, formononetin, ononin, calycosin, and calycosin‐7‐O‐β‐d‐glucoside, have been employed to enhance hematopoietic functions by regulating erythropoietin (EPO) expression; hence, possibly could be used in treating anemia 18, 19, 20. TCM employs a combinatorial therapeutic approach: this is essential to identify an optimal drug cocktail in terms of efficacy and low side effects. This is a challenge due to the large number of possible combinations of such herbal mixtures. Recently, an engineering approach, namely feedback system control (FSC) scheme, has been developed to search potent drug combinations in tens of iterations out of a hundred thousand possible trials 21, 22. By using this novel FSC screening method, we found the best combination of formononetin, ononin, calycosin, and calycosin‐7‐O‐β‐d‐glucoside in activating the transcriptional activity of EPO gene 20. To verify the usage of combined flavonoids, CYP‐induced anemic rats were treated with the flavonoids. The serum parameters of hematology, oxidation and immunology were systematically compared in CYP‐induced rats.
Materials and methods
Materials and chemicals
Formononetin, ononin, calycosin, and calycosin‐7‐O‐β‐d‐glucoside were purchased from Weikeqi Biotechnology Co. (Sichuan, China). HPLC was employed to determine the purities of these marker chemicals, which showed over 98.0% of purity. Analytical‐ and HPLC‐grade reagents were purchased from Merck (Darmstadt, Germany).
Optimization of flavonoid combination
The FSC scheme was performed by an iterative closed‐loop of three processes which includes drug combination preparation, experimental readout, and search algorithm. To determine the optimal flavonoid combination, the maximal transcriptional activity of EPO gene in cultured human embryonic kidney fibroblast (HEK 293) was measured by using luciferase assay 20. The readouts from luciferase assay were fed into stochastic search algorithm (DE, differential evolution), which used the readout as fitness index and generated the new flavonoid combination for next luciferase assay. This process was repeated until stable optimal flavonoid combination was found. The optimal combination 0.08, 0.08, 0.4, and 0.08 μm (a ratio of 1 : 1 : 5 : 1) of four flavonoids, that is, formononetin, ononin, calycosin, and calycosin‐7‐O‐β‐d‐glucoside, was established that demonstrated ~ 3‐fold increase in transcriptional activity as compared to control 20. The same ratio of combined flavonoids was used for animal experiments.
Animals and experimental design
Male Sprague–Dawley rats (4 weeks old) weighing 180–220 g were provided by Laboratory Animal Services Center, China Pharmaceutical University (Nanjing, China). The rats were housed under controlled environmental conditions of temperature (25 ± 2 °C) and 12 h of light and dark cycle. All animal‐handling procedures were performed in strict accordance with China Legislation on The Use and Care of Laboratory Animals, with the guidelines established by Institute for Experimental Animal of China Pharmaceutical University, and which were approved by the college committee for animal experiments. Rats were divided into five major groups, each group contained eight rats: normal (treated with saline only), control (treated with CYP), EPO (treated with CYP + EPO), CF (treated with CYP + combined flavonoids at high and low doses). All rats were first intraperitoneally injected with CYP (25 mg·kg−1) for 4 days to induce anemia, except the normal rats. After 4 days, the control group was treated with saline, EPO group was intraperitoneally injected with EPO (75 IU·kg−1·day−1), the flavonoid group was intraperitoneally injected with a low dosage of flavonoid combination (11.2 μmol·kg−1·day−1) or with a high dosage of flavonoid combination (32 μmol·kg−1·day−1), for 14 days. The flavonoid mixture (32 μmol), containing 4 μmol formononetin, 4 μmol ononin, 20 μmol calycosin, and 4 μmol calycosin‐7‐O‐β‐d‐glucoside, that is, 1 : 1 : 5 : 1 ratio, was dissolved in saline.
Biochemical measurement
Twenty‐four hours after the last injection, the blood samples were collected from the eye pit, centrifuged at 3000 g for 15 min at 4 °C, so as to obtain serum for hematopoietic function test. Then, all rats were weighed and sacrificed by cervical dislocation; spleen, liver, kidney, and thymus were removed immediately and weighed. Super oxidase dismutase (SOD), malondialdehyde (MDA) and total antioxidant capacity (T‐AOC) were determined spectrophotometrically according to the instructions for commercial kits, while serum IL‐2 level was determined by ELISA using commercially available kits (Jiancheng Bioengineering Institute, Nanjing, China). Red blood count, white blood count, hemoglobin, and hematocrit levels were revealed according to the commercial instructions for the automatic biochemical analyzer (Biochemical Analytic Center of Zhongda Hospital, Nanjing, China).
Statistical analysis
The data are expressed as mean ± SD value, n = 6–8. Statistical tests were performed by two‐way ANOVA. Statistically significant changes were classed as [*] where P < 0.05; [**] where P < 0.01 and; [***] where P < 0.001.
Results
Before performing the biological assay, the weight index of spleen, liver, kidney, and thymus did not change significantly, as shown in Table S1. The blood hematological parameters in anemic rats are shown in Fig. 1. The levels of red blood cells, white blood cells, hemoglobin, and hematocrit were significantly decreased in CYP‐induced anemic rats, indicating that CYP injection successfully induced anemia in rats. The anemic rats were treated with EPO and combined flavonoids for 14 days. In both cases, the hematological parameters of anemic rats were restored to normal level (Fig. 1). High dose of combined flavonoids (32 μmol·kg−1·day−1) showed better effects, which fully restored the reduced hematological parameters in anemic rats, that is, 6.37 ± 0.73 × 1012 L−1 in red blood count, 11.85 ± 1.94 × 109 L−1 in white blood count, 127.01 ± 0.93 g·L−1 in hemoglobin, and 41.72 ± 3.69% in hematocrit count (Fig. 1). These parameters were very similar to the EPO‐treated anemic rats, except the white blood count, which was much better in the scenario of flavonoid treatment. These parameters suggested that the therapeutic effect of a novel flavonoid mixture could replace the function of EPO in anemic rats.
Figure 1.
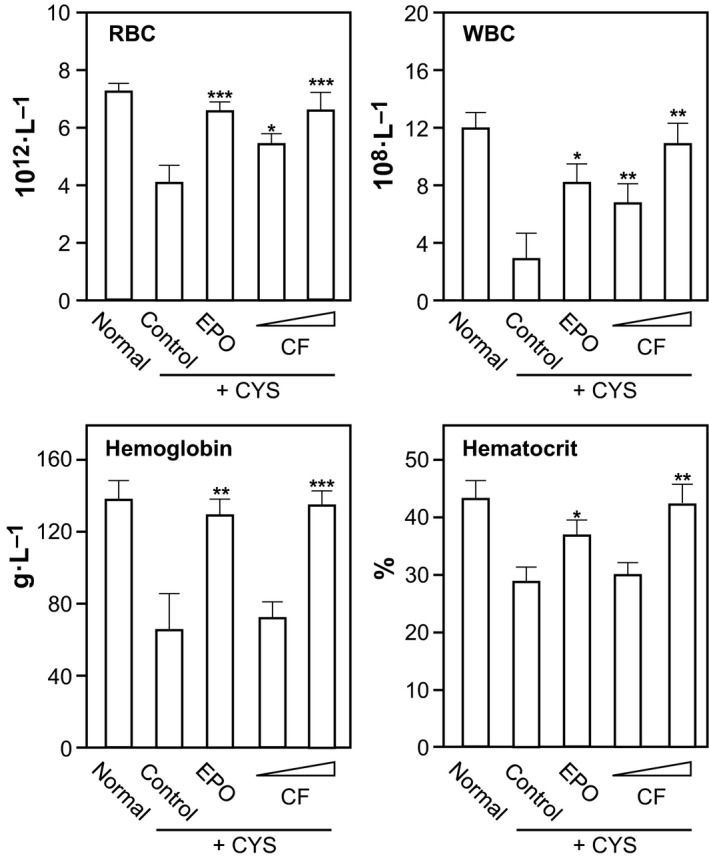
Combined flavonoids on hematological parameters in anemic rats. Rats were injected with sterile water or with CYP (25 mg·kg−1) for 4 days. Then, the rats were divided into five groups. Normal: normal rats treated with sterile water (normal); Control: CYP‐treated rats with saline; EPO: CYP‐treated rats with EPO (75 IU·kg−1·day−1 body weight); CF: CYP‐treated rats with low dose (11.2 μmol·kg−1·day−1) or with a high dose of flavonoid combination (32 μmol·kg−1·day−1). The flavonoid mixture (32 μmol), containing 4 μmol formononetin, 4 μmol ononin, 20 μmol calycosin, and 4 μmol calycosin‐7‐O‐β‐d‐glucoside, was dissolved in saline. The number of red blood cell (RBC), white blood cell (WBC), amount of hemoglobin, and the percentage of hematocrit were determined. Data are expressed as Mean ± SD, where n = 6–8. [*] where P < 0.05; [**] where P < 0.01 and; [***] where P < 0.001 as compared with control group.
The nonenzymatic antioxidant defense system capacity is reflected by T‐AOC. In the anemic rat model, CYP dramatically decreased markedly the serum levels of T‐AOC and SOD. In contrast, the injection of CYP increased the level of MDA (Fig. 2). After the administration of combined flavonoids, the altered levels of T‐AOC, SOD, and MDA in rat serum were restored back to normal. Again, a high dose of combined flavonoids showed a robust effect (Fig. 2). EPO showed similar effects to that of the flavonoids in restoring the parameters back to normal.
Figure 2.
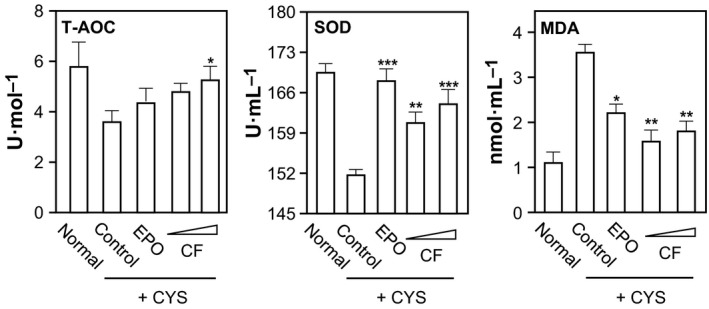
Combined flavonoids on antioxidative properties in anemic rats. The treatment of anemic rats, as well as the grouping, was carried out as in Fig. 1. The levels of antioxidant capacity (T‐AOC), super oxidase dismutase (SOD), and malondialdehyde (MDA) were measured in the rat serum. Data are expressed as Mean ± SD, where n = 6–8. [*] where P < 0.05; [**] where P < 0.01 and; [***] where P < 0.001 as compared with control group.
Long‐term administration of CYP could result in immunosuppression, that is, because of the deficiency of EPO. Interleukin (IL)‐2, secreted by T lymphocytes, is an important factor to promote immune cell proliferation and differentiation 23. A significant decrease (~ 26%) in IL‐2 was revealed in the rat serum after CYP administration (Fig. 3). The administration of combined flavonoids, or EPO, restored the reduced level of IL‐2 back to normal (Fig. 3). High dose of flavonoids showed a significantly better effect, as compared to EPO control. Our results clearly showed that flavonoids not only recovered the hematological parameters but also possessed oxidant inhibition and immunological enhancement activities.
Figure 3.
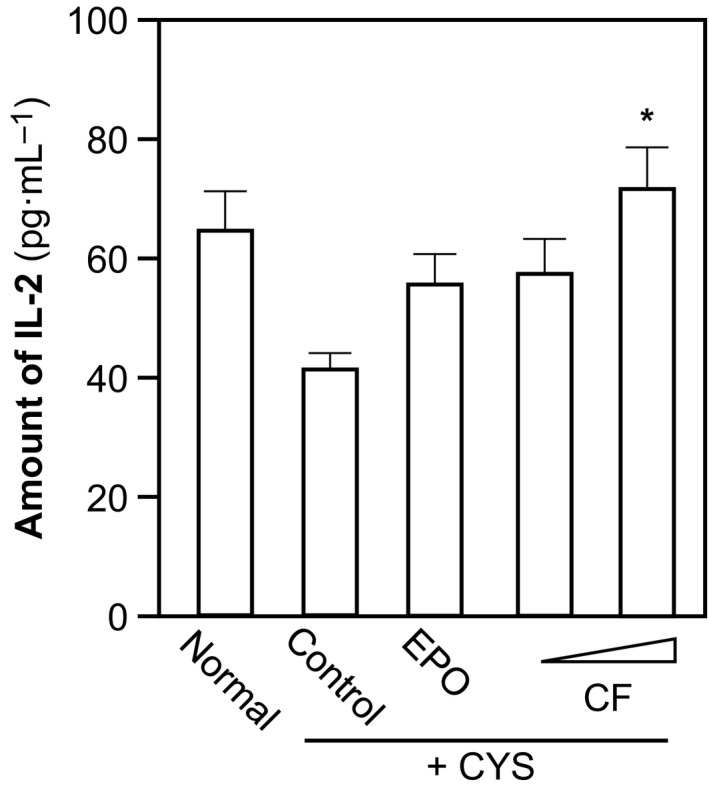
Combined flavonoids on serum level of IL‐2. The treatment of anemic rats, as well as the grouping, was carried out as in Fig. 1. The amount of IL‐2 was measured by ELISA. Data are expressed as Mean ± SD, where n = 6–8. [*] where P < 0.05 as compared with control group.
Discussion
Traditional Chinese medicine is a style of traditional Asian medicine building on a foundation of more than 2500 years of Chinese medical practice, which includes various forms of herbal medicine, acupuncture, massage, exercise, and dietary therapy. Astragali Radix is the most common herbal medicine on the market. Four major flavonoids, derived from Astragali Radix, formononetin, ononin, calycosin, and calycosin‐7‐O‐β‐d‐glucoside, have been shown to be effective in regulating EPO expression, which in turn might improve hematopoietic functions 18, 19. The low levels of these flavonoids being used here could not fully account for the induction activity of EPO, suggesting a possible synergistic interaction among the four flavonoids. Different experimental approaches have been used for the optimization of combinatorial therapies. But these methods require numerous experiments, additional cost, and are time consuming. FSC scheme has been introduced to search for optimized drug combinations using iterative stochastic search in terms of iterations out of a hundred thousand possible trials 21, 22. By using cell culture as a model, we have adopted this novel methodology in optimizing the four flavonoids in combination, which shows strong activation on transcriptional activity of hypoxia responsive element 20.
We have utilized DE as a stochastic search algorithm in FSC scheme. We started with an initial flavonoid combination and measured maximal transcriptional activity of EPO gene in cultured HEK293 using luciferase assay. The results from this experiment were fed back into the algorithm, which used these as fitness index to generate a new flavonoid combination for the next step. These three steps were repeated until we explored a stable optimal flavonoid combination with the highest efficacy and the lowest toxicity 20.
The cytotoxicity of CYP is known due to its reaction with DNA, resulting in CYP‐specific DNA adducts. These adducts are responsible for the formation of interstrand crosslinks, blocking DNA replication and causing bone marrow suppression. Experimental results indicated that there was a decrease in the red blood count, white blood count, hemoglobin, and hematocrit levels after CYP administration. Liver and kidney are responsible for the production of EPO that is an erythrocyte‐specific hematopoietic growth factor 24. A critical regulator for EPO transcription is a hypoxia responsive element, the promoter region of EPO gene 19. EPO expression is initiated when the activated hypoxia‐induced factor (HIF) is being bound onto the DNA promoter 25. These four flavonoids could induce the expression of EPO and therefore, enhance hematological parameters 26. Optimized flavonoid combination after CYP injection showed that hematological parameters have recovered to normal.
Free radical formation is associated with inflammatory and cardiovascular diseases 27. Different organisms have defense systems or detoxification enzymes i.e. SOD and glutathione, to protect us from radical damages 27. Due to the production of free radicals, CYP causes cytotoxicity and oxidative stress 28. SOD is a key enzyme that helps in the clearance of free radicals 29. Here, the injection of flavonoids could increase the activity of SOD in CYP‐treated rats. These data indicated that the combined flavonoids may play a vital role in protecting immune organs from damage by inhibiting the CYP‐induced oxidative stress.
Optimizing the combination of these four flavonoids by the FSC scheme could provide hints in the study of TCM formulae. According to TCM theory, the herbal formulae should be prepared in a unique methodology having a specific combination of different herbs (named as Fu Fang). The combination among different herbs within a decoction will directly affect the pharmacological properties of a herbal formula. Indeed, our previous work has supported the usage of the best combination of mixed herbs in an ancient herbal decoction. Thus, the FSC scheme could be a promising method to optimize the combination of a herbal formula, which accelerates the exploration of the veil of TCM.
Author contributions
LZ and AG conceived, designed and did experiments. KR and JYD performed FSC calculating, CMH, HQL, TTD, YKL and KWT were responsible for drafting the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Supporting information
Table S1. Effects of flavonoids on liver, kidney, spleen, and thymus index.
Acknowledgements
Supported by Hong Kong Research Grants Council Theme‐Based Research Scheme (T13‐607/12R), GRF (663012, 662713, M‐HKUST604/13), TUYF15SC01, The Hong Kong Jockey Club Charities Trust (HKJCCT12SC01) to Karl Tsim.
References
- 1. Borrows R, Loucaidou M, Chusney G, Borrows S, Tromp JV, Cairns T, Griffith M, Hakim N, McLean A, Palmer A et al (2008) Anaemia and congestive heart failure early post‐renal transplantation. Nephrol Dial Transplant 23, 1728–1734. [DOI] [PubMed] [Google Scholar]
- 2. Dunne JR, Malone D, Tracy JK, Gannon C and Napolitano LM (2002) Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 102, 237–244. [DOI] [PubMed] [Google Scholar]
- 3. Gianni L, Cole BF, Panzini I, Snyder R, Holmberg SB, Byrne M, Crivellari D, Colleoni M, Aebi S, Simoncini E et al (2008) Anemia during adjuvant non‐taxane chemotherapy for early breast cancer: incidence and risk factors from two trials of the International Breast Cancer Study Group. Support Care Cancer 16, 67–74. [DOI] [PubMed] [Google Scholar]
- 4. Claster S (2002) Biology of anemia, differential diagnosis, and treatment options in human immunodeficiency virus infection. J Infect Dis 185, 105–109. [DOI] [PubMed] [Google Scholar]
- 5. Sloand E, Kumar P, Klein HG, Merritt S and Sacher R (1994) Transfusion of blood components to persons infected with human immunodeficiency virus type 1: relationship to opportunistic infection. Transfusion 34, 48–53. [DOI] [PubMed] [Google Scholar]
- 6. Faris PM, Spence RK, Larholt KM, Sampson AR and Frei D (1999) The predictive power of baseline hemoglobin for transfusion risk in surgery patients. Orthopedics 22, 135–140. [DOI] [PubMed] [Google Scholar]
- 7. Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE and Haley NR (1989) Recombinant human erythropoietin in anemic patients with end‐stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 111, 992–1000. [DOI] [PubMed] [Google Scholar]
- 8. Henry DH and Abels RI (1994) Recombinant human erythropoietin in the treatment of cancer and chemotherapy‐induced anemia: results of double‐blind and open‐label follow‐up studies. Semin Oncol 21, 21–28. [PubMed] [Google Scholar]
- 9. Nordstrom BL, Luo W, Fraeman K, Whyte JL and Nordyke RJ (2008) Use of erythropoiesis‐stimulating agents among chemotherapy patients with hemoglobin exceeding 12 grams per deciliter. J Manag Care Pharm 14, 858–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS et al (2009) A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361, 2019–2032. [DOI] [PubMed] [Google Scholar]
- 11. Houssiau F (2007) Thirty years of cyclophosphamide: assessing the evidence. Lupus 16, 212–216. [DOI] [PubMed] [Google Scholar]
- 12. Sorbe B, Andersson H, Boman K, Rosenberg P and Kalling M (2008) Treatment of primary advanced and recurrent endometrial carcinoma with a combination of carboplatin and paclitaxel‐long‐term follow‐up. Int J Gynecol Cancer 18, 803–808. [DOI] [PubMed] [Google Scholar]
- 13. Woo S, Krzyzanski W and Jusko WJ (2008) Pharmacodynamic model for chemotherapy‐induced anemia in rats. Cancer Chemother Pharmacol 62, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oboh G, Akomolafe TL and Adetuyi AO (2010) Inhibition of cyclophosphamide‐induced oxidative stress in brain by dietary inclusion of red dye extracts from sorghum (Sorghum bicolor) stem. J Med Food 13, 1075–1080. [DOI] [PubMed] [Google Scholar]
- 15. Xue T and Roy R (2003) Studying traditional Chinese medicine. Science 300, 740–741. [DOI] [PubMed] [Google Scholar]
- 16. Ma XQ, Shi Q, Duan JA, Dong TT and Tsim KW (2002) Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. J Agric Food Chem 50, 4861–4866. [DOI] [PubMed] [Google Scholar]
- 17. Qi LW, Yu QT, Li P, Li SL, Wang YX, Sheng LH and Yi L (2006) Quality evaluation of Radix Astragali through a simultaneous determination of six major active isoflavonoids and four main saponins by high‐performance liquid chromatography coupled with diode array and evaporative light scattering detectors. J Chromatogr A 1134, 162–169. [DOI] [PubMed] [Google Scholar]
- 18. Xu ZB, Wu J, Chen HC, Lu YJ and Gong MC (1987) Radix Codonopsis Pilosulae and Radix Astragali promote erythrocyte glycolysis. Chin Med J 100, 654–657. [PubMed] [Google Scholar]
- 19. Zheng KY, Choi RC, Cheung AW, Guo AJ, Bi CW, Zhu KY, Fu Q, Du Y, Zhang WL, Zhan JY et al (2011) Flavonoids from Radix Astragali induce the expression of erythropoietin in cultured cells: a signaling mediated via the accumulation of hypoxia‐inducible factor‐1α. J Agric Food Chem 59, 1697–1704. [DOI] [PubMed] [Google Scholar]
- 20. Yu H, Zhang WL, Ding X, Zheng KY, Ho CM, Tsim KW and Lee YK (2013) Optimizing combinations of flavonoids deriving from Astragali Radix in activating the regulatory element of erythropoietin by a feedback system control scheme. Evid Based Complement Alternat Med 541436. doi: 10.1155/2013/541436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong PK, Yu F, Shahangian A, Cheng G, Sun R and Ho CM (2008) Closed‐loop control of cellular functions using combinatory drugs guided by a stochastic search algorithm. Proc Natl Acad Sci USA 105, 5105–5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsutsui H, Valamehr B, Hindoyan A, Qiao R, Ding X, Guo S, Witte ON, Liu X, Ho CM and Wu H (2011) An optimized small molecule inhibitor cocktail supports long‐term maintenance of human embryonic stem cells. Nat Commun 2, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao QT, Cheung JK, Li J, Chu GK, Duan R, Cheung AW, Zhao KJ, Dong TT and Tsim KW (2006) A Chinese herbal decoction, Danggui Buxue Tang, prepared from Radix Astragali and Radix Angelicae Sinensis stimulates the immune responses. Planta Med 72, 1227–1231. [DOI] [PubMed] [Google Scholar]
- 24. Dame C, Kirschner KM, Bartz KV, Wallach T, Hussels CS and Scholz H (2006) Wilms tumor suppressor, Wt1, is a transcriptional activator of the erythropoietin gene. Blood 107, 4282–4290. [DOI] [PubMed] [Google Scholar]
- 25. Maxwell P (2003) HIF‐1: an oxygen response system with special relevance to the kidney. J Am Soc Nephrol 14, 2712–2722. [DOI] [PubMed] [Google Scholar]
- 26. Zheng KY, Choi RC, Xie HQ, Cheung AW, Guo AJ, Leung KW, Chen VP, Bi CW, Zhu KY, Chan GK et al (2010) The expression of erythropoietin triggered by Danggui Buxue Tang, a Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis, is mediated by the hypoxia‐inducible factor in cultured HEK293T cells. J Ethnopharmacol 132, 259–267. [DOI] [PubMed] [Google Scholar]
- 27. Hu Y, Chen X, Duan H, Hu Y and Mu X (2009) Pulsatilla decoction and its active ingredients inhibit secretion of NO, ET‐1, TNF‐alpha, and IL‐1 alpha in LPS‐induced rat intestinal microvascular endothelial cells. Cell Biochem Funct 27, 284–288. [DOI] [PubMed] [Google Scholar]
- 28. Tripathi DN and Jena GB (2009) Intervention of astaxanthin against cyclophosphamide‐induced oxidative stress and DNA damage: a study in mice. Chem Biol Interact 180, 398–406. [DOI] [PubMed] [Google Scholar]
- 29. Ravid K, Smith‐Mungo LI, Zhao Z, Thomas KM and Kagan HM (1999) Upregulation of lysyl oxidase in vascular smooth muscle cells by cAMP: role for adenosine receptor activation. J Cell Biochem 75, 177–185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Effects of flavonoids on liver, kidney, spleen, and thymus index.


