Abstract
Aim
There is emerging concern that antipsychotics may be associated with an increased risk of myocardial infarction (MI). A previous review identified five observational studies that did not provide an accurate estimate of the association between antipsychotic drug use and MI risk. More recent studies have produced variable results.
Methods
We performed a systematic review and meta‐analysis of observational studies to determine whether antipsychotic use affects the risk for MI. Our analysis included all observational studies that compared MI incidence among patients receiving antipsychotics vs. no treatment.
Results
Nine observational studies were included in the analysis. The odds for developing MI were 1.88‐fold higher (odds ratio (OR) 1.88, 95% confidence interval (CI) 1.39, 2.54) in antipsychotic users compared with individuals who had not taken antipsychotics. Subgroup analyses found an OR of 2.48 (95% CI 1.66, 3.69) among patients with schizophrenia and an OR of 2.64 (95% CI 2.48, 2.81) among short term (<30 days) antipsychotic users.
Conclusion
The findings of this meta‐analysis support an increased risk of MI in antipsychotic drug users. The present systematic review expands previous knowledge by demonstrating an increased and more pronounced risk in short term users.
Keywords: antipsychotic agents, coronary disease, myocardial infarction
Introduction
Antipsychotic drugs are commonly prescribed for the treatment of schizophrenia, bipolar disorder, acute mania, depression, behavioural and psychological symptoms of dementia and delirium 1, 2, 3, 4, 5. The safety of these drugs has been questioned. Several different types of adverse events have been associated with antipsychotics such as tardive dyskinesia, neuroleptic malignant syndrome, pneumonia, diabetes and more 6, 7, 8, 9.
Cardiovascular adverse events associated with the use of antipsychotics are well documented 10. However, it remains controversial whether antipsychotic therapy is associated with an increased risk of myocardial infarction (MI). A number of epidemiologic cohort and case–control studies have investigated this possible association. The most recent systematic review 11 identified five observational studies that provided variable results. One study 12 with a large sample size reported no increased risk of MI in antipsychotic users, whereas four studies 13, 14, 15, 16 with small events did. The inconsistent conclusions may be attributed to heterogeneity of these studies (sample size, exposure time, type of antipsychotics and so on). The data included by previous systematic reviews were limited to studies conducted before 2006. Many studies 17, 18, 19, 20 have been published since, which allow for a more detailed analysis of the association between antipsychotic use and MI risk.
Given the high prevalence of antipsychotic use worldwide, it is important to determine whether there is a relationship between antipsychotics and the risk of MI. Therefore, the aim of this study was to conduct a systematic review and meta‐analysis of all observational studies to estimate MI risk with antipsychotic medication use in adults.
Methods
Data sources and searches
We followed the guidelines published by the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) group 21 to complete the meta‐analysis. We conducted a comprehensive literature search of the Cochrane Library, PubMed and Embase databases up to July 2015, using the following terms ‘antipsychotic agents’, ‘antipsychotic drugs’ and ‘antipsychotics’ AND ‘acute coronary syndrome’ and ‘myocardial infarction’. In addition, reference lists of the retrieved articles were hand‐searched for further relevant articles.
Study selection
Abstracts were considered eligible for full manuscript data extraction if they fulfilled the following criteria: (1) a case–control or case–crossover or self‐controlled case series (SCCS) or cohort study, (2) antipsychotics were compared with non‐antipsychotics, (3) measurement of MI was a primary or secondary outcome and (4) risk estimates with confidence intervals (CIs) or sufficient information to calculate these values were included.
Data extraction and quality assessment
All data were extracted independently by two authors using predesigned electronic data extraction and a third author resolved any discrepancies before the final analysis. Raw data, unadjusted odds ratios (OR) or relative risks (RR) with 95% CIs and adjusted OR or RR with 95% CIs were recorded when possible. If a study reported more than one measure of MI, each measure was extracted separately (Table S1). The most adjusted estimate was included when a study reported more than one risk estimate. Two authors assessed the quality of the included studies based on the Newcastle‐Ottawa Scale (NOS), as recommended by the Cochrane Collaboration 22 (Table S2 and S3).
Outcomes assessed
The primary analysis focused on assessing the risk of MI among users of antipsychotics. We performed a post hoc sensitivity analysis by eliminating one study from the same database. We also ran a sensitivity analysis by including one estimate of study involved two study designs one at a time. In an attempt to explain possible heterogeneity between studies, we performed subgroup analyses based on study design (case–control, cohort, SCCS or case–crossover), type of antipsychotic (typical or atypical), diagnostic categories (schizophrenia, dementia or mood disorders) and exposure duration (30, 60 or 90 days). If one study involved two study designs, the estimates were respectively pooled in the subgroup analysis based on study design.
Data synthesis and analysis
STATA 10.0 software (StataCorp LP, College Station, TX, USA) was utilized for all statistical analyses. The Cochran Q chi‐square test and the I2 statistic were used to assess heterogeneity among studies 23. I2 values of > 50% or P values of < 0.05 for the Q‐statistic were taken to indicate significant heterogeneity. Random effects models were used to analyze pooled effects when statistic heterogeneity existed. Otherwise, fixed effects models were used. The association between antipsychotic use and MI risk was estimated using ORs and corresponding 95% CIs generated from comparisons between cases and controls. Since the outcomes were relatively uncommon, ORs were considered approximations of RR. Publication bias of the studies included in the final analysis was analyzed using the Begg funnel plot and the Egger test 24.
Results
Search results
By searching the three databases using the keywords as well as the relevant reference sections, a total of 1428 potentially eligible articles were identified. Of these, 1335 articles were excluded after reading the titles and abstracts and the remaining 93 articles underwent detailed full text evaluation. Nine observational studies 12, 13, 14, 15, 16, 17, 18, 19, 20 were eligible for inclusion and were assessed for quality. The number of studies that were excluded from the review and meta‐analysis are shown in Figure 1.
Figure 1.
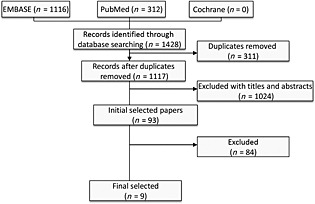
Flow chart of the studies considered and finally selected for review
Characteristics of included studies
The main characteristics of the studies included are shown in Table 1. The earliest study 13 began in 1992 and the most recent of the included studies 20 ended in 2015. Of the included studies, three 12, 13, 14 were case–control studies, two 15, 16 cohort studies and two 18, 20 case–crossover studies. One 17 study used SCCS and cohort study design and the remaining one 19 used SCCS and case–control study design. Seven 12, 15, 16, 17, 18, 19, 20 of the studies identified patients from databases, while two 13, 14 used medical records or interview data. In terms of diagnostic categories, one study 17 evaluated patients with dementia only, four 16, 18, 19, 20 evaluated patients with schizophrenia, mood disorder or dementia and the others 12, 13, 14, 15 included patients with any diagnosis.
Table 1.
Characteristics of observational studies assessing the risk of myocardial infarction associated with exposure to antipsychotic drug
| Study, year | Location, setting | Study design | Study period | Population characteristics | Total population | Astertainment of antipsychotic drug exposure | Case or outcome definition | High‐ quality |
|---|---|---|---|---|---|---|---|---|
| Thorogo‐od et al. 13 | UK, hospital‐ based | Case–control | 1986 –1988 | Women aged 16‐39 years, died during 1986‐1988 (cases were matched by age, marital status and general practitioner) | 161 cases and 309 controls | Interviews with the general practioners of the cases and patient records | Death certificates supplied by the Office of Population Censuses and Surveys, verified by copies of post mortem reports and relevant hospital records. | No |
| Penttinen & Valonen 14 | Finland, hospital‐ based | Case–control | 1980–1992 | NA (cases were matched by age, smoking habit, social status and county of residence) | 83 cases and 249 controls | Patients records | Hospital discharge registries and copies of death certificates from the Finnish Statistics Bureau | No |
| Pratt et al. 15 | USA, population‐based | Cohort | 1993–1994 | Patients >18 years old, with a history of depression or dysphoria or no depression or dysphoria | 8 cases in 71 antipsychotic drug users and 55 cases in 1551 non‐antipsychotic drug users | Interview data on self‐reported drug use assessed with colour photographs of pills. | Self‐reported MI | No |
| Enger et al. 16 | USA, population‐based | Cohort | 1995–1999 | Patients with schizophrenia, defined by a visit to a healthcare provider or inpatient hospital stay and an antipsychotic prescription (cases were matched by age, gender, date and health plan) | 12 cases in 1920 antipsychotic drug user and 28 cases in 9600 no‐ antipsychotic drug users | Private Health Insurance Database | Private Health Insurance Database | Yes |
| Nakagawa et al. 12 | Denmark, population‐based | Case–control | 1992–2003 | Aged 15 years and older and residents (cases were matched by age, gender and residence) | 21 377 cases and 106 885 controls | Population‐based prescription databases in the three counties | Hospital discharge registries in the three counties | Yes |
| Pariente et al. 17 | Canada, population‐based | Cohort | 2000–2009 | Dementia patients aged 66 years and older | 138 cases in 10 969 antipsychotic drug users and 126 cases in 10 969 no‐ antipsychotic drug users | Prescription database | A location of service at an emergency department | Yes |
| Self‐ controlled case series | 804 cases | |||||||
| Lin et al. 18 | Taiwan, population‐based | Case–crossover | 1997–2009 | Schizophrenic disorders, mood disorders, dementia patients aged 18 years and older | 59 806 cases | Taiwan's National Health Insurance Research Database | Taiwan's National Health Insurance Research Database | Yes |
| Brauer et al. 19 | UK, population‐based | Self‐controlled case series | 1999–2011 | Schizophrenic disorders, mood disorders, dementia, other psychiatric patients aged 18 years and older | 1468 cases | Clinical Practice Research Datalink | MINAP‐linked CPRD | Yes |
| Case–control | 27 861 cases and 108 234 controls | |||||||
| Wu et al. 20 | Taiwan, population‐based | Case–crossover | 1996–2007 | Hospitalized schizophrenia or schizoaffective disorder; hospitalized bipolar disorder; at least three ambulatory instances of the above diagnoses patients | 834 cases | Taiwan's National Health Insurance Research Database | Taiwan's National Health Insurance Research Database | Yes |
On the basis of the methodologic quality assessment scores, six studies were of high quality and three were of low quality. The breakdown of each score is given in Table S2 and S3.
Main results
Upon meta‐analysis of all included studies, the use of antipsychotics significantly increased the risk of MI (OR 1.88, 95% CI 1.39, 2.54, P < 0.001). There was, however, considerable heterogeneity observed across studies (I2 98%, P < 0.001) (Figure 2). The sensitivity analysis showed no substantial change in pooled risk estimates upon exclusion of any single study from the same database or inclusion of one estimate from one study involved two study designs.
Figure 2.
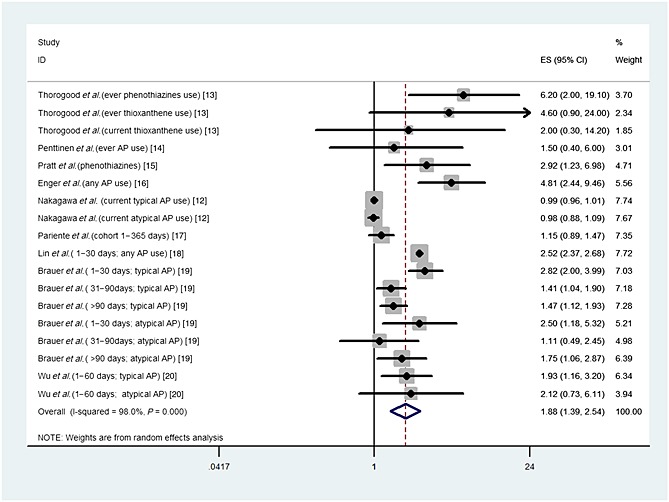
Relative risk of myocardial infarction in antipsychotic drug users
Subgroup meta‐analyses
Table 2 presents the results of subgroup analyses. When studies were grouped by study design, significant associations were observed in case–control studies (OR 1.20, 95% CI 1.03, 1.40), SCCS studies (OR 1.62, 95% CI 1.34, 1.95), and case–crossover studies (OR 2.51, 95% CI 2.36, 2.67). Although this association was not significantly found (OR 2.42, 95% CI 0.89, 6.60), there was a trend toward an increase in MI risk in cohort studies.
Table 2.
Subgroup analysis for studies included in the analysis
| Subgroup analysis | Number of studies | Number of estimates | Pooled OR (95% CI), I2 statistics (%), P value for the heterogeneity Q test |
|---|---|---|---|
| All estimates combined | 9 | 21 | 1.88 (1.39, 2.54); I2 = 98%, P < 0.001 |
| Elimating Lin et al.'s study [18] | 8 | 20 | 1.65 (1.38, 1.97); I2 = 85.3%, P < 0.001 |
| Elimating Wu et al.'s study [20] | 8 | 19 | 1.85 (1.40, 2.46); I2 = 97.9%, P < 0.001 |
| Study design | |||
| Case–control | 4 | 12 | 1.20 (1.03, 1.40); I2 = 79.4%, P < 0.001 |
| Cohort | 3 | 3 | 2.42 (0.89, 6.60); I2 = 88.8%, P < 0.001 |
| SCCS | 2 | 10 | 1.62 (1.34, 1.95); I2 = 55.2%, P = 0.017 |
| Case–crossover | 2 | 3 | 2.51 (2.36, 2.67); I2 = 0%, P = 0.565 |
| Type of antipsychotic drugs | |||
| typical | 7 | 11 | 2.19 (1.46, 3.28); I2 = 98.4%, P < 0.001 |
| atypical | 5 | 7 | 1.72 (0.96, 3.07); I2 = 97%, P < 0.001 |
| Diagnostic category | |||
| Schizophrenia | 3 | 3 | 2.48 (1.66, 3.69); I2 = 94.7%, P < 0.001 |
| Mood disorder | 2 | 2 | 1.66 (0.86, 3.22); I2 = 71.2%, P < 0.001 |
| Dementia | 3 | 7 | 1.82 (1.16, 2.84); I2 = 92.1%, P < 0.001 |
| Exposure time | |||
| 1–30 days | 3 | 4 | 2.64 (2.48, 2.81); I2 = 0%, P = 0.904 |
| 1–60 days | 2 | 3 | 1.59 (1.17, 2.18); I2 = 0%, P = 0.447 |
| 1–90 days | 3 | 7 | 1.35 (1.09, 1.67); I2 = 0%, P = 0.447 |
In a subgroup analysis by type of antipsychotics, a significant association was observed among those using typical (OR 2.19, 95% CI 1.46, 3.28) or atypical (OR 1.72, 95% CI 0.96, 3.07) antipsychotic drugs. Only a few studies provided data on individual drugs. In one study, a significantly higher risk of MI was observed with amisulpride (OR 5.65, 95C I% 2.97, 10.76). Two studies found that antipsychotic drug use was associated with a dose‐dependent increase in MI risk, but one study did not.
Grouping the studies by diagnostic categories revealed a significantly higher risk of MI among patients with schizophrenia (OR 2.48, 95% CI 1.66, 3.69) or dementia (OR 1.82, 95% CI 1.16, 2.84). However, no significant associations were observed among patients with mood disorders (OR 1.66, 95% CI 0.86, 3.22).
When we grouped studies by exposure duration, the association between antipsychotic use and MI risk weakened over time, with ORs decreasing from 2.64 (95% CI 2.48, 2.81) to 1.59 (95% CI 1.17, 2.18) to 1.35 (95% CI 1.09, 1.67) from 30 to 60 to 90 days, respectively.
Publication bias
Although we observed no statistical evidence of publication bias (Begg's test, P = 0.33; Egger's test, P for bias = 0.74) (Figure 3), it should be noted that the funnel plot showed the distribution was deviated. The funnel plot revealed an apparent asymmetry that suggested the presence of a potential publication bias, a language bias, inflated estimates by a flawed methodologic design in smaller studies and/or a lack of publication of small trials with opposite results.
Figure 3.
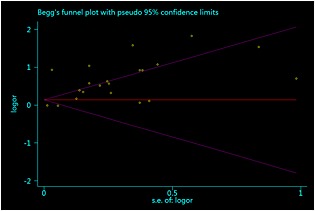
The Begg funnel plot and Egger test for identifying publication bias in a meta‐analysis of observational studies
Discussion
This meta‐analysis of current observational evidence suggests that antipsychotic medications are associated with a modest increase in the risk of MI. Most of the results of the subgroup analyses were consistent with the overall results. A more pronounced risk of MI was found among patients with schizophrenia or in patients with antipsychotic drug use within a 30 day period. As considerable heterogeneity was observed in the marked study, we can be less certain about this result.
Several explanations for the increased risk of MI with the use of antipsychotics are hypothesized, but the underlying mechanisms remain speculative. It is suggested that weight gain and metabolic syndrome induced by antipsychotic use could be risk factors for MI 25. If such factors are involved, the risk would be expected to increase progressively with antipsychotic drug use duration. However, our analysis showed an acute decrease in MI risk after 30 days of use. A second plausible explanation refers to the finding that, in one study, a highest increased risk for MI has been observed with antipsychotics having a high affinity for the D3 receptor (amisulpride). Preclinical studies 26, 27, 28 demonstrated that aberrant D3 receptor expression in the heart and peripheral vascular system may increase intimal permeability, vascular remodelling and atherosclerosis formation. Additionally, a recent meta‐analysis 29 found a link between the use of antipsychotics and venous thromboembolism, suggesting that D3 receptor systems may be involved in platelet aggregation and the secretion of procoagulant factors 30, 31. Hence, antipsychotic use could predispose patients to the formation of acute thrombosis in stenotic coronary arteries, contributing to MI. Finally, antipsychotic medicines have been shown to activate 5‐HT2A receptors at sites of coronary atherosclerosis 32, leading to thrombus formation and vascular contraction. Such an effect might play a role in the pathogenesis of MI.
Although the modifying effects of antipsychotics on MI are biologically plausible, the included studies have reported conflicting results, as reflected in the significant heterogeneity in our meta‐analysis. This heterogeneity could not be explained by study design and quality. The existence of clinical heterogeneity would be expected to lead to some degree of statistical heterogeneity in the results. The inconsistent conclusions may be owing to differences in enrolment criteria and exposure definition. First, most of included patients were diagnosed with dementia, schizophrenia or affective disorders and these patients were at different risks of cardiovascular diseases. To minimize the heterogeneity, subgroup analyses based on diagnostic categories found different risks of MI among these patients. A highest risk seen in patients with schizophrenia may be explained by more use of co‐medication and a worse underlying health state 33, 34. Furthermore, lifestyle factors such as alcoholism and smoking are also associated with a higher risk of MI 35. However, antipsychotic use was found to increase, but not significantly, the risk of MI in patients with affective disorders. This might be explained by direct cardioprotective effects of mood stabilizers which have been suggested in other research 36.
Second, the different degree of receptor binding affinity by antipsychotic medicines may have been a potential source of heterogeneity. Most of the included studies explored the effects of typical and atypical antipsychotics as a whole, and a similar risk of MI was found between them. However, only one study 18 provided varied risk data of individual drugs, finding the highest MI risk in amisulpride users. Besides, it is of paramount relevance to understand if there are dose‐dependent effects. It should be acknowledged that these concerns are not addressable by means of meta‐analyses of aggregate data and, therefore, only tentative suggestions can be made. Three studies 12, 18, 20 evaluated the effect of dosage on the MI risk. Two studies 18, 20 found a positive association, while one 12 found no association. Hence, our results of individual or dosage of antipsychotic drugs on risk of MI may be limited by sample size and need further investigation to clarify this issue.
Third, definition of antipsychotic exposure was inconsistent across the included studies. In our subgroup analysis based on exposure time, there was 0% heterogeneity among studies. Our results expand on previous knowledge by demonstrating a substantial MI risk in short term users, thus suggesting an acute effect of antipsychotics. This finding is further reinforced by a relatively lower but significant risk observed in longer term users. Such lower risk observed in more long term antipsychotic users might be related to the effects of tolerance and cross‐tolerance to antipsychotic drugs.
Although antipsychotic use is associated with a modestly increased risk of MI, the population impact of antipsychotic‐associated MI is likely to be substantial because of the large number of users. Clinicians should ensure that antipsychotics are prescribed only for patients with a clear indication and be cautious when prescribing antipsychotics to patients who have an underlying increased MI risk. As antipsychotics are an effective intervention for some major psychiatric conditions, the relatively modest increased absolute risk of MI is unlikely to alter their benefit–risk balance when used appropriately. Nonetheless, all patients prescribed these medications should be monitored during the course of antipsychotic treatment if MI‐related signs and symptoms are identified, considering the possibility of treatment withdrawal.
This systematic review and meta‐analysis was limited by the inclusion of only observational studies, which are susceptible to confounding. Unfortunately, there have been no randomized controlled trials that evaluated the risk of MI with antipsychotic use. Such studies are usually underpowered to detect rare events. Second, there was evidence of heterogeneity for the association between antipsychotic use and MI risk. Thus, subgroup analyses were performed to examine the source of the heterogeneity, but the variables evaluated did not thoroughly explain the source of heterogeneity. When the exposure time period was taken into account, there was 0% heterogeneity among studies, suggesting that antipsychotic use has a time‐dependent effect on MI risk. Different individual antipsychotic drugs may also have been a source of heterogeneity and this issue merits further exploration. Third, all included studies focused on adult patients. Given that there is an elevation in antipsychotic use among children and youth 37, more data about cardiovascular safety are needed in this population. Fourth, the Begg's test is known to lack power, reducing our ability to detect potential publication bias. Finally, we could not extract enough data to run a subgroup analysis based on concurrent drug use. Antidepressants are commonly prescribed concurrently with antipsychotics for treatment of diverse psychiatric disorders. These drugs are considered to be associated with an increased risk of cardiovascular events 38. Future studies should also investigate the risk of MI when antipsychotic medications are used together with these drugs.
Conclusion
In summary, the present meta‐analysis suggests that antipsychotic use is significantly associated with MI risk, especially among patients with schizophrenia or with drug use during the first 30 days. Clinicians should ensure that antipsychotics are prescribed only for patients with clear indications.
Competing Interests
The authors declare no competing interests.
Contributors
ZHY and BR conceived the study and revised the manuscript critically for important intellectual content. ZHY and HYJ made substantial contributions to its design, acquisition, analysis and interpretation of data. LS, YYZ and HYS participated in the design, acquisition, analysis and interpretation of data. All authors read and approved the final manuscript.
Funding
This study was supported by the Fundamental Research Funds for the Central Universities, China (2014XZZX008, 2013XZZX00).
Supporting information
Table S1 All effect estimates extracted from the nine included studies
Table S2 Newcastle Ottawa Scale for assessment of quality of included studies: case–control studies
Table S3 Newcastle Ottawa Scale for assessment of quality of included studies: cohort studies
Supporting info item
Yu, Z. , Jiang, H. , Shao, L. , Zhou, Y. , Shi, H. , and Ruan, B. (2016) Use of antipsychotics and risk of myocardial infarction: a systematic review and meta‐analysis. Br J Clin Pharmacol, 82: 624–632. doi: 10.1111/bcp.12985.
References
- 1. Kuipers E, Yesufu‐Udechuku A, Taylor C, Kendall T. Management of psychosis and schizophrenia in adults: summary of updated NICE guidance. BMJ 2014; 348: g1173. [DOI] [PubMed] [Google Scholar]
- 2. Taylor DM, Cornelius V, Smith L, Young AH. Comparative efficacy and acceptability of drug treatments for bipolar depression: a multiple‐treatments meta‐analysis. Acta Psychiatr Scand 2014; 130: 452–69. [DOI] [PubMed] [Google Scholar]
- 3. Ogawa Y, Tajika A, Takeshima N, Hayasaka Y, Furukawa TA. Mood stabilizers and antipsychotics for acute mania: a systematic review and meta‐analysis of combination/augmentation therapy versus monotherapy. CNS Drugs 2014; 28: 989–1003. [DOI] [PubMed] [Google Scholar]
- 4. Declercq T, Petrovic M, Azermai M, Vander Stichele R, De Sutter AI, van Driel ML, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev 2013; 3: CD007726. [DOI] [PubMed] [Google Scholar]
- 5. Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev 2007; CD005594. [DOI] [PubMed] [Google Scholar]
- 6. Ryu S, Yoo JH, Kim JH, Choi JS, Baek JH, Ha K, et al. Tardive dyskinesia and tardive dystonia with second‐generation antipsychotics in non‐elderly schizophrenic patients unexposed to first‐generation antipsychotics: a cross‐sectional and retrospective study. J Clin Psychopharmacol 2015; 35: 13–21. [DOI] [PubMed] [Google Scholar]
- 7. Trollor JN, Chen X, Chitty K, Sachdev PS. Comparison of neuroleptic malignant syndrome induced by first‐ and second‐generation antipsychotics. Br J Psychiatry 2012; 201: 52–6. [DOI] [PubMed] [Google Scholar]
- 8. Nose M, Recla E, Trifiro G, Barbui C. Antipsychotic drug exposure and risk of pneumonia: a systematic review and meta‐analysis of observational studies. Pharmacoepidemiol Drug Saf 2015; 24: 812–20. [DOI] [PubMed] [Google Scholar]
- 9. Smith M, Hopkins D, Peveler RC, Holt RI, Woodward M, Ismail K. First‐ v. second‐generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta‐analysis. Br J Psychiatry 2008; 192: 406–11. [DOI] [PubMed] [Google Scholar]
- 10. Correll CU, Joffe BI, Rosen LM, Sullivan TB, Joffe RT. Cardiovascular and cerebrovascular risk factors and events associated with second‐generation antipsychotic compared to antidepressant use in a non‐elderly adult sample: results from a claims‐based inception cohort study. World Psychiatr 2015; 14: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brauer R, Douglas I, Smeeth L. The association between antipsychotic agents and the risk of myocardial infarction: a systematic review. Br J Clin Pharmacol 2011; 72: 871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakagawa S, Pedersen L, Olsen ML, Mortensen PB, Sorensen HT, Johnsen SP. Antipsychotics and risk of first‐time hospitalization for myocardial infarction: a population‐based case‐control study. J Intern Med 2006; 260: 451–8. [DOI] [PubMed] [Google Scholar]
- 13. Thorogood M, Cowen P, Mann J, Murphy M, Vessey M. Fatal myocardial infarction and use of psychotropic drugs in young women. Lancet 1992; 340: 1067–8. [DOI] [PubMed] [Google Scholar]
- 14. Penttinen J, Valonen P. Use of psychotropic drugs and risk of myocardial infarction: a case‐control study in Finnish farmers. Int J Epidemiol 1996; 25: 760–2. [DOI] [PubMed] [Google Scholar]
- 15. Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaton WW. Depression, psychotropic medication, and risk of myocardial infarction. Prospective data from the Baltimore ECA follow‐up. Circulation 1996; 94: 3123–9. [DOI] [PubMed] [Google Scholar]
- 16. Enger C, Weatherby L, Reynolds RF, Glasser DB, Walker AM. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis 2004; 192: 19–27. [DOI] [PubMed] [Google Scholar]
- 17. Pariente A, Fourrier‐Reglat A, Ducruet T, Farrington P, Beland SG, Dartigues JF, et al. Antipsychotic use and myocardial infarction in older patients with treated dementia. Arch Intern Med 2012; 172: 648–53 .discussion 54‐5 [DOI] [PubMed] [Google Scholar]
- 18. Lin ST, Chen CC, Tsang HY, Lee CS, Yang P, Cheng KD, et al. Association between antipsychotic use and risk of acute myocardial infarction: a nationwide case‐crossover study. Circulation 2014; 130: 235–43. [DOI] [PubMed] [Google Scholar]
- 19. Brauer R, Smeeth L, Anaya‐Izquierdo K, Timmis A, Denaxas SC, Farrington CP, et al. Antipsychotic drugs and risks of myocardial infarction: a self‐controlled case series study. Eur Heart J 2015; 36: 984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu SI, Kao KL, Chen SC, Juang JJ, Lin CJ, Fang CK, et al. Antipsychotic exposure prior to acute myocardial infarction in patients with serious mental illness. Acta Psychiatr Scand 2015; 131: 213–22. [DOI] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–12. [DOI] [PubMed] [Google Scholar]
- 22. Higgins. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane collaboration. [online]. Available at: http://www.cochrane‐handbook.org (last accessed 6 December 2014).
- 23. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M. Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta‐analysis of first episode, untreated and treated patients. Schizophr Bull 2013; 39: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeng C, Asico LD, Wang X, Hopfer U, Eisner GM, Felder RA, et al. Angiotensin II regulation of AT1 and D3 dopamine receptors in renal proximal tubule cells of SHR. Hypertension 2003; 41: 724–9. [DOI] [PubMed] [Google Scholar]
- 27. Yu C, Yang Z, Ren H, Zhang Y, Han Y, He D, et al. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. Am J Hypertens 2009; 22: 877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang H, Han Y, Wang X, Chen C, Yu C, He D, et al. Inhibitory effect of the D(3) dopamine receptor on insulin receptor expression and function in vascular smooth muscle cells. Am J Hypertens 2011; 24: 654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barbui C, Conti V, Cipriani A. Antipsychotic drug exposure and risk of venous thromboembolism: a systematic review and meta‐analysis of observational studies. Drug Saf 2014; 37: 79–90. [DOI] [PubMed] [Google Scholar]
- 30. De Keyser J, De Waele M, Convents A, Ebinger G, Vauquelin G. Identification of D1‐like dopamine receptors on human blood platelets. Life Sci 1988; 42: 1797–806. [DOI] [PubMed] [Google Scholar]
- 31. Ricci A, Bronzetti E, Mannino F, Mignini F, Morosetti C, Tayebati SK, et al. Dopamine receptors in human platelets. Naunyn Schmiedebergs Arch Pharmacol 2001; 363: 376–82. [DOI] [PubMed] [Google Scholar]
- 32. Yamada S, Akita H, Kanazawa K, Ishida T, Hirata K, Ito K, et al. T102C polymorphism of the serotonin (5‐HT) 2A receptor gene in patients with non‐fatal acute myocardial infarction. Atherosclerosis 2000; 150: 143–8. [DOI] [PubMed] [Google Scholar]
- 33. Weinbrenner S, Assion HJ, Stargardt T, Busse R, Juckel G, Gericke CA. Drug prescription patterns in schizophrenia outpatients: analysis of data from a German health insurance fund. Pharmacopsychiatry 2009; 42: 66–71. [DOI] [PubMed] [Google Scholar]
- 34. Jeste DV, Gladsjo JA, Lindamer LA, Lacro JP. Medical comorbidity in schizophrenia. Schizophr Bull 1996; 22: 413–30. [DOI] [PubMed] [Google Scholar]
- 35. Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med 1999; 29: 697–701. [DOI] [PubMed] [Google Scholar]
- 36. Lee TM, Lin SZ, Chang NC. Antiarrhythmic effect of lithium in rats after myocardial infarction by activation of Nrf2/HO‐1 signaling. Free Radic Biol Med 2014; 77: 71–81. [DOI] [PubMed] [Google Scholar]
- 37. Olfson M, King M, Schoenbaum M. Treatment of Young People With Antipsychotic Medications in the United States. JAMA Psychiatr 2015; 72: 867–74. [DOI] [PubMed] [Google Scholar]
- 38. Oh SW, Kim J, Myung SK, Hwang SS, Yoon DH. Antidepressant use and risk of coronary heart disease: meta‐analysis of observational studies. Br J Clin Pharmacol 2014; 78: 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 All effect estimates extracted from the nine included studies
Table S2 Newcastle Ottawa Scale for assessment of quality of included studies: case–control studies
Table S3 Newcastle Ottawa Scale for assessment of quality of included studies: cohort studies
Supporting info item


