Abstract
Objective
Study on the application of high pressure processing (HPP) for dark-firm-dry (DFD) beef was conducted to observe whether HPP has any impact on physical properties and to evaluate oxidative deterioration during refrigerated storage under vacuum.
Methods
The longissimus lumborum muscles obtained from Friesian Holstein steers (33±0.5 months old) with 24-h postmortem pH higher than 6.0 were vacuum-packed and subjected to pressurization at 200, 400, and 600 MPa for 180 s at 15°C±2°C; the samples were then stored for 9 days at 4°C±1°C and compared with control (0.1 MPa).
Results
HPP increased meat pH by 0.1 to 0.2 units and the tenderness of cooked DFD beef significantly with no significant effects on meat texture profile. The stability of meat pH was well maintained during refrigerated storage under vacuum. No clear effects were found on the activity of catalase and superoxide dismutase, however, glutathione peroxidase activity was significantly reduced by high pressure. HPP and storage time resulted in aroma changes and the increasing amount of malondialdehyde and metmyoglobin relative composition.
Conclusion
Although the increasing amount of malondialdehyde content, metmyoglobin formation and aroma changes in HPP-treated samples could not be avoided, HPP at 200 MPa increased L* and a* values with less discoloration and oxidative deterioration during storage.
Keywords: Beef, Color, Dark-Firm-Dry, High Pressure Processing, Oxidation
INTRODUCTION
High pressure processing (HPP) has been widely applied as a cold-pasteurization process in the food industry without deteriorating nutritional content and to obtain extended stability of food freshness. In meat industry, HPP is commonly used to extend the shelf-life of ready-to-eat meat products. Many studies have been conducted to observe whether HPP gives any impact on functional properties, nutritive values and microbiological quality of the meat and meat products [1,2].
Color is one of the most important raw meat quality attributes that affect consumer preferences when purchasing [3]. Dark-firm-dry (DFD) beef is known for having undesirable color [4]. On the other hand, color stability in high pressurized meat has been an issue for decades. Studies on the discoloration and myoglobin oxidation of normal minced beef treated with HPP have been conducted previously by Carlez et al [5]. However, the effect of HPP on the surface color of DFD beef remains unknown.
The oxidation of lipid and protein generates off-flavors and color changes in pressurized muscle foods [6]. A recent comprehensive review by Medina-Meza et al [7] has highlighted temperature and pressurization time as main factors affecting the lipid oxidation of food treated with this technology. Meat antioxidant enzymes such as catalase, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) are naturally produced to inhibit the potential oxidation from endogenous pro-oxidants that are mostly derived from reactive oxygen species, such as superoxide anion radical (O2−). These enzymes are considered major antioxidant enzymes in muscle and their concentrations influence lipid oxidation rate [8]. The decline rate of antioxidant enzyme activity was found in HPP-treated dry-cured ham [9]. To the best of our knowledge, no study has yet investigated the effects of high pressure on the activity of these antioxidant enzymes in DFD beef.
It is important to further observe the use of HPP for DFD beef to better understand whether it provides benefits. The objective of this study was to investigate the effects of HPP on physical properties of DFD beef strip loin, particularly surface color and its oxidative deterioration during refrigerated storage.
MATERIALS AND METHODS
Sample and high pressure treatments
Beef strip loin (longissimus lumborum) was purchased from local slaughterhouse (Hoengsong, Korea). The strip loins were obtained from the carcasses of Friesian Holstein steers finished on pasture system and slaughtered at a similar age (33±0.5 months old). Carcasses with 24-h postmortem pH higher than 6.0 were selected and evaluated by Korean Institute for Animal Products Quality Evaluation as under grade with yield grade D [10]. The strip loin was cut into slices (350 g and 3 cm thick), vacuum-packaged in polyethylene vacuum bags (30×20 cm) and trans-ported to a HPP plant (Hyungkuk F&B, Eumseong, Korea) in ice box within 1 h. Subsequently, vacuum-packaged samples (n = 12) were randomly separated and treated at 200, 400, and 600 MPa in a 350-L chamber (QFP 350L-600, Avure Technologies, Inc., Kent, WA, USA) using a pressurization medium of water at 15°C±2°C. Pressurization, holding and depressurization times were 56.3 s, 180 s, and 12.2 s, respectively. Samples were then stored at 4°C±1°C for 9 days under vacuum. Chemical analyses were performed in triplicate using samples at 0, 3, 6, and 9 days and compared with a non-treated sample, 0.1 MPa (atmospheric pressure), as a control.
Chemicals
HPLC-grade chloroform was purchased from Daejung Chemical & Metals Co. Ltd., Siheung, Korea. Distilled water (DW) was purified with a glass still (Mega-Pure System MP-11A, Corning Inc., Corning, NY, USA), and the other reagents were purchased from Sigma-Aldrich Corp., LLC., St. Lois, MO, USA.
Cooking loss, shear force and texture profile analysis
The 2.5 cm thick-samples in triplicate were cooked in the polyethylene zipper bags by immersing in water bath set at 80°C for 45 min. The cooked samples were then removed off the polyethylene bags immediately, stored overnight at 2°C±2°C and weighed. Cooking loss was expressed as the percentage of weight loss during heating. The cooked samples were cut (1× 1×1 cm) into 8 pieces for each sample and subjected to texture profile analysis (TPA) and shear force measurement using TA-XT2i Plus (Stable Micro Systems Ltd., Godalming, Surrey, UK). For shear force measurement, the cut sample was placed on the table, under the V blade, and was cut through as the blade moved down with a constant speed through the slit of the table (assay parameters were: pre-test speed: 2.0 mm/s; test speed: 2.0 mm/s; post-test speed: 5.0 mm/s). A cylindrical 10 mm-diameter probe was used for all TPA tests in this study at a compression rate of 80%. The sample was placed under the probe that moved downwards at a constant speed of 2.0 mm/s (pre-test), 2.0 m/s (test), and 5.0 mm/s (post-test).
Meat pH measurement
Sample pH was determined using meat slurry. First, 5 g of sample was added with 45 mL of DW in triplicate, and the samples were then homogenized at 10,000 rpm for 60 s using a homogenizer (PH91, SMT Co., Ltd., Chiba, Japan). The pH values of the homogenized samples were recorded using a pH meter (Seven Easy pH, Mettler-Toledo GmbH, Greifensee, Switzerland).
Instrumental surface color
The instrumental surface color was recorded by measuring CIE lightness (L*), redness (a*), and yellowness (b*) using a chromameter (CR-400, Konica Minolta Inc., Tokyo, Japan). The light source of illuminant C (2° observer) was calibrated with a white plate (Y = 93.6, X = 0.3134, y = 0.3194). Hue angle (h° = arctan [b*×a*−1]) were calculated using a data processor (DP-400, Konica Minolta Inc., Japan). Each sample was assessed at 5 different locations immediately after being removed from the vacuum bag to determine the actual retail condition of vacuum-packaged beef.
Myoglobin determination
Myoglobin content was determined from absorbance measurements of the sarcoplasmic extract, dissolved in 40 mM phosphate buffer (pH 6.8), at 525, 545, 565, and 572 nm (UV-2401 PC, Shimadzu Corp., Kyoto, Japan). Briefly, 5 g of sample was homogenized with cold phosphate buffer using a homogenizer (Ultra Turrax T25 basic, IkaWerke GmbH and Co., Staufen, Germany) at 13,000 rpm for 30 s. The homogenate was kept at 4°C for an hour, then was centrifuged at 2,800×g for 30 min (1248R, 117 Labogene, Denmark). Supernatant was filtered through 0.45 syringe filter (Hydrophilic PTFE, Advantec MFS, Inc., Dublin, CA, USA). The relative proportions (%) of oxymyoglobin (OxyMb) and metmyoglobin (MetMb) were calculated according to Krzywicki’s method [11]. The presented values are the mean of triplicate measurements per sample.
Lipid oxidation
Lipid oxidation was analyzed using the 2-thiobarbituric acid reactive substances (TBARS) method by Sinnhuber and Yu [12] with slight modification. A total of 0.5 g of sample was vortex-mixed with 0.1 g of antioxidant mixture (consisting of 54% propylene glycol, 40% Tween 20, 3% butylated hydroxytoluene, and 3% butylated hydroxyanisole), 3 mL of 1% TBA in 0.3% NaOH, and 17 mL of 2.5% trichloroacetic acid in 36 mM HCl. The sample was heated in a water bath (BW-20G, Biotechnical Services, Inc., North Little Rock, AR, USA) at 100°C for 30 min and then immersed in ice water for 15 min. Subsequently, 5 mL of aqueous sample was mixed with 3 mL of chloroform. The absorbance value of the upper layer was recorded at 532 nm (UV Mini 1240 PC, Shimadzu Corp., Japan) against blank after centrifuging at 2,400×g for 30 min at 4°C (1248R, Labogene, Lynge, Denmark). The result was expressed in mg of malondialdehyde (MDA) per kg of meat. Each sample was analyzed in triplicate.
Antioxidant enzymes activity measurement
Catalase (E.C. 1.11.1.6), SOD (E.C. 1.15.1.1), and GSH-Px (E.C. 1.11.1.9) were measured in aqueous meat extract dissolved in 50 mM phosphate buffer (pH 7.0 at 25°C). The extraction method was according to a procedure by Renerre et al [13]. Catalase activity was measured using a method described by Aebi [14]. First, 100 μL of filtered supernatant was mixed with 2.9 mL of 30 mM H2O2 within a crystal cuvette (light path: 1 cm). The decrease in absorbance at 240 nm was recorded every 10 s for 2 min (UV-2401 PC, Shimadzu Corp., Japan). Catalase activity was expressed as U/g sample. SOD activity was determined according to the pyrogallol autoxidation method described by Marklund and Marklund [15] with modification. The optical density of a mixture consisting of 50 μL of filtered supernatant, 3.025 mL of 50 mM Tris-cacodylate-diethylenetriaminepentaacetic acid buffer (pH 8.2 at 25°C) and 50 μL of 24.8 mM pyrogallol was recorded at 420 nm every 15 s for 2 min against a blank (UV-2401 PC, Shimadzu Corp., Japan). One unit of SOD represents the amount of SOD required for the auto oxidation of 50% of the pyrogallol in the meat extract at pH 8.2 and 25°C. GSH-Px activity measurement was performed according to the enzymatic protocol by Flohé and Günzler [16] with slight modification. A total of 100 μL of filtered supernatant was mixed with 0.5 mL of phosphate buffer containing 0.001 M ethylenediaminetetraacetic acid and 0.1 M NaN3, 100 μL of the assay mixture containing 5 U/mL glutathione reductase in 50 mM phosphate buffer, 100 μL of 10 mM L-glutathione reduced, 100 μL of 1.5 mM nicotinamide adenine dinucleotide phosphate (NADPH) in NaHCO3, and 100 μL of 1.5 mM H2O2 within a 10-mm precision cell (104-QS, Hellma Analytics, Müllheim, Germany) and incubated for 5 min. GSH-Px activity was measured by recording the decrease in absorbance of the incubated mixture at 340 nm over 2 min (UV-2401 PC, Shimadzu Corp., Japan). GSH-Px activity was expressed as U/g sample. One unit represents the amount of meat extract needed to alter 1 μmol of NADPH with extinction coefficient of 6.3 at 340 nm.
Aroma pattern analysis
An electronic nose (FOX3000, Alpha MOS, Toulouse, France) was used to distinguish the effect of different levels of high pressure treatment on the aroma pattern. A total of 2 g of day 9 sample was weighed into a 10 mL-headspace vial and five replications were prepared for each level of pressure. The vials were then sealed with rubber septum (Supelco 29176-U, Sigma-Aldrich Corp., LLC., USA). The samples were heated at 40°C for 180 s with an agitation speed of 500 rpm. The 2.5 mL-gas in the headspace of the samples was extracted by automatic sampler syringe (HS 100, Alpha MOS, France) at 45°C, flow-injected into the carrier gas (synthetic air with a purity quotient >99.99%, pressure set at 0.5 bar) flow (150 mL/min) of the electronic nose and detected using a metal oxide sensor (MOS) array system. The sensor array was composed of 12 MOSs. The discrimination index and two-dimensional principal component analysis (PCA) were used for data processing with the Alpha Soft version 8.01.
Statistical analysis
A 4×4 factorial design with three replicates was employed with pressure rates and storage period as the main effects, using two-way analysis of variance (ANOVA) for pH, color, myoglobin relative proportion, lipid oxidation and antioxidant enzyme activity. Cooking loss, shear force and texture profile data were statistically analyzed using one-way ANOVA with pressure rates as the main effect. The statistical significance of the differences between the means from different treatments was determined by Duncan’s multiple range test. The p values of less than 0.05 were regarded as significant. Analyses were performed using R-version 3.2.0 with the “Agricolae” library (The R-foundation for Statistical Computing, Vienna, Austria).
RESULTS AND DISCUSSION
Cooking loss, tenderness and texture profile
The significant effect of HPP on cooking loss is shown in Table 1. The cooking loss increases as the level of pressure increases. HPP at 600 MPa led to higher weight loss (43.7%) after cooking at 80°C for 30 min than those treated at 200 MPa and control. McArdle et al [1] reported that higher cooking loss was observed in beef pectoralis profundus treated at 300 and 400 MPa and at lower temperature set during pressurization (20°C). The effect of HPP itself on cooking loss was found to be dependent on temperature used during pressurization. The water-binding ability of meat is related with the solubility of sarcoplasmic protein. Marcos et al [17] found that HPP significantly lowered sarcoplasmic protein solubility in beef longissimus muscles. In addition, the reduction of reactive sulfihydril content by HPP decreases sarcoplasmic protein solubility [18].
Table 1.
Cooking loss, shear force value and texture profile of DFD strip loin subjected to different levels of pressure
| Parameter | Pressure (Mpa) | |||
|---|---|---|---|---|
|
| ||||
| 0.1 | 200 | 400 | 600 | |
| Cooking loss (%)1) | 35.63±2.37c | 39.41±1.36b | 39.84±1.85b | 43.70±0.76a |
| Shear force value (kgf) | 9.89±1.96a | 8.73±1.65b | 7.14±1.41c | 4.82±0.53d |
| Hardness (kg/cm2) | 22.86±4.23 | 21.40±3.53 | 20.91±4.03 | 19.779±3.04 |
| Springiness (cm) | 0.50±0.06 | 0.50±0.06 | 0.49±0.07 | 0.47±0.04 |
| Cohesiveness (ratio) | 0.48±0.04 | 0.49±0.04 | 0.53±0.02 | 0.47±0.01 |
| Gumminess (kg/cm2) | 11.14±2.78 | 10.44±2.14 | 11.04±2.21 | 9.37±1.44 |
| Chewiness (kg/cm) | 5.60±1.52 | 5.24±1.42 | 5.36±1.30 | 4.43±1.00 |
DFD, dark-firm-dry.
Cooked at 80°C for 45 min.
Values are presented as mean±standard deviation (n = 3).
Means within each row are significantly different (p<0.05).
No significant differences were found on hardness, springiness, cohesiveness, gumminess and chewiness of both non-treated and treated cooked samples. However, the significant decrease in shear force value was found in all HPP-treated samples as shown in Table 1. In contrast with our results, Jung et al [19] reported that HPP at 325 MPa and higher at 10°C with holding-time of 260 s resulted muscle shortening through ultrastructural modifications in post-rigor beef biceps femoris such as M-line and H-zone breakdown, an increase in solubilization of myosin light chains and an increase in myofibrillar size that were associated with toughness. The differences in meat part, pH (rigor-phase) and longer holding-time may contribute to meat toughening. Sun and Holley [20] noted that the tenderizing effect of HPP at low temperature only occurred in pre-rigor meat, while no benefits were found in post-rigor meat. Although the DFD beef used in this study were treated post-rigor, the meat pH remained higher than 6.0, which was more-likely normal meat in pre-rigor state. Macfarlane [21] reported that HPP at 103 MPa at low temperature resulted tender but less juicy than non-treated meat due to myofibrillar proteins disruption during pressurization and moisture loss during cooking. Cooking temperature and time also play a role on this matter. Vasanthi et al [22] reported significant decrease in shear force value of cooked buffalo meat with raw meat pH higher than 6.0 as temperature and cooking time increased. Thus, we suggest that the tenderizing effect of HPP also occurs to DFD beef which shows an advantage for under grade beef we used in this experiment.
Meat pH
The pH of HPP-treated samples was significantly higher than non-treated samples (p<0.05). The highest pH value was recorded in samples treated at either 400 Mpa or 600 MPa. Ma and Ledward [23] mentioned that the myofibrillar protein denatures under high pressure and at the same time, pressure increases ionization, sequestering free hydrogen ions and decreasing the acidic groups. The effect of HPP on meat pH in this study is in accordance with previous finding by McArdle et al [1] regardless the use of different temperature. However, there were no significant changes in meat pH (p>0.05) until 9 days of refrigerated storage under vacuum and no significant interaction was found (Table 2).
Table 2.
The effects of different levels of pressure and storage period on meat pH, TBARS, instrumental surface color, myoglobin relative proportion and antioxidant enzyme activity of DFD strip loin
| Parameter | Pressure | Storage time | Interaction |
|---|---|---|---|
| pH | * | ns | ns |
| L* | *** | * | ** |
| a* | *** | *** | *** |
| b* | *** | *** | *** |
| h° | *** | * | * |
| OxyMb | *** | *** | * |
| MetMb | *** | *** | * |
| TBARS | *** | *** | ** |
| Catalase | ns | ** | ns |
| SOD | ns | ** | ns |
| GSH-Px | * | ** | ns |
TBARS, 2-thiobarbituric acid reactive substances; DFD, dark-firm-dry; ns, not significant; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase.
p<0.05,
p<0.01,
p<0.001.
Surface color, myoglobin and TBARS value
Supplemental Figure 1 shows that HPP at 200 MPa modified the dark characteristic of DFD beef to be brighter and redder than control. This could be explained by their higher L* and a* values (Table 3). HPP significantly led to higher lightness values (L*) than non-treated samples. DFD beef subjected to HPP at 600 MPa had the highest L* value than those subjected at 200 MPa and control (p<0.001). Although HPP seems to have discoloration effect to meat color in general, HPP at 200 MPa in this study increased the redness value (a*) by approximately 2 units than control. However, the redness decreased when the samples treated at 600 MPa. The increases of a* value were also found in beef biceps femoris, which were treated at pressure rate lower than 600 MPa [24].
Table 3.
Changes in meat pH and instrumental surface color of DFD strip loin subjected with different levels of pressure during refrigerated storage under vacuum
| Parameter | Storage period (d) | Pressure (Mpa) | |||
|---|---|---|---|---|---|
|
| |||||
| 0.1 | 200 | 400 | 600 | ||
| pH | 0 | 6.17±0.14c | 6.25±0.24b | 6.32±0.01a | 6.30±0.07a |
| 3 | 6.13±0.06c | 6.29±0.05b | 6.36±0.01a | 6.33±0.12a | |
| 6 | 6.18±0.09c | 6.24±0.17b | 6.29±0.15a | 6.31±0.03a | |
| 9 | 6.12±0.12c | 6.24±0.10b | 6.32±0.07a | 6.32±0.16a | |
| L* | 0 | 34.05±3.08cy | 40.92±6.62bx | 48.46±3.78a | 50.18±3.71a |
| 3 | 38.86±4.62bx | 39.32±1.80bxy | 49.44±3.75a | 49.44±3.88a | |
| 6 | 37.33±2.37bx | 39.58±5.47bxy | 48.93±3.50a | 51.10±3.61a | |
| 9 | 36.64±1.68bx | 37.22±3.08by | 48.73±3.12a | 48.55±4.15a | |
| a* | 0 | 15.27±1.68bx | 17.59±1.85ax | 16.28±1.63bx | 13.83±1.29cx |
| 3 | 13.74±1.45by | 16.12±1.43axy | 14.66±1.73by | 11.49±1.78cy | |
| 6 | 13.23±1.98by | 16.05±1.32axy | 14.50±1.14by | 10.29±1.32cy | |
| 9 | 11.69±1.35cy | 15.83±2.30ay | 13.85±1.97by | 10.22±1.26cy | |
| b* | 0 | 1.46±0.86c | 2.35±1.12c | 3.53±1.71b | 6.92±1.26az |
| 3 | 1.48±0.72c | 2.19±0.59c | 3.60±1.20b | 9.51±1.98ay | |
| 6 | 1.65±0.56c | 2.06±1.24c | 4.25±0.94b | 10.23±1.39axy | |
| 9 | 1.62±0.53c | 1.48±0.57c | 3.73±0.63b | 10.22±1.26ax | |
| h° | 0 | 7.03±4.01c | 8.51±3.77c | 11.97±5.35b | 21.58±4.20ay |
| 3 | 6.11±2.41c | 8.48±2.25c | 13.35±6.04b | 39.54±7.49ay | |
| 6 | 6.73±2.02c | 8.68±6.37c | 14.96±3.85b | 44.83±4.66ax | |
| 9 | 6.66±2.16c | 8.74±1.89c | 14.26±2.02b | 46.54±4.58ax | |
DFD, dark-firm-dry.
Values are presented as mean±standard deviation (n = 3).
Means within each row are significantly different (p<0.05).
Means within each column are significantly different (p<0.05).
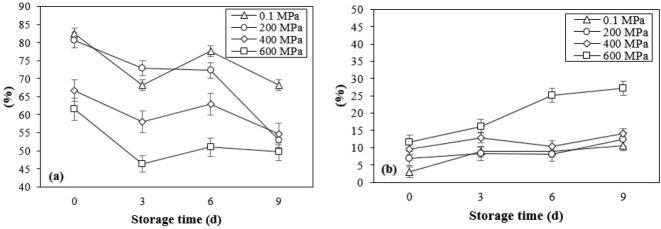
The discoloration effect was found in samples treated at 600 MPa in which the highest L* and b* values and hue-angle (h°) were recorded. The significant increase in b* value and hue-angle demonstrate the browning effect of high pressure on the surface of the meat. This was due to the accumulation of metmyoglobin, while significant decrease in proportion of oxymyoglobin was observed in samples treated at 600 MPa. HPP decreased the relative proportion of OxyMb, in contrast with MetMb. Changes in meat color due to pressurization are related to myoglobin denaturation. Zipp and Kauzmann [25] suggested that the complete myoglobin denaturation of meat with pH 6 was reached with pressurization rate of 350 MPa at 10°C or 450 MPa at 20°C. Then, a whitening effect on the surface of HPP-treated meat occurs as depolymerized actin starts to re-aggregate after the release of pressure, forming aggregates that enhance light scattering. Storage until 9 days significantly increased and decreased the proportions of MetMb and OxyMb (Figure 1), respectively, resulting in the decline of redness on the surface of either non-treated or treated samples (p<0.001). Significant interactions between level of pressure and duration of storage were found on the relative proportion of MetMb and OxyMb (p<0.05).
Figure 1.
Changes in oxymyoglobin (a) and metmyoglobin (b) relative proportion of DFD strip loin subjected with different levels of pressure during refrigerated storage under vacuum. These data are presented as mean±standard deviation. DFD, dark-firm-dry.
Lipid oxidation is one of the quality deteriorations in beef. It can be determined by an increase in the MDA content. The off-flavor generated by lipid oxidation influences consumer acceptance. As expected, HPP led to higher TBARS values than the control in this study (Figure 2). HPP and refrigerated storage led to the development of lipid oxidation, with significant differences compared to the control (p<0.001). Interaction was found between different levels of pressure and storage times (p<0.01). The highest TBARS values were found in samples treated at 600 MPa at day 3 of storage. However, this study shows that HPP below 600 MPa at 15°C±2°C did not enhance the amount of MDA over that of previous finding and still in acceptable amount for raw beef [6]. The lower temperature during pressurization in this study may cause these differences. Cheftel and Culioli [26] mentioned that the melting temperature of lipid increases by more than 10°C per 100 MPa, causing them to crystallize under pressure. Furthermore, the melting point of lipids has negative correlation with lipid oxidation [27]. Thus, to minimize the risk of lipid oxidation in pressurized meat, the temperature used in the pressurization chamber should be maintained below 20°C.
Figure 2.
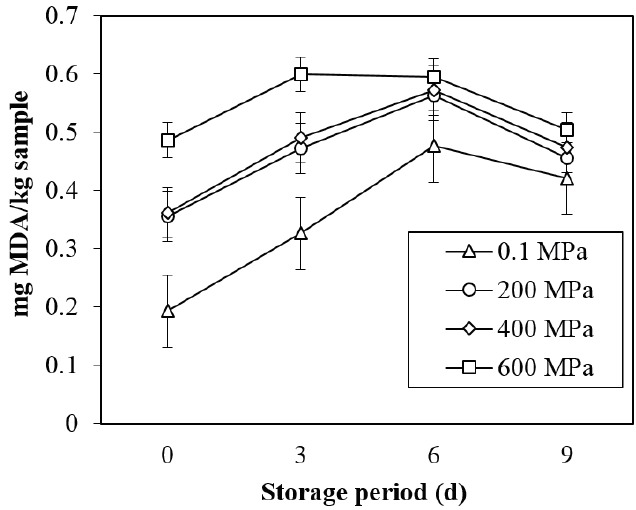
Changes in TBARS values of DFD strip loin subjected with different levels of pressure during refrigerated storage under vacuum. These data are presented as mean±standard deviation. TBARS, 2-thiobarbituric acid reactive substances; DFD, dark-firm-dry; MDA; malondialdehyde.
Antioxidant enzyme activity
Muscle cells have their own defense system against lipid oxidation, which is related to aging with slow oxidative processes. The activity of self-defense enzymes against free radicals can reveal the mechanisms of oxidation in meat post-mortem. In this study, the activity levels of catalase, SOD and GSH-Px were observed in vitro. HPP along with refrigerated storage reduced the activity of GSH-Px (Figure 3). No clear effects of HPP on the activity of catalase and SOD were found in this study. Catalase and SOD activity significantly weakened over 9 days of storage for both non-treated and treated samples (p<0.01). However, no interactions between different levels of pressure and storage time were found on the activity of antioxidant enzymes. As HPP has potential application for food preservation purposes through inactivating microorganism growth and enzyme activity, the activity of antioxidant enzymes in meat might be affected as well. This study showed that pressurization up to 600 MPa at a temperature of 15°C±2°C did not fully inactivate those enzymes in DFD beef, although it weakened the activity of GSH-Px significantly. Miyagawa et al [28] distinguished four types of enzyme inactivation based on activity loss and recovery under pressure; completely and irreversibly inactivated, completely and reversibly inactivated, incompletely and irreversibly inactivated, and incompletely and reversibly inactivated. Our results suggest that catalase and SOD were incompletely and reversibly inactivated under high pressure.
Figure 3.
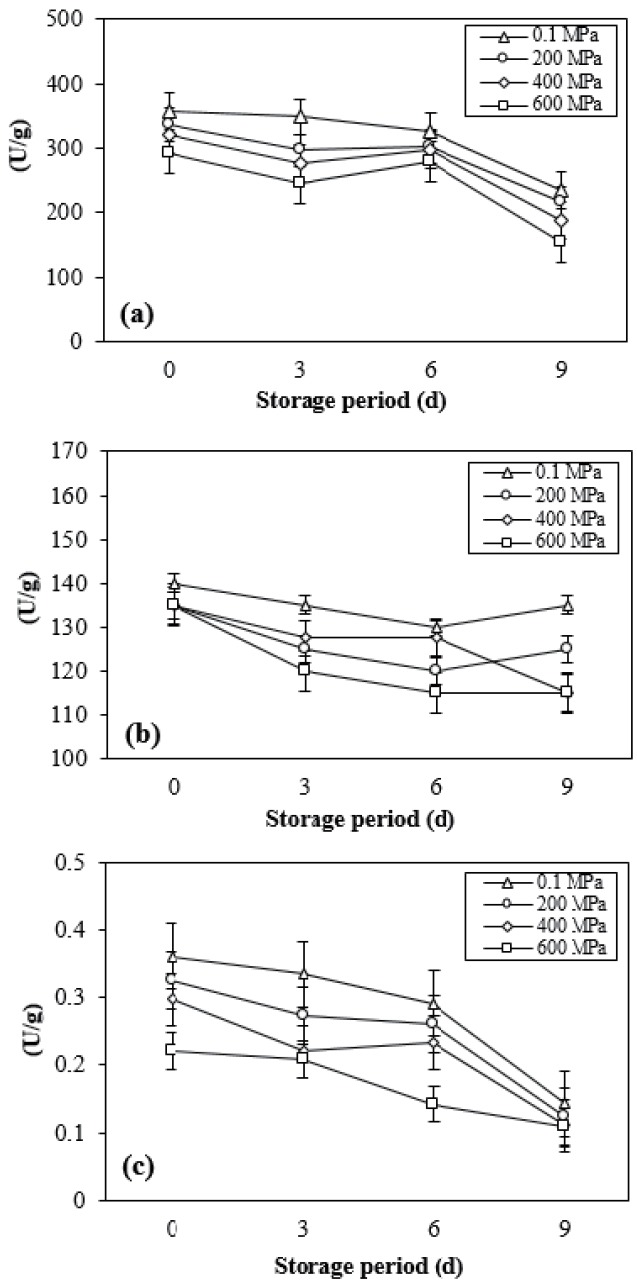
Changes in the activity of catalase (a), superoxide dismutase (b), and glutathione peroxidase (c) of DFD strip loin subjected with different levels of pressure during refrigerated storage under vacuum. These data are presented as mean±standard deviation. DFD, dark-firm-dry.
Aroma pattern
In present study, the aroma pattern of DFD beef subjected to different level of HPP changed during refrigerated storage for 9 days under vacuum (Figure 4) as TBARS values changed as well. About 99.93% of total variance was explained by first two components, in which the first (C1) and second component (C2) explain 96.31% and 3.62%, respectively. As the total variance contribution rate of the first two component of PCA is more than 85%, the model can be used to explain most of information. In addition, the successful discrimination model should have the index of 85% to 100% [29]. The discrimination index in this study (85%) shows that discrimination was achieved among samples. The aroma pattern of non-treated samples was always discriminated from samples subjected to HPP during storage. Because no changes in pH were detected during storage, another factor such as lipid oxidation might contribute to aroma changes. At day 9, the distances between treatments are closer as the TBARS values are not statistically different. Brewer and Vega [30] reported that lipid oxidation products such as pentanal and heptanal are responsible for off-odor released by oxidized ground beef.
Figure 4.
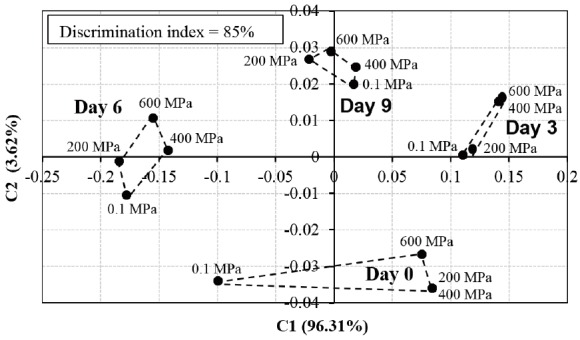
Principle component analysis of aroma pattern revealed by electronic nose for DFD beef strip loin subjected to different levels of pressure during refrigerated storage under vacuum. DFD, dark-firm-dry.
CONCLUSION
The HPP at 15°C±2°C lowered the shear force value, modified the surface color of DFD beef and maintained the stability of meat pH during refrigerated storage under vacuum. However, the oxidative deterioration such as metmyoglobin formation, lipid oxidation, the decline rate of antioxidant enzyme activity and changes in aroma pattern could not be avoided during storage. HPP at 200 MPa is considered giving advantage in color modification of DFD beef with less oxidative deterioration. However, the occurrence of DFD beef is relatively rare nowadays and HPP does not guarantee cost efficiency, thus this technology is not worth for preserving raw DFD beef.
Supplementary Information
ACKNOWLEDGMENTS
This research was supported by High Value-added Food Technology Development Program from Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.McArdle RA, Marcos B, Kerry JP, Mullen AM. Monitoring the effects of high pressure processing and temperature on selected beef quality attributes. Meat Sci. 2010;86:629–34. doi: 10.1016/j.meatsci.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Jung S, Yun H, Kim HJ, et al. Inactivation efficiency of Escherichia coli and Listeria monocytogenes in ground pork by combination of natural food ingredients and high pressure processing. Korean J Food Sci Anim Resour. 2012;32:1–5. [Google Scholar]
- 3.Carpenter CE, Cornforth DP, Whittier D. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 2001;57:359–63. doi: 10.1016/s0309-1740(00)00111-x. [DOI] [PubMed] [Google Scholar]
- 4.Newton KG, Gill CO. The microbiology of DFD fresh meats: A review. Meat Sci. 1981;5:223–32. doi: 10.1016/0309-1740(81)90005-X. [DOI] [PubMed] [Google Scholar]
- 5.Carlez A, Veciana-Nogues T, Cheftel JC. Changes in colour and myoglobin of minced meat due to high pressure processing. Lebenson Wiss Technol. 1995;28:528–38. [Google Scholar]
- 6.Ma H, Ledward DA, Zamri AI, Frazier RA, Zhou GH. Effects of high pressure/thermal treatment on lipid oxidation in beef and chicken muscle. Food Chem. 2007;104:1575–9. [Google Scholar]
- 7.Medina-Meza IG, Barnaba C, Barbosa-Cánovas GV. Effects of high pressure processing on lipid oxidation: A review. Innov Food Sci Emerg Technol. 2014;22:1–10. [Google Scholar]
- 8.Utama DT, Lee SG, Baek KH, et al. Correlation between antioxidant enzyme activity, free iron content and lipid oxidation in four lines of Korean native chicken meat. Korean J Food Sci Anim Resour. 2016;36:44–50. doi: 10.5851/kosfa.2016.36.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clariana M, Guerrero L, Sárraga C, Garcia-Regueiro JA. Effects of high pressure application (400 and 900 MPa) and refrigerated storage time on the oxidative stability of sliced vacuum packed dry-cured ham. Meat Sci. 2012;90:323–9. doi: 10.1016/j.meatsci.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 10.The Beef Carcass Grading [Internet] Korea Institute for Animal Products Quality Evaluation; c2010. [cited 2015 Dec 21]. Available from: http://www.ekape.or.kr/view/eng/system/beef.asp. [Google Scholar]
- 11.Krzywicki K. The determination of haem pigments in meat. Meat Sci. 1982;7:29–36. doi: 10.1016/0309-1740(82)90095-X. [DOI] [PubMed] [Google Scholar]
- 12.Sinnhuber RO, Yu TC. The 2-thiobarbituric acid reaction, an objective measure of the oxidative deterioration occurring in fat and oil. J Oleo Sci. 1977;26:259–67. [Google Scholar]
- 13.Renerre M, Dumont F, Gatellier P. Antioxidant enzyme activities in beef in relation to oxidation of lipid and myoglobin. Meat Sci. 1996;43:111–21. doi: 10.1016/0309-1740(96)84583-9. [DOI] [PubMed] [Google Scholar]
- 14.Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 15.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 16.Flohé L, Günzler WA. Assays of glutathione peroxidase. Meth Enzymol. 1984;105:114–20. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 17.Marcos B, Kerry JP, Mullen AM. High pressure induced changes on sarcoplasmic protein fraction and quality indicators. Meat Sci. 2010;85:115–20. doi: 10.1016/j.meatsci.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Chan JTY, Omana DA, Betti M. Application of high pressure processing to improve the functional properties of pale, soft, and exudative (PSE)-like turkey meat. Innov Food Sci Emerg Technol. 2011;12:216–25. [Google Scholar]
- 19.Jung S, de Lamballerie-Anton M, Ghoul M. Textural changes in bovine meat treated with high pressure. High Press Res. 2000;19:69–74. [Google Scholar]
- 20.Sun XD, Holley RA. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr Rev Food Sci Food Saf. 2011;10:33–51. [Google Scholar]
- 21.Macfarlane JJ. Pre-rigor pressurisation of muscle: Effect of pH, shear value and taste panel assessment. J Food Sci. 1973;38:294–8. [Google Scholar]
- 22.Vasanthi S, Venkataramanujam V, Dushyanthan K. Effect of cooking temperature and time on the physico-chemical, histological and sensory properties of female carabeef (buffalo) meat. Meat Sci. 2007;76:274–80. doi: 10.1016/j.meatsci.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Ma H, Ledward DA. High pressure processing of fresh meat — Is it worth it? Meat Sci. 2013;95:897–903. doi: 10.1016/j.meatsci.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Jung S, Ghoul M, de Lamballerie-Anton M. Influence of high pressure on the color and microbial quality of beef meat. Lebenson Wiss Technol. 2003;36:625–31. [Google Scholar]
- 25.Zipp A, Kauzmann W. Pressure denaturation of metmyoglobin. Biochemistry. 1973;12:4217–28. doi: 10.1021/bi00745a028. [DOI] [PubMed] [Google Scholar]
- 26.Cheftel JC, Culioli J. Effects of high pressure on meat: A review. Meat Sci. 1997;46:211–36. doi: 10.1016/s0309-1740(97)00017-x. [DOI] [PubMed] [Google Scholar]
- 27.Sanz M, Flores A, Lopez-Bote CJ. Effect of fatty acid saturation in broiler diets on abdominal fat and breast muscle fatty acid composition and susceptibility to lipid oxidation. Poult Sci. 1999;78:378–82. doi: 10.1093/ps/78.3.378. [DOI] [PubMed] [Google Scholar]
- 28.Miyagawa K, Sannoe K, Suzuki K. Studies on Taka-amylase A under high pressure treatment: II. Recovery of enzymatic activity of pressure inactivated Taka-amylase A and its enhancement by retreatment at moderate pressure. Arch Biochem Biophys. 1964;106:467–74. doi: 10.1016/0003-9861(64)90217-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhu L, Seburg RA, Tsai E, Puech S, Mifsud JC. Flavor analysis in a pharmaceutical oral solution formulation using an electronic-nose. J Pharm Biomed Anal. 2004;34:453–61. doi: 10.1016/s0731-7085(03)00651-4. [DOI] [PubMed] [Google Scholar]
- 30.Brewer MS, Vega JD. Detectable odor thresholds of selected lipid oxidation compounds in a meat model system. J Food Sci. 1995;60:592–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.