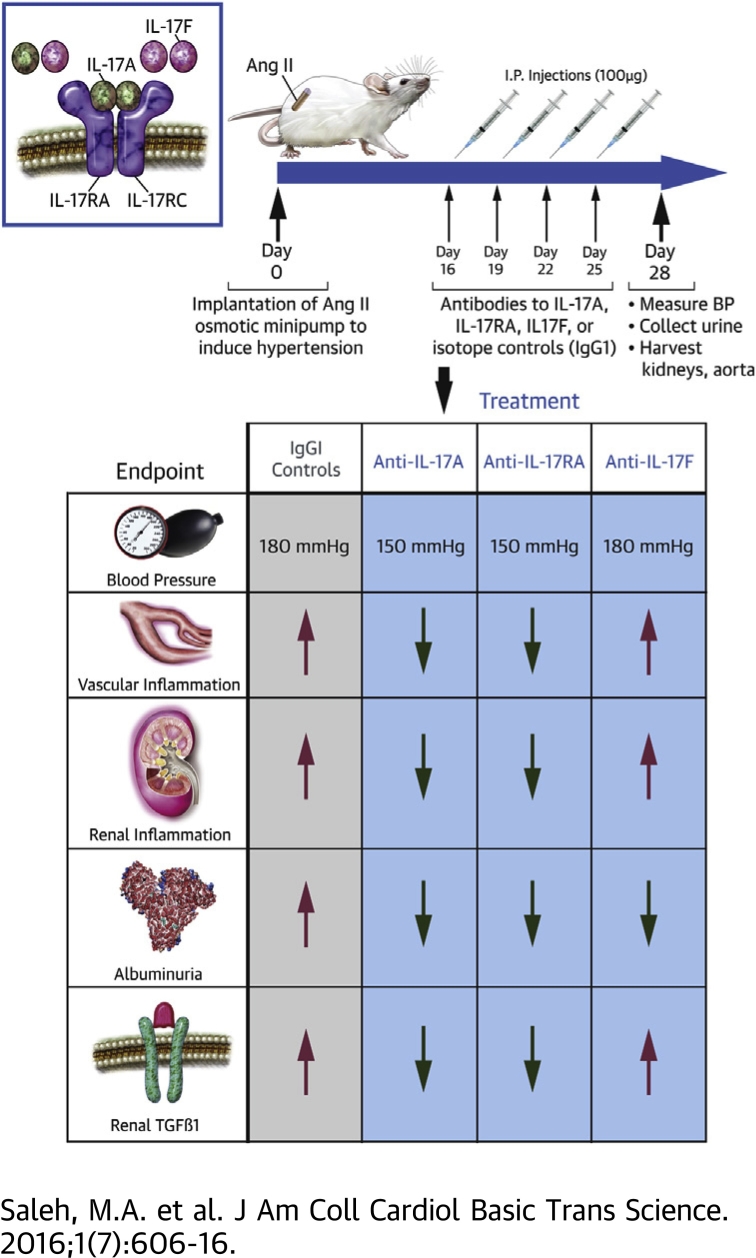
Visual Abstract
Key Words: angiotensin II, gamma delta T cells, hypertension, interleukin-17A, interleukin-17F, interleukin-17RA receptor, T lymphocytes
Abbreviations and Acronyms: Ang II, angiotensin II; ANOVA, analysis of variance; DOCA-salt, deoxycorticosterone acetate and high-salt diet; ELISA, enzyme-linked immunosorbent assay; FDA, Food and Drug Administration; Ig, immunoglobulin; IL, interleukin; IL-17RA, interleukin-17 receptor A; TGF, transforming growth factor
Highlights
-
•
Hypertension is associated with an increase in T-cell–derived cytokines such IL-17A and IL-17F.
-
•
Monoclonal antibodies to IL-17A, IL-17F, IL-17RA, or isotype control antibodies (IgG1) were administered twice weekly during the last 2 weeks of a 4-week angiotensin II infusion protocol in mice.
-
•
Antibodies to IL-17A or IL-17RA, but not IL-17F, lowered blood pressure by 30 mm Hg, attenuated renal and vascular inflammation, and reduced renal transforming growth factor beta levels (a marker of renal fibrosis) compared with control IgG1 antibodies. All 3 experimental antibodies blunted the progression of albuminuria.
-
•
Monoclonal antibodies to IL-17A or IL-17RA may be a useful adjunct treatment for hypertension and the associated end-organ dysfunction.
Summary
Inflammatory cytokines play a major role in the pathophysiology of hypertension. The authors previously showed that genetic deletion of interleukin (IL)-17A results in blunted hypertension and reduced renal/vascular dysfunction. With the emergence of a new class of monoclonal antibody–based drugs for psoriasis and related autoimmune disorders that target IL-17 signaling, the authors sought to determine whether these antibodies could also reduce blood pressure, renal/vascular inflammation, and renal injury in a mouse model of hypertension. The authors show that antibodies to IL-17A or the IL-17RA receptor subunit, but not IL-17F, may be a novel adjunct treatment for hypertension and the associated end-organ dysfunction.
There is now strong evidence that hypertension is an inflammatory disease in which T cells and T-cell–derived cytokines contribute to blood pressure elevation and end-organ damage 1, 2. Our group was the first to demonstrate the critical role of the proinflammatory cytokine interleukin (IL)-17A in hypertension and the accompanying vascular dysfunction, using mice with genetic deletion of IL-17A (3). Subsequently, Nguyen et al. (4) showed that infusion of recombinant IL-17A into mice raised blood pressure and impaired vascular relaxation through phosphorylation of endothelial nitric oxide synthase and reduced vascular nitric oxide production. Recently, we showed that IL-17A contributes to angiotensin II (Ang II)-induced renal injury and modulates the expression of renal sodium transporters resulting in altered pressure natriuresis, whereas the related cytokine, IL-17F, had little or no effect on blood pressure, renal injury, and renal sodium chloride cotransporter activity 5, 6.
The IL-17 cytokine family consists of 6 isoforms (IL-17A through IL-17F) of which IL-17A and IL-17F share the highest (50%) sequence homology (7). Both IL-17A and IL-17F are produced by similar subsets of immune cells and bind as homo- or heterodimers to the same receptor complex composed of IL-17RA and IL-17RC subunits (8). Although IL-17A has been widely studied and found to play critical roles in the pathogenesis of many autoimmune diseases such as psoriasis and multiple sclerosis (9), the role of IL-17F is less well characterized. IL-17F has a lower affinity for the IL-17RA receptor subunit and has been found to have overlapping, as well as distinctive functions, from IL-17A (10).
IL-17A is the signature cytokine of a relatively recently described subset of CD4+ T-helper cells designated TH17 cells. However, a subset of CD8+ T cells as well as gamma delta (γδ) T cells (innate-like unconventional T cells with a distinct T-cell receptor) can produce IL-17A under various conditions (11). In fact, γδ T cells are thought to be the most prominent producers of IL-17A under certain pathological conditions 12, 13, 14. Interestingly, recent studies have demonstrated that salt can promote the production of pathogenic TH17 cells, thus providing a potential link between high salt intake and the development of hypertension 15, 16.
In the current study, we sought to determine the cellular source of IL-17 isoforms in the kidney and vasculature of hypertensive animals, and determine whether pharmacological inhibition of IL-17 signaling can reduce blood pressure and ameliorate renal/vascular inflammation and renal injury in a mouse model of hypertension. A number of human monoclonal antibodies to IL-17A, IL-17F, or the IL-17RA receptor subunit are in development, clinical testing, or Food and Drug Administration (FDA)-approved for the treatment of psoriasis and related autoimmune disorders (reviewed in Beringer et al. [9]). However, to date, these drugs have not been tested in humans for their efficacy in hypertension or other cardiovascular disorders. We hypothesized that inhibition of IL-17A or the IL-17RA receptor, but not IL-17F, would lower blood pressure and end-organ damage in a mouse model of Ang II–induced hypertension.
Methods
Animals and blood pressure measurement
Wild-type C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine). All protocols were approved by the Institutional Animal Care and Use Committee at Vanderbilt University. At approximately 10 to 12 weeks of age, osmotic minipumps (Alzet, Model 2004, Cupertino, California) were implanted subcutaneously for infusion of Ang II (490 ng/kg/min). Blood pressure was measured at baseline and weekly during the 4 weeks of Ang II infusion using a computerized, noninvasive, tail-cuff system (MC4000 Blood Pressure Analysis System, Hatteras Instruments, Cary, North Carolina). Mice were sacrificed at the end of all experiments by CO2 inhalation.
Antibody treatments
Two weeks after induction of hypertension with Ang II infusion, mice were randomly allocated to the following 5 experimental groups: 1) control mouse IgG1 antibody (mouse IgG1 K isotype control functional grade purified, Clone P3.6.2.8.1 [eBioscience, San Diego, California] or mouse IgG1, PL-31545 [Amgen, Thousand Oaks, California]); 2) control rat IgG1 antibody (rat IgG1 K isotype control functional grade purified, Clone eBRG1 [eBioscience]); 3) IL-17A neutralizing antibody (mouse anti-mouse IL-17A functional grade purified, Clone eBioMM17F3 [eBioscience]); 4) IL-17F neutralizing antibody (rat anti-mouse IL-17F functional grade purified, Clone RN17 [eBioscience]); and 5) IL-17RA receptor antagonist (murine muIL-17RA-M751, PL-31280 [Amgen]). Mice were injected intraperitoneally with 100 μg of antibodies twice weekly during the last 2 weeks of Ang II infusion (days 16, 19, 22, and 25). This dose and frequency was chosen based on prior studies from our group and others 17, 18, 19, 20.
Flow cytometry
Mice were euthanized with CO2 and transcardially perfused with phosphate buffered saline–heparin (10 U/ml) before the collection of organs. Kidney and thoracic aortic leukocytes were isolated as described previously (21) and stained using the antibodies listed in Supplemental Table 1. For intracellular staining, single-cell suspensions of kidneys or the whole aortae in RPMI medium supplemented with 5% fetal bovine serum were stimulated with 2 μl of BD Leukocyte Activation Cocktail (ionomycin, brefeldin A, and phorbol myristic acetate) at 37°C for 3 h. Cells were then stained using the antibodies listed in Supplemental Table 2.
Enzyme-linked immunosorbent assays
Urine was collected over a 24-h period at baseline, after 2 weeks, and after 4 weeks of Ang II infusion by placement of mice in metabolic cages. Urinary albumin concentrations were determined by enzyme-linked immunosorbent assay (ELISA) (Exocell M, Exocell, Philadelphia, Pennsylvania). Total urinary volume was recorded and multiplied by the albumin concentration to determine the daily albumin excretion rate. Renal transforming growth factor beta (TGFβ) levels were quantified from whole-kidney homogenates using a mouse/rat/porcine/canine TFG-β1 Quantikine ELISA kit (R&D Systems, Minneapolis, Minnesota) and normalized to total kidney weight.
Statistical analyses
Data are expressed as mean ± SEM. The assumption of normality was made because most continuous physical characteristics follow a Gaussian distribution. Differences between 2 groups were compared using the Student t test. Differences between 3 or more groups were compared using 1-way analysis of variance (ANOVA) followed by the Holm-Sidak post hoc test. Blood pressure and albuminuria data were analyzed at day 28 using 1-way ANOVA followed by the Holm-Sidak post hoc test. p < 0.05 was considered significant.
Results
Ang II–induced hypertension is associated with increased T-cell production of IL-17A and IL-17F in the kidney and vasculature
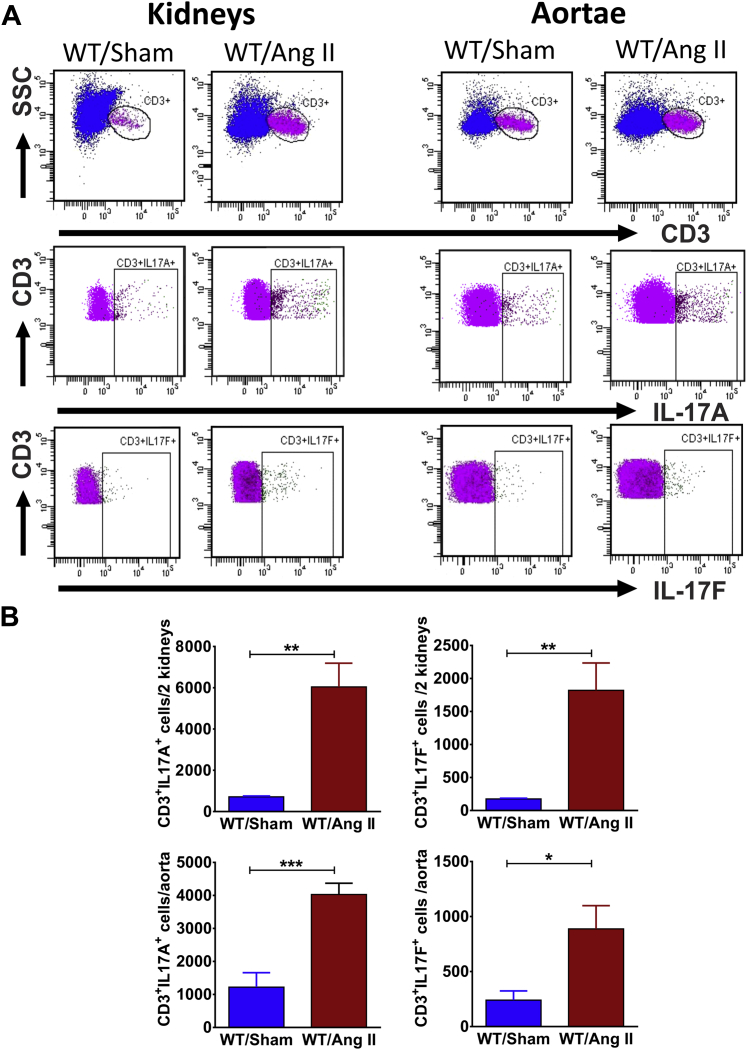
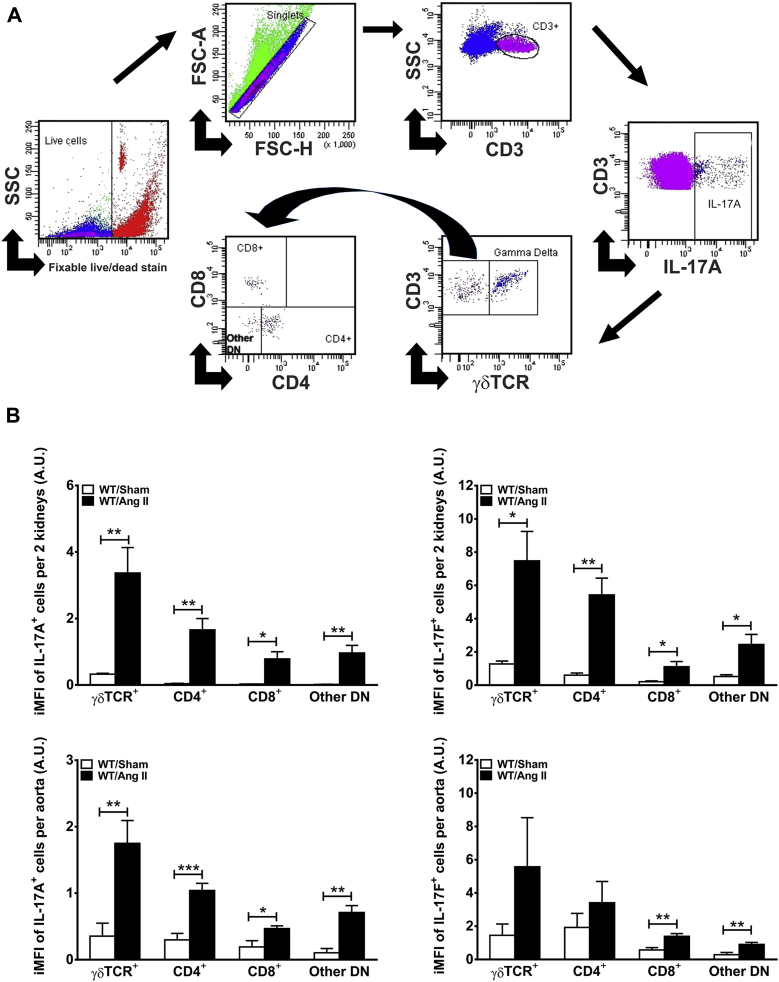
We first determined whether there was an increase in IL-17A– and IL-17F–producing T cells in the kidney and aortae of mice following 4 weeks of Ang II (490 ng/kg/min) infusion. Intracellular staining indicated that Ang II–induced hypertension was associated with a significant increase in CD3+IL-17A+ and CD3+IL-17F+ cells in the kidney and aortae (Figure 1). To determine the specific T-cell subsets producing IL-17A and IL-17F, we employed the gating strategy depicted in Figure 2A. CD3+ T cells were selected from the live singlets. Intracellular staining with IL-17A or IL-17F identified the total T cells producing these cytokines. Gamma delta (γδ) T cells possess a distinct T-cell receptor that can be distinguished from conventional alpha beta (αβ) T-cell receptors by flow cytometry. We therefore gated on the presence of the γδ T-cell receptor to quantify the IL-17–producing γδ T cells. Finally, we gated on the γδ T-cell receptor negative cells (which are presumably conventional αβ T cells) and further classified these into CD4+, CD8+, or other double-negative cells. To determine the relative T-cell subset contribution to IL-17A and IL-17F production, we used the integrated mean fluorescence intensity, which is obtained by multiplying the number of cells expressing a particular marker with the mean fluorescence intensity in that channel (22). We found that γδ T cells and CD4+ TH17 cells are the predominant sources of IL-17A and IL-17F in the kidney and aorta, particularly following Ang II infusion (Figure 2B).
Figure 1.
Angiotensin II Increases IL-17A– and IL-17F–Producing T Cells in Mouse Kidneys and Aortae
(A) Representative flow cytometry analysis of renal and aortic T cells isolated from mice after 28 days of vehicle (Sham) or angiotensin II (Ang II) infusion. (B) Quantification of the total number of interleukin (IL)-17A– or IL-17F–producing cells in kidneys and aortae (n = 5 to 6 per group). Data were analyzed using Student t test and expressed as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001 vs. WT/Sham. WT = wild type.
Figure 2.
γδ T Cells and TH17 Cells are the Major Sources of IL-17A and IL-17F in the Kidney and Vasculature Following Ang II Infusion
(A) Representative example of flow cytometry gating strategy for the quantification of IL-17A–producing CD3+ T cells in the kidney of an Ang II–treated mouse. A similar strategy was used for IL-17F and for aortic samples. (B) Quantification of the integrated mean fluorescence intensity (iMFI) of IL-17A– or IL-17F–producing subsets of T cells (γδ T cell receptor [TCR]+, CD4+, CD8+, and other double-negative T cells [other DN]). Data were analyzed using Student t test and expressed in arbitrary units (A.U.) as mean ± SEM (n = 5 to 6 per group). *p < 0.05; **p < 0.01; ***p < 0.001 versus WT/Sham. Abbreviations as in Figure 1.
Administration of monoclonal antibodies to IL-17A or the IL-17RA receptor subunit, but not IL-17F, lowers blood pressure in response to Ang II infusion
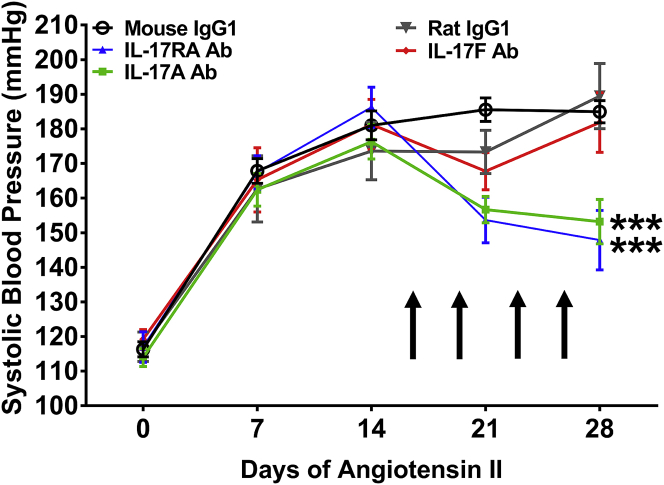
Monoclonal antibodies to human IL-17A, IL-17F, and the IL-17RA receptor subunit are in various phases of development, testing, and FDA approval for the treatment of psoriasis and related IL-17A–mediated autoimmune diseases. As proof of concept, we sought to determine whether these antibodies would have a beneficial effect in a mouse model of hypertension. Ang II was infused for 4 weeks into wild-type C57Bl/6J mice. During the final 2 weeks of Ang II infusion, antibodies to IL-17A, IL-17F, IL-17RA, or corresponding IgG1 control antibodies were injected intraperitoneally twice weekly as depicted in Figure 3. Administration of monoclonal antibodies to IL-17A and IL-17RA resulted in a 30 mm Hg decrease in blood pressure. By contrast, the anti–IL-17F antibody and both IgG1 control antibodies had no effect on blood pressure during the final 2 weeks of Ang II infusion (Figure 3). It should be noted that although blood pressure was reduced from approximately 180 mm Hg to 150 mm Hg with anti–IL-17A and anti–IL-17RA antibodies, the pressures were still elevated compared with the baseline blood pressures of 110 to 120 mm Hg.
Figure 3.
Monoclonal Antibodies to IL-17A or the IL-17RA Receptor Subunit, But Not IL-17F, Lowers Blood Pressure in Response to Ang II Infusion
Systolic blood pressure was measured at baseline and weekly during 28 days of Ang II infusion. Arrows indicate timing of antibody administration. Data are expressed as mean ± SEM and were analyzed at day 28 using 1-way analysis of variance followed by Holm-Sidak post hoc test (n = 7 to 20 per group). ***p < 0.001 vs. mouse IgG1. Ab = antibody; other abbreviations as in Figures 1 and 2.
Administration of monoclonal antibodies to IL-17A or the IL-17RA receptor subunit, but not IL-17F, reduces renal and vascular inflammation
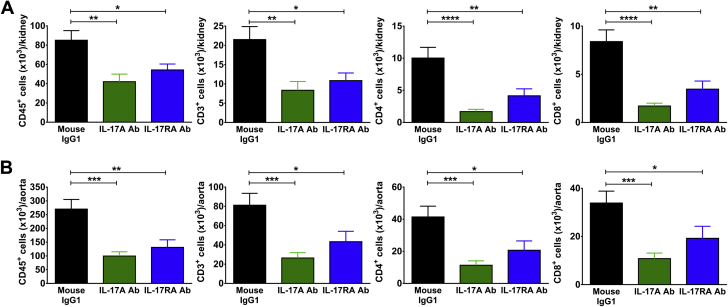
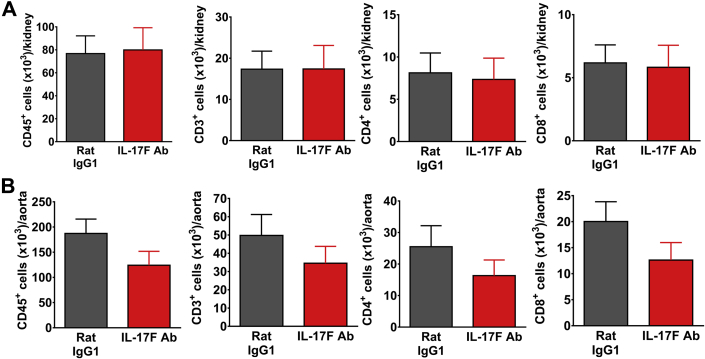
To determine the effect of IL-17 blockade on renal and vascular inflammation, Ang II was infused for 4 weeks, and monoclonal antibodies to IL-17A, IL-17F, IL-17RA, or corresponding IgG1 control antibodies were injected intraperitoneally twice weekly during the last 2 weeks of Ang II infusion as described in the preceding text. Flow cytometry was performed on single-cell suspensions from 1 kidney and the thoracic aorta to quantify degree of inflammation. Surface staining was performed for CD45 (total leukocytes), CD3 (total T cells), CD4 (T helper cells), CD8 (cytotoxic T cells), and F4/80 (a monocyte/macrophage marker). Mice treated with monoclonal antibodies to IL-17A and IL-17RA exhibited significantly reduced levels of total leukocytes, total T cells, CD4+ T cells, and CD8+ T cells in the kidney compared with mice treated with a corresponding IgG1 control antibody (Figure 4A). Of note, there was no change in the number of double-negative T cells or monocytes/macrophages in the kidney with either of these antibody treatments (Supplemental Figure 1A). Similar results were observed in the aorta (Figure 4B, Supplemental Figure 1B). By contrast, mice that received the IL-17F antibody exhibited no change in total leukocyte, T-cell, or monocyte/macrophage numbers in the kidney or aorta compared to IgG1-treated mice, consistent with a lack of blood pressure effect with this antibody (Figure 5, Supplemental Figure 2). To confirm that the administration of isotype control antibodies did not affect renal or vascular immune cell infiltration, we compared levels of total leukocytes, total T cells, T-cell subsets, and monocytes/macrophages in untreated wild-type mice following 4 weeks of Ang II infusion to mice that received mouse or rat IgG1 during the last 2 weeks of Ang II infusion and found no significant differences in the numbers of these cells in both the kidney and aorta (data not shown).
Figure 4.
Monoclonal Antibodies to IL-17A or the IL-17RA Receptor Subunit Reduce Renal and Vascular Inflammation in Response to Ang II Infusion
Quantification of total leukocytes (CD45+ cells), total T lymphocytes (CD3+ cells), and T-cell subsets (CD4+ and CD8+) per kidney (A) or thoracic aorta (B) in mice infused with Ang II for 4 weeks and treated with either IL-17A neutralizing antibody, IL-17RA receptor antagonist, or corresponding mouse IgG1 isotype control twice weekly during the last 2 weeks of Ang II infusion. Data were analyzed using 1-way analysis of variance followed by Holm-Sidak post hoc test and expressed as mean ± SEM (n = 12 to 20 per group). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs. mouse IgG1. Abbreviations as in Figures 1, 2, and 3.
Figure 5.
Monoclonal Antibodies to IL-17F Have No Effect on Renal and Aortic Inflammation
Quantification of total leukocytes (CD45+ cells), total T lymphocytes (CD3+ cells), and T-cell subsets (CD4+ and CD8+) per kidney (A) or thoracic aorta (B) in mice infused with Ang II for 4 weeks and treated with either IL-17F neutralizing antibody or corresponding rat IgG1 isotype control twice weekly during the last 2 weeks of Ang II infusion. Data were analyzed using Student t test and expressed as mean ± SEM (n = 12 per group). Abbreviations as in Figures 1, 2, and 3.
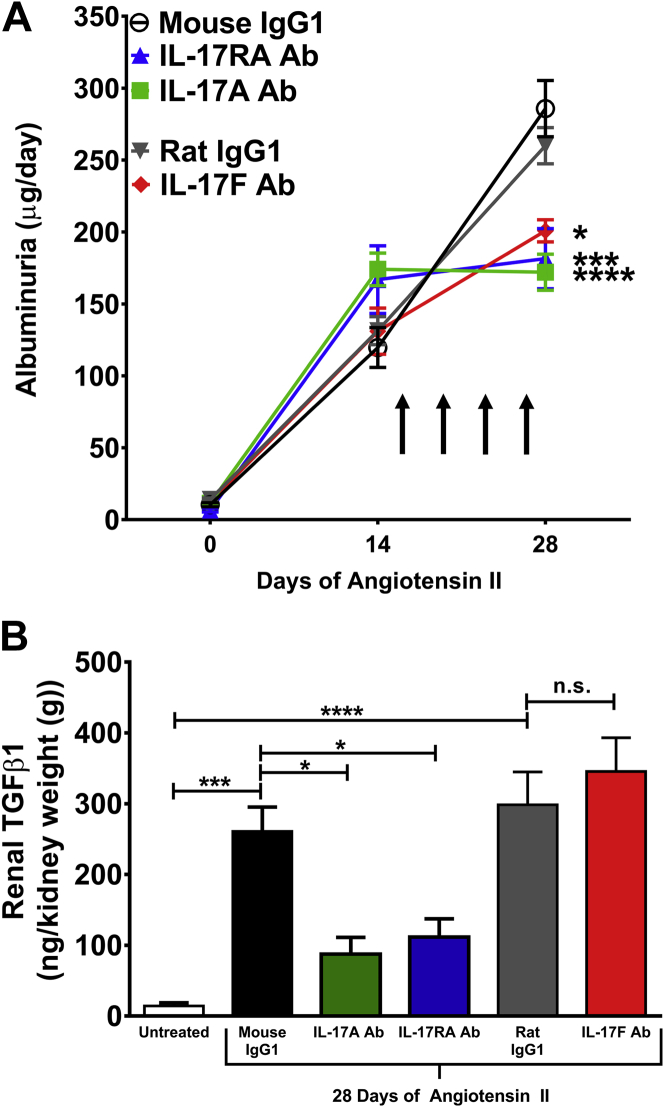
Administration of monoclonal antibodies to IL-17A, IL-17F, or IL-17RA blunts the progression of albuminuria in response to Ang II infusion
To assess the effect of IL-17 blockade on renal injury, 24-h urine was collected at baseline, after 2 weeks of Ang II infusion (before antibody administration), and after 4 weeks of Ang II infusion (with twice weekly antibody injections given between weeks 2 and 4). Urinary albumin concentration was measured by ELISA. At baseline, there was very little albuminuria and no significant differences among the groups. Albuminuria increased markedly after 2 weeks of Ang II infusion. Interestingly, treatment with any of the IL-17 antibodies, including the IL-17F antibody, halted the progression of albuminuria, whereas mice that received either control antibody had continued progression of albuminuria (Figure 6A). Thus, blockade of IL-17 signaling protects from Ang II–induced glomerular injury.
Figure 6.
Effect of Monoclonal Antibodies to IL-17A, IL-17F, or the IL-17RA Receptor Subunit on Glomerular Injury and Renal Fibrosis in Response to Ang II Infusion
(A) Glomerular injury was assessed by quantifying 24-h urinary excretion of albumin at baseline and after 14 or 28 days of Ang II infusion. Arrows indicate timing of antibody administration. Data are expressed as mean ± SEM and were analyzed at day 28 by 1-way analysis of variance followed by Holm-Sidak post hoc test (n = 7 to 15 per group). *p < 0.05; ***p < 0.001; ****p < 0.0001 vs. corresponding IgG1 control. (B) Renal fibrosis was assessed by quantifying transforming growth factor β1 (TGFβ1) from renal homogenates. Data from mice that received no antibodies and no Ang II (Untreated) are shown as a reference control. The other groups were infused with Ang II for 28 days and treated with the indicated antibodies twice weekly during the last 2 weeks of Ang II infusion. Data were analyzed by 1-way analysis of variance followed by Holm-Sidak post hoc test and expressed as mean ± SEM (n = 5 per group). *p < 0.05; ***p < 0.001; ****p < 0.0001. Abbreviations as in Figures 1, 2, and 3.
Administration of monoclonal antibodies to IL-17A or the IL-17RA receptor subunit, but not IL-17F, reduces renal TGFβ levels
Renal fibrosis is another hallmark of renal damage from hypertension and is mediated by TGFβ signaling (23). To assess renal fibrosis, we measured TGFβ levels from whole-kidney homogenates following 4 weeks of Ang II infusion. Antibodies to IL-17A, IL-17RA, IL-17F, or corresponding isotype controls were injected twice weekly during the last 2 weeks of Ang II infusion as depicted in Figure 6A. Kidneys from mice that did not receive Ang II or antibodies were used as a reference control (untreated). Renal TGFβ1 levels markedly increase in response to Ang II infusion in isotype control–treated mice. Antibodies to IL-17A or IL-17RA, but not IL-17F, significantly reduced TGFβ levels (Figure 6B). Thus, inhibition of IL-17A and IL-17RA reduces blood pressure, renal/vascular inflammation, and markers of renal injury and fibrosis.
Discussion
We and others have previously demonstrated that the proinflammatory cytokine IL-17A is elevated in hypertensive animals and humans, and that IL-17A promotes sustained hypertension, as well as renal and vascular dysfunction 3, 4, 5, 6, 24. However, the specific T-cell subsets that contribute to IL-17 production in the kidney and vessels were previously unknown. From a translational perspective, there are few data on whether neutralizing antibodies to IL-17A, IL-17F, or the IL-17RA receptor subunit can lower blood pressure and ameliorate hypertension-associated end-organ damage. In the current study, we show that innate-like γδ T cells and CD4+ TH17 cells are the primary sources of IL-17A and IL-17F in the aortae and kidneys of Ang II–infused mice. Importantly, we show that acute pharmacological inhibition of IL-17A or the IL-17RA receptor subunit can reduce blood pressure by ∼30 mm Hg, attenuate renal/vascular inflammation, and decrease markers of renal injury and fibrosis, making these potentially attractive therapeutic options for the management of hypertension.
Our finding that γδ T cells are a major source of IL-17 isoforms in the aorta and kidney of hypertensive animals is consistent with a recent report by Li et al. (14) showing that γδ T cells are a major source of IL-17A in the heart and contribute to Ang II–induced cardiac fibrosis and injury. Similar to our findings, these authors showed that whereas γδ T cells were one of the smallest cardiac infiltrating T-cell populations, their secretion of IL-17A was the greatest. These unconventional T cells are particularly interesting in that they do not seem to require antigen processing and major histocompatibility complex presentation of peptide antigens. Moreover, they play an important role in the recognition of lipid antigens and may be triggered by alarm signals such as heat shock proteins. In autoimmune disease, these cells appear to be driven by self molecules that arise during inflammation. In many inflammatory processes, they appear to be the dominant early source of IL-17 with antigen-specific CD4+ TH17 cells developing later and contributing to the sustained immune response 13, 25. Further studies are needed to determine the role of γδ T cells in hypertension and whether specific pharmacological inhibition of these cells may prove beneficial in treating hypertensive end-organ damage.
In an elegant study, Amador et al. (19) demonstrated that spironolactone treatment prevented TH17 cell activation and increased numbers of anti-inflammatory T regulatory cells in rats in response to deoxycorticosterone acetate and high-salt diet (DOCA-salt). The effect of spironolactone on γδ T cells was not investigated in this study, but it does raise the question of whether the beneficial effects of spironolactone, one of the most effective drugs on the market for resistant hypertension, could be due to inhibition of the IL-17A axis. Consistent with our results, these authors showed that administration of an anti–IL-17A antibody to DOCA-salt–treated rats lowered blood pressure by ∼20 mm Hg and reduced the expression of some profibrotic and proinflammatory mediators in the heart and kidney (19). However, in this study, the authors noted no improvement in proteinuria with anti–IL-17A treatment. Our findings confirm and extend this study by using a different animal model of hypertension and examining the effects of inhibition of IL-17A, IL-17F, and the IL-17RA receptor. We found that inhibition of both IL-17A and the IL-17RA receptor significantly lowered blood pressure and end-organ damage from hypertension, including inflammation, albuminuria, and renal TGFβ levels.
The mechanism by which IL-17A promotes immune cell infiltration into the aorta or kidney is likely through the up-regulation of chemokines that attract all types of immune cells (not just IL-17–producing immune cells) into the target organ. For example, Paust et al. (26) showed that in mouse mesangial cells, IL-17A up-regulates CCL2, CCL3, and CCL20—chemokines involved in the recruitment of T cells and monocytes. We previously showed that IL-17A induces the expression of a number of chemokines such as CCL8, CXCL2, and CCL7 in human aortic smooth muscle cells (3). Thus, by blocking the effects of IL-17, we reduced overall inflammation in the aorta and kidney. However, double-negative cells were not reduced (Supplemental Figure 1). These cells are a small subset of the total immune cell population in these organs, so it is possible that we were not able to detect a decrease or there was no significant decrease in this small population of cells. Moreover, there may be a feedback mechanism in effect, by which double-negative cell numbers are maintained to compensate for the reduction in IL-17 signaling by the antibodies.
Of note, the anti–IL-17F antibody caused a modest reduction in albuminuria without a reduction in blood pressure or other parameters of renal inflammation/fibrosis. We previously showed that mice genetically deficient in IL-17F had no reduction in Ang II–induced hypertension or albuminuria (6). Moreover, we showed in that study that IL-17A, but not IL-17F, regulates the renal sodium chloride cotransporter (NCC) through a serum and glucocorticoid regulated kinase 1 (SGK1) pathway. Taken together, IL-17A signaling through the IL-17RA subunit appears to play a critical role in Ang II–induced hypertension and end-organ damage with little or no contribution of the IL-17F isoform.
Given that IL-17A and IL-17F are so closely related and signal through the same receptor complex, the reason for the differential effects of IL-17A and IL-17F in hypertension is unclear. However, there is precedence for this in other chronic inflammatory diseases. For example, in a chronic asthma model, IL-17A positively regulates asthmatic allergic responses, whereas IL-17F has a negative role. In dextran sulfate sodium–induced colitis, IL-17F–deficient mice exhibit reduced colitis, whereas IL-17A deficiency is associated with more severe disease (10). Thus, it is important to determine the precise role of these related cytokines in hypertensive models.
Our results are timely and clinically relevant because there are currently several monoclonal antibody–based drugs that are in various phases of development, testing, and FDA approval for the treatment of psoriasis and other IL-17A–mediated autoimmune diseases (reviewed in Beringer et al. [9]). Specifically, secukinumab has recently been FDA approved for psoriasis, psoriatic arthritis, and ankylosing spondylitis. Brodalumab (a human monoclonal anti–IL-17RA antibody) showed promising results in Phase III clinical trials for psoriasis. For our study, we used a murine anti–IL-17RA antibody (a generous gift from Amgen). Interestingly, there is strong epidemiological evidence for a link between psoriasis and hypertension 27, 28, 29, which may be due to a shared pathophysiology of increased IL-17A signaling and inflammation. Psoriasis patients tend to have more difficult-to-control hypertension compared with their non-psoriatic counterparts (27). Through a population-based study in the United Kingdom, Takeshita et al. (29) found that among patients with hypertension, psoriasis is associated with a greater likelihood of uncontrolled hypertension.
Study limitations
One limitation of this study is that we only utilized a mouse model of Ang II-induced hypertension. Further studies are needed to determine whether our results are applicable to other animal models of hypertension and ultimately to human hypertension. Another limitation is that we did not have a vehicle infused (normotensive) control group for our flow cytometry experiments. Thus, while we observed a reduction in renal and vascular inflammation in mice treated with anti-IL-17A and anti-IL-17RA antibodies during the last two weeks of a 4- week Ang II infusion protocol, we do not know if the levels of inflammation in these tissues returned to baseline. Finally, we only tested one dose, frequency, and duration of antibody administration. Thus, it is unknown whether alternative doses, frequency, and treatment durations would result in greater reductions in blood pressure and end-organ damage.
Conclusions
Our results suggest that anti–IL-17A/RA–based therapies for psoriasis could have a dual benefit by also reducing hypertension severity and cardiovascular risk. Moreover, the benefit of these IL-17A/RA inhibitors could apply to the general hypertensive population as well. Of course, the risk-to-benefit ratio of these drugs needs to be determined in a cardiovascular disease cohort. There is an increased risk of mild-to-moderate infections in patients treated with IL-17 inhibitors. In addition, it should be noted that in a few experimental models of renal injury, IL17 inhibition has been shown to exacerbate disease 30, 31, 32. Nevertheless, our provocative data support the use of IL-17A/RA inhibitors in pilot proof-of-principle clinical trials in humans for the treatment of hypertension and its devastating complications.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The major T-cell sources of IL-17A and IL-17F in the kidney and vasculature in response to angiotensin II–induced hypertension are specialized subsets of innate-like γδ T cells and CD4+ TH17 cells. Monoclonal antibodies to IL-17A or the IL-17RA receptor subunit lower blood pressure, attenuate renal/vascular inflammation, and decrease markers of renal injury and fibrosis in a mouse model of hypertension.
TRANSLATIONAL OUTLOOK: Monoclonal antibodies to IL-17 isoforms and the IL-17 receptor are in various phases of development, clinical testing, and FDA approval for the treatment of psoriasis and related autoimmune disorders. There is a strong epidemiological link between psoriasis and hypertension severity. Thus, this new class of drugs could have an added benefit of reducing blood pressure and end-organ damage in psoriasis patients. Importantly, our results suggest that these drugs could also have a benefit in the management of hypertension in the general population. Thus it is imperative that small clinical trials and eventually larger randomized studies are conducted to determine the safety and efficacy of these drugs in human hypertension.
Footnotes
This work was supported by an American Heart Association Fellowship Award (14POST20420025) to Dr. Saleh, a training grant from the National Institutes of Health (NIH T32 HL069765-11A1) and an NIH NRSA Award (F31 HL127986) to Dr. Norlander, and an NIH K08 award (HL121671) to Dr. Madhur. Amgen provided some reagents used in this study. Dr. Madhur has received a research grant from Gilead Sciences, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
References
- 1.Harrison D.G., Marvar P.J., Titze J.M. Vascular inflammatory cells in hypertension. Front Physio. 2012;3:128. doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMaster W.G., Kirabo A., Madhur M.S., Harrison D.G. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhur M.S., Lob H.E., McCann L.A. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen H., Chiasson V.L., Chatterjee P., Kopriva S.E., Young K.J., Mitchell B.M. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamat N.V., Thabet S.R., Xiao L. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma-/- and interleukin-17A-/- mice. Hypertension. 2015;65:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norlander A.E., Saleh M.A., Kamat N.V. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension. 2016;68:167–174. doi: 10.1161/HYPERTENSIONAHA.116.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal S., Gurney A.L. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 8.Wright J.F., Bennett F., Li B. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol. 2008;181:2799–2805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 9.Beringer A., Noack M., Miossec P. IL-17 in chronic inflammation: from discovery to targeting. Trends Mol Med. 2016;22:230–241. doi: 10.1016/j.molmed.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Yang X.O., Chang S.H., Park H. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu S., Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y., Usui T., Kobayashi S. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60:2294–2303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- 13.Roark C.L., Simonian P.L., Fontenot A.P., Born W.K., O'Brien R.L. Gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Wu Y., Zhang C. gammadeltaT Cell-derived interleukin-17A via an interleukin-1beta-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension. 2014;64:305–314. doi: 10.1161/HYPERTENSIONAHA.113.02604. [DOI] [PubMed] [Google Scholar]
- 15.Wu C., Yosef N., Thalhamer T. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinewietfeld M., Manzel A., Titze J. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan A.J., Alikhan M.A., Odobasic D. Innate IL-17A-producing leukocytes promote acute kidney injury via inflammasome and Toll-like receptor activation. Am J Pathol. 2014;184:1411–1418. doi: 10.1016/j.ajpath.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Cheng X., Taleb S., Wang J. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011;215:471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Amador C.A., Barrientos V., Pena J. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. 2014;63:797–803. doi: 10.1161/HYPERTENSIONAHA.113.02883. [DOI] [PubMed] [Google Scholar]
- 20.Madhur M.S., Funt S.A., Li L. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saleh M.A., McMaster W.G., Wu J. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest. 2015;125:1189–1202. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shooshtari P., Fortuno E.S., 3rd, Blimkie D. Correlation analysis of intracellular and secreted cytokines via the generalized integrated mean fluorescence intensity. Cytometry A. 2010;77:873–880. doi: 10.1002/cyto.a.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branton M.H., Kopp J.B. TGF-beta and fibrosis. Microbes Infect. 1999;1:1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 24.Yao W., Sun Y., Wang X., Niu K. Elevated serum level of interleukin 17 in a population with prehypertension. J Clin Hypertens (Greenwich) 2015;17:770–774. doi: 10.1111/jch.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzik T.J., Mikolajczyk T. In search of the T cell involved in hypertension and target organ damage. Hypertension. 2014;64:224–226. doi: 10.1161/HYPERTENSIONAHA.114.03340. [DOI] [PubMed] [Google Scholar]
- 26.Paust H.J., Turner J.E., Steinmetz O.M. The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol. 2009;20:969–979. doi: 10.1681/ASN.2008050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong A.W., Lin S.W., Chambers C.J., Sockolov M.E., Chin D.L. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:e18227. doi: 10.1371/journal.pone.0018227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernste F.C., Sanchez-Menendez M., Wilton K.M., Crowson C.S., Matteson E.L., Maradit Kremers H. Cardiovascular risk profile at the onset of psoriatic arthritis: a population-based cohort study. Arthritis Care Res (Hoboken) 2015;67:1015–1021. doi: 10.1002/acr.22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeshita J., Wang S., Shin D.B. Effect of psoriasis severity on hypertension control: a population-based study in the United Kingdom. JAMA Dermatol. 2015;151:161–169. doi: 10.1001/jamadermatol.2014.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebs C.F., Lange S., Niemann G. Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate+angiotensin II-induced hypertension. Hypertension. 2014;63:565–571. doi: 10.1161/HYPERTENSIONAHA.113.02620. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed R., Jayakumar C., Chen F. Low-dose IL-17 therapy prevents and reverses diabetic nephropathy, metabolic syndrome, and associated organ fibrosis. J Am Soc Nephrol. 2016;27:745–765. doi: 10.1681/ASN.2014111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamour S., Gan P.Y., Pepper R. Local IL-17 production exerts a protective role in murine experimental glomerulonephritis. PLoS One. 2015;10:e0136238. doi: 10.1371/journal.pone.0136238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.