Abstract
Background:
The decrease in intraocular pressure (IOP) within exercise has been recently suggested; however, this change remained ambiguous following exercise test. The present study aimed to assess changes in IOP induced by exercise test in patients who suspected to coronary artery disease (CAD) and indicated for exercise test evaluation.
Methods:
In a cross-sectional study at the cardiovascular research center of Amin Heart Hospital in Isfahan, 101 eyes from 51 consecutive patients suspected to CAD aged 30–70 years referred for exercise testing were evaluated. IOP was measured at the three time points of before exercise test as well as 5 and 20 min after completing exercise test using Schiotz tonometer. All exercise tests were programmed by the treadmill.
Results:
The mean IOP in all assessed eyes was 16.12 ± 2.61 mmHg initially that was gradually decreased to 13.79 ± 2.40 mmHg 5 min after the exercise test, but elevated to 15.67 ± 2.26 mmHg 20 min after the test. Assessing IOP following exercise testing showed a significant decrease in IOP in 75 eyes (74.3%), remained unchanged in 19.8% of eyes, and even elevated in 5.9% of eyes. There was a significant direct association between patients' age and IOP changes assessed by the Pearson's correlation test (r = 0.350,P = 0.009). No significant difference was revealed in the trend of the changes in IOP after exercise test between men and women, between left-sided and right-sided eyes as well as between different body mass index subgroups.
Conclusion:
IOP temporarily reduced after exercise test, but return to baseline value shortly after test. This lowering is more evident in advanced aging.
Keywords: Age, exercise test, intraocular pressure
Introduction
A well-known physiological response to changes in blood pressure or vascular resistance during physical activities is an autoregulatory system that aims to keep constant local vascular blood flow by changing arteriolar blood flow.[1,2,3] One of the most important changes in blood pressure occurring within exercise is decrease in intraocular pressure (IOP) that temporarily occurs up to 2–5 mmHg within a mild exercise activity. Recent studies could show that an aerobic exercise for 3–6 months results in lowering IOP in patients with glaucoma; however, it can be returned to baseline level after 3–5 weeks of quitting exercise.[4,5] Because of the major role of ocular blood flow to regulate metabolic needs of retina, physiological reducing IOP within exercise, especially in glaucoma patients can be an important reflex response and even a therapeutic tool.[6] However, different factors may affect the IOP reducing by exercise including advanced age, sedentary lifestyle pattern, and the presence of any vascular disorder such as diabetes or cardiovascular diseases. Thus, meaningful changes are expected in IOP within exercise-based diagnostic programs such as exercise test in patients with or suspected to cardiovascular disorders.[7] Hence, the present study aimed to assess changes in IOP induced by exercise test in patients who suspected to coronary artery disease (CAD) and indicated for exercise test evaluation.
Methods
In a cross-sectional study at the cardiovascular research center of Amin Heart Hospital in Isfahan, 61 consecutive patients aged 30–70 years with chest pain with and without cardiovascular risk factors who were suspected to CAD and needing exercise test for further assessment that referred for exercise testing were enrolled in the study. Those who did not attend or refuse participating the test, had a recent history of ocular infectious disorders or under-treated with antibiotics, had previous history of any ophthalmologic surgeries, receiving any medication which influence IOP, or those who could not continue complete exercise test (achieving exercise heart rate to 80% of maximum heart rate) were all excluded from the study. All patients had well-known CAD risk factors such as positive family history of CAD, diabetes, smoking, hyperlipidemia. Thus, 51 patients (30 men and 21 women) were finally included in the study. Approval from the University Human Research Ethics Committee was obtained before the commencement of the study. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. All patients underwent an ophthalmic examination before their participation in the study. They were also interviewed to collect baseline data regarding demographic characteristics, medical history, and medications. All study population were asked to discontinue using beta blockers, Ca-blockers, and nitrates in 48 h before exercise test to prevent any effect on result of test. IOP was measured at the three time points of before exercise test as well as 5 and 20 min after completing exercise test using Schiotz tonometer (Hako system, Germany). All ocular measures were conducted on 101 left and right eyes (one right-sided eye in a man was excluded because of the existence of a rigid lens). All exercise tests were programmed by treadmills (Quinton and Formula tools, NJ, USA) by a single trained technician. Within the test, patients were monitored and electrocardiograms from three leads of D2, V2 and V5 were recorded.
We compared categorized variables using Chi-square test or Fisher's exact test if required. Continuous variables were also compared using independent t-test. Changes in IOP following exercise test were assessed using the paired t-test. Correlation between the changes in IOP and other continuous variables including age and body mass index (BMI) were assessed using the Pearson's correlation test. P < 0.05 was considered statistically significant. All the statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
The mean age of the participants was 48.50 ± 10.01 years and the mean BMI was also 25.60 ± 3.57 kg/m2. Assessing IOP following exercise testing showed a significant decrease in IOP in 75 eyes (74.3%) from 16.43 mmHg before test to 13.16 mmHg 5 min after completing the test. Furthermore, IOP remained unchanged in 19.8% of the eyes and even elevated in 5.9% of the eyes after exercise test from 13.66 mmHg before test to 15.33 mmHg 5 min after that. In total, the mean IOP in all assessed eyes was 16.12 ± 2.61 mmHg initially that was gradually decreased to 13.79 ± 2.40 mmHg 5 min after the exercise test, but elevated to 15.67 ± 2.26 mmHg 20 min after the test. The change in IOP 5 min after the exercise test was statistically significant (mean change 2.33 ± 2.03 mmHg, P < 0.001), but this change after the 20 min of completing test compared to before test was not significant (mean change 0.45 ± 1.03 mmHg, P > 0.05). As shown in Figure 1, no significant difference was revealed in the trend of the changes in IOP after exercise test between men and women (P = 0.367). The mean IOP in men at three time points of the study (before exercise test, and 5 and 20 min after the test) was 15.95 ± 2.89 mmHg, 13.82 ± 2.37 mmHg, and 15.51 ± 2.38 mmHg, respectively. The same measurements in women at the three study time points were 16.36 ± 2.21 mmHg, 13.72 ± 2.43 mmHg, and 15.90 ± 2.20 mmHg, respectively. The mean values of IOP at different cutoffs of BMI are shown in Figure 2. The trend of the changes in IOP was similar at different BMI subgroups. The Pearson's correlation test was also showed no significant association between BMI and IOP changes after completing exercise test (r = 0.070, P = 0.310). Also as presents in Figure 3 with regard to the changes in IOP following exercise test at different age subgroups, there was a significant direct association between patients' age and IOP changes assessed by the Pearson's correlation test (r = 0.350, P = 0.009). Comparison of the mean IOP before and after exercise test between left-sided and right-sided eyes showed no significant difference (P = 0.910) [Figure 4].
Figure 1.

Trend of the changes in intraocular pressure after exercise test in men and women
Figure 2.

Trend of the changes in intraocular pressure after exercise test in different body mass index cutoffs
Figure 3.
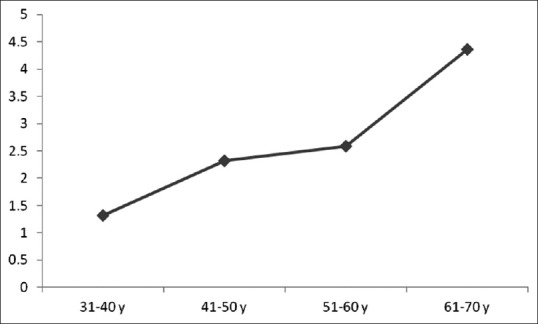
Trend of the changes in intraocular pressure after exercise test by increasing age
Figure 4.
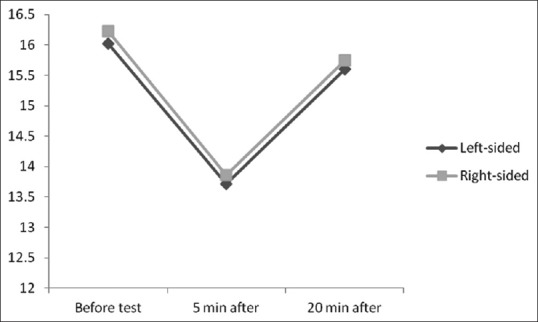
Trend of the changes in intraocular pressure after exercise test in left-sided and right-sided eyes
Discussion
The present study attempted to assess changes in IOP following exercise test. To the best of our knowledge, this is the first study that focused on changing IOP following exercise test as a programmed exercise tool for confirming or ruling out ischemic cardiac state in those who were suspected to CAD. Thus, our findings can be very valuable because of considering a special target population including suspected CAD and indicated for exercise test. It is also very important because majority of these participants suffered from peripheral vascular events that may potentially affect peripheral vascular blood flow and vascular resistance. Moreover, in notable number of patients with coronary ischemic involvement suffered concurrently from ischemic events in other peripheral arteries that can impair vascular blood flow autoregulation.
Our study had two important findings. first, we showed a significant decrease in IOP early after exercise test that is consistent with most previous studies on the effects of exercise training on IOP. However, this change was not constant and IOP was shown to return to baseline value within 20 min of completing exercise test. In fact, the change in IOP following exercise test was temporary that may be very important and even vital in ischemic heart disease patients. Conte et al. similarly showed a relevant decrease of IOP after the strength test in both left-sided and right-sided eyes.[8] Similarly, Price et al. found that IOP was found to decrease significantly with strenuous exercise and recovered gradually toward baseline over 30 min.[9] Qureshi revealed that physical fitness reduces IOP and causes significant attenuation in the IOP response to a 3-month physical exercise.[10] In another study by Era et al. on the effect of bicycle ergometer test on IOP in elderly athletes, the results indicated a decrease (more than 2 mmHg) in 34% of the patients, no change in 57%, and an increase in 9%. The decrease was more pronounced in patients with higher pretest values.[11] According to our findings and similarly previous observations, exercise can lower significantly IOP; however, this change is reversible and return to baseline value early after quitting exercise. The change in ocular vascular blood flow referred to both endocrinological responses and even genetic variants. It has been well demonstrated that the stimulation of the sympathetic nervous system in anticipation of and during the stress of exercise can be accompanied with production and secretion of large quantities of adrenaline and noradrenaline from the adrenal medulla. The release of these mediators reduces IOP by lowering outflow resistances and moreover, by lowering the rate of aqueous formation. In this context, these hormones as therapeutic regimen are widely used as ocular hypotensive drugs for the treatment of glaucoma.[12,13,14] In some other studies, it has been evidenced that progressive exercise lower IOP and elevate ocular perfusion pressure. Within the retinal circulation, this exercise tended to raise mean dye velocity as it significantly narrowed the superior temporal artery and vein; as a result, calculated retinal blood flow was unchanged. In fact, the normal retinal hemodynamic response to increase in perfusion pressure on dynamic exercise includes vasoconstriction that normalizes flow and faster capillary and overall retinal blood transit.[15] Some single nucleotide polymorphisms related to the beta 2-adrenergic receptors (beta 2ARs) have been shown to be related to lowering IOP within exercise. Güngör et al. assessed the effects of mutations in the beta 2AR gene on IOP, in response to acute dynamic exercise and showed that reductions in mean IOP values were found in patients with the Gly16Gly and Arg16Gly genotypes, but these values remained low in the patients with the Gly16Gly genotype 3 h postexercise, whereas they returned to baseline within 1 h in eight patients with the Arg16Gly genotype.[16] These genetic and physiologic mechanisms can well explain temporary reduce in IOP after exercise.
Conclusion
In the present study, changes in IOP after exercise test were more highlighted in advance aging. Age-related maculopathy and also retinopathy have been previously described.[17] These pathological changes and thus different features on ocular condition in the elderly can lead to progressive changes in ocular blood flow as well as vascular resistance[18,19] that may explain more reduce in IOP following exercise in older population.
As a potential limitation, we did not categorize our samples to two groups with and without CAD that can be a good recommendation in further studies.
Financial support and sponsorship
This work was financially supported by Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pal S, Radavelli-Bagatini S, Ho S. Potential benefits of exercise on blood pressure and vascular function. J Am Soc Hypertens. 2013;7:494–506. doi: 10.1016/j.jash.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Duncker DJ, Bache RJ, Merkus D. Regulation of coronary resistance vessel tone in response to exercise. J Mol Cell Cardiol. 2012;52:802–13. doi: 10.1016/j.yjmcc.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Horiuchi M, Okita K. Blood flow restricted exercise and vascular function. Int J Vasc Med. 2012;2012:543218. doi: 10.1155/2012/543218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckingham T, Young R. The rise and fall of intra-ocular pressure: The influence of physiological factors. Ophthalmic Physiol Opt. 1986;6:95–9. [PubMed] [Google Scholar]
- 5.Passo MS, Goldberg L, Elliot DL, Van Buskirk EM. Exercise conditioning and intraocular pressure. Am J Ophthalmol. 1987;103:754–7. doi: 10.1016/s0002-9394(14)74388-0. [DOI] [PubMed] [Google Scholar]
- 6.Passo MS, Goldberg L, Elliot DL, Van Buskirk EM. Exercise training reduces intraocular pressure among subjects suspected of having glaucoma. Arch Ophthalmol. 1991;109:1096–8. doi: 10.1001/archopht.1991.01080080056027. [DOI] [PubMed] [Google Scholar]
- 7.Natsis K, Asouhidou I, Nousios G, Chatzibalis T, Vlasis K, Karabatakis V. Aerobic exercise and intraocular pressure in normotensive and glaucoma patients. BMC Ophthalmol. 2009;9:6. doi: 10.1186/1471-2415-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte M, Scarpi MJ, Rossin RA, Beteli HR, Lopes RG, Marcos HL. Intraocular pressure variation after submaximal strength test in resistance training. Arq Bras Oftalmol. 2009;72:351–4. doi: 10.1590/s0004-27492009000300013. [DOI] [PubMed] [Google Scholar]
- 9.Price EL, Gray LS, Humphries L, Zweig C, Button NF. Effect of exercise on intraocular pressure and pulsatile ocular blood flow in a young normal population. Optom Vis Sci. 2003;80:460–6. doi: 10.1097/00006324-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi IA. Effects of exercise on intraocular pressure in physically fit subjects. Clin Exp Pharmacol Physiol. 1996;23:648–52. doi: 10.1111/j.1440-1681.1996.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 11.Era P, Pärssinen O, Kallinen M, Suominen H. Effect of bicycle ergometer test on intraocular pressure in elderly athletes and controls. Acta Ophthalmol (Copenh) 1993;71:301–7. doi: 10.1111/j.1755-3768.1993.tb07139.x. [DOI] [PubMed] [Google Scholar]
- 12.Guyton AC, editor. Guyton Textbook of Medical Physiology. 8th ed. Philadelphia: WB Saunders Company; 1991. The adrenocortical hormones. Ch. 77. [Google Scholar]
- 13.Richards JS, Drance SM. The effect of 2 percent epinephrine on aqueous dynamics in the human eye. Can J Ophthalmol. 1967;2:259–65. [PubMed] [Google Scholar]
- 14.Nagataki S, Brubaker RF. Early effect of epinephrine on aqueous formation in the normal human eye. Ophthalmology. 1981;88:278–82. doi: 10.1016/s0161-6420(81)35039-8. [DOI] [PubMed] [Google Scholar]
- 15.Harris A, Arend O, Bohnke K, Kroepfl E, Danis R, Martin B. Retinal blood flow during dynamic exercise. Graefes Arch Clin Exp Ophthalmol. 1996;234:440–4. doi: 10.1007/BF02539410. [DOI] [PubMed] [Google Scholar]
- 16.Güngör K, Beydagi H, Bekir N, Arslan C, Süer C, Erbagci I, et al. The impact of acute dynamic exercise on intraocular pressure: Role of the beta 2-adrenergic receptor polymorphism. J Int Med Res. 2002;30:26–33. doi: 10.1177/147323000203000105. [DOI] [PubMed] [Google Scholar]
- 17.Wei L, Chen P, Lee JH, Nussenblatt RB. Genetic and epigenetic regulation in age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 2013;2:269–74. doi: 10.1097/apo.0b013e31829e2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrlich R, Kheradiya NS, Winston DM, Moore DB, Wirostko B, Harris A. Age-related ocular vascular changes. Graefes Arch Clin Exp Ophthalmol. 2009;247:583–91. doi: 10.1007/s00417-008-1018-x. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich R, Harris A, Kheradiya NS, Winston DM, Ciulla TA, Wirostko B. Age-related macular degeneration and the aging eye. Clin Interv Aging. 2008;3:473–82. doi: 10.2147/cia.s2777. [DOI] [PMC free article] [PubMed] [Google Scholar]


