Abstract
Asthma phenotypes based on age-of-onset may be differently influenced by the interaction between variation in toll-like receptor (TLR)/CD14 genes and environmental microbes. We examined the associations between single-nucleotide polymorphisms (SNP) in the TLR/CD14 genes and asthma, and their interaction with proxies of microbial exposure (childhood farm exposure and childhood rural environment). Ten SNPs in four genes (TLR2, TLR4, TLR6, CD14) were genotyped for 1,116 participants from the Tasmanian Longitudinal Health Study (TAHS). Using prospectively collected information, asthma was classified as never, early- (before 13 years) or late-onset (after 13 years). Information on childhood farm exposure/childhood rural environment was collected at baseline. Those with early-onset asthma were more likely to be males, had a family history of allergy and a personal history of childhood atopy. We found significant interaction between TLR6 SNPs and childhood farm exposure. For those with childhood farm exposure, carriers of the TLR6-rs1039559 T-allele (p-interaction = 0.009) and TLR6-rs5743810 C-allele (p-interaction = 0.02) were associated with lower risk of early-onset asthma. We suggest the findings to be interpreted as hypothesis-generating as the interaction effect did not withstand correction for multiple testing. In this large, population-based longitudinal study, we found that the risk of early- and late-onset asthma is differently influenced by the interaction between childhood farming exposure and genetic variations.
Farm and rural environment are associated with high load of microbial compounds from gram-negative and gram-positive bacteria and fungi1 and is thought to reduce the risk of asthma through modulation of the immune system. High exposure to microbial compounds such as lipopolysaccharide (LPS)/endotoxin, lipopeptide and muramic acid may deviate the Th1/Th2 immune balance by activating the Th1 response that in turn suppresses the Th2 response2. However, in a low microbe environment, the immune response is redirected to favour a Th2 response, therefore predisposing the host to allergic diseases3. These microbial compounds initiate a response through interaction with innate immunity receptors such as the Toll-like receptors (TLRs) and CD144,5.
The TLRs and CD14 receptors are pattern recognition receptors (PRR) found on the surface of macrophages and dendritic cells6. The CD14 is also found in a soluble form in the serum and confers LPS specificity to cells lacking CD14 receptors on the surface7. Recognition of microbial products by TLRs and CD14 induces production and release of Th1 proinflammatory cytokines such as IL-1 and TNF-α8. As TLRs and CD14 were heavily involved in the recognition of microbial compounds, genetic variation of these receptors can have a substantial effect on Th1/Th2 immune balance that directly influence the risk of asthma. Single nucleotide polymorphisms (SNPs) in the TLRs and CD14 genes have been associated with a change in amino acid sequence leading to a change in protein structure9, the level of gene expression4 and serum IgE levels10. Despite evidence of a possible biological mechanism by which genetic variations in these SNPs may influence asthma risk, studies examining the association between the SNPs in the TLRs and CD14 genes and asthma risk have been inconclusive11.
Given the complex interaction between TLR/CD14 genes and the environment, studies that have looked at either factor individually have produced inconsistent results. We recently conducted a systematic review of gene-environmental interactions for the CD14 gene and markers of microbial exposure. We found that high endotoxin exposure in early-life was associated with a lower risk of atopy in children with the CC genotype of the CD14-rs2569190 SNP12. Our review also identified a clear differential pattern of association based on childhood versus adult disease. The majority of gene-environmental interaction studies have focused on intermediate phenotypes of allergic disease such as IgE level and atopy with few studies in asthma13,14 and none have explored different phenotypes of asthma based on age. Refinement of asthma phenotype is critical to the examination of gene-environmental interactions as different asthma phenotypes such as those based on age of onset, are known to have distinct characteristics15.
Based on their importance in the immune response to microbial exposure we aimed to investigated the gene-environmental interaction between ten SNPs in the TLR2 (rs4696480, rs1898830, rs3804100), TLR4 (rs1927911, rs46986790), TLR6 (rs1039559, rs5743810) and CD14 (rs2915863, rs5744455, rs2569190) genes and exposure to childhood farm exposure or childhood rural environment on the risk of early-onset asthma or late-onset asthma. The selection of SNPs were based on previous candidate gene studies that had examined the relationship with either asthma, hay fever or atopic sensitisation (Table S1). The selection of CD14 rs2569190, rs5744455 and rs2915863 SNPs were based on their relationship with asthma or wheeze, sensitisation or hay fever12. The TLR6 rs5743810 and rs1039559 SNPs was selected because they have been shown to be associated with hay fever and sensitisation in three large GWAS studies16,17,18. The SNPs from the TLR2 and TLR4 were selected based on the associations shown with asthma and atopy, and their effect modification by farming exposure in a previous study19.
We utilised data from the Tasmanian Longitudinal Health Study (TAHS)20, a population-based cohort followed from childhood to adulthood to examine the associations. We investigated if exposure to a farming environment or living in a rural area in childhood would modify the association.
Results
Characteristics of the study population
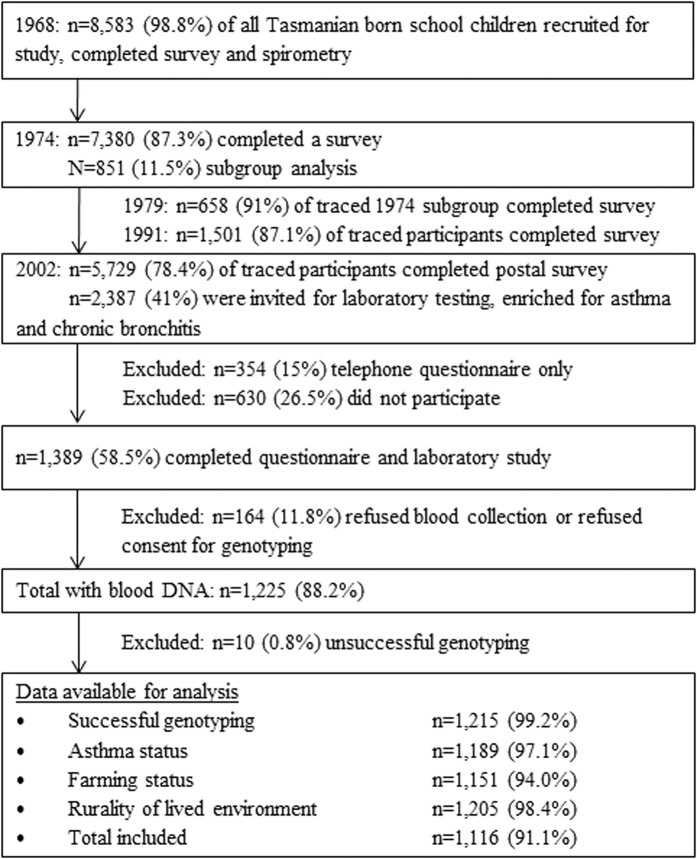
A total of 1,116 participants had both genotyping and data collected asthma and early-life environmental exposures and were included in this analysis (Fig. 1). General characteristics of participants who were included in this study is shown in Table 1. About forty-eight percent of the participants (n = 534) had early-onset asthma, 21.8% (n = 243) had late-onset asthma and 30.4% (n = 339) had never reported asthma over their lifetime. The median age of asthma for early-onset asthma was 3 years (interquartile range [IQR] 1–5) and 30 years (IQR 25–38) for late-onset asthma. Those with early-onset asthma were more likely to be males, had a history of maternal and paternal asthma and/or hay fever and a personal history of hay fever and eczema at age 7 years. Those with late-onset asthma were more likely to have current asthma and were using asthma medication (inhaled corticosteroid) at the 2002 follow-up.
Figure 1. Study design of the TAHS with data available for this study.
Adapted from Matheson et al. Cohort Profile: The Tasmanian Longitudinal Health Study (TAHS), 201620. The differences in the data available for analysis is due to missing data.
Table 1. General characteristics of the asthma phenotypes.
| Baseline characteristics | Never asthma n/N (%) | Early-onset asthma n/N (%) | Late-onset asthma n/N (%) |
|---|---|---|---|
| Childhood personal factors | |||
| Sex – Male | 174/356 (48.9) | 312/579 (53.9) | 106/260 (40.8) |
| Hay fever at 7 years | 32/350 (9.1) | 172/559 (30.8) | 26/256 (10.2) |
| Eczema at 7 years | 25/351 (7.1) | 149/565 (26.4) | 32/256 (12.5) |
| Parental Factors at baseline | |||
| Maternal asthma and/or hay fever | 80/350 (22.9) | 215/553 (38.9) | 64/256 (25.0) |
| Paternal asthma and/or hay fever | 62/343 (18.1) | 197/539 (36.6) | 51/248 (20.6) |
| Parental smoking | 219/338 (64.8) | 371/543 (68.3) | 159/250 (63.6) |
| Maternal age at birth of proband (years ± s.d.) | 27.7 ± 5.8 | 27.1 ± 5.9 | 27.2 ± 5.6 |
| Social class | |||
| Managers/administrators | 73/337 (21.7) | 133/541 (24.6) | 54/245 (22.0) |
| Associate professionals | 26/337 (7.7) | 52/541 (9.6) | 17/245 (6.9) |
| Tradesperson/advance clerical | 90/337 (26.7) | 163/541 (30.1) | 85/245 (34.7) |
| Intermediate production/sales/clerical | 104/337 (30.9) | 139/541 (25.7) | 65/245 (26.5) |
| Labourer/house person | 44/337 (13.1) | 54/541 (9.9) | 24/245 (9.8) |
| Adult personal factors | |||
| Current asthma at 45 years | – | 132/575 (22.9) | 148/258 (57.4) |
| Medication – current use of inhaled corticosteroid | – | 111/575 (19.3) | 85/258 (33.0) |
| Any sensitisation at 45years* | 138/349 (39.5) | 364/563 (64.7) | 156/258 (60.5) |
| Highest education | |||
| Grade 9 or below | 17/353 (4.8) | 33/574 (5.7) | 18/257 (7.0) |
| Grade 10 or 11 | 116/353 (32.9) | 146/574 (25.4) | 78/257 (30.4) |
| Grade 12 or equivalent | 35/353 (9.9) | 57/574 (9.9) | 26/257 (10.1) |
| Trades/apprenticeship | 55/353 (15.6) | 97/574 (16.9) | 33/257 (12.8) |
| Certificate of diploma | 63/353 (17.9) | 124/574 (21.6) | 58/257 (22.6) |
| University degree | 53/353 (15.0) | 81/574 (14.1) | 28/257 (10.9) |
| Higher university degree | 14/353 (4.0) | 36/574 (6.3) | 16/257 (6.2) |
*Positive skin prick test to one or more of the following allergens using standard techniques: Dermatophagoides pteronyssinus (house dust mite), cat pelt, Homodendrum, Alternaria tenuis, Penicillium mix, Aspergillus fumigatus, and mixed grasses20.
Association between proxies of microbial exposure and asthma onset
We did not find an association between childhood rural environment and early- nor late-onset asthma (Table 2). There was also no evidence of an association between childhood farm exposure and either early-onset asthma or late-onset asthma.
Table 2. The association between proxies of microbial exposures and asthma onset.
| Proxies of microbial exposures | Never asthma n/N (%) | Early-onset asthma n/N (%) | Late-onset asthma n/N (%) | Early-onset asthma vs Never asthma |
Late-onset asthma vs Never asthma |
||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value for OR | OR (95% CI) | p-value for OR | ||||
| Childhood rural environment | 125/354 (35.3) | 204/572 (35.7) | 98/257 (38.1) | 0.94 (0.69–1.27) | 0.67 | 1.04 (0.72–1.50) | 0.67 |
| Childhood farm exposure | 26/340 (7.7) | 52/545 (9.5) | 23/247 (9.3) | 1.21 (0.71–2.07) | 0.48 | 1.23 (0.67–2.25) | 0.50 |
*All analyses were adjusted for sex, maternal and paternal history of asthma and/or hay fever, parental smoking, maternal age, and childhood history of eczema and/or hay fever.
Association between TLR2, TLR4, TLR6 and CD14 polymorphisms and asthma onset
The characteristics and distributions of the genotypes for each SNP is shown in Table 3. For the genotype frequencies for each of the ten SNPs, there was no marked deviation from that expected under the HWE. The AIC and BIC scores was used to determine the best fitting genetic model for each SNP (Table S3). The association between each SNP and asthma risk was examined however we did not find any association between the SNPs and early-onset or late-onset asthma (Tables 4 and S2).
Table 3. The location, genotype distribution, Hardy-Weinberg equilibrium and minor allele frequencies of the SNPs included in this study.
| Gene | SNP | Position | Location in gene | Genotype distributiona |
HWE p-value | MAF |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| No asthma | Early-onset asthma | Late-onset asthma | No asthma | Early-onset asthma | Late-onset asthma | |||||
| TLR2 | rs4696480 | −16934 A > T | Intron | 98/173/85 | 148/298/125 | 64/131/63 | 0.60 | 0.482 | 0.479 | 0.504 |
| rs1898830 | −15607 A > G | Intron | 163/145/49 | 268/242/62 | 113/107/38 | 0.08 | 0.340 | 0.319 | 0.355 | |
| rs3804100 | +1349 T > C | Coding region | 312/42/2 | 484/80/10 | 229/29/1 | 0.65 | 0.065 | 0.087 | 0.060 | |
| TLR4 | rs1927911 | −4953 C > T | Intron | 199/130/26 | 314/218/38 | 142/99/17 | 0.48 | 0.256 | 0.258 | 0.258 |
| rs4986790 | +295 A > G | Exon | 321/34/0 | 517/53/2 | 241/16/1 | 0.34 | 0.048 | 0.050 | 0.035 | |
| TLR6 | rs1039559 | −502 T > C | Promoter | 89/183/74 | 133/208/156 | 69/121/67 | 0.28 | 0.478 | 0.532 | 0.496 |
| rs5743810 | +744 C > T | Exon | 106/192/55 | 165/280/130 | 89/116/53 | 0.06 | 0.428 | 0.470 | 0.430 | |
| CD14 | rs2915863 | −1721 T > C | Promoter | 137/154/66 | 191/286/96 | 89/135/35 | 0.06 | 0.401 | 0.417 | 0.396 |
| rs5744455 | −651 C > T | Promoter | 204/130/22 | 339/205/27 | 140/109/8 | 0.88 | 0.244 | 0.227 | 0.243 | |
| rs2569190 | −260 G > A | Promoter | 107/166/83 | 148/286/138 | 64/139/54 | 0.24 | 0.466 | 0.491 | 0.481 | |
aHomozygous major allele/heterozygous/homozygous minor allele; HWE: Hardy-Weinberg equilibrium; MAF: Minor allele frequency.
Table 4. Association between TLR2, TLR4, TLR6 and CD14 SNPs for genetic models with the lowest AIC and BIC scores and early- and late-asthma.
| Gene | SNP | Genetic model type | Genotype | Early-onset asthma vs never asthma | Late-onset asthma vs never asthma |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||||
| TLR2 | rs1898830 | Additive | A allele | ref | ref |
| Per G allele | 0.96 (0.78–1.19) | 1.12 (0.87–1.42) | |||
| rs3804100 | Dominant | TT | ref | ref | |
| CT/CC | 1.25 (0.82–1.93) | 0.88 (0.52–1.48) | |||
| rs4969480 | Dominant | AA | ref | ref | |
| AT/TT | 1.12 (0.81–1.56) | 1.17 (0.80–1.71) | |||
| TLR4 | rs1927911 | Recessive | CC/CT | ref | ref |
| TT | 0.80 (0.45–1.45) | 0.87 (0.45–1.69) | |||
| rs4986790 | Dominant | AA | ref | ref | |
| AG/GG | 1.23 (0.75–2.04) | 0.76 (0.40–1.44) | |||
| TLR6 | rs1039559 | Dominant | CC | ref | ref |
| CT/TT | 0.79 (0.56–1.12) | 0.78 (0.53–1.16) | |||
| rs5743810 | Dominant | TT | ref | ref | |
| CT/CC | 0.75 (0.51–1.10) | 0.73 (0.47–1.12) | |||
| CD14 | rs2569190 | Recessive | AA/AG | ref | ref |
| GG | 0.80 (0.58–1.10) | 0.70 (0.48–1.02) | |||
| rs5744455 | Dominant | CC | ref | ref | |
| CT/TT | 0.90 (0.67–1.21) | 1.06 (0.76–1.48) | |||
| rs2915863 | Dominant | TT | ref | ref | |
| CT/CC | 1.13 (0.77–1.67) | 1.29 (0.81–2.05) |
All adjusted for sex, maternal and paternal history of asthma and/or hay fever, and atopy at 7 years.
Interactions between genes and environment
We investigated whether childhood farm exposure or childhood rural environment modified the association between the TLR and CD14 SNPs and risk of early- and late-onset asthma. There was strong evidence for interactions between the TLR6-rs1039559 and TLR6-rs5743810 SNPs and childhood farm exposure for early-onset asthma (p = 0.009 and p = 0.02 respectively, Tables 5 and S5). For those with childhood farm exposure, the TLR6-rs1039559 T-allele was associated with a markedly reduced risk of early-onset asthma (additive genetic model for the T-allele OR = 0.34, 95%CI 0.16–0.73), but not for those without childhood farm exposure. Again in those with childhood farm exposure having the TLR6-rs5743810 C-allele was associated with a lower risk of early-onset asthma (additive genetic model for the C-allele OR = 0.41, 95%CI 0.19–0.86). No interactions were observed for those with late-onset asthma (Tables 5 and S4).
Table 5. Association between TLR6 polymorphisms in an additive genetic model and childhood farm exposure on early-onset and late-onset asthma.
| TLR6 SNP | Early-onset asthma vs. never asthma |
Late-onset asthma vs. never asthma |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No childhood farm exposure | Childhood farm exposure | No childhood farm exposure | Childhood farm exposure | p-interaction between SNP and farm exposure | No childhood farm exposure | Childhood farm exposure | No childhood farm exposure | Childhood farm exposure | p-interaction between SNP and farm exposure | |
| n/N | n/N | OR (95% CI) | OR (95% CI) | n/N | n/N | OR (95% CI) | OR (95% CI) | |||
| rs1039559 | ||||||||||
| C allele | 126/483 | 22/53 | ref | ref | 0.009 | 58/221 | 4/23 | ref | ref | 0.38 |
| Per T allele | 357/483* | 31/53* | 0.79 (0.44–1.41) | 0.34 (0.16–0.73) | 163/221* | 19/23* | 0.77 (0.40–1.47) | 0.68 (0.30–1.56) | ||
| rs5743810 | ||||||||||
| T allele | 52/311 | 3/26 | ref | ref | 0.02 | 102/488 | 21/53 | ref | ref | 0.52 |
| Per C allele | 259/311# | 23/26# | 0.77 (0.43–1.36) | 0.41 (0.19–0.86) | 386/488# | 32/53# | 0.76 (0.40–1.46) | 0.77 (0.34–1.74) | ||
All models were adjusted for sex, maternal and paternal history of asthma and/or hay fever, and personal history of atopy at baseline.
*The number represents those with one or two T-alleles.
#The number represents those with one or two C-alleles.
There was no evidence for an interaction between TLR6 polymorphisms and childhood rural environment for either early- or late-onset asthma (data not shown). Nor was there any evidence for an interaction between any of the SNPs and either childhood farm exposure or childhood rural environment in relation to the risk of early- or late- onset asthma (Table S5).
Reanalyses of the interaction effects in this study did not withstand correction for multiple testing (data not shown). The p-values for the analyses are presented without adjustment and the results should be viewed as hypothesis-generating rather than proof of a causal association.
Sensitivity analysis for significant interactions
We performed a sensitivity analysis that further refined early-onset asthma phenotypes into early-onset transient asthma and early-onset persistent asthma. Specifically, we examined the interaction between TLR6 SNPs and childhood farm exposure for these early-onset asthma phenotypes and found comparable findings for both phenotypes. The findings for each groups were comparable to the combined early-onset asthma group (Table S6). Those with the TLR6-rs1039559 T-allele with childhood farm exposure had lower risk of early-onset transient and early-onset persistent asthma compared to those without childhood farm exposure. The findings were similar for the TLR6-rs5743810 whereby those with the C-allele and childhood farm exposure had reduced risk of early-onset transient asthma and early-onset persistent asthma. We then removed individuals who had asthma before age 5 years and repeated the analysis of our main findings (Table S7). The findings for the interaction between TLR6 and childhood farm exposure for asthma were strongly comparable to the findings with all participants included.
Discussion
Using a longitudinal cohort that has been followed from childhood (age 7 years) into adult-life (age 45 years when this clinical study was conducted), we identified an effect modification by farm exposure in childhood on the association between TLR6 SNPs and asthma phenotypes based on age of onset. Being exposed to a farm environment in childhood was protective against early-onset asthma for those with TLR6-rs1039559 T-allele and TLR6-rs5743810 C-allele, showing a clear difference in the pattern of interaction for early- and late-onset asthma. We did not observe any association between TLR2, TLR4 or CD14 SNPs and asthma onset, nor was there any modification of these relationships by childhood farm exposure/childhood rural environment.
The TLR6 is located on chromosome 4p14, a region that was consistently associated with atopy in three GWAS16,17,18. Early-onset asthma is more likely to be attributable to atopy15 and therefore it is not surprising to observe some evidence for the association between the two TLR6 SNPs and early-onset asthma but not late-onset asthma in our study. In our data, early-onset asthma were more likely to have a history of atopy in childhood than those with late-onset asthma. Only about 22% of those with early-onset asthma had adult current asthma whereas about 59% of late-onset asthma had adult current asthma by age 44 years. We have also shown previously that early- and late-onset asthma exhibit different clinical characteristics in our study21. Our findings were also consistent with several other studies that have also found some evidence of an association between TLR6-rs5743810 T-allele and atopic asthma9, allergic rhinitis22 and allergic sensitisation23 in children.
We are aware of only one previous study that examined the interaction between TLR6 SNPs and proxies of microbial exposure and allergic disease outcomes23. In contrast to our finding of a lower risk of asthma in individuals with the TLR6-rs1039559 T-allele and early farm exposure, their study found higher IgE levels at age two years in individuals with the TLR6-rs1039559 CC-genotype and exposure to two or more older siblings. The inconsistency with our findings may be due to the difference between our proxy measures of microbial exposure (farm exposure and rural environment) and that used by Reijmerink et al. (siblings). Reijmerink et al. finding with regard to exposure to siblings leading to higher IgE is in contrast to most existing literature.
There is some evidence from in vitro studies of a variable response to bacterial cell products with the TLR6-rs5743810 SNP. A polymorphism of C to T in the TLR6-rs5743810 resulted in a change in amino acid from proline to serine at position 249 in the extracellular domain of the receptor. Although the effect on the structure is still unknown, studies using peripheral blood cells shown that the TLR6-rs5743810 C-allele showed greater NF-κB signalling activity in response to stimulation by lipopeptide when compared to the TLR6-rs5743810 T-allele24. The higher NF-κB signalling in turn resulted in higher TLR6 mRNA gene expression and higher IFN-γ levels, thereby promoting greater Th1-immunity with a matching reduction of Th2-immunity9. This mechanism could explain the observed reduced risk of asthma in those with the TLR6-rs5743810 C-allele who were exposed to a farm environment in our study.
We did not find any evidence of modification by childhood farm exposure or childhood rural environment on the relationship between any of the CD14 SNPs and asthma risk. This is consistent with a case-control study of adults that found no association with the CD14-rs2569190 SNPs and asthma for those who lived in the country in childhood13. A systematic review of GxE in the CD14 gene showed consistent evidence of a decreased risk of allergic sensitisation in childhood when exposed to high levels of endotoxin in carriers of the CD14-rs2569190 CC-genotype12. Importantly this consistent effect was only seen for atopy assessed in childhood, and no consistent effect was observed for outcomes other than allergic sensitisation. Unfortunately we did not have an objective marker of microbial exposure such as endotoxin levels in the current study to fully examine the relationship between CD14 and microbial exposure and asthma risk.
At present, any effect of the TLR2 and TLR4 gene polymorphisms on asthma is still largely undetermined. A recent meta-analysis showed no evidence of association between TLR4-rs4986790 and asthma25. For TLR2, two studies found a reduced risk of asthma in children with A-alleles of the rs4696480 SNP19,26. Another study found a reduced risk of doctor-diagnosed asthma in adults with a T-allele of the TLR2-rs4696480 SNP27. Two other studies, however, reported no association between asthma risk and TLR2-rs4696480 in children9 and adult28. We found no evidence of association between TLR2-rs4696480 and asthma, and the association was not modified by childhood farm exposure or childhood rural environment. The inconsistency in the findings across studies may be due to variation in the definition of asthma across studies.
Farm exposure is commonly used as a proxy for microbial exposure1 and has been consistently reported as protective against atopy and asthma, albeit mainly in cross-sectional studies29. We did not find any association between childhood farm exposure and asthma per se in the current analysis. Past studies of childhood farming exposure have used a variety of ways to define “farming exposure” including “growing up on a farm/farm residence”30, “physical contact with farm animals in childhood”31, “regular consumption of unpasteurised farm milk”14 and “parental farming occupation”32. Of these, “farm residence” and “parental farming occupation” have been most utilised in epidemiological studies29. We used father’s reported occupation when the child started school to define exposure. Since information on father’s occupation was collected prior to parental report of asthma status in the child, any misclassification of true exposure to a “farm environment” is likely to be non-differential. We did not find an association between childhood rural environment and early-onset and late-onset asthma. Classification of childhood rural environment was based on the postcode of the school that the child attended in year 1968, and hence, it may not capture the true effect of microbial exposures.
Strengths of our study include the large cohort of individuals and the prospective data collection enabling us to accurately classify participants into different asthma phenotypes based on age of onset of disease and also to define the proxies of microbial exposure prior to the baseline assessment of asthma. The use of self/parental-reported asthma is a limitation as it is likely to be less reliable than a physician’s diagnosis. However our definitions of self/parent-reported asthma has been validated against a definition that included bronchial hyper-responsiveness33.
In conclusion, we have shown for the first time that farming exposure in early-life modified the relationship between polymorphisms in the TLR6 gene and asthma that started in early life. Our finding may provide some explanation to the lack of consistent evidence in genetic and gene by environment interaction studies especially when asthma phenotypes based on age of onset were not clearly defined. Replication of our finding using longitudinal studies with repeated measurements of objective markers such as endotoxin and distinct phenotypes of asthma are needed to fully explore these relationships. Our work has provided some indication of a possible mechanism in which microbial products may interaction with TLR in a well-defined asthma group.
Methods
Study design – Tasmanian Longitudinal Health Study (TAHS)
The design of the TAHS, as well as the prevalence of asthma at baseline and follow-up, has been reported elsewhere20. In brief, the TAHS commenced in 1968 with recruitment of all school children in Tasmania born in the year 1961 and 8,583 (99% of all eligible subjects) were enrolled. These children (probands) underwent clinical examinations including lung function measurement, and parents completed a respiratory health survey for each child. The parents also completed separate surveys for themselves. Follow-up surveys were completed in 1974, 1981 and 1992 at the ages of 13, 20 and 31 years, respectively.
The 2002 follow-up survey traced 7,380 (87.31%) of the original 1968 cohort to an address34 and achieved a response from 5,729 (78.4%) to a postal survey. A subgroup of these respondents, enriched for cases of asthma or cough reported in childhood or adulthood, were invited to participate in a more detailed laboratory study. Of the 2,387 invited, 1,389 (58.5%) took part in a full laboratory visit, 354 (15%) completed a telephone questionnaire or laboratory visit only, and 630 (26.5%) withdrew. This analysis was based on those who had successful genotyping (n = 1,215). Of those who provided their blood sample for genotyping, majority of them (97.6%) were born in Tasmania (93.4%) or other states in Australia (4.2%) and are of Anglo-Celtic ancestry.
Ethics
This study was conducted in accordance with the amended Declaration of Helsinki and approved by the Human Ethics Review Committees at The Universities of Melbourne (approval number 040375), Tasmania (040375.1) and New South Wales (08094), the Alfred Hospital (1118/04), and Royal Brisbane & Women’s Hospital Health Service District (2006/037). Informed written consent was obtained from all study participants at each follow-up.
Early- and Late-onset Asthma
Asthma was defined by an affirmative response to the question: “Has he/she at any time in his/her life suffered from attacks of asthma or wheezy breathing?” This question was asked in all follow-up surveys and in the 1968 and 1974 studies the parents answered on behalf of their children, but in 1992 and 2004 the participants answered for themselves. Participants who reported asthma in the 1968 or 1974 surveys were classified as having “Early-onset asthma” and asthma reported in the 1992 or 2004 surveys was classified as “Late-onset asthma”. The reference group was those who did not report asthma at any survey. The cut-off for early onset was set at the age of 13 years which has been consistently used in previous studies15 and we have shown some differences in characteristics within the TAHS using this cut-off 21.
Definitions of Exposure
Two proxy measures of exposure to a farming or rural environment in our study:
“Childhood farm exposure” was defined from the father’s occupation obtained from the school medical record completed by the parents before the child commenced primary school when aged 5 years. Occupations were coded using the Australian Standard Classification of Occupations (ASCO) four-digit classification35 and codes 1311–1314, 4611–4614, and 9211 were defined as “Farmer”.
“Childhood rural environment” was measured by residential remoteness using the postcode of the school that the child attended in 1968 and was based on the Australian Standard Geographical Classification (ASGC)36. “Rural environment” was defined as those in groups ‘outer regional Australia’, ‘remote Australia’ and ‘very remote Australia’.
Definitions of baseline characteristics
‘Maternal asthma and/or hay fever’ and ‘paternal asthma and/or hay fever’ were defined by a parent having asthma and/or hay fever from the 1968 survey. ‘Parental smoking’ was defined as probands with either or both parents who reported a history of smoking in the 1968 survey. Childhood eczema and hay fever was reported by parents at the 1968 survey.
Genotyping
DNA samples were isolated from whole blood samples obtained from each participant. Genotyping was performed for the following genes: TLR2 (rs4696480, rs1898830, rs3804100), TLR4 (rs1927911, rs46986790), TLR6 (rs1039559, rs5743810) and CD14 (rs2915863, rs5744455, rs2569190). The selection of SNPs were based on previous candidate gene studies that had examined the relationship with either asthma, hay fever or atopic sensitisation12,16,19,37. Genotyping was performed using Taq-Man allelic discrimination 5′nuclease assays (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. Fluorescence was measured by an ABI7900HT Fast Real-Time PCR System (Perkin-Elmer, Waltham, MA, USA).
Sensitivity analysis
To further explore other phenotypes within early-onset asthma, we have categorised early-onset asthma into transient early-onset asthma and persistent early-onset asthma. Individuals with early-onset asthma who also reported having asthma at age 45 years was categorised into early-onset persistent asthma while individuals who did not have asthma at age 45 years was group into early-onset transient asthma. Asthma under 5 years of age may not be true asthma and hence we repeated our main analysis by excluding participants who had their first asthma attack before the age of 5 years.
Statistical analyses
All analyses were performed using STATA ver13.1 (StataCorp LP, College Station, TX). Deviation from Hardy-Weinberg equilibrium was tested using a Pearson chi-squared test in the reference group (never asthma).
The association between proxies of microbial exposure or SNPs and early-onset/late-onset asthma was assessed using multinomial logistic regression. All analyses were adjusted for sex, maternal and paternal asthma and/or hay fever at baseline, and atopy at age 7 years.
The association between the SNP and asthma outcomes was examined using all four types of genetic models (dominant, recessive, additive and codominant). To determine the best fitting genetic model for the interaction analysis, we compared the Akaike’s information criteria (AIC) and Bayesian information criteria (BIC) for each genetic model for each SNP (Table S3). The model with the lowest AIC and BIC scores was selected and presented in the manuscript. The interaction between SNPs and childhood farm exposure or childhood rural environment was examined using a multinomial logistic regression while adjusting for confounders. The interaction was examined by comparing each model with the interaction term to the same model without an interaction term using a likelihood ratio test. A p-value of less than 0.1 from the likelihood ratio test indicates that the models were different and the estimates for the interaction in each subgroup were presented. The p-value from the interaction odds ratio was used to determine the significance of interaction term. For sensitivity analysis, we have only analysed models with significant interaction. The association was analysed in the same manner using multinomial logistic regression.
Additional Information
How to cite this article: Lau, M. Y. Z. et al. The interaction between farming/rural environment and TLR2, TLR4, TLR6 and CD14 genetic polymorphisms in relation to early- and late-onset asthma. Sci. Rep. 7, 43681; doi: 10.1038/srep43681 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank the TAHS participants and all previous investigators of the TAHS. We acknowledge the Tasmanian State Achives for providing access to the school medical records and all the respiratory scientists who collected laboratory data in Tasmania, Victoria, Queensland and New South Wales. All participant blood samples were processed and DNA was extracted in the Genetic Epidemiology Laboratory (southeylab.org), The University of Melbourne. This study was funded by the National Health and Medical Research Council of Australia (NHMRC). M.Y.Z. Lau is supported by Marjory Edwards OAM PhD Scholarship from the Asthma Australia. M.C. Matheson, S.C. Dharmage, A. J. Lowe, J.A. Burgess, and A. K. Win are supported by the NHMRC.
Footnotes
Michael Abramson holds investigator initiated grants for unrelated research from Pfizer and Boehringer-Ingelheim. He undertook an unrelated consultancy for AstraZeneca. He has received assistance with conference attendance from Boehringer-Ingelheim and Sanofi.
Author Contributions Study concept and design: M.Y.Z.L., S.C.D., M.C.M. Acquisition of data: S.C.D., J.H., P.S.T., S.C.M., G.G.G., J.L.H., M.J.A., E.H.W., M.C.M. Statistical analysis and interpretation of data: M.Y.Z.L., A.K.W., S.C.D., M.C.M. Drafting of the manuscript: M.Y.Z.L., S.C.D., J.A.B., A.K.W., M.C.M. Critical revision of the manuscript for important intellectual content: M.Y.Z.L., S.C.D., J.A.B., J.H., P.S.T., S.C.M., G.G.G., J.L.H., M.J.A., E.H.W., M.C.M., A.K.W., A.J.L., C.J.L., J.P. Obtained funding: S.C.D., P.S.T., S.C.M., G.G.G., J.L.H., M.J.A., E.H.W., M.C.M. Study supervision: S.C.D., J.A.B., A.K.W., M.C.M. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- von Mutius E. & Vercelli D. Farm living: effects on childhood asthma and allergy. Nature reviews. Immunology 10, 861–868, doi: 10.1038/nri2871 (2010). [DOI] [PubMed] [Google Scholar]
- Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. The Journal of allergy and clinical immunology 113, 395–400, doi: 10.1016/j.jaci.2003.11.025 (2004). [DOI] [PubMed] [Google Scholar]
- Okada H., Kuhn C., Feillet H. & Bach J. F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clinical and experimental immunology 160, 1–9, doi: 10.1111/j.1365-2249.2010.04139.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss G. et al. Prenatal and early-life exposures alter expression of innate immunity genes: the PASTURE cohort study. The Journal of allergy and clinical immunology 130, 523–530 e529, doi: 10.1016/j.jaci.2012.05.049 (2012). [DOI] [PubMed] [Google Scholar]
- Kanagaratham C., Camateros P., Flaczyk A. & Radzioch D. Polymorphisms in TOLL-like receptor genes and their roles in allergic asthma and atopy. Recent patents on inflammation & allergy drug discovery 5, 45–56 (2011). [DOI] [PubMed] [Google Scholar]
- Hemmi H. & Akira S. TLR signalling and the function of dendritic cells. Chemical immunology and allergy 86, 120–135, doi: 10.1159/000086657 (2005). [DOI] [PubMed] [Google Scholar]
- Kim J. I. et al. Crystal structure of CD14 and its implications for lipopolysaccharide signaling. The Journal of biological chemistry 280, 11347–11351, doi: 10.1074/jbc.M414607200 (2005). [DOI] [PubMed] [Google Scholar]
- Akira S., Takeda K. & Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature immunology 2, 675–680, doi: 10.1038/90609 (2001). [DOI] [PubMed] [Google Scholar]
- Kormann M. S. et al. Toll-like receptor heterodimer variants protect from childhood asthma. The Journal of allergy and clinical immunology 122, 86–92, 92 e81-88, doi: 10.1016/j.jaci.2008.04.039 (2008). [DOI] [PubMed] [Google Scholar]
- Leung T. F. et al. The C-159T polymorphism in the CD14 promoter is associated with serum total IgE concentration in atopic Chinese children. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology 14, 255–260 (2003). [DOI] [PubMed] [Google Scholar]
- Klaassen E. M., Thonissen B. E., van Eys G., Dompeling E. & Jobsis Q. A systematic review of CD14 and toll-like receptors in relation to asthma in Caucasian children. Allergy, asthma, and clinical immunology: official journal of the Canadian Society of Allergy and Clinical Immunology 9, 10, doi: 10.1186/1710-1492-9-10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M. Y. et al. CD14 polymorphisms, microbial exposure and allergic diseases: a systematic review of gene-environment interactions. Allergy 69, 1440–1453, doi: 10.1111/all.12454 (2014). [DOI] [PubMed] [Google Scholar]
- Smit L. A. et al. CD14 and toll-like receptor gene polymorphisms, country living, and asthma in adults. American journal of respiratory and critical care medicine 179, 363–368, doi: 10.1164/rccm.200810-1533OC (2009). [DOI] [PubMed] [Google Scholar]
- Bieli C. et al. A polymorphism in CD14 modifies the effect of farm milk consumption on allergic diseases and CD14 gene expression. The Journal of allergy and clinical immunology 120, 1308–1315, doi: 10.1016/j.jaci.2007.07.034 (2007). [DOI] [PubMed] [Google Scholar]
- Tan D. J. et al. Age-of-asthma onset as a determinant of different asthma phenotypes in adults: a systematic review and meta-analysis of the literature. Expert review of respiratory medicine 9, 109–123, doi: 10.1586/17476348.2015.1000311 (2015). [DOI] [PubMed] [Google Scholar]
- Bonnelykke K. et al. Meta-analysis of genome-wide association studies identifies ten loci influencing allergic sensitization. Nature genetics 45, 902–906, doi: 10.1038/ng.2694 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds D. A. et al. A genome-wide association meta-analysis of self-reported allergy identifies shared and allergy-specific susceptibility loci. Nature genetics 45, 907–911, doi: 10.1038/ng.2686 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson D. et al. Replication of genomewide associations with allergic sensitization and allergic rhinitis. Allergy 69, 1506–1514, doi: 10.1111/all.12495 (2014). [DOI] [PubMed] [Google Scholar]
- Eder W. et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. The Journal of allergy and clinical immunology 113, 482–488, doi: 10.1016/j.jaci.2003.12.374 (2004). [DOI] [PubMed] [Google Scholar]
- Matheson M. C. et al. Cohort Profile: The Tasmanian Longitudinal Health STUDY (TAHS). International journal of epidemiology, doi: 10.1093/ije/dyw028 (2016). [DOI] [PubMed] [Google Scholar]
- Tan D. J. et al. Clinical and functional differences between early-onset and late-onset adult asthma: a population-based Tasmanian Longitudinal Health Study. Thorax, doi: 10.1136/thoraxjnl-2015-208183 (2016). [DOI] [PubMed] [Google Scholar]
- Koponen P. et al. The association of genetic variants in toll-like receptor 2 subfamily with allergy and asthma after hospitalization for bronchiolitis in infancy. The Pediatric infectious disease journal 33, 463–466, doi: 10.1097/INF.0000000000000253 (2014). [DOI] [PubMed] [Google Scholar]
- Reijmerink N. E. et al. Toll-like receptors and microbial exposure: gene-gene and gene-environment interaction in the development of atopy. The European respiratory journal 38, 833–840, doi: 10.1183/09031936.00099210 (2011). [DOI] [PubMed] [Google Scholar]
- Shey M. S. et al. Single nucleotide polymorphisms in toll-like receptor 6 are associated with altered lipopeptide- and mycobacteria-induced interleukin-6 secretion. Genes and immunity 11, 561–572, doi: 10.1038/gene.2010.14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizaoui K., Kaabachi W., Hamzaoui K. & Hamzaoui A. Association of Single Nucleotide Polymorphisms in Toll-like Receptor Genes With Asthma Risk: A Systematic Review and Meta-analysis. Allergy, asthma & immunology research 7, 130–140, doi: 10.4168/aair.2015.7.2.130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhof M. et al. Toll-like receptor 2 and 4 genes influence susceptibility to adverse effects of traffic-related air pollution on childhood asthma. Thorax 65, 690–697, doi: 10.1136/thx.2009.119636 (2010). [DOI] [PubMed] [Google Scholar]
- Potaczek D. P. et al. An association of TLR2-16934A >T polymorphism and severity/phenotype of atopic dermatitis. Journal of the European Academy of Dermatology and Venereology: JEADV 25, 715–721, doi: 10.1111/j.1468-3083.2010.03812.x (2011). [DOI] [PubMed] [Google Scholar]
- Smit L. A. et al. Atopy and new-onset asthma in young Danish farmers and CD14, TLR2, and TLR4 genetic polymorphisms: a nested case-control study. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 37, 1602–1608, doi: 10.1111/j.1365-2222.2007.02831.x (2007). [DOI] [PubMed] [Google Scholar]
- Genuneit J. Exposure to farming environments in childhood and asthma and wheeze in rural populations: a systematic review with meta-analysis. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology 23, 509–518, doi: 10.1111/j.1399-3038.2012.01312.x (2012). [DOI] [PubMed] [Google Scholar]
- Svanes C. What has the ECRHS told us about the childhood risks of asthma, allergy and lung function? The clinical respiratory journal 2 Suppl 1, 34–44, doi: 10.1111/j.1752-699X.2008.00082.x (2008). [DOI] [PubMed] [Google Scholar]
- Riedler J. et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 358, 1129–1133, doi: 10.1016/S0140-6736(01)06252-3 (2001). [DOI] [PubMed] [Google Scholar]
- Braun-Fahrlander C. et al. Prevalence of hay fever and allergic sensitization in farmer’s children and their peers living in the same rural community. SCARPOL team. Swiss Study on Childhood Allergy and Respiratory Symptoms with Respect to Air Pollution. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 29, 28–34 (1999). [DOI] [PubMed] [Google Scholar]
- Jenkins M. A. et al. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. International journal of epidemiology 25, 609–616 (1996). [DOI] [PubMed] [Google Scholar]
- Wharton C. et al. Tracing 8,600 participants 36 years after recruitment at age seven for the Tasmanian Asthma Study. Australian and New Zealand journal of public health 30, 105–110 (2006). [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics. Australian Standard Classification of Occupations (ASCO). Australian Bureau of Statistics, Canberra Second Edition (1997).
- Australian Bureau of Statistics. Statistical geography, volume 1: Australian Standard Geographical Classification (ASGC). Australian Bureau of Statistics, Canberra (2001).
- Baldini M. et al. A Polymorphism* in the 5′ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. American journal of respiratory cell and molecular biology 20, 976–983, doi: 10.1165/ajrcmb.20.5.3494 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.