Abstract
Oxidative stress suppresses animal health, performance, and production, subsequently impacting economic feasibility; hence, maintaining and improving oxidative status especially through natural nutrition strategy are essential for normal physiological process in animals. Phytochemicals are naturally occurring antioxidants that could be considered as one of the most promising materials used in animal diets in various forms. In this review, their antioxidant effects on animals are discussed as reflected by improved apparent performance, productivity, and the internal physiological changes. Moreover, the antioxidant actions toward animals further describe a molecular basis to elucidate their underlying mechanisms targeting signal transduction pathways, especially through the antioxidant response element/nuclear factor (erythroid-derived 2)-like 2 transcription system.
Keywords: Oxidative Stress, Phytochemicals, Antioxidant Molecules, Animals
INTRODUCTION
Oxidative stress, an imbalance condition when reactive oxygen species (ROS) formation exceed cellular antioxidant capacity, has become a major issue and the subject of production concerns and related research in the domestic animal industry. ROS are constantly produced in aerobic organisms as byproducts of normal oxygen metabolism. For example, chick embryos and hatchlings may be subjected to oxidative stress caused by their rapid growth and oxidative metabolism associated with the production of large quantities of free radicals and other reactive oxygen metabolites [1,2]. On the other hand, exogenous stressors may exacerbate the ROS levels more dramatically. Heat stress is a one of the debilitating problems that compromises performance and productivity regarding the present animal agricultural area and physical defect such as feather in birds or losses of sweat gland in porcine [3,4].
The use of synthetic antioxidants in animals to improve health, performance and products quality have been seriously considered. However, the potential adverse effects of these compounds make their innocuousness questioned [5,6]. A growing body of research has been devoted to natural antioxidants that are currently receiving considerable attention in animal nutrition fields. Phytochemicals have been shown to exert their positive antioxidant benefits toward animals in terms of favored performance, production quality [7–9], and enhanced endogenous antioxidant system, possibly by directly affecting specific molecular targets or indirectly as stabilized conjugates affecting metabolic pathway [10,11]. Accordingly, dissecting the antioxidant effects and the underlying mechanism of dietary phytochemicals is an important area.
Recently, much attention is being focused on a new wave of nutrigenomics [12]. Both external and endogenous stimuli turn on or switch off critical events in intracellular relays, thereby transmitting proper signaling to diverse downstream target molecules in a highly sophisticated fashion to fine-tune cellular homeostasis [13]. The cellular signaling network often goes awry due to excessive ROS; moreover, the protective effects that most dietary phytochemicals exert are likely to be the sum of several distinct mechanisms [12]. Several studies have been dedicated to understanding and formulating mechanistic pathways by which these naturally-derived substances could alter the fate of the cell. Particularly those antioxidant properties of phytochemicals that have been implicated as stress-alleviation agents [14–17]. One of the promising approaches is targeting intracellular signaling cascades for elucidating molecular mechanisms underlying the antioxidant protecting actions of dietary phytochemicals.
PHYTOCHEMICALS
Phytochemicals, plant-derived non-nutritive compounds, are one of the many different types of the dietary factors which play an important role in various functions of the animal body [18]. Given the advantage of using dietary components with relatively low toxicity, abundance of materials and low cost; nutritional therapy provides an important strategy for preventing and/or treating numerous diseases and contributing to the welfare of individuals [19]. The discovery of the promising potential benefits of changing dietary habits shed light on naturally occurring compounds from plants in health promotion and maintenance [20]. The protective effects of these phytochemicals were found in many human diseases and ailments [21–23], and research in nutraceuticals and functional foods and natural health products have been hot topics in recent years.
A large number of natural compounds present in food materials have been reported to possess antioxidant properties [18]. Aeschbach et al [24] reported that thymol, carvacrol and 6-gingerol possess useful antioxidant properties and may become very important in the search for “natural” replacements of “synthetic” antioxidant food additives. Despite prevalence of antioxidants such as vitamin C and E, the majority of the antioxidant activity of fruits, vegetables, spices and herbs may be from compounds such as phenolic acids and flavonoids considered to be much greater than that of the essential vitamins [25,26]; besides, the antioxidant activity of these compounds is predominantly determined by their structures, in particular the electron delocalization over an aromatic nucleus, in those based on a phenolic structure [20]. With over 8,000 structural variants, the polyphenols are a group of chemical substances found in plants that are characterized by having aromatic ring(s) bearing one or more hydroxyl moieties. The polyphenols are broadly divided into four classes including flavonoids (e.g., flavonols, flavones, isoflavones, and anthocyanidins), stilbenes, lignans, and phenolic acids [27,28] (Figure 1). When these compounds react with a free radical, the scavenging action results in the delocalization of the gained electron over the phenolic antioxidant and the stabilization by the resonance effect of the aromatic nucleus, which averts the continuation of the free radical chain reaction [20]. However, this is merely one of the antioxidant properties exerted by polyphenolic compounds, they inhibit oxidation through a variety of mechanisms rather than single mode of action typical of synthetic antioxidants [29,30]. Furthermore, the significant biological actions such as subduing oxidative stress, protection from degenerative disease, and reducing risk of cardiovascular disease could be attributed to their intrinsic antioxidant capabilities [31].
Figure 1.
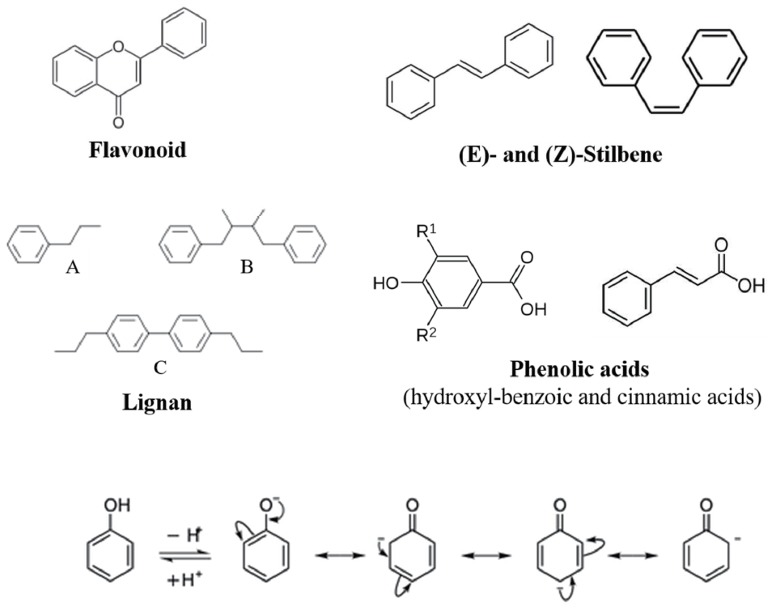
Chemical structures of the different classes of polyphenols and the resonance antioxidant effects of their aromatic ring. They are classified on the basis of the number of phenol ring and the structural elements binding the rings, broadly four classes; flavonoids, stilbenes, lignans, and phenolic acids.
EFFECTS OF DIFFERENT PHYTOCHEMICALS ON OXIDATIVE STATUS IN ANIMALS
The livestock industry would considerably benefit if natural antioxidants were used as substitutes for synthetic antioxidants, thus allowing consumer demands for food products without harmful residual substances to be satisfied. In view of several reports to date, phytogenic feed additives comprising single or combinations of components offer promising feed additives with pronounced antioxidant capacity for progressive animal production (Table 1). Research over the last decade has shown that several phytochemicals improve performance and products quality [32]. For example, Akdemir et al [33] evaluated the effects of tomato powder supplementation on performance, productive traits and the peroxidation level of laying hens (mid-lay). Hens fed 10 g/kg tomato powder in their diet showed a significant increase in feed intake in comparison with those in control group (116.3 vs 114.9 g/d); moreover, egg production increased from 89.94% to 93.11%, and the egg weight also had a profound increase (from 63.73 g to 66.59 g). Birds fed 200 g/kg dry tomato pulp (DTP) had the highest final body weight especially in comparison with those hens without DTP (1,993 vs 1,861 g), these results could also be proved by the improved feed intake and feed conversion ratio (FCR). However, there was no difference found among all the groups regarding the egg yield (%), and most of the egg characteristics, such as shell weight, shell thickness and shell strength, were similar, indicating that there was no adverse effects from these re-used materials when applied in hens’ diet [34]. In addition, to the concomitant benefits, in terms of reducing negative effects of stress [14–17,35], the additives were considered safe and environmentally friendly.
Table 1.
Active ingredients in phytogenic materials and their mode of antioxidant actions in animals
| Materials | Total phenolics (mg GAE/g DW) | Total flavonoid (mg QE/g DW) | Active ingredients | antioxidant capacities | References |
|---|---|---|---|---|---|
| TS | 22.23±1.13 | 1.38±0.06 | DPPH scavenging activity, superoxide anion radical scavenging activity, Inhibition of lipid peroxidation acitivity | Lin et al [5] | |
| 81.86±1.48 | 62.23±4.62 | Quercetin, Myricetin, Kaempferol | DPPH scavenging activity, oxygen radical absorption activity | Chao et al [53] | |
| Superoxide anion scavenging activity, Reducing power, Metals ion chelating effect | Hseu et al [54] | ||||
| Quercetrin, Kaempferol-3-O-α-L-rhamopyranoside, Astragalin, Quercetin, Kaempferol, Methyl gallate, Ethyl gallate, 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucopyranose | DPPH scavenging activity, Superoxide anion scavenging activity, Reducing power, Metals ion chelating effect | Yang et al [55] | |||
| EP | 55.9±1.1 | ND | Chlorogenic acid, Echinacoside, Alkamide8/9, Cynarin, Caftaric acid, Cichoric acid | Lee et al [8] Lee et al [9] | |
| GP | Delphinidin 3-O-glucoside, Cyanidin 3-O-glucoside, Petunidin 3-O-glucoside, Peonidin 3-O-glucoside, Malvidin 3-O-glucoside, Delphinidin 3-O-(600-O-p-coumaryl)-glucoside, Petunidin 3-O-(600-O-p-coumaryl)-glucoside, Peonidin 3-O-(600-O-p-coumaryl)-glucoside, Malvidin 3-O-(600-O-p-coumaryl)-glucoside, Malvidin 3-O-(600-O-acetyl)-glucoside, Malvidin 3-O-(600-O-caffeyl)-glucoside, Quercetin 3-O-glucuronide, Quercetin 3-O-glucoside, Quercetin 3-O-rutinoside, Kaempferol 3-O-glucoside, Isorhamnetin 3-O-glucoside, Isorhamnetin 3-O-glucuronide, Myricetin, Quercetin, Laricitrin, Kaempferol, Isorhamnetin | DPPH scavenging activity | Ruberto et al [45] | ||
| Resveratrol, catechin, epicatechin, gallocatechin, gallic acid, ellagic acid | Peroxyl radical scavenging activity | Yilmaz and Toledo [46] | |||
| ML | 7.4±0.15 | 4.4±0.19 | Gallic acid, Caffeic acid, Epicatechin, Gallocatechin gallate | DPPH scavenging activity, Reducing capacity, Metals ion chelating effect, Inhibition of lipid peroxidation acitivity | Lin et al [96] |
| DPPH/NO/superoxide anion scavenging activity, Reducing power | Andallu et al [95] |
GAE, gallic acid equivalent; QE, quercetin equivalent; DW, dry weight; TS, Toona sinensis; DPPH, 2,2-Diphenyl-1-picrylhydrazyl; EP, Echinacea purpurea L.; ND, non-detected; GP, grape seeds and/or skins; ML, Mulberry leave.
Oxidative rancidity, typically resulting from ROS-induced lipid peroxidation, is one of the major causes of deterioration of feed or food for consumption and the associated oxidative stress in animals may lead to metabolic disorders [5] followed by depressed growth and production performance [5,36]. Natural medicinal herbs have been suggested good antioxidants and have been used as feed additives in animal husbandry for over 2,000 years [37]. For example supplementation of dietary essential oil (Zataria mulriflora) could delay the peroxidation and microbial spoilage of chicken breast fillets [38]. Several phytochemical materials have been shown to benefit animal performance as provided in animal diet.
Echinacea purpurea L
Lee et al [9] evaluated Echinacea purpurea L. (EP), a herbal medicine whose secondary metabolites could improve cellular oxidative status and anti-inflammatory activity, as antioxidant supplement in broiler diets. Results showed that 0.5% EP supplemental group had higher body weight gain at 0 to 35 days of age compared with the non-supplemented (control) group (1,793 g/bird vs 1,687 g/bird). Feed efficiency of broilers in the 0.5 EP supplemented group was higher than the control group at 22 to 35 days (1.85 vs 1.90) and 0 to 35 days of age (1.60 vs 1.66). Moreover, 2% EP addition group, as comparing with the control group, showed that dietary supplementation of EP could improve meat quality, as reflected by increased pH value (6.18 vs 6.39 and 6.2 vs 6.33, for breast and thigh respectively), lower drip loss in breast (4.15% vs 3.89%) and thigh (5.02% and 4.42%) muscles, and increased water holding capacity (65.1% vs 70.6% and 69.9% and 75.6%, for breast and thigh muscle respectively); these positive benefits were due to decreasing lipid peroxidation and ameliorating oxidative status in broilers at 35 days of age, further proven by the elevated catalase (CAT) and superoxide dismutase (SOD) activities and reduced malaldehyde (MDA) levels. Previous studies also suggested the antioxidant potentiality of EP as reflected by the similar antioxidant capacity as ascorbic acid [39] attributed to those phenolic compounds that are important antioxidants sources for animals [40–42]. Jahanian et al [43] further applied the similar concept and material to laying hens, generally, the egg production and egg mass were increased by dietary supplementation of EP powder; yet these effects were diverse among different EP powder supplemented levels and feeding periods. However, the numerically reduced MDA level in egg yolk, although statistically insignificant, indicated a stable oxidative status of yolk largely owing to the Echinacea components, such as echinacoside, cichoric acid, and caffeic acid derivatives characterized by their strong antioxidant activity [44]. The slight difference in performance may reveal that the origin of materials and the targeted animal species are also important determining factors influencing the traits presented.
Grape and the related processing by-products
Recent investigations have stressed the importance of by-products recycling since the residues generated usually cause multi-faces problems. Hence, useful materials from by-products may represent an interesting advance in the maintenance of the environmental equilibrium and also an economic revaluation in either animal agriculture or animal husbandry. In the areas of wine production, great quantities of residues, such as grape pomace composed mainly of peels, stems and seed, are generated. Grape skin and seeds are well-known polyphenol-rich materials having strong capacity to act as powerful antioxidants by scavenging free radicals and terminating oxidative reactions [45,46]. Plant extracts rich in polyphenols are good candidates for bio-efficient antioxidants [47]. Ahn et al [48] suggested that grape seed extract could improve oxidative stability of cooked beef, similar effects was also found in other meat types such as turkey patties and cooled stored turkey meat. However, use of such natural antioxidants in animal nutrition could be limited due to the possibility of growth suppression [49,50] resulting from the loss of part of their antioxidant capacity in vivo [51]. Nevertheless, research done by Brenes et al [47] showed that when polyphenols present in grape pomace concentrate (GPC) were used in sufficient quantity to produce an effect, performance like weight gain and FCR were not affected by GPC inclusion, while the ileal and fecal digestibility of hydrolyzed polyphenols were 56.73% and 76.16%, and 55.62% vs 77.78% (for 60 g/kg diet supplementation of GPC vs control group) respectively. These results suggest the possible poorly absorbed characteristics of polyphenols in grapes could further exert the protection of membrane lipid oxidation and modulate the antioxidant activity in ileal content (increased from 15.8 to 43.5 μmol of Trolox equivalent/g dry weight (DW), as dietary supplementation with 60 g/kg control diet), and muscle tissue MDA level (reduced from 0.72 to 0.46 mg/kg meat, as dietary supplementation with 60 g/kg control diet) in broilers.
Toona sinensis
Currently, Chinese medical herbs have received considerable attention due to not only their therapeutic effects in human disorders but their positive association with animals and products quality [7,52]. Herbal remedies or botanicals were added to the animal diets to stimulate the performance and/or to enhance the antioxidant system [7,9,53]. Toona sinensis (syn. Cedrela sinensis A. Juss.; TS), a well-known traditional Chinese medicine herb, has been reported to possess a wide range of biological functions, including antioxidant activities [5]. Chao et al [53] and Hseu et al [54] found that TS exerts an effective antioxidant capacity against various oxidative systems in vitro, including the scavenging of free radicals and superoxide anion radicals, metal chelation and reducing power. Moreover, Yang et al [55] pointed out five flavonols and three derivatives of gallic acid which have strong antioxidant activity and scavenge superoxide free radicals. Lin et al [5] applied dried TS leaves (TSL) into the diet of White Roman geese. Despite no significant effect being found in body weight gain (1.89 vs 1.83 kg/bird), feed intake (12 vs 12 kg feed/bird) or FCR (6.43 vs 6.58 kg feed /kg gain) especially between the control and the highest dosage (0.2% addition) group during 7 to 12 week, it was noticeable that there was a weight difference between male and female geese. Tilki et al [56] reported that the live weight became statistically significant only when sexes were included; these results suggested that sex may be one of the factors contributing to the discrepancy. The last but not least, the serum biochemical parameters in study done by Lin et al [5] revealed that in comparison with the control group, serum SOD content was higher in birds fed 0.2% TSL, suggesting the potential role of its antioxidant compounds such as phenolics or flavonoids, and the growth parameters were not negatively affected by TSL supplementation.
POSSIBLE INTERPLAY BETWEEN CERTAIN PHYTOCHEMICALS AND ROS-RELATED MOLECULAR TARGET IN ANIMALS
In order to combat and neutralize the deleterious effects of ROS, the cells develop various sophisticated mechanisms for maintaining redox homeostasis which could be classified into two types of antioxidants. First, direct antioxidants have redox activity and short half-lives that should be supplemented or regenerated during the process [57]. Moreover, they should be administrated frequently and in relatively high dosages to sustain their physiological efficacy. Alternatively, indirect antioxidants act through the augmentation of cellular antioxidant capacity by enhancing specific genes encoding antioxidant proteins through the key transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2), that is known as a master regulator of the antioxidant response [36,57], so their physiological effects last longer than those of directs antioxidants. Further, indirect antioxidants are unlikely to evoke pro-oxidant effects which have been a problem in the use of high dose vitamin E therapy [58].
Nrf2 is a basic leucine zipper-containing transcription factor that activates phase II/detoxifying and many other genes through the cis-antioxidant response element (ARE), which contains a conserved core sequence (5′-A/GTGAC/GNNNGCa/c-3′) located in the promoter region [59–61], including NAD(P)H:quinone oxidoreductase 1 (NQO1), glutathione S-transferase (GST), heme oxigenase-1 (HO-1), glutathione peroxidase (GSH-Px), glutamatecysteine ligase (GCL), CAT, SOD, uridine diphosphate-glucuronosyltransferase glucuronosyltransferase, and the thioredoxin/peroxiredoxin system, which plays a crucial role in cell defense by improving the removal of ROS and, thus, has a protective role against oxidative stress [62]. Under normal conditions, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH associating protein 1 (Keap1), a cytoskeleton binding protein, which in turn associates with Cullin 3 to form an E3 ubiquitin ligase complex that targets Nrf2 for constant proteasomal degradation [19]. Disruption of the interaction between the Nrf2 and Keap1, possibly by covalent modification or oxidation of critical cysteine residues in Keap1, makes unbound Nrf2 translocate into the nucleus where it heterodimerizes with the small musculoponeurotic fibrosarcoma protein and co-activator proteins that binds to ARE leading to transcription of cytoprotective genes [13,62–64].
A number of studies demonstrated that many dietary phytochemicals derived from various vegetables, fruits, spices and herbal medicines can activate Nrf2 and induce expression of antioxidant or phase II detoxifying enzymes [13,36,65]. Recently, epigallocatechin gallate (EGCG), lycopene and resveratrol have become the subject of more intensive investigation in animals [15,17,36]. Further evidence showed that numerous phytochemicals can interfere with multiple cell-signaling pathways and they could be used in their natural form in larger doses for alleviation of stress in animals [4]. In this review, the five selected phytochemicals (catechin, lycopene, curcumin, resveratrol and mulberry leaf) are described and their potential modulating mechanism toward Nrf2/ARE signaling pathways in animals are presented (Figure 2).
Figure 2.
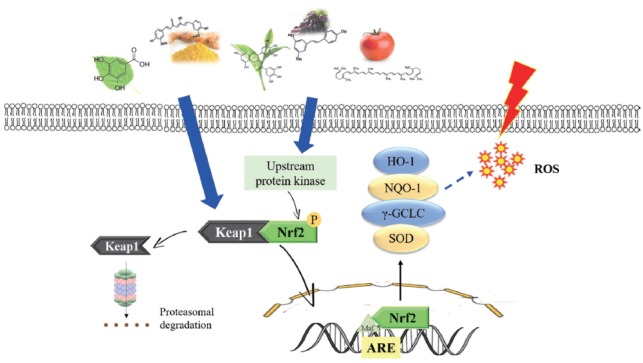
The potential molecular mechanism of catechin, lycopene, curcumin, resveratrol, and mulberry leaf modulating Nrf2/ARE pathway and its downstream antioxidant protein. Nrf2/ARE, nuclear factor (erythroid-derived 2)-like 2/antioxidant response element.
Catechin
Catechin are monomers of flavonols with a variety of similar compounds comprising catechin, epigallocatechin, epicatechin gallate (EGC) and EGCG; among the compounds, EGCG is the major component of polyphenols in green tea [36,66,67] exerting numerous biological effects that are pertinent to human medicine including an antioxidant attributed with possessing high reductive powers capable of quenching singlet molecular oxygen and peroxyl radicals. Sahin et al [36]) supplemented EGCG in quail diets to elucidate the action mode of EGCG in alleviation of oxidative stress. Decreases in hepatic MDA levels and increases in Nrf2 expression were found in birds fed increasing EGCG supplementation levels under heat stress. It is notable that the oxidative biomarker levels were strongly correlated with expression of hepatic nuclear transcription factors. Although there were no analytic data concerning the direct interaction between performance and molecular change, there were still linear increases in feed intake (4.3%) and egg production (6.9%) in response to increasing supplemental EGCG level. Romeo et al [68] also showed that EGCG increase HO-1 activity in rat neurons to protect from oxidative stress, which is associated with Nrf2 activation and translocation. Antioxidant actions of EGCG have also found in human skin suffering from oxidative stress by UV radiation [69]; as well as human fibroblasts exposed to hydrogen peroxide [70,71].
Lycopene
Lycopene is a non-provitamin A carotenoid abundant in tomato and its products even byproducts [72], which is a powerful antioxidant that provides protection against damage to the cells due to free radicals with a singlet-oxygen-quenching ability twice as high as that of β-catotene and ten fold higher than that of α-tocophenrol. It has been reported that lycopene can regulate redox-sensitive molecular targets such as the activation of mitogen-activated protein kinases and Nrf2 further affecting enzyme activity [73]. The supplementation of lycopene in rats increased the expression of Nrf2, HO-1, glutathione and antioxidant enzymes (CAT, GSH-Px, and SOD) [74]. Sahin et al [14] investigated the possible protective role of lycopene in the form of tomato powder in quail reared under heat stress and found that tomato powder supplementation decreased MDA concentration in serum, liver and muscle in all birds of both thermoneutral and heat stress groups. In addition, they further reported that lycopene might scavenge ROS and activate the antioxidant machinery through Nrf2 activation [14,35]. The benefit effects may be attributed to the accumulation of natural lycopene in tissue [14,15]. Lian and Wang [75] and Linnewiel et al [74] evaluated the effects of lycopene metabolites on the expression of the ARE/Nrf2-regulated antioxidant system, reporting that they have the ability to stimulate ARE transcription system and induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes, including HO-1, NQO1, GCL, glutathione reductase, and GST. Moreover, treatment with lycopene metabolites also increased intracellular GSH levels and decreased ROS levels in the cells [75].
Curcumin
Curcumin, a polyphenol phytochemical found in turmeric, has been shown to attenuate oxidative stress and preserve the activity of several antioxidant enzymes by modulating Nrf2 [76,77]. Garg and Maru [78] indicated that curcumin increased ARE-binding of Nrf2 and induced the activity as well as expression of GST and NQO1 and their mRNA transcripts; additionally, the liver and lung of mice treated with dietary curcumin had reduced oxidative stress. Curcumin could augment antioxidant status reflected by elevated expression of the SOD gene in chickens fed turmeric [79]. Similarly, Ahmadi [80] reported that CAT and SOD activities increased when basal diet of broilers was supplemented with 0.3 and 0.6 g/kg turmeric powder. Several studies determined that curcumin disrupts the Nrf2/Keap1 complex, leading to increased Nrf2 binding to ARE and was associated with a significant increase in the activity and expression of HO-1 [81–84]. Recently, Sahin et al [16] found that Nrf2 expression level in heat-stressed quail was linearly increased in response to increasing supplemental curcumin level, whilst the HO-1 level was upregulated. The positive antioxidant effects on the transcription level could be further demonstrated in the decreased MDA level. The HO-1 gene is redox regulated and the curcumin-induced HO-1 overexpression was correlated with the activation of transcription factors Nrf2 [85].
Resveratrol
Several studies shown that resveratrol, another polyphenol phytochemical found in grapes, cranberries and peanuts, prevents the cytotoxic effects of oxidative stress and can cease the deleterious effects of free radicals and concomitant generation of ROS in case of heat stress [12,15]. Liu et al [86] observed that resveratrol protected human keratinocytes from ultraviolet A-induced oxidative stress and increased antioxidant enzyme activities by increased Nrf2 expression and its accumulation in the nucleus. In addition, treatment of resveratrol can normalize and increase expression of Nrf2/Keap1 and the Nrf2-targeted genes such as SOD, CAT, NQO1, HO-1, and γ-GCS in both in vitro and in vivo studies in challenged mice [87,88]. Recently, the cytoprotective virtues of resveratrol were applied in the animal nutrition field to conquer negative effects of oxidative stress. Sahin et al [15] reported that resveratrol supplementation resulted in increases in hepatic antioxidant enzyme activities, for example, the SOD and CAT activity for quail receiving 0 and 400 mg/kg diet supplementation were 131.9 and 162.3 U/mg, and 46.6 and 60.5 U/mg, respectively. Moreover, the reduction of MDA concentration was also confirmed to be significant (2.6 and 1.66 nmol/g for 0 and 400 mg/kg diet resveratrol addition), which may associated with improved egg production (87.1% and 93.2%, 0 and 400 mg supplementation per kg diet) and feed intake (30.1 and 31.7 g/d, 0 and 400 mg supplementation per kg diet) in quails under a heat-stressed environment. Regarding the correlation between the surveyed antioxidant index and the final performance traits, there were positive correlation between performance and hepatic antioxidant enzymes’ activities; and negative correlation rather found between performance and hepatic MDA level. Similar effects were found in serum and egg yolk oxidation status in laying quails fed resveratrol diet. These may attributed to the scavenging effects on free radicals [89]; futhermore, research has proved that resveratrol can stimulate Nrf2 leading to upregulation of downstream antioxidant/phase II enzymes [90,91] accompanied by reduced expression of heat shock protein, a classical sign of stress in animals [15].
Mulberry leaves
Mulberry, genus Morus (Moraceae), is a plant with valuable but low-cost leaves that have therapeutic applications in traditional medicine [92]; these medical effects are mainly linked to the leaves’ phenolic composition, which have effective antioxidant properties [93,94]. Andallu et al [95] showed that mulberry leaf (ML) extract effectively scavenged free radicals such as NO, superoxide and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radicals, and exhibited significant reducing power as well. Moreover, suppressed lipid peroxidation was also found in erythrocyte membranes treated with ML extract; the in vivo study further supporting the antioxidant role of ML. We recently identified critical polyphenolic components in ML and demonstrated that the antioxidant properties exerted in vitro could act and be reflected in vivo from not only the improved oxidative status markers in serum, e.g. decreased MDA level and increased SOD activity, but also the underlying gene expression changes such as increased Nrf2 and its downstream antioxidant molecules (HO-1 and GST), as well as decreased ROS production factors (ROS modulator protein 1 and nicotinamide adenine dinucleotide phosphate-oxidase 1 [NADPH oxidase 1]) in laying hens fed ML-supplemented diet [96]. The increased egg shell weight and shell strength with ML addition in our study may be attributed to improved oxidative status, as indicated by the underlying indirect molecular changes and the direct serum oxidative indices. In the light of the present research results, the animal industry is paying attention to local, natural and safe materials not only for reducing the feed cost but also for improving the animals’ condition during raising and their final performance traits. It’s worth to considering the possible interplay between the antioxidant benefits derived from phytochemicals and the growth and productive parameter of animals.
According to literature discussed above, the discrepancy between trials may be attributed to the origin of the plant materials, including the variety and the planting even the storing condition and time. Furthermore, for the environment of husbandry, the temperature, humidity and the presence of stress factors may also influence the results; the last but not the least, may be the animal, their health status, gender, even the species are potential contributing factors.
In spite of that, it can be summed that phytochemicals possess various kinds and levels of antioxidant components and activities, exerting different “strength” in the animal body that can give raise to diverse performance outcomes without doing harm. The increased antioxidant capacity or the stabilization effects while in adverse conditions provided to animals by phytochemicals would act as a value-added benefit from these natural, available and even cost-beneficial materials. Moreover, as increasing numbers of phytochemicals become the target of research, there may be more and more plants reveled as a source of promising natural-derived antioxidants to be applied in animal diets.
CONCLUSION
Today’s domestic animals producers are confronted with numerous challenges to prevent oxidative stress-related situation and maintain health without using sub-therapeutic antibiotics, not only for proper animal health, growth and production but for feasible economic outcomes. Therefore, it’s necessary to support the animals, producers and consumers through a variety of positive measures that are effective, valuable, and the prime importance, natural. Several studies have shown the antioxidant activity of phytochemicals in vitro; however it’s more pertinent to employ intracellular-signaling cascades as molecular targets for elucidating and corroborating the actual efficacy in animal body. The aforementioned finding demonstrated that despite the ability of these functional phytochemicals to induce the expression of antioxidant/phase II enzymes, it appears that their major role is acting as modifiers of signal transduction pathways to elicit cytoprotective responses through suppressing stress-induced protein activation and enhancing Keap1 dissociation from Nrf2 in response to stressors. Therefore, suppression of abnormally amplified oxidation signaling and restoration of function as well as activation of antioxidant machinery can provide important strategies for prevention of oxidative stress and augmentation of antioxidant defense in animals.
ACKNOWLEDGMENTS
The authors would like to express the sincere gratitude to the Ministry of Science and Technology (MOST 103-2628-B-005-001-MY3) for financially supporting the study to Prof. Tzu-Tai Lee.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–96. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 2.von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H. Good genes, oxidative stress and condition-dependent sexual signals. Proc Biol Sci. 1999;266:1–12. doi: 10.1098/rspb.1999.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajakaiye JJ, Perez-Bello A, Mollineda-Trujillo A. Impact of heat stress on egg quality in layer hens supplemented with l-ascorbic acid and dl-tocopherol acetate. Vet Arhiv. 2011;81:119–32. [Google Scholar]
- 4.Sahin K, Orhan C, Smith MO, Sahin N. Molecular targets of dietary phytochemicals for the alleviation of heat stress in poultry. Worlds Poult Sci J. 2013;69:113–24. [Google Scholar]
- 5.Lin MJ, Chang SC, Jea YS, et al. In vitro antioxidant capability and performance assessment of White Roman goose supplemented with dried Toona sinensis. J Appl Anim Res. 2016;44:395–402. [Google Scholar]
- 6.Joseph AM, Anthony TT. Food additive toxicology. NY: Marcel Dekker; 1994. pp. 89–110. [Google Scholar]
- 7.Ansari J, Khan SH, Haq Au, Yousaf M. Effect of the levels of Azadirachta indica dried leaf meal as phytogenic feed additive on the growth performance and haemato-biochemical parameters in broiler chicks. J Appl Anim Res. 2012;40:336–45. [Google Scholar]
- 8.Lee TT, Yu B. Application of biologics to feedstuff. Afr J Biotechnol. 2013;12:526–30. [Google Scholar]
- 9.Lee TT, Ciou JY, Chen CL, Yu B. Effect of Echinacea purpurea L. on oxidative status and meat quality in Arbor Acres broilers. J Sci Food Agric. 2013;93:166–72. doi: 10.1002/jsfa.5745. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Kelloff GJ, Crowell JA, Steele VE, et al. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130( Suppl):E467–71. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese V, Cornelius C, Mancuso C, et al. Cellular stress response: A novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 3:2444–71. doi: 10.1007/s11064-008-9775-9. 32008. [DOI] [PubMed] [Google Scholar]
- 13.Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46:1271–8. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Sahin K, Orhan C, Akdemir F, et al. Tomato powder supplementation activates Nrf-2 via ERK/Akt signaling pathway and attenuates heat stress-related responses in quails. Anim Feed Sci Technol. 2011;165:230–7. [Google Scholar]
- 15.Sahin K, Orhan C, Akdemir F, Tuzcu M, Sahin N. Resveratrol protects quail hepatocytes against heat stress: modulation of the Nrf2 transcription factor and heat shock proteins. J Anim Physiol Anim Nutr. 2012;96:66–74. doi: 10.1111/j.1439-0396.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 16.Sahin K, Orhan C, Tuzcu Z, Tuzcu M, Sahin N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem Toxicol. 2012;50:4035–41. doi: 10.1016/j.fct.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Tuzcu M, Sahin N, Karatepe M, et al. Epigallocatechin-3-gallate supplementation can improve antioxidant status in stressed quail. Br Poult Sci. 2008;49:643–8. doi: 10.1080/00071660802298336. [DOI] [PubMed] [Google Scholar]
- 18.Abbas ZK, Saggu S, Sakeran MI, et al. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J Biol Sci. 2015;22:322–6. doi: 10.1016/j.sjbs.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardozo LF, Pedruzzi LM, Stenvinkel P, et al. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie. 2013;95:1525–33. doi: 10.1016/j.biochi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Tsao R, Deng Z. Separation procedures for naturally occurring antioxidant phytochemicals. J Chromatogr B. 2004;812:85–99. doi: 10.1016/j.jchromb.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 21.Barnes S. Role of phytochemicals in prevention and treatment of prostate cancer. Epidemiol Rev. 2001;23:201–5. doi: 10.1093/oxfordjournals.epirev.a000773. [DOI] [PubMed] [Google Scholar]
- 22.Bergman M, Varshavsky L, Gottlieb HE, Grossman S. The antioxidant activity of aqueous spinach extract: chemical identification of active fractions. Phytochemistry. 2001;58:143–52. doi: 10.1016/s0031-9422(01)00137-6. [DOI] [PubMed] [Google Scholar]
- 23.Toniolo P, Van Kappel AL, Akhmedkhanov A, et al. Serum carotenoids and breast cancer. Am J Epidemiol. 2001;153:1142–7. doi: 10.1093/aje/153.12.1142. [DOI] [PubMed] [Google Scholar]
- 24.Aeschbach R, Loliger J, Scott BC, et al. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol. 1994;32:31–6. doi: 10.1016/0278-6915(84)90033-4. [DOI] [PubMed] [Google Scholar]
- 25.Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds—biochemistry and functionality. J Med Food. 2003;6:291–9. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agric Food Chem. 1996;44:701–5. [Google Scholar]
- 27.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–52. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radical Bio Med. 2007;42:1690–703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Cuvelier ME, Richard H, Berset C. Comparison of the antioxidative activity of some acid-phenols: structure-activity relationship. Biosci Biotechnol Biochem. 1992;56:324–5. [Google Scholar]
- 30.Kahkonen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–62. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 31.Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8:950–88. [Google Scholar]
- 32.Lokaewmanee K, Yamauchi K, Komori T, Saito K. Effects on egg yolk colour of paprika or paprika combined with marigold flower extracts. Ital J Anim Sci. 2010;9:356–9. [Google Scholar]
- 33.Akdemir F, Orhan C, Sahin N, Sahin DrK, Hayirli A. Tomato powder in laying hen diets: effects on concentrations of yolk carotenoids and lipid peroxidation. Br Poult Sci. 2012;53:675–80. doi: 10.1080/00071668.2012.729142. [DOI] [PubMed] [Google Scholar]
- 34.Calislar S, Uygur G. Effects of dry tomato pulp on egg yolk pigmentation and some egg yield characteristics of laying hens. J Anim Vet Adv. 2010;9:96–8. [Google Scholar]
- 35.Sahin N, Orhan C, Tuzcu M, Sahin K, Kucuk O. The effects of tomato powder supplementation on performance and lipid peroxidation in quail. Poult Sci. 2008;87:276–83. doi: 10.3382/ps.2007-00207. [DOI] [PubMed] [Google Scholar]
- 36.Sahin K, Orhan C, Tuzcu M, et al. Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult Sci. 2010;89:2251–8. doi: 10.3382/ps.2010-00749. [DOI] [PubMed] [Google Scholar]
- 37.Wang RJ, Li DF, Bourne S. Can 2000 years of herbal medicine history help us solve problems in the year 2000?. Biotechnology in the Feed Industry Proceedings of Alltech’s 14th Annual Symposium; Nottingham, UK: Nottingham University Press; 1998. pp. 273–291. [Google Scholar]
- 38.Jebelli AJ, Ghazvinian K, Mahdavi A, Vayeghan AJ, Staji H, Khaligh SG. The effect of dietary Zataria multiflora boiss: Essential oil supplementation on microbial growth and lipid peroxidation of broiler breast fillets during refrigerated storage. J Food Process Preserv. 2013;37:881–8. [Google Scholar]
- 39.Lee TT, Chen CL, Shieh ZH, Lin JC, Yu B. Study on antioxidant activity of Echinacea purpurea L. extracts and its impact on cell viability. Afr J Biotechnol. 2009;8:5097–105. [Google Scholar]
- 40.Matthias A, Banbury L, Bone KM, Leach DN, Lehmann RP. Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia. 2008;79:53–8. doi: 10.1016/j.fitote.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Percival SS. Use of Echinacea in medicine. Biochem Pharmacol. 60:155–8. doi: 10.1016/s0006-2952(99)00413-x. 200. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan AM, Laba JG, Moore JA, Lee TD. Echinacea induced macrophage activation. Immunopharmacol Immunotoxicol. 2008;30:553–74. doi: 10.1080/08923970802135534. [DOI] [PubMed] [Google Scholar]
- 43.Jahanian E, Jahanian R, Rahmani HR, Alikhani M. Dietary supplementation of Echinacea purpurea powder improved performance, serum lipid profile, and yolk oxidative stability in laying hens. J Appl Anim Res. 2017;45:45–51. [Google Scholar]
- 44.Thygesen L, Thulin J, Mortenson A, Skibsted LH, Molgaard P. Antioxidant activity of cichoric acid and alkamides from Echinacea purpurea, alone and in combination. Food Chem. 2007;101:74–81. [Google Scholar]
- 45.Ruberto G, Renda A, Daquino C, et al. Polyphenols constituents and antioxidant activity of grape pomace from five Sicilian red grape cultivars. Food Chem. 2007;100:203–10. [Google Scholar]
- 46.Yilmaz Y, Toledo RT. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric Food Chem. 2004;52:255–60. doi: 10.1021/jf030117h. [DOI] [PubMed] [Google Scholar]
- 47.Brenes A, Viveros A, Goni I, et al. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult Sci. 2008;87:307–16. doi: 10.3382/ps.2007-00297. [DOI] [PubMed] [Google Scholar]
- 48.Ahn JH, Grun IU, Fernando LN. Antioxidant properties of natural plant extracts containing polyphenolic compounds in cooked ground beef. J Food Sci. 2002;67:1364–9. [Google Scholar]
- 49.Hughes RJ, Brooker JD, Smyl C. Growth rate of broiler chickens given condensed tannins extracted from grape seed. Aust Poult Sci Symp. 2005;67:65–8. [Google Scholar]
- 50.Lau DW, King AJ. Pre- and post-mortem use of grape seed extract in dark poultry meat to inhibit development of thiobarbituric acid reactive substances. J Agric Food Chem. 2003;51:1602–7. doi: 10.1021/jf020740m. [DOI] [PubMed] [Google Scholar]
- 51.Manach C, Scalbert C, Morand C, Remezy C, Jiménez L. Polyphenols: Food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 52.Huang CW, Lee TT, Shih YC, Yu B. Effects of dietary supplementation of Chinese medicine herbs on polymorphonuclear neutrophil immune activity and small intestinal morphology in weanling pigs. J Anim Physiol Anim Nutr. 2012;96:285–94. doi: 10.1111/j.1439-0396.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 53.Chao PY, Lin SY, Lin KH, et al. Antioxidant activity in extracts of 27 indigenous Taiwanese vegetables. Nutrients. 2014;6:2115–30. doi: 10.3390/nu6052115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hseu YC, Chang WH, Chen CS, et al. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem Toxicol. 2008;46:105–14. doi: 10.1016/j.fct.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Yang H, Gu Q, Gao T, et al. Flavonols and derivatives of gallic acid from young leaves of Toona sinensis (A. Juss.) Roemer and evaluation of their anti-oxidant capacity by chemical methods. Pharmacogn Mag. 2014;10:185–90. doi: 10.4103/0973-1296.131034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tilki M, Saatci M, Kirmizibayrak T, Aksoy A. Effect of age on growth and carcass composition of native Turkish geese. Archiv für Geflügelkunde. 2005;69(Suppl):E77–83. [Google Scholar]
- 57.Jung KA, Kwak MK. The Nrf2 System as a potential target for the development of indirect antioxidant. Molecules. 2010;15:7266–91. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mamede AC, Tavares SD, Abrantes AM, et al. The role of vitamins in cancer: a review. Nutr Cancer. 2011;63:479–94. doi: 10.1080/01635581.2011.539315. [DOI] [PubMed] [Google Scholar]
- 59.Barve A, Khor TO, Hao X, et al. Murine prostate cancer inhibition by dietary phytochemicals - curcumin and phenyethylisothiocyanate. Pharm Res. 2008;25:2181–9. doi: 10.1007/s11095-008-9574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu R, Saw CL, Yu R, Kong AN. Regulation of NF-E2-related factor 2 signaling for cancer chemoprevention: antioxidant coupled with anti-inflammatory. Antioxid Redox Signal. 2010;13:1679–98. doi: 10.1089/ars.2010.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen GG, Kong AN. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm Drug Dispos. 2009;30:345–55. doi: 10.1002/bdd.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–72. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 63.Kobayashi A, Kang MI, Watai Y, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–9. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takaya K, Suzuki T, Motohashi H, et al. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radical Bio Med. 2012;53:817–27. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saw CL, Yang AY, Cheng DC, et al. Pharmacodynamics of ginsenosides: antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem Res Toxicol. 2012;25:1574–80. doi: 10.1021/tx2005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beeche GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr. 2003;133(Suppl):E3248–54. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- 67.Sahin K, Tuzcu M, Gencoglu H, et al. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010;87:240–5. doi: 10.1016/j.lfs.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 68.Romeo L, Intrieri M, D’Agata V, et al. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J Am Coll Nutr. 2009;28(Suppl_):E492–9. doi: 10.1080/07315724.2009.10718116. [DOI] [PubMed] [Google Scholar]
- 69.Katiyar SK, Afaq F, Perez A, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–94. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 70.Meng Q, Velalar CN, Ruan R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radical Bio Med. 2008;44:1032–41. doi: 10.1016/j.freeradbiomed.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 71.Wei H, Zhang X, Zhao JF, et al. Scavenging of hydrogen peroxide and nhibition of ultraviolet light-induced oxidative DNA damage by aqueous extracts from green and black teas. Free Radical Bio Med. 1999;26:1427–35. doi: 10.1016/s0891-5849(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 72.Ali NAL, Mohammed AB, Allow AA. Effect of adding different levels of Lycopene to the ration on some lipid profile traits of the Laying hens ISA-Brown. J Biol Agric Healthc. 2014;4:10–9. [Google Scholar]
- 73.Palozza P, Catalano A, Simone R, Cittadini A. Lycopene as a guardian of redox signalling. Acta Biochim Pol. 2012;59:221–5. [PubMed] [Google Scholar]
- 74.Linnewiel K, Ernst H, Caris-Veyrat C, et al. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic Biol Med. 2009;47:659–67. doi: 10.1016/j.freeradbiomed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer. 2008;123:1262–8. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He HJ, Wang GY, Gao Y, et al. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes. 2012;3:94–104. doi: 10.4239/wjd.v3.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tapia E, Zatarain-Barron ZL, Hernandez-Pando R, et al. Curcumin reverses glomerular hemodynamic alterations and oxidant stress in 5/6 nephrectomized rats. Phytomedicine. 2013;20:359–66. doi: 10.1016/j.phymed.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 78.Garg R, Maru G. Dietary curcumin enhances benzo(a)pyrene-induced apoptosis resulting in a decrease in BPDE-DNA adducts in mice. J Environ Pathol Toxicol Oncol. 2009;28:121–31. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i2.40. [DOI] [PubMed] [Google Scholar]
- 79.Cheng H, Liu W, Ai X. Protective effect of curcumin on myocardial ischemia reperfusion injury in rats. Zhong Yao Cai. 2005;28:920–2. [PubMed] [Google Scholar]
- 80.Ahmadi F. Effect of Turmeric (Curcumin longa) powder on performance, oxidative stress state and some of blood parameters in broilers fed on diets containing aflatoxin. Glob Vet. 2010;5:312–7. [Google Scholar]
- 81.Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–95. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003;17:473–5. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 83.Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase–1. Food Chem Toxicol. 2008;46:1279–87. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 84.Surh YJ, Chun KS. Cancer chemopreventive effects of curcumin. Adv Exp Med Biol. 2007;595:149–72. doi: 10.1007/978-0-387-46401-5_5. [DOI] [PubMed] [Google Scholar]
- 85.Mancuso C, Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab. 2009;10:579–94. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, Chan F, Sun H, et al. Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression. Eur J Pharmacol. 2011;650:130–7. doi: 10.1016/j.ejphar.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 87.Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta. 2011;1812:719–31. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 88.Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lopez-Velez M, Martínez-Martínez F, Del Valle-Ribes C. The study of phenoliccompounds as natural antioxidants in wine. Crit Rev Food Sci Nutr. 2003;43:233–44. doi: 10.1080/10408690390826509. [DOI] [PubMed] [Google Scholar]
- 90.Cao Z, Li Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. Eur J Pharmacol. 2004;489:39–48. doi: 10.1016/j.ejphar.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 91.Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 92.Wu CH, Chen SC, Ou TT, Chyau CC, Chang YC, Wang CJ. Mulberry leaf polyphenol extracts reduced hepatic lipid accumulation involving regulation of adenosine monophosphate activated protein kinase and lipogenic enzymes. J Funct Foods. 2013;5:1620–32. [Google Scholar]
- 93.Chan KC, Ho HH, Huang CN, Chen MC, Wang CJ. Mulberry leaf extract inhibits vascular smooth muscle cell migration involving a block of small GTPase and Akt/NF-kappaB signals. J Agric Food Chem. 2009;57:9147–53. doi: 10.1021/jf902507k. [DOI] [PubMed] [Google Scholar]
- 94.Gundogdu M, Muradoglu F, Gazioglu Sensoy RI, Yilmaz H. Determination of fruit chemical properties of M. nigra L., M. alba L. and M. rubra L. by HPLC. Sci Hortic. 2011;132:37–41. [Google Scholar]
- 95.Andallu B, Shankaran M, Ullagaddi R, Iyer U. In vitro free radical scavenging and in vivo antioxidant potential of mulberry (Morus indica L.) leaves. J Herb Med. 2014;4:10–17. [Google Scholar]
- 96.Lin WC, Lee MT, Chang YL, Shih CH, Chang SC, Yu B, Lee TT. Effects of mulberry leaves on production performance and the potential modulation of antioxidative status in laying hens. Poult Sci. 2016 doi: 10.3382/ps/pew350. [DOI] [PubMed] [Google Scholar]


