Abstract
Objective
The current study compared the effects of dietary levels of two phase stoned olive cake (OC) in form of silage (OCS) on milk production and quality of Saanen goats.
Methods
The OCS included in total mixed ration (TMR) at dry matter proportions of 0.0 (OC0), 0.10 (OC10), and 0.20 (OC20). The TMR were fed to a total of 18 goats in a completely randomized design for a period of 5 weeks.
Results
Dietary treatments had no effect on the milk yield of Saanen goats, but the daily milk fat production was greater (p<0.05) at feeding OC20. The total phenolic (TP) compounds contents increased (p<0.01) in each increment of OCS in TMR and this was also reflected in the TP contents of milk. The C8:0, C10:0, C12:0, and C14:0 saturated fatty acids (FAs) in milk fat decreased (p<0.01) with increasing dietary level of OCS, but the decrease (p<0.001) in C16:0 and the increase (p<0.01) in C18:0 in milk fat occurred similarly at each inclusion level of OCS. Only OC20 reduced (p<0.05) the total saturated FA, yet the reduction (p<0.01) in n6/n3 ratio and atherogenicity index occurred in both OC10 and OC20.
Conclusion
Two phase stoned OCS increases milk quality not only through modifying the milk FA composition, but also by increasing the milk TP content. These favorable changes in milk quality are closely associated with the dietary level of OCS.
Keywords: Stoned Olive Cake Silages, Fatty Acid Composition, Goat, Milk, Phenolic Compounds
INTRODUCTION
The positive effects of olive cake (OC) on fatty acid (FA) composition of milk fat include the reduced total saturated fatty acids (SFA) and the increased total monounsaturated FAs (MUFA) in milk FA. While, this effect was unequivocally observed with sheep [1–4], it was not evident with buffalos [5], camel [6] and goat [7] probably due to insufficient inclusions of OC to the diets. Moreover, the inconsequential responses to feeding OC on the yield parameters of dairy ruminants suggest that both milk quality and animal performance could be affected by the dietary level of OC. However, there is insufficient information about the dietary level of OC that could affect these parameters for dairy goats.
Oil extraction from olives is performed with two or three-phase centrifugation extraction procedures. As two-phase system is a more efficient and environmentally friendly, the number of extraction plants which use two-phase system has been increasing in Turkey and worldwide. Both systems produce a similar amount of OC on a dry matter (DM) basis but two-phase OC has approximately 4 to 5 and 2 to 3 fold higher sugar and total phenolic (TP) compounds, respectively [8], since two-phase system does not produce vegetation water during the oil extraction that three-phase system produces. Elevated TP in dairy milk, associated with the intake of feed sources that are rich in TP were reported by Kuhnen et al [9] and Kälber et al [10]. These studies suggest that feeding dairy animals with feed sources rich in TP has a potential to increase milk TP depending on the nature of TP. Therefore, two-phase OC could also offer increased TP in milk. But, there is no work to indicate if two-phase OC could increase the TP content of milk of dairy goats.
Thus, the main objectives of this study was to evaluate the effects of three dietary levels of olive cake silages (OCS) obtained from two-phase oil extraction on the animal performance, milk composition and FA profile of milk fat in dairy goats.
MATERIALS AND METHODS
Animal and diets
Olive cake produced from a two-phase oil extraction process was passed through a 3.5 mm industrial screen in fresh form and ensiled in 120 L drums for 6 m. No silage additives were added to OC when ensiling. When ready to feed the silages were sampled for the DM, pH and nutritive values. Three treatments including a control which was formulated according to NRC [11] recommendations for dairy goats and fed as a total mixed ration (TMR) ad libitum. The OCS was proportionally included at 0.0 in the control diet (OC0) and in at 0.1 and 0.2 in the other formulated TMR (OC10) and (OC20) based on DM, respectively. The ingredients of three experimental TMR are presented in Table 1.
Table 1.
Ingredients composition of the experimental TMR (g/kg DM)
| Ingredients | TMR1) | ||
|---|---|---|---|
|
| |||
| OC0 | OC10 | OC20 | |
| Maize silage | 250 | 250 | 250 |
| Alfalfa hay | 300 | 300 | 300 |
| Wheat straw | 150 | 150 | 150 |
| Olive cake silage (OCS) | - | 100 | 200 |
| Barley | 204 | 113 | 23 |
| Soybean meal | 10 | 30 | 50 |
| Cotton seed meal | 70 | 41 | 11 |
| Urea | 1.4 | 1.6 | 1.9 |
| Limestone | 3.0 | 1.5 | - |
| Dicalcium phosphate | 6.0 | 7.0 | 8.0 |
| Salt | 4.0 | 4.0 | 4.0 |
| Vitamin-mineral mix2) | 2.0 | 2.0 | 2.0 |
TMR, total mixed ration; DM, dry matter; OC, olive cake.
OC, control, without OCS; OC10, 100 g/kg OCS; OC20, 200 g/kg OCS.
Each kilogram contains: 1,300,000 IU vitamin A, 260,000 IU vitamin D3, 3,000 mg vitamin E, 120,000 mg niacin, 5,000 mg Mn, 5,000 mg Fe, 5,000 mg Zn, 1,000 mg Cu, 15 mg Co, 80 mg I, and 15 mg Se.
These TMR were fed to a total of 18 Saanen goats in a completely randomized design after receiving the approval from the Animal Research Ethics Committee of Adnan Menderes University with a number of 050.04/2012/053 in 05 July to 07 August 2014. The goats, in late of their 1st and 4th lactations were first divided into two groups according to the lactation numbers (6 goats at first and 12 goats at fourth lactation). The two groups were further divided into three sub groups according to yield parameters and one goat from each of the sub-groups was randomly allocated to one of three dietary treatments (6 goats in each treatment equal in lactation number and yield). Goats were kept in individual pens (1.2×1.7 m) equipped with identical plastic feeders and water troughs during the 5 week experiment and fed individually. Goats were allowed 2-d to adapt for their pens and further 12-d for their TMR. The TMR was prepared daily and offered ad libitum once a day at 0900 h, after refusals were removed and weighed. The individual TMR offered and refusals were recorded and collected during 6 days in 3th and 5th week of the experiment. These collected samples were pooled for each animal, and analyzed to measure DM and nutrient intake. Milk yield and composition were also measured at the third and fifth week of the experiment. Milk was sampled at the 33th day of the experiment for determining FA composition of milk fat.
Chemical analysis
Samples of OCS, feedstuff used in TMR, TMR and refusals were dried in an air-forced oven at 55°C for a minimum period of 48 h to reach a constant weigh. Dried samples were then ground to pass a 2-mm screen (MF 10 B, IKA werke, Staufen, Germany), and analyzed for DM (102°C for 4 h), ash (550°C for 4 h) and ether extract (EE, with hexan). Kjeldahl method according to AOAC [12] was applied to determine crude protein (CP, N×6.25) content (Gerhardt, Vapodest 45s, with automated distillation and titration, Königswinter, Germany). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were assayed according to the methods prescribed by Van Soest et al [13] using with Ankom200/220 Fiber Analyzer (Ankom Technology, Macedon, NY, USA). The NDF and ADF residues were further analyzed for their CP content for neutral detergent insoluble CP (NDICP) and acid detergent insoluble CP (ADICP). The acid detergent lignin (ADL) was determined by incubating ADF residues in diluted H2SO4 (1.634 g/L) in 20°C) for 3 hours in Daisy Incubator (Ankom Technology, USA). The metabolizable energy (ME) contents of feedstuff were calculated according to NRC [14]. The pH of OC silages was determined in filtrate of 20 grams of sampled silages with 180 ml of distilled water. The pH of the filtrate was measured with a pH meter (HI 2211 pH/ORP Hanna instruments, Leighton Buzzard, Bedfordshire, UK). The TP, non-tannin phenols and condensed tannin contents of OCS and TMR were analyzed according the method of Makkar [15], and were expressed as tannic acid equivalent. The chemical compositions of the analyzed experimental TMR are shown in Table 2.
Table 2.
Nutritive value of the experimental TMR and OCS (g/kg DM)
| Analyzed nutrient (n = 6) | TMR | OCS | ||
|---|---|---|---|---|
|
| ||||
| OC01) | OC10 | OC20 | ||
| Organic matter | 914 | 919 | 925 | 964 |
| Crude protein | 121 | 118 | 117 | 75 |
| NDICP (g/kg CP) | 116 | 201 | 247 | 748 |
| ADICP (g/kg CP) | 77 | 159 | 192 | 626 |
| Ether extract | 17 | 34 | 41 | 97 |
| NDF | 489 | 527 | 551 | 673 |
| ADF | 338 | 385 | 394 | 530 |
| Hemicellulose | 151 | 143 | 157 | 143 |
| Cellulose | 283 | 290 | 292 | 301 |
| ADL | 55 | 94 | 102 | 229 |
| NFC2) | 287 | 240 | 216 | 119 |
| TDN | 567 | 524 | 520 | 475 |
| ME (Mcal/kg DM) | 1.99 | 1.80 | 1.78 | 1.47 |
| Total phenols3) | 13.5 | 14.8 | 15.7 | 20.3 |
| Non-tannin phenols3) | 8.1 | 8.6 | 9.0 | 11.6 |
| Condensed tannin3) | 0.93 | 0.76 | 0.58 | 0.52 |
TMR, total mixed ration; OCS, olive cake silage; DM, dry matter; CP, crude protein; NDICP, neutral detergent insoluble CP; ADICP, acid detergent insoluble CP; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; NFC, non-fiber carbohydrates; TDN, total digestible nutrients; ME, metabolizable energy.
OC, control, without OCS; OC10, 100 g/kg OCS; OC20, 200 g/kg OCS.
NFC = 1,000−ash−CP−EE−NDF.
Expressed as tannic acid equivalent.
Total solids, protein (N×6.38), fat (Gerber method) and ash contents of milk were determined according to AOAC [12]. Milk lactose was calculated by subtracting the sum of protein, milk and ash from total solids. For determining TP content of milk samples, 2 mL of defrosted milk was mixed with 6 mL of methanol, and centrifuged at 3.000×g for 20 min. The 0.1 mL supernatant was then moved into 10 mL test tube, and in sequence, 0.4 mL distillated water, 0.25 mL of Folin-Ciocalteu reagent and 1.25 mL of the sodium carbonate solution was added and vortexed. The tubes were incubated in dark for 40 min and transferred to spectrophotometer tube. The absorbance was recorded at 725 nm (V-1200 Spectrophotometer, VWR International bvba, Leuven, Belgium). The calculation of TP content was performed as described by Makkar [15], and expressed as tannic acid equivalents.
Proportional FA compositions of milk were determined in their FA methyl esters (FAME). Defrosted milk samples (0.3 to 0.5 mL) were mixed with 1.5 mL of 0.5 N methanolic NaOH for 7 min at 115°C. After cooling, 2 mL of boron trifluoride was added and heated for another 5 min at the same temperature. Test tubes were cooled and 2 mL of iso-octane and 3 mL of saturated NaCl solution were added, and mixed for 30 second. The samples were then allowed to separate organic phase. The FAME’s were extracted from the top layer, and transferred into the amber vial for further gas chromatography (GC) analysis. FAME extracts were kept in freezer at −20°C until GC analysis. The FAME analyzed by a GC (Agilent 7697A, Agilent Technologies, USA) fitted with a flame ionization detector. The FAME separated with a capillary HP-FFAP column (J&W 19091F – 433, Agilent Technologies, USA; 30 m×0.25 mm i.d; 0.25 μm film thickness). Hydrogen was the carrier gas at a flow of 3 mL/min. The initial set oven temperature was 100°C. It was programmed to increase up to 240°C at a rate 10°C/min. The sample volume was 2 μL, and inlet temperature was 225°C. The split ratio was 100:1. The identification of individual FAME was done by retention time compared to standard FAME mix (Supelco 37 component FAME mix, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), and was reported as proportional of the total FAME. Each sample was injected twice by the GC auto sampler.
Statistical analysis
Intake parameters (DM and nutrients), milk yield and compositions were analyzed by repeated measurement. The animal effects were nested within the weeks (goats were the random factor). Proportional FA composition of milk fat was analyzed by analysis of variance (ANOVA) in a completely randomized design accounted for main effect of TMR using SPSS 10 [16]. Where ANOVA was significant, means were separated by Fisher’s protected least significant difference test. Significance was declared at p<0.05, p<0.01, p<0.001.
RESULTS
The daily intake of DM and nutrients are presented in Table 3. Overall, goats had more (p<0.05) DM intake (DMI) in the first week than they did in the rest of the experiment. Feeding OC10 reduced (p<0.05) the DMI compared to control group yet, g DM or g NDF intake relative to live weight (LW) did not differ (p<0.05) among the groups. The OM, EE, NDF, and ADL intake increased (p<0.001), but CP and non-fiber carbohydrates intake decreased (p<0.001) with each increment of OCS in TMR. The ADF intake was higher (p<0.001), but energy intake was lower (p<0.05) by feeding both level of OCS. Regardless of the diet groups, goats gained higher (p<0.05) live weight during the third week of the experiment.
Table 3.
Effect of dietary treatment and sampling time (third and fifth week of the experiment) on intake (g/kg DM, except DM) and live weight change (g/d) of dairy goats
| Third (week) | Fifth (week) | SEM2) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| OC01) | OC10 | OC20 | OC0 | OC10 | OC20 | W | OC | W×OC | ||
| Intake | ||||||||||
| Dry matter (kg/d) | 2.27 | 2.06 | 2.32 | 2.1 | 2.02 | 2.03 | 0.059 | * | * | NS |
| g DM 100 kg LW | 4.7 | 4.1 | 4.5 | 4.2 | 3.9 | 3.7 | 0.26 | * | NS | NS |
| g NDF 100 kg LW | 2.2 | 2.1 | 2.4 | 1.9 | 2.0 | 2.0 | 0.17 | NS | NS | NS |
| Organic matter | 912 | 917 | 923 | 912 | 917 | 923 | 0.7 | NS | *** | NS |
| Crude protein | 128 | 126 | 121 | 129 | 126 | 123 | 1.2 | NS | *** | NS |
| Ether extract | 17.4 | 32.5 | 41.8 | 17.4 | 32.5 | 42.3 | 0.80 | NS | *** | NS |
| NDF | 461 | 504 | 535 | 457 | 503 | 527 | 13.1 | NS | *** | NS |
| ADF | 312 | 358 | 376 | 309 | 357 | 366 | 8.7 | NS | *** | NS |
| ADL | 50 | 90 | 103 | 50 | 90 | 104 | 4.3 | NS | *** | NS |
| Hemicellulose | 148 | 146 | 159 | 148 | 146 | 161 | 8.0 | NS | NS | NS |
| Cellulose | 262 | 268 | 273 | 259 | 267 | 262 | 7.3 | NS | NS | NS |
| NFC | 306 | 254 | 225 | 308 | 255 | 230 | 12.1 | NS | *** | NS |
| ME (Mcal/kg) | 2.06 | 1.85 | 1.81 | 2.07 | 1.85 | 1.82 | 0.034 | NS | *** | NS |
| LWC (g/d) | 49 | 21 | 11 | 112 | 85 | 81 | 33.5 | * | NS | NS |
DM, dry matter; SEM, standard error of the mean; LW, live weight; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; NFC, non-fiber carbohydrates; ME, metabolizable energy; LWC, live weight changes; OCS, olive cake silage.
OC, control, without OCS; OC10, 100 g/kg OCS; OC20, 200 g/kg OCS.
For the two-way interactions.
Addition of 100 or 200 g/kg DM OCS to the TMR of the dairy goats had no (p<0.05) effect on milk yield and production protein, but daily fat production was higher (p<0.05) by feeding OC20 (Table 4). The efficiency of TMR when expressed as both milk and energy corrected milk (ECM) yield per DMI and the efficiency of feed N to the milk N were not affected (p<0.05) by the dietary treatment. The fat content of the milk was greater (p<0.01) in the last week than it was in the previous weeks. Milk fat content was higher (p<0.001) by feeding OC20.
Table 4.
Effect of dietary treatments and sampling time (third and fifth week of the experiment) on milk yield, compositon and feed effiencies of dairy goats
| Third (week) | Fifth (week) | SEM2) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| C1) | OC10 | OC20 | C | OC10 | OC20 | W | OC | W×OC | ||
| Yield (kg/d) | ||||||||||
| Milk | 1.11 | 1.11 | 1.06 | 1.02 | 1.07 | 1.00 | 0.046 | NS | NS | NS |
| FCM3) | 1.02 | 1.06 | 1.10 | 0.99 | 1.06 | 1.11 | 0.053 | NS | NS | NS |
| ECM3) | 1.04 | 1.07 | 1.09 | 1.00 | 1.07 | 1.10 | 0.052 | NS | NS | NS |
| Fat | 0.39 | 0.41 | 0.45 | 0.39 | 0.42 | 0.48 | 0.026 | NS | * | NS |
| Protein | 0.37 | 0.36 | 0.36 | 0.34 | 0.36 | 0.34 | 0.019 | NS | NS | NS |
| Composition (g/kg) | ||||||||||
| Total solids | 121 | 121 | 126 | 125 | 125 | 130 | 3.7 | NS | NS | NS |
| Fat | 36 | 37 | 42 | 39 | 40 | 47 | 1.5 | ** | *** | NS |
| Protein | 33 | 33 | 33 | 34 | 34 | 34 | 1.1 | NS | NS | NS |
| Lactose | 43 | 43 | 43 | 44 | 43 | 40 | 2.4 | NS | NS | NS |
| MUN | 14.0 | 14.3 | 12.0 | 14.7 | 15.5 | 12.8 | 1.79 | NS | NS | NS |
| Feed efficiency | ||||||||||
| MY/DMI | 0.49 | 0.54 | 0.46 | 0.48 | 0.53 | 0.49 | 0.027 | NS | NS | NS |
| FCM/DMI | 0.46 | 0.52 | 0.47 | 0.48 | 0.53 | 0.54 | 0.030 | NS | NS | NS |
| Milk N/N intake | 0.21 | 0.24 | 0.21 | 0.21 | 0.24 | 0.23 | 0.013 | NS | NS | NS |
SEM, standard error of the mean; NS, non-significant; MUN, milk ürea N; MY, milk yield; DMI, dry matter intake; OCS, olive cake silage.
OC, control, without OCS; OC10, 100 g/kg OCS; OC20, 200 g/kg OCS.
For the two-way interactions.
FCM, 4% fat corrected milk; ECM, International energy corrected milk standardized at 4% and 3.3% protein.
The proportional FA compositions of milk fat are presented in Table 5. The addition of OCS to the TMR reduced (p<0.05) the most of the individual SFA in milk fat. The reduction (p<0.001) in C8:0, C10:0, C12:0, and C14:0 proportions was occurred by feeding both level of OCS, while reduction (p<0.001) in C16:0 proportion occurred at only OC20 compared to OC0. Within SFA, only C18:0 was proportionally higher (p<0.01) with feeding OCS. The increase in C18:1n9c proportion occurred (p<0.01) only at OC20, while C18:2n6c proportion in milk fat was lower (p<0.001) at both levels of OCS. The proportion of total SFA decreased (p<0.05), but proportion of total MUFA increased (p<0.01) with OC20. The proportions of the total polyunsaturated FA (PUFA) tended to decrease (p = 0.06) with OC20. Addition of OCS at two level had similar effects on the n6/n3 ratio and atherogenicity index with the value being lower (p<0.01) than control group.
Table 5.
Effects of two levels of OCS on proportional fatty acid composition of milk fat (proportions of the total fatty acid methyl esters)
| Fatty acids proportions | OC01) | 0C10 | 0C20 | SEM | p-value |
|---|---|---|---|---|---|
| C4:0 | 0.628 | 0.588 | 0.523 | 0.032 | NS |
| C6:0 | 1.02 | 0.95 | 0.76 | 0.055 | * |
| C8:0 | 1.45 | 1.24 | 0.93 | 0.056 | *** |
| C10:0 | 5.72 | 4.69 | 3.63 | 0.256 | *** |
| C11:0 | 0.159 | 0.162 | 0.115 | 0.017 | NS |
| C12:0 | 3.17 | 2.37 | 1.80 | 0.135 | *** |
| C13:0 | 0.252 | 0.278 | 0.319 | 0.021 | NS |
| C14:0 | 7.11 | 6.14 | 5.26 | 0.270 | *** |
| C15:0 | 0.613 | 0.613 | 0.536 | 0.038 | NS |
| C15:1 | 0.238 | 0.259 | 0.207 | 0.021 | NS |
| C16:0 | 23.9 | 21.7 | 20.5 | 0.44 | *** |
| C16:1 | 0.357 | 0.302 | 0.344 | 0.031 | NS |
| C18:0 | 11.2 | 13.2 | 14.5 | 0.54 | ** |
| C18:1n9c | 20.4 | 21.9 | 24.9 | 0.76 | ** |
| C18:2n6c | 5.16 | 4.35 | 4.01 | 0.167 | *** |
| C18:2n6t | 0.342 | 0.380 | 0.316 | 0.030 | NS |
| C18:3n6 | 0.183 | 0.180 | 0.187 | 0.019 | NS |
| C18:3n3 | 0.859 | 0.890 | 0.909 | 0.048 | NS |
| C20:0 | 0.330 | 0.299 | 0.304 | 0.036 | NS |
| C20:4n6 | 0.281 | 0.292 | 0.297 | 0.023 | NS |
| C20:5 | 0.357 | 0.361 | 0.382 | 0.025 | NS |
| SFA | 66.6 | 65.1 | 61.1 | 1.32 | * |
| MUFA | 25.2 | 28.0 | 31.8 | 1.08 | ** |
| PUFA | 8.64 | 8.05 | 7.60 | 0.281 | NS |
| n6/n3 | 4.69 | 3.98 | 3.52 | 0.190 | ** |
| Atherogenicity index | 1.66 | 1.36 | 1.11 | 0.089 | *** |
OCS, olive cake silage; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
OC, control, without OCS; OC10, 100 g/kg OCS; OC20, 200 g/kg OCS.
Total phenolic compounds increased (p<0.01) with the advance of experiment, and an increase (p<0.001) in milk TP with the addition of OCS occurred with increasing level of OCS in TMR (Figure 1).
Figure 1.
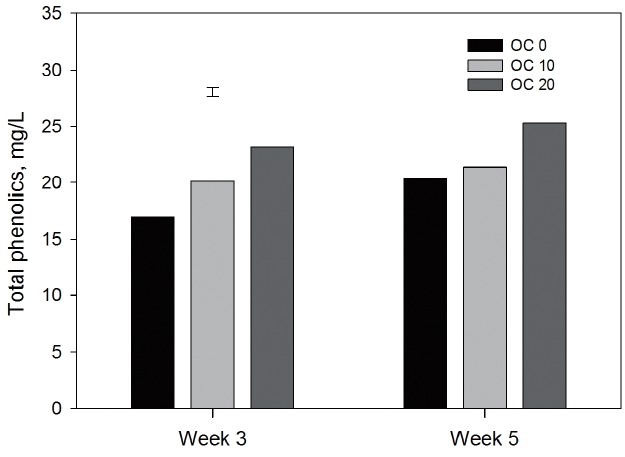
Total phenolic content of goat milk fed different dietary level of olive cake silage (OCS) (OC, control; OC10, 100 g/kg dry matter OCS; OC20, 200 g/kg dry matter OCS; standard error of the mean = 0.82; n = 18, p<0.001 for dietary level of OCS, p<0.01 for week).
DISCUSSION
Fiber content of OCS is considerably high and ADL content of OCS is greater than many other feed sources (roughage or concentrate feed). Therefore, it is not surprising that the higher inclusion (340 g/kg DM) of OC to the diets of sheep have been reported to reduce the DMI [17]. However, Hadjipanayiotou [18] reported no adverse effect when OCS included in sheep, goat or cow diets at the levels of 140 to 150 g/kg DM. Similar to this report, the inclusion of 59 g/kg DM OC in the buffalo diets [5] or 170 g/kg DM OC in the camel diets [6] did not reduce the DMI. In the present study, the addition of 100 g/kg DM OCS to TMR of dairy goats suppressed the DMI yet, goats fed OC20 had the highest fiber consumption. This was related to LW of goats, as goats consumed similar DM or NDF relative to their LW. Without any given actual digestible nutrient intake in published studies which seems to be the more reliable data to evaluate the value of a feed source with elevated ADL content like OC, it is difficult to perform a reliable comparison between these studies as highest NDF intake did not suppress the DMI of goats in the present study.
Inconsistent results on production parameters (milk production, fat, and protein yield) were reported by several others, as Hadjipanayiotou [18], Faye et al [6] and Chiofalo et al [1] reported improved production parameters, whereas Molina-Alcaide et al [7] and Abbeddou et al [3] reported adverse effects. Different reports for production parameters seem mostly related to different animal species with different production levels and nutritive value of OC used (stoned or not). The higher fat content of milk from the goats that were fed OC20 can be attributed to high fiber intake in the current research as all three TMR had the same roughage ingredients. Within the similar yield parameters, feeding OCS did not affect the feed efficiency. As indicated by the comparable milk urea-N levels which is a good predictor of N utilization, the efficiency of use of feed N to milk N was not affected by the dietary N in each group.
All proportional individual FA in milk fat in the current study was in range or close to the ranges reported by Tsiplakou et al [19]. The main effects of OCS on FA composition of milk fat were decreased total SFA, but increased MUFA as reported for sheep [1–4] and for goats [7]. The only increase in individual SFA by feeding OCS was in C18:0 and this was in line with the other works [1–4]. This could be attributed to higher EE intake of OCS goats as C18:0 is final product of ruminal biohydrogenation. Reduced C16:0 was related to its level in TMR as this FA incorporated via blood from diets and storage tissue [20]. From human health perspective increased C18:0, but decreased 12:0, 14:0, and 16:0 was promising as C18:0 is neutral with respect to low density lipoprotein (LDL)-cholesterol compared to other SFAs [21] while the others are known as hypercholesterolemic saturated FA [22]. Increase in C18:1c9 with high level of inclusions of OCS could be associated with the high level of TP and ADL contents of TMR, supporting the hypothesizes of Chiofalo et al [1] that there is a lack of biohydrogenation from oleic to stearic when feeding by-product rich in ADL and TP. Alternatively this FA, rich in OCS, can be directly incorporated in the mammary gland. Similar to the results, Abbeddou et al [3–4] and Gómez-Cortés et al [23], who included supplementary olive oil to ewe’s diet, reported that C18:2c9 proportions were lower in milk fat of goats that were fed TMR containing OCS. This could have resulted from the different ingredients of TMR, even when OCS was replaced with mostly barley in the control diet, and thereby had a probable lower content of linoleic acid in TMR. The effects of OCS on ratio of n6/n3 and atherogenicity index were unrelated from dietary level of OCS in TMR and even low application of OCS had favorable effect on these two parameters which are related to human health.
Silanikove et al [24] described the goat milk rich in phenolic compounds derived from their diets as a ‘treasure trove’ for developing functional foods. Thus, transfer of phenolic contents to milk is very important as these phenols contain high antioxidant properties [25–26]. The increase in TP was reported by Kuhnen et al [9] in milk of cows that were fed pasture rich in TP. Similarly, Kälber et al [10] reported an increased TP in cow milk that was obtained from animals that consumed diets containing chicory and buckwheat. This was similar to the TP content of OCS used in the present experiment. The increase in OCS in TMR also increased the TP content of milk, and this was closely associated with the increase in TP of the TMR. This indicates that including two phase OCS in the diets of dairy animals has a potential to increase milk antioxidant capacity and hence the quality, not only by changing the FA composition of milk fat, but also through increasing the TP content of milk. This increase seems closely related to their levels in the diets. Ultimately, the more OCS added to the diets the more TP occurs in milk.
In conclusions, the feeding value of OCS was satisfactory in this research where low-yielding goats in late lactation were used. However, DMI and NDF intakes are not good parameters for assessing feeding value of OCS, as published research indicated inconsequential results. Therefore, more reliable intake parameters (i.e digestible NDF intake) were needed to better evaluate the feeding value of OCS. The current study showed that OCS improved milk quality by increasing the TP content of milk and by modifying the FA profile of milk fat towards a healthier profile for human consumption. Increase of the TP in milk depended on the level of inclusion, but valuable effects of OCS on FA composition did not seem to be related to the level of inclusion used in this study.
ACKNOWLEDGMENTS
This work was supported by the Scientific Research Project Council of Adnan Menderes University under Grant number: ZRF-14008.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Chiofalo B, Liotta L, Zumbo A, Chiofalo V. Administration of olive cake for ewe feeding: effect on milk yield and composition. Small Rumin Res. 2004;55:169–76. [Google Scholar]
- 2.Vargas-Bello-Perez E, Vera RR, Aguilar C, et al. Feeding olive cake to ewes improves fatty acid profile of milk and cheese. Anim Feed Sci Technol. 2013;184:94–9. [Google Scholar]
- 3.Abbeddou S, Rischkowsky BA, El-Dine Hilali M, et al. Supplementing diets of Awassi ewes with olive cake and tomato pomace: on-farm recovery of effects on yield, composition and fatty acid profile of the milk. Trop Anim Health Prod. 2015;47:145–52. doi: 10.1007/s11250-014-0699-x. [DOI] [PubMed] [Google Scholar]
- 4.Abbeddou S, Rischkowsky BA, Richter EK, Hess HD, Kreuzer M. Modification of milk fatty acid composition by feeding forages and agro-industrial by-products from dry areas to Awassi sheep. J Dairy Sci. 2011;94:4657–68. doi: 10.3168/jds.2011-4154. [DOI] [PubMed] [Google Scholar]
- 5.Terramoccia S, Bartocci S, Taticchi A, et al. Use of dried stoned olive pomace in the feeding of lactating buffaloes: effect on the quantity and quality of the milk produced. Asian-Australas J Anim Sci. 2013;26:971–80. doi: 10.5713/ajas.2012.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faye B, Konuspayeva G, Narmuratova M, et al. Effect of crude olive cake supplementation on camel milk production and fatty acid composition. Dairy Sci Technol. 2013;93:225–39. [Google Scholar]
- 7.Molina-Alcaide E, Morales-García EY, Martín-García AI, et al. Effects of partial replacement of concentrate with feed blocks on nutrient utilization, microbial N flow, and milk yield and composition in goats. J Dairy Sci. 2010;93:2076–87. doi: 10.3168/jds.2009-2628. [DOI] [PubMed] [Google Scholar]
- 8.Keles G. The nutritive and feeding value of olive cake for ruminants. Turkish J Agric-Food Sci Technol. 2015;3:780–9. [Google Scholar]
- 9.Kuhnen S, Moacyr JR, Mayer JK, et al. Phenolic content and ferric reducing-antioxidant power of cow’s milk produced in different pasture-based production systems in southern Brazil. J Sci Food Agric. 2014;94:3110–3117. doi: 10.1002/jsfa.6654. [DOI] [PubMed] [Google Scholar]
- 10.Kälber T, Kreuzer M, Leiber F. Silages containing buckwheat and chicory: quality, digestibility and nitrogen utilization by lactating cows. Arch Anim Nutr. 2012;66:50–65. doi: 10.1080/1745039x.2011.630213. [DOI] [PubMed] [Google Scholar]
- 11.NRC. Sheep, Goats, Cervids and New World Camelids. National Research Council; Washington, DC: National Academy Press; 2007. Nutrient requirements of small ruminants. [Google Scholar]
- 12.AOAC. Official Methods of Analysis Association of Official Analytical Chemists. Arlington, VA: AOAC International; 1990. [Google Scholar]
- 13.Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 14.NRC. Nutrient Requirements of Dairy Cattle. 7th ed. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 15.Makkar HPS. Quantification of tannins in tree and shrub foliage: a laboratory manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2003. [Google Scholar]
- 16.SPSS. SPSS for Windows, Version 17. Chicago IL: SPSS Inc; 2010. [Google Scholar]
- 17.Abbeddou S, Riwahi S, Iniguez L, et al. Ruminal degradability, digestibility, energy content, and influence on nitrogen turnover of various Mediterranean by-products in fat-tailed Awassi sheep. Anim Feed Sci Tech. 2011;163:99–110. [Google Scholar]
- 18.Hadjipanayiotou M. Feeding ensiled crude olive cake to lactating Chios ewes, Damascus goats and Friesian cows. Livest Prod Sci. 1999;59:61–6. [Google Scholar]
- 19.Tsiplakou E, Mountzouris KC, Zervas G. Concentration of conjugated linoleic acid in grazing sheep and goat milk fat. Livest Sci. 2006;103:74–84. [Google Scholar]
- 20.Schreiner M, Windisch W. Supplementation of cow diet with rapeseed and carrots: Influence on fatty acid composition and carotene content of the butter fat. J Food Lipids. 2006;13:434–44. [Google Scholar]
- 21.Hunter JE, Zhang J, Kris-Etherton P. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr. 2010;91:46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- 22.Mensink RP. Effects of stearic acid on plasma lipid and lipoproteins in humans. Lipids. 2005;40:1201–5. doi: 10.1007/s11745-005-1486-x. [DOI] [PubMed] [Google Scholar]
- 23.Gómez-Cortés P, Frutos P, Mantecón AR, et al. Addition of olive oil to dairy ewe diets: effect on milk fatty acid profile and animal performance. J Dairy Sci. 2008;91:3119–27. doi: 10.3168/jds.2007-0954. [DOI] [PubMed] [Google Scholar]
- 24.Silanikove N, Leitner G, Merin U, Prosser CG. Resent advances in exploiting goat’s milk: Quality, safety and production aspects. Small Rumin Res. 2010;89:110–24. [Google Scholar]
- 25.Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1977;2:152–9. [Google Scholar]
- 26.Roleira FMF, Tavares-da-Silva EJ, Varela CL, et al. Plant derived and dietary phenolic antioxidants: anticancer properties. Food Chem. 2015;183:235–58. doi: 10.1016/j.foodchem.2015.03.039. [DOI] [PubMed] [Google Scholar]


