Abstract
Aims
This study characterized the pharmacokinetics of ramosetron and compared prophylactic anti‐emetic efficacy with that of ondansetron in a large population.
Methods
Fifty‐eight patients consented to the pharmacokinetic analysis and were assigned randomly to receive 0.3, 0.45 or 0.6 mg ramosetron after induction of anaesthesia. Blood samples were acquired at preset intervals. Non‐compartmental and population pharmacokinetic analyses were performed. In total, 1102 patients consented to the evaluation of prophylactic anti‐emetic efficacy and were allocated randomly to receive 0.3 mg ramosetron or 4 mg ondansetron at the end of surgery. An additional 16 mg ondansetron were mixed in the intravenous patient‐controlled analgesia pump of the ondansetron group. Post‐operative nausea and vomiting (PONV) were evaluated 6, 24 and 48 h post‐operatively using the Rhodes index of nausea, vomiting and retching (RINVR). Administration of rescue anti‐emetics and adverse events were evaluated.
Results
The pharmacokinetic parameter estimates were V 1 (l) = 5.12, V 2 (l) = 108, CL (l⋅min−1) = 0.08 + (59⋅age−1) × 0.09, Q (l⋅min−1) = 1.42. The incidences of PONV in the ramosetron and ondansetron groups were 77 (13.9%) and 113 (20.6%) and 44 (7.9%) and 66 (12.0%) at 24 and 48 h post‐operatively, respectively (P = 0.004, 0.030). RINVR was significantly lower in the ramosetron than the ondansetron group 24 and 48 h post‐operatively (P = 0.003, 0.025). Use of rescue anti‐emetics and incidence of adverse events were comparable.
Conclusions
A two compartment mammillary model was used to describe ramosetron pharmacokinetics. Prophylactic anti‐emetic efficacy of ramosetron was significantly better 24 and 48 h post‐operatively than that of ondansetron, particularly when the Apfel score was ≥ 3.
Keywords: anti‐emetics, pharmacokinetics, post‐operative nausea and vomiting, ramosetron
What is Already Known about this Subject
Ramosetron is more potent and has a longer efficacy compared with previous 5‐HT3 antagonists, but few population pharmacokinetic results are available.
Previous studies compared the prophylactic anti‐emetic efficacy of ramosetron with that of ondansetron, but none has been performed in large populations or considered risk stratification during enrolment.
What this Study Adds
A two compartment mammillary model was used to describe ramosetron pharmacokinetics and age was a significant covariate in metabolic clearance.
Prophylactic administration of a 0.3 mg ramosetron bolus significantly reduced the incidence of late post‐operative nausea and vomiting compared with prophylactic 4 mg ondansetron mixed with 16 mg ondansetron in the i.v. patient‐controlled analgesia.
Introduction
Post‐operative nausea and vomiting (PONV) is one of the most common post‐anaesthetic complications and is affected by patient, surgical and anaesthetic factors 1, 2, 3, 4. History of motion sickness or PONV, female gender, non‐smoking status and use of post‐operative opioids are predictive of PONV 3. Selective 5‐hydroxytryptamine (5‐HT3) antagonists have been widely used as prophylactics or rescue anti‐emetics 5. Ramosetron is a new 5‐HT3 antagonist that is more potent and longer acting compared with previous 5‐HT3 antagonists 6. Although widely used for PONV prophylaxis, few population pharmacokinetic results for ramosetron are available. One study showed that age and weight were significant covariates of the metabolic clearance of ramosetron 7. This suggests that the ramosetron dosing strategy can change according to covariates such as age and weight. A thorough investigation of the pharmacokinetic characteristics of ramosetron would help establish an individualized dosing strategy.
In earlier studies that compared the prophylactic efficacies of ramosetron and ondansetron, 0.3 mg ramosetron demonstrated better efficacy for reducing PONV and increased the complete response rate compared with 4 mg ondansetron 8, 9, 10, 11, 12, 13, 14. However, those studies included small to moderate sample sizes and only a single type of surgery 8, 9, 10, 11, 12, 13, 14, and comparative studies in low risk groups have not been performed 9, 10, 11, 12. Anti‐emetic efficacy could be exaggerated if the drug is evaluated selectively in a population with a high incidence of PONV, because PONV occurs more frequently in populations with higher Apfel scores 3. Therefore, a study in a larger population undergoing multiple types of surgery is needed to consider risk stratification during patient enrolment to confirm previous results on the efficacy of ramosetron.
The aims of this study were to characterize the pharmacokinetics of ramosetron using non‐compartmental analysis and non‐linear mixed effects modelling and to compare the prophylactic anti‐emetic efficacies of ramosetron and ondansetron in a large population.
Methods
Patient population
This study included two study populations. The pharmacokinetic analysis of the ramosetron population was designed as a randomized, double‐blind study. This study was performed at Asan Medical Centre, the study protocol was approved by the institutional review board (approval number: 2012–0091) and registered at the international clinical research information system (http://cris.nih.go.kr, KCT0001500). We enrolled patients ≥19 years of age with an American Society of Anesthesiologists (ASA) physical classification of I or II, scheduled for stomach or colorectal surgery between October 2012 and April 2013.
The evaluation of the prophylactic anti‐emetic efficacy of ramosetron was designed as a randomized, double‐blind, active‐controlled study. The primary endpoint of this analysis was the incidence of PONV. This study was performed at Asan Medical Centre, Severance Hospital, Seoul National University Hospital and Chonnam National University Hwasun Hospital in Korea. The study protocol was approved by the institutional review board of each hospital and registered on the international clinical research information system (http://cris.nih.go.kr, KCT0001499). We enrolled patients ≥19 years of age with an ASA physical classification I or II, scheduled for general, gynaecological or major orthopaedic surgery at the four hospitals between November 2010 and November 2011.
In both analyses, exclusion criteria included an allergic history of the study drugs, administration of drugs that affect emesis within 24 h of the operation, use of a post‐operatively maintained nasogastric tube, early feeding within 8 h post‐operatively, blood donation >450 ml within 1 month before surgery, active hepatitis and alcoholics or patients who binge drink alcohol. Written informed consent was obtained from all patients.
Pharmacokinetic study and analysis design
Patients who consented to arterial blood sampling were allocated and assigned randomly to receive a bolus of 0.3, 0.45 or 0.6 mg ramosetron intravenously after induction of anaesthesia. Arterial blood (5 ml) was sampled before (0 min) and 2, 5, 10, 15, 30 min and 1, 2, 4, 8, 12 and 24 h after administration of ramosetron. An additional 3 ml of arterial blood was obtained during the operation for analysis of the CYP2D6 single nucleotide polymorphism. The efficacy of 0.3, 0.45 and 0.6 mg ramosetron on PONV was evaluated using the Rhodes index of nausea, vomiting and retching (RINVR) 6, 24 and 48 h post‐operatively 15. Genotyping of the CYP2D6 cytochrome P450 enzyme was performed to evaluate its effect on ramosetron pharmacokinetics 16, 17, 18. The patients were classified into four phenotypes based on CYP2D6 activity: poor, intermediate, extensive and ultrarapid metabolizers.
Blood samples and assays for pharmacokinetic analysis
Blood was sampled and collected into ethylenediaminetetraacetic acid‐containing tubes and then centrifuged at 252 × g for 10 min. Until assay, the plasma was collected and kept at −70°C. A validated high performance liquid chromatography–tandem mass spectrometry was used to analyze plasma concentrations of ramosetron. Briefly, 200 μl plasma and 20 μl of an internal standard (5 ng∙ml−1 risperidone) were mixed. After that, 1.5 ml methyl tert‐butyl ether was added and the mixture was vortexed for 2 min and centrifuged at 12 000 rev min–1 for 2 min. The supernatants were transferred and evaporated under a stream of nitrogen gas. After reconstituting the residue in 100 μl 40% acetonitrile for 1 min, 10 μl were injected into the h.p.l.c.‐MS/MS system (HPLC‐MS/MS; Shimadzu HPLC system, Shimadzu Co., Kyoto, Japan/API 4000Q‐TRAP mass spectrometer; AB SCIEX, Framingham, MA, USA). The column was a Gemini C18 100 × 2.0 mm, 3.0 μm particle size (Phenomenex, Torrance, CA, USA), and a mixture of ammonium acetate (10 mm) and acetonitrile (60/40, v/v) was used as a mobile phase. The flow rate was maintained at 0.25 ml∙min−1. The calibration curve was linear over the range of 0.1–100 ng∙ml−1 for ramosetron (r 2 ≥ 0.98). The intra and interday precision and accuracy were within 15% and 85–115%, respectively.
Non‐compartmental analysis
WinNonlin 6.3 (Pharsight, St Louis, MO, USA) was used to calculate pharmacokinetic parameters by non‐compartmental methods. Linear trapezoidal integration was used to calculate the area under the curve (AUC) from administration to the last measurement (AUC(0,t last)). The AUC from administration to infinity (AUC(0,∞)) was calculated by adding AUC(0,t last) and C last∙λz −1. C last is the last measured concentration and λz is the apparent terminal rate constant. λz was estimated by unweighted linear regression for the linear portion of the terminal log concentration–time curve. Dose linearity was evaluated by comparing dose‐normalized AUC(0,∞) values using one way analysis of variance (anova). Dose linearity was confirmed if the differences among three groups were not significant. Additionally, a power model and confidence interval (CI) criteria approach was used 19:
| (1) |
where dose linearity implies that β1 = 1 in Equation (1) and PK denotes a pharmacokinetic variable.
Population pharmacokinetic analysis
The pharmacokinetic modelling was performed with nonmem VII (ICON Development Solutions, Ellicott City, MD, USA) using the ADVAN 6 subroutine and the first order conditional estimate with interaction. A log‐normal or additive model was used to estimate the inter‐individual random variability of pharmacokinetic parameters. Diagonal matrices were estimated for the distributions of inter‐individual random variability (η). Additive, constant and combined additive and constant coefficients of variation residual error models were evaluated during the model building process. Using nonmem software, minimum value of the objective function was computed, which is a statistical equivalent of the −2 log likelihood of the model. A reduction of 3.84 in objective function value (OFV) (chi‐square distribution, degrees of freedom =1, P < 0.05), was used to discriminate between hierarchical models 20. In addition, diagnostic goodness‐of‐fit plots were used to evaluate the improvements between hierarchical models.
One, two and three compartment disposition models with first order elimination were tested. Age, height, weight, lean body mass, body mass index, body surface area, gender and CYP2D6 phenotype were analyzed as covariates 21, 22, 23. Non‐parametric bootstrap analysis was used for internal validation of the model (fit4NM 3.6.0, Eun‐Kyung Lee and Gyu‐Jeong Noh, http://www.fit4nm.org/download, last accessed November 4, 2013) 24. Bootstrap replicate values were generated 2000 times in total, by random sampling with replacement. The median values and the 95% confidence interval of the non‐parametric bootstrap replicates were compared with parameter estimates of the final pharmacokinetic model. Predictive checks were performed by simulating 2000 iterations and comparing the 95% prediction intervals with those of the original data (fit4NM 3.6.0) 25. Differences in the OFVs between the model without the covariate tested (reference model) and the model containing the covariate tested (covariate model) fitted to the permuted datasets were sorted in ascending order and the fifth percentile was set as δ. If the change in the OFVs between the reference and covariate models of the original data was greater than the δ, the covariate efficacy was considered statistically significant 26.
Evaluation of prophylactic anti‐emetic efficacy
In an earlier study that compared ramosetron and ondansetron, the incidences of nausea were 62% and 70%, respectively 10. To allow detection of an 8% difference in the incidence of PONV between the two groups with 80% power and an α value of 0.05, 546 patients were needed per group. The dropout rate was assumed to be 10%. In total, 1102 patients were enrolled in the PONV analysis. Risk stratification of the enrolled patients was performed according to their Apfel score 3.
Patients who consented to the PONV analysis were allocated randomly to either the ramosetron or ondansetron group. The randomization codes for the four medical centres were created by a statistician who was not involved in the study. Random assignments were made with a 1 : 1 allocation ratio using block randomization based on the Apfel score and surgery type. All patients received sevoflurane anaesthesia during surgery. At the end of surgery, either 0.3 mg ramosetron (Nasea®, Astellas, Tokyo, Japan) or 4 mg ondansetron (Zofran®; GlaxoSmithKline, London, UK) was administered intravenously according to the allocated group. The intravenous patient‐controlled analgesia (i.v. PCA) was a 200 ml solution consisting of fentanyl, ketorolac and normal saline. For the ondansetron group, an additional 16 mg ondansetron were mixed with the i.v. PCA, according to the current clinical practice in the four medical centres. PONV was evaluated by incidence and the RINVR 6, 24 and 48 h post‐operatively. The patients in the study and clinicians who evaluated PONV were blinded to the allocated group. The number needed to treat (NNT) was calculated to measure the treatment effects of ramosetron compared with ondansetron 27, 28, 29, 30. NNT indicates the number of patients needed to undergo the experimental therapy to prevent one additional adverse event, which can be used as an indicator of the overall clinical impact of the intervention 30, 31. When a patient first complained of PONV, 10 mg metoclopramide were administered intravenously. If the symptoms persisted after 15 min, 1 mg ondansetron was administered intravenously up to five times until symptoms subsided. PONV was assessed before and 1 h after administration of the rescue anti‐emetic to evaluate its efficacy in relieving symptoms. Early and late PONV were defined as occurring 0–6 and 6–48 h post‐operatively, respectively.
Based on the final pharmacokinetic model, the plasma ramosetron concentration was simulated using the Asan Pump ver. 2.1.3 software (Dr G.J. Noh and Bionet, Seoul, South Korea) and a non‐compartmental analysis was performed in patients allocated to the ramosetron group for the PONV analysis. A simulation was also performed based on the pharmacokinetic parameters from a recent study of the pharmacokinetics of ramosetron 7.
Statistics
The statistical analysis was performed using R ver. 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) or SigmaStat 3.5 (Systat Software, Chicago, IL, USA). Continuous variables were analyzed using the t‐test or rank‐sum test and categorical variables were analyzed using the chi‐square test or Fisher's exact test. Comparisons between more than two groups were analyzed using one way anova or the Kruskal–Wallis test. Data are presented as means (SDs), medians (ranges) and counts and percentages for normally distributed continuous variables, non‐normally distributed continuous variables, and categorical variables, respectively. A P value < 0.05 was considered significant.
Results
Pharmacokinetic analysis
For the pharmacokinetic analysis, 67 patients were enrolled and assigned randomly, nine of whom were excluded due to withdrawal of consent or violation of the study protocol. Seven hundred and thirty‐nine plasma concentration measurements from the remaining 58 patients were used to determine the pharmacokinetics. Patient characteristics were comparable among the 0.3, 0.45 and 0.6 mg ramosetron groups (Table 1). According to the CYP2D6 phenotype, 1, 12, 45 and 0 patients were poor, intermediate, extensive and ultrarapid metabolizers, respectively.
Table 1.
Characteristics of the patients included in the ramosetron pharmacokinetic analysis
| 0.3 mg (n = 17) | 0.45 mg (n = 19) | 0.6 mg (n = 22) | |
|---|---|---|---|
| Age (years) | 55.9 ± 9.3 | 61.5 ± 9.1 | 58.4 ± 13.9 |
| Gender (male/female) | 10/7 | 12/7 | 13/9 |
| Weight (kg) | 65.0 ± 9.1 | 64.7 ± 10.2 | 59.4 ± 8.2 |
| Height (cm) | 162.9 ± 7.7 | 162.4 ± 7.7 | 159.1 ± 7.8 |
| Lean body mass * (kg) | 47.0 (38.1–56.1) | 51.6 (37.4–52.7) | 46.0 (35.7–50.0) |
| Body mass index (kg∙m−2) | 24.5 ± 2.5 | 24.5 ± 3.2 | 23.4 ± 2.2 |
Data are means ± SD, medians (25–75%), percentages or counts, as appropriate.
Calculated by the Janmahasatian formula. No statistical differences in parameters between groups (P > 0.05).
The non‐compartmental pharmacokinetic parameters are shown in Table 2. The differences in the dose‐normalized AUC(0,t last) or AUC(0,∞) were not significant. The dose‐normalized AUC(0,t last) was 2.63, 7.28 (25–75%; 5.19–11.24), and 7.27 (4.86–8.79) min l−1 in poor, intermediate and extensive metabolizers, respectively (P = 0.244, Kruskal–Wallis test). The relationship between the total dose and total AUC after log transformation revealed a linear pharmacokinetic relationship (slope = 1.291; 95% CI 0.941, 1.642).
Table 2.
Non‐compartmental pharmacokinetic parameters for ramosetron after bolus administration
| 0.3 mg (n = 17) | 0.45 mg (n = 19) | 0.6 mg (n = 22) | |
|---|---|---|---|
| AUC(0,tlast_D (min∙l−1) | 4.5 ± 1.7 | 4.4 ± 1.3 | 5.0 ± 1.7 |
| AUC(0,∞_D) (min∙l−1) | 5.5 (4.5–6.7) | 5.4 (4.1–6.1) | 7.0 (5.2–8.0) |
| CL(l∙min−1) | 0.18 (0.15–0.22) | 0.18 (0.17–0.25) | 0.15 (0.12–0.19) |
| λZ (l∙min−1) | 0.0011 (0.0009–0.0015) | 0.0012 (0.0010–0.0014) | 0.0010 (0.0007–0.0011) |
Data are means ± SD or medians (25–75%), as appropriate. AUC(0,t last_D) dose‐normalized AUC(0,t last) (AUC(0,t last) area under the curve from administration to the last measured concentration); AUC(0,∞_D) dose‐normalized AUC(0,∞) (AUC (0,∞) area under the curve from administration to infinity); CL clearance; λZ terminal elimination rate constant.
A two compartment mammillary model with first order elimination best described the pharmacokinetics of ramosetron. Age was a significant covariate in metabolic clearance, resulting in an improved OFV (5.43, P < 0.001, degrees of freedom 1), compared with the basic model (number of model parameters 9). The CYP2D6 phenotype had no influence on metabolic clearance when incorporated into the pharmacokinetic model. The δ value between the basic and final models in the randomization test was 0.17. The pharmacokinetic parameter estimates and the non‐parametric bootstrap results of the final model are listed in Table 3. The population parameter estimates and the bootstrap median values were close and the 95% CIs of these parameters were relatively small, indicating the accuracy of the final pharmacokinetic parameter estimates. Figure 1 shows the goodness‐of‐fit plots of the final model. The predicted values and the measured values were consistent and the conditional weighted residuals were generally distributed around zero. The predictive check results are shown in Figure 2. The observed values were mostly within the 95% prediction interval. A small proportion of the data was distributed outside the 95% prediction intervals of 2.0%, 3.0% and 3.8% in the ramosetron 0.3, 0.45 and 0.6 mg groups, respectively, indicating that the final pharmacokinetic model adequately described the time courses of the plasma ramosetron concentrations.
Table 3.
Population pharmacokinetic parameter estimates, inter‐individual variability and median parameter values (2.5–97.5%) of the non‐parametric bootstrap replicates for the final ramosetron pharmacokinetic model
| Model | Parameters | Estimates (RSE, %) | CV (%) | Median (2.5–97.5%) | |
|---|---|---|---|---|---|
| Basic | V 1(l) | 5.2 (9.5) | 46.2 | − | |
| V 2 (l) | 108 (3.6) | 27.8 | − | ||
| CL(l∙min−1) | 0.17 (3.9) | 27.3 | − | ||
| Q (l∙min−1) | 1.43 (7.3) | 47.4 | − | ||
| σ2 (%) | 0.274 (7.3) | − | − | ||
| Final | V 1 (l) | 5.12 (9.2) | 45.6 | 5.17 (4.05–6.34) | |
| V 2 (l) | 108 (6.3) | 27.5 | 108 (99.4–117) | ||
| CL (l∙min−1) θ1 + (59∙age−1) × θ2 | θ1 | 0.08 (47.5) | 26.3 | 0.06 (0.006–0.16) | |
| θ2 | 0.09 (45.9) | 0.11 (0.02–0.33) | |||
| Q(l∙ min−1) | 1.42 (10.2) | 47.0 | 1.41 (0.33–1.66) | ||
| σ 2, % | 0.274 (3.1) | − | 0.27 (0.24–0.32) | ||
A log‐normal distribution of inter‐individual random variability was assumed. Residual random variability was modelled using a constant coefficient of variation (CV) model. Non‐parametric bootstrap analysis was repeated 2000 times. The shrinkage value of V 1, V 2, CL and Q was 19.98, 9.26, 9.94 and 7.99%, respectively. V 1 central volume of distribution; V 2 peripheral volume of distribution; CL clearance; Q inter‐compartmental clearance; RSE relative standard error = SE∙mean−1 × 100 (%); σ2 variance of residual random variability.
Figure 1.
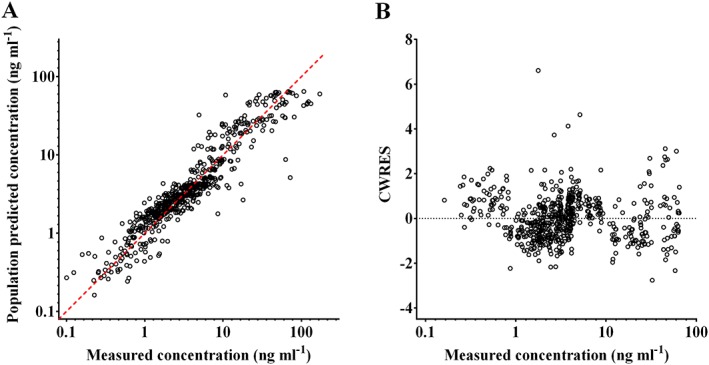
Goodness‐of‐fit plots for the final ramosetron pharmacokinetic model. A) Population predicted vs. measured plasma ramosetron concentrations and B) conditional weighted residuals (CWRES) vs. predicted plasma ramosetron concentration
Figure 2.
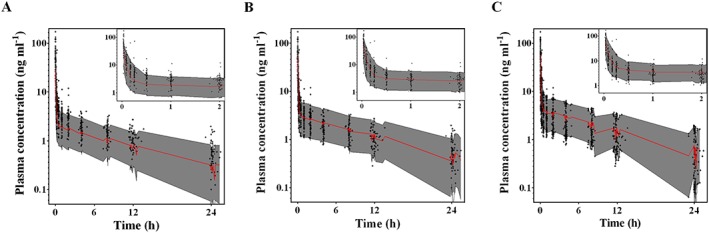
Predictive checks of the final ramosetron pharmacokinetic model with A) 0.3, B) 0.45 and C) 0.6 mg ramosetron. Grey‐filled area represents the model's 95% prediction interval. Red solid line indicates the 50% prediction interval. +, measured plasma ramosetron concentration
Evaluation of prophylactic anti‐emetic efficacy
A total of 1236 patients were enrolled and assigned randomly for the PONV assessment and 134 patients were excluded due to withdrawal of consent, loss during follow‐up, omission of the allocated intervention, violation of the study protocol or adverse events. In total, 1102 patients were included in the final analysis. The characteristics of the patients used for the PONV analysis are summarized in Table 4.
Table 4.
Characteristics of the patients included in the anti‐emetic efficacy analysis
| Ramosetron (n = 554) | Ondansetron (n = 548) | |
|---|---|---|
| Age (years) | 53 (44–63) | 53 (45–65) |
| Gender (male/female) | 204/350 | 218/330 |
| Weight (kg) | 60.0 (53.0–67.2) | 60.0 (54.0–68.6) |
| Height (cm) | 160.0 (156.0–166.3) | 160.1 (156.0–167.0) |
| ASA class 1/2/3 (n) | 379/165/9 | 388/155/6 |
| Duration of anaesthesia (min) | 150 (106–215) | 145 (107–210) |
| Type of surgery (%) | ||
| General surgery | 59.6 | 61.7 |
| Gynaecologic surgery | 39.0 | 37.8 |
| Major orthopaedic surgery | 1.4 | 1.4 |
| Apfel score 1/2/3/4 (n) | 76/106/306/66 | 80/117/293/58 |
Data are means ± SD, medians (25–75%), percentages or counts, as appropriate.
General surgery included colon, stomach, laparoscopic general and breast surgery. Gynaecological surgery included laparoscopic gynaecologic surgery, myomectomy, radical hysterectomy, abdominal hysterectomy, vaginal hysterectomy and salpingo‐oophorectomy. Major orthopaedic surgery included shoulder surgery, spinal surgery using instruments and total hip replacement surgery. ASA American Society of Anesthesiologists; PONV post‐operative nausea and vomiting. No differences were detected in any of the parameters between the ramosetron and ondansetron groups.
The incidence of PONV and the RINVR subscores (experience score for total PONV experience, occurrence score for objective symptoms and distress score for subjective symptoms) demonstrated significant differences between the two groups 24 and 48 h post‐operatively (Table 5) 15. The NNTs at 6, 24 and 48 h post‐operatively were 32.4 (95% CI 12.3, 74.1), 14.9 (8.7, 52.2) and 24.4 (14.7, 70.8), respectively. Patients with an Apfel score of 3 showed significant differences on the RINVR 24 and 48 h post‐operatively (Figure 3). The differences in the RINVR 6 and 24 h post‐operatively were marginally significant in patients with an Apfel score of 4 (P = 0.069 and 0.084, respectively). The NNTs calculated for patients with an Apfel score of 4 were 7.4 (95% CI 4.4, 24.0) and 6.4 (3.0, 18.0) at 6 and 24 h post‐operatively. Fentanyl consumption 48 h post‐operatively was not different between the groups (P = 0.507; rank‐sum test).
Table 5.
Incidence and Rhodes index of post‐operative nausea, vomiting and retching
| Ramosetron (n = 554) | Ondansetron (n = 548) | |
|---|---|---|
| 6 h post‐operative | ||
| Incidence | 86 (15.5) | 102 (18.6) |
| RINVR | ||
| Experience | 0 (0–20) | 0 (0–18) |
| Occurrence | 0 (0–12) | 0 (0–12) |
| Distress | 0 (0–9) | 0 (0–8) |
| 24 h post‐operative | ||
| Incidence * | 77 (13.9) | 113 (20.6) |
| RINVR | ||
| Experience * | 0 (0–15) | 0 (0–32) |
| Occurrence * | 0 (0–10) | 0 (0–20) |
| Distress * | 0 (0–9) | 0 (0–12) |
| 48 h post‐operative | ||
| Incidence * | 44 (7.9) | 66 (12.0) |
| RINVR | ||
| Experience * | 0 (0–16) | 0 (0–20) |
| Occurrence * | 0 (0–16) | 0 (0–12) |
| Distress * | 0 (0–7) | 0 (0–8) |
| Overall incidence * | 145 (26.2) | 179 (32.7) |
Data are numbers (percentages) or medians (ranges), as appropriate. RINVR, Rhodes index of nausea, vomiting and retching.
P < 0.05 vs. the ondansetron group.
Figure 3.
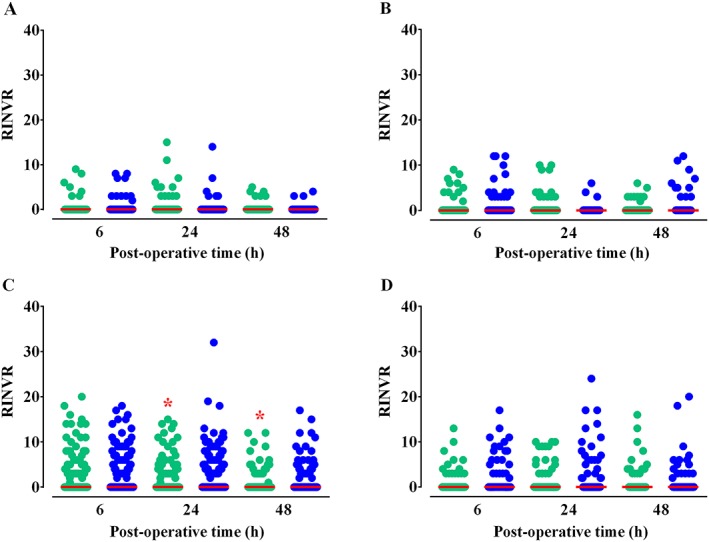
Rhodes index of nausea, vomiting and retching (RINVR) scores for patients with A) an Apfel score of 1 (ramosetron/ondansetron: n = 76/81), B) an Apfel score of 2 (106/118), C) an Apfel score of 3 (308/294) or D) an Apfel score of 4 (67/60). Red solid lines indicate the median values, which were 0 for all. The RINVR ranges for patients with an Apfel score of 1 at 6, 24, and 48 h post‐operatively were 0–9/0–8, 0–15/0–14, and 0–5/0–4, respectively. For patients with an Apfel score of 2, 0–9/0–12, 0–12/0–10 and 0–6/0–6; for patients with an Apfel score of 3, 0–20/0–18, 0–15/0–32, and 0–12/0–17; for patients with an Apfel score of 4, 0–13/0–17, 0–10/0–24 and 0–16/0–20, respectively. *P < 0.05 vs. ondansetron according to the rank‐sum test.  ramosetron,
ramosetron,  ondansetron
ondansetron
The incidence rates of rescue anti‐emetic use were 8.2% and 11.2% in the ramosetron and ondansetron groups, respectively (P = 0.117, chi‐square test). The reductions in total distress after administration of a rescue anti‐emetic in the ramosetron and ondansetron groups were 1 (1–3) and 2 (1–3), respectively (P = 0.308, rank‐sum test). Overall, 194 adverse events were reported during the study period. The incidence of adverse events was comparable between the two groups (P = 0.981, chi‐square test). Adverse events included sedation (ramosetron n = 11/ondansetron n = 8), headache (38/34), and dizziness (44/51). Other less common (<1%) adverse events were considered unrelated to the investigational drugs, including hypotension, arrhythmia, atelectasis, hypoglycaemia and shivering.
Table 6 shows the age and dose‐normalized AUC(0,t last) calculated from the simulated plasma concentration in the ramosetron group using the pharmacokinetic models in this and a previous study. The age and dose‐normalized AUC(0,t last) did not differ significantly between patients with and without PONV.
Table 6.
Dose‐normalized AUC(0,t last) calculated from the simulated plasma concentrations from different pharmacokinetic models of ramosetron
| Age (years) | Present study AUC(0,t last_D) (min∙l−1) | Lee et al. 7 AUC(0,t last_D ) (min∙l−1) | |
|---|---|---|---|
| 6 h | |||
| Patients with PONV | 51 (41–63) | 6.12 (6.02–6.24) | 5.40 (4.80–6.04) |
| Patients without PONV | 54 (44.5–63) | 6.17 (6.07–6.24) | 5.39 (4.85–5.88) |
| 24 h | |||
| Patients with PONV | 52.5 (46–63) | 8.59 (8.25–8.88) | 7.85 (7.11–8.76) |
| Patients without PONV | 53 (44–63) | 8.54 (8.16–8.88) | 7.89 (7.03–8.76) |
| 48 h | |||
| Patients with PONV | 54.5 (47–67) | 9.20 (8.78–9.78) | 8.82 (7.62–9.60) |
| Patients without PONV | 53 (43.5–63) | 9.12 (8.60–9.61) | 8.53 (7.61–9.49) |
Data are medians (25–75%). The simulation was performed for patients who had received prophylactic ramosetron, based on pharmacokinetic parameters. AUC(0,t last_D) dose‐normalized AUC(0,t last) (AUC(0,t last) area under the curve from administration to the last measured concentration); PONV post‐operative nausea and vomiting. There were no significant differences between patients with and without PONV in age or AUC(0,t last_D). P < 0.05 was considered significant.
Discussion
In this study, the pharmacokinetics of ramosetron were well described by a two compartment mammillary model, and age was a significant covariate on metabolic clearance. Prophylactic anti‐emetic efficacy of ramosetron was significant 24 and 48 h post‐operatively, compared with that of ondansetron and this difference was significant in patients with an Apfel score of 3. This study extended the results of the previous pharmacokinetics study of ramosetron in two ways. First, our study revealed dose linearity for the pharmacokinetics of ramosetron. Second, we evaluated the prophylactic anti‐emetic efficacy of ramosetron in a large population, considering risk stratification based on the Apfel score at enrolment.
Our results demonstrated that age was a significant covariate on metabolic clearance. In a previous study on ramosetron pharmacokinetics, age was the most significant covariate on metabolic clearance, which coincides with our study 7. The effect of age on metabolic clearance can be readily explained by the reduction of liver function with increasing age, as 5‐HT3 antagonists usually undergo extensive hepatic metabolism 32. However, based on the pharmacokinetic parameters, the simulation of the prophylactic anti‐emetic efficacy evaluation in the ramosetron group performed using Asan Pump software revealed a comparable dose‐normalized AUC(0,t last) between patients with and without PONV. There are several possible explanations for this result. First, age is a predictable risk factor for PONV in the adult population, which is compatible with the simulation result 3, 33, 34. Second, although age as a covariate improved the OFV of the pharmacokinetic model, the improvement was minimal, suggesting a weak influence of age on the plasma ramosetron concentration. Therefore, comparable ages among subjects, regardless of PONV, may have prevented the significant difference in the dose‐normalized AUC(0,t last). Moreover, the correlation between PONV and the plasma ramosetron concentration may be an all‐or‐none pattern rather than a linear pattern, due to its high receptor affinity 35, 36.
The difference compared with the previous study is that body weight was not a significant covariate in this study. However, there are two clinical reasons why body weight was not a covariate. First, although body weight was a significant covariate in the previous study, the inclusion of body weight decreased the interindividual variability by less than 5%, leaving a large portion of the variability unexplained 7. Second, a study of the pharmacokinetics of ondansetron suggested that it is unlikely for weight to cause dose adjustment of ondansetron due to the wide therapeutic index, although their data revealed that weight was a significant covariate 37. Since ramosetron has greater receptor affinity than ondansetron, we suggest that it is clinically more acceptable not to include body weight as a covariate.
The pharmacokinetics of ramosetron were best described by a two compartment model, whereas a three compartment model was used in a recent study 7. The discrepancy between the two studies might be explained by the additional 48 h post‐operative sample acquired in the previous study. During the early stages of ramosetron development in 1991, the last sample point used in the phase 1 trial was 24 h. However, the high receptor affinity of ramosetron may have required a sampling time longer than 24 h to allow identification of a possible third compartment 35.
Our results demonstrate similar anti‐emetic efficacy 6 h post‐operatively between the ramosetron and ondansetron groups, whereas i.v. bolus administration of 0.3 mg ramosetron showed better anti‐emetic efficacy than that of 4 mg i.v. ondansetron in earlier studies 13, 14. This may be due to the additional ondansetron that was mixed in the i.v. PCA. Previous reports suggest that mixing anti‐emetics in i.v. PCA reduces the incidence of PONV after surgery, and that bolus 4 mg administration with 12 mg ondansetron mixed in the i.v. PCA has similar anti‐emetic efficacy to that of a 0.3 mg ramosetron bolus 0–6 h post‐operatively 8, 10. However, in our study, although additional ondansetron was mixed in the i.v. PCA, the total incidence of PONV was higher in the ondansetron group 24 and 48 h post‐operatively than in the ramosetron group. The longer efficacy of ramosetron compared with ondansetron has been demonstrated in a previous study 10. This may be due to the higher receptor affinity of ramosetron, suggesting that the prophylactic anti‐emetic efficacy of a bolus of 0.3 mg ramosetron lasts longer than 24 h post‐operatively 35, 36.
The comparable efficacies between the two anti‐emetics in patients with Apfel scores of 1 or 2 may be due to the relatively low incidence of PONV in the population. However, despite a relatively higher incidence of PONV in patients with an Apfel score of 4, the differences in the RINVR score between the two groups 6 and 24 h post‐operatively were marginally significant. Therefore, we considered NNT to account for this result. NNT reflects the efficacy of an intervention compared with the control using a simple and intuitive estimate, and a positive value or confidence interval (CI) is interpreted as a benefit of the intervention, whereas a value of ∞ indicates no effect and a negative value a harmful effect 30. A low NNT value indicates a highly effective intervention, but values <5 are rare in clinical practice 30. In our study, the NNT values 6 and 24 h post‐operatively in patients with an Apfel score of 4 were close to 5, which was low enough to favour the anti‐emetic efficacy of ramosetron. This result suggests that the size of the group with an Apfel score of 4 may have been insufficient to show a difference between the two anti‐emetics. NNT has been used as a measure to report results from randomized controlled trials investigating PONV prevention and treatment 27, 28, 29, 38.
A limitation of our study was that insufficient numbers of poor and ultrarapid metabolizers were included in the pharmacokinetic analysis, so we could not compare all four CYP2D6 phenotypic classes. The frequencies of the CYP2D6 phenotypic classes differ widely among ethnic groups 39. Extensive and intermediate metabolizers comprise most of the East Asian population, whereas the phenotypic frequencies for poor and ultrarapid metabolizers are extremely low 40. Further investigation is required to determine the impact of the CYP2D6 genotype.
In conclusion, a two compartment mammillary model adequately described the pharmacokinetics of ramosetron, and age was a significant covariate on metabolic clearance. Prophylactic bolus administration of 0.3 mg ramosetron significantly reduced the incidence of late PONV compared with prophylactic 4 mg ondansetron with 16 mg ondansetron mixed in the i.v. PCA, particularly in patients with Apfel scores ≥3.
Competing Interests
All authors have completed the unified competing interest form. None of the authors has had any financial relationships during the past 3 years with any organization with a potential interest in the submitted work and none has any other relationships or activities that could have influenced the submitted work. Astellas Pharma Korea, Inc. had no role in the study design, data interpretation, data analysis or report writing.
We are grateful to Ga‐Eun Kang, Ph.D., from the Clinical Trial Centre of Chonnam National University Hospital, for performing the ramosetron concentration measurements. We are also deeply grateful to Jeong‐Sim Yang, AS of Asan Medical Centre, for her efforts as clinical research coordinator.
Funding
This study was supported by Astellas Pharma Korea and a Student Research Grant (14–15) of University of Ulsan College of Medicine, Seoul, Korea.
Contributors
YHL, JHS, BMC and GJN designed the study, KTM, YJL, BMC and SWJ collected the data and YHL, JHS, EKL and BMC performed the data analysis and interpretation. All authors contributed to manuscript writing, provided critical revisions and approved the final version.
Lee, Y.‐H. , Seo, J.‐H. , Min, K.‐T. , Lim, Y.‐J. , Jeong, S.‐W. , Lee, E.‐K. , Choi, B.‐M. , and Noh, G.‐J. (2016) Population pharmacokinetics and prophylactic anti‐emetic efficacy of ramosetron in surgical patients. Br J Clin Pharmacol, 82: 762–772. doi: 10.1111/bcp.13010.
References
- 1. Gan T, Sloan F, Dear Gde L, El‐Moalem HE, Lubarsky DA. How much are patients willing to pay to avoid postoperative nausea and vomiting? Anesth Analg 2001; 92: 393–400. [DOI] [PubMed] [Google Scholar]
- 2. Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg 1999; 89: 652–8. [DOI] [PubMed] [Google Scholar]
- 3. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross‐validations between two centers. Anesthesiology 1999; 91: 693–700. [DOI] [PubMed] [Google Scholar]
- 4. Gan TJ, Ginsberg B, Grant AP, Glass PS. Double‐blind, randomized comparison of ondansetron and intraoperative propofol to prevent postoperative nausea and vomiting. Anesthesiology 1996; 85: 1036–42. [DOI] [PubMed] [Google Scholar]
- 5. Habib AS, Gan TJ. Evidence‐based management of postoperative nausea and vomiting: a review. Can J Anaesth 2004; 51: 326–41. [DOI] [PubMed] [Google Scholar]
- 6. Shi Y, He X, Yang S, Ai B, Zhang C, Huang D, et al. Ramosetron versus ondansetron in the prevention of chemotherapy‐induced gastrointestinal side effects: A prospective randomized controlled study. Chemotherapy 2007; 53: 44–50. [DOI] [PubMed] [Google Scholar]
- 7. Lee SH, Cho SY, Yoo KY, Jeong S. Population pharmacokinetics of ramosetron. J Pharmacokinet Pharmacodyn 2016; 43: 73–83. [DOI] [PubMed] [Google Scholar]
- 8. Choi DK, Chin JH, Lee EH, Lim OB, Chung CH, Ro YJ, et al. Prophylactic control of post‐operative nausea and vomiting using ondansetron and ramosetron after cardiac surgery. Acta Anaesthesiol Scand 2010; 54: 962–9. [DOI] [PubMed] [Google Scholar]
- 9. Choi YS, Shim JK, Ahn SH, Kwak YL. Efficacy comparison of ramosetron with ondansetron on preventing nausea and vomiting in high‐risk patients following spine surgery with a single bolus of dexamethasone as an adjunct. Korean J Anesthesiol 2012; 62: 543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi YS, Shim JK, Yoon DH, Jeon DH, Lee JY, Kwak YL. Effect of ramosetron on patient‐controlled analgesia related nausea and vomiting after spine surgery in highly susceptible patients: comparison with ondansetron. Spine (Phila Pa 1976) 2008; 33: E602–6. [DOI] [PubMed] [Google Scholar]
- 11. Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic antiemetic efficacy of ramosetron and ondansetron in patients at high‐risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia 2010; 65: 500–4. [DOI] [PubMed] [Google Scholar]
- 12. Lee JW, Park HJ, Choi J, Park SJ, Kang H, Kim EG. Comparison of ramosetron's and ondansetron's preventive antiemetic effects in highly susceptible patients undergoing abdominal hysterectomy. Korean J Anesthesiol 2011; 61: 488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryu J, So YM, Hwang J, Do SH. Ramosetron versus ondansetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc 2010; 24: 812–7. [DOI] [PubMed] [Google Scholar]
- 14. Ryu JH, Lee JE, Lim YJ, Hong DM, Park HP, Han JI, et al. A prospective, randomized, double‐blind, and multicenter trial of prophylactic effects of ramosetronon postoperative nausea and vomiting (PONV) after craniotomy: comparison with ondansetron. BMC Anesthesiol 2014; 14: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rhodes VA, McDaniel RW. The Index of Nausea, Vomiting, and Retching: a new format of the Index of Nausea and Vomiting. Oncol Nurs Forum 1999; 26: 889–94. [PubMed] [Google Scholar]
- 16. Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet 2009; 48: 761–804. [DOI] [PubMed] [Google Scholar]
- 17. Ingelman‐Sundberg M. Genetic susceptibility to adverse effects of drugs and environmental toxicants. The role of the CYP family of enzymes. Mutat Res 2001; 482: 11–9. [DOI] [PubMed] [Google Scholar]
- 18. Kaiser R, Sezer O, Papies A, Bauer S, Schelenz C, Tremblay PB, et al. Patient‐tailored antiemetic treatment with 5‐hydroxytryptamine type 3 receptor antagonists according to cytochrome P‐450 2D6 genotypes. J Clin Oncol 2002; 20: 2805–11. [DOI] [PubMed] [Google Scholar]
- 19. Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res 2000; 17: 1278–83. [DOI] [PubMed] [Google Scholar]
- 20. Beal S. SLNUsGIPVIG. San Francisco: NONMEM Project Group, University of California, 1987; 48. [Google Scholar]
- 21. Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med 1987; 317: 1098. [DOI] [PubMed] [Google Scholar]
- 22. Hallynck TH, Soep HH, Thomis JA, Boelaert J, Daneels R, Dettli L. Should clearance be normalised to body surface or to lean body mass? Br J Clin Pharmacol 1981; 11: 523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet 2005; 44: 1051–65. [DOI] [PubMed] [Google Scholar]
- 24. Kern SE, Xie G, White JL, Egan TD. A response surface analysis of propofol‐remifentanil pharmacodynamic interaction in volunteers. Anesthesiology 2004; 100: 1373–81. [DOI] [PubMed] [Google Scholar]
- 25. Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther 2007; 82: 17–20. [DOI] [PubMed] [Google Scholar]
- 26. Wählby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn 2001; 28: 231–52. [DOI] [PubMed] [Google Scholar]
- 27. Eberhart LH, Morin AM, Bothner U, Georgieff M. Droperidol and 5‐HT3‐receptor antagonists, alone or in combination, for prophylaxis of postoperative nausea and vomiting. A meta‐analysis of randomised controlled trials. Acta Anaesthesiol Scand 2000; 44: 1252–7. [DOI] [PubMed] [Google Scholar]
- 28. Tramer M, Moore RA, McQuay H. Prevention of vomiting after paediatric strabismus surgery: a systematic review using the numbers‐needed‐to‐treat method. Br J Anaesth 1995; 75: 556–61. [DOI] [PubMed] [Google Scholar]
- 29. Tramer MR, Moore RA, Reynolds DJ, McQuay HJ. A quantitative systematic review of ondansetron in treatment of established postoperative nausea and vomiting. BMJ 1997; 314: 1088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tramer MR, Walder B. Number needed to treat (or harm). World J Surg 2005; 29: 576–81. [DOI] [PubMed] [Google Scholar]
- 31. Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995; 310: 452–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gan TJ. Selective serotonin 5‐HT3 receptor antagonists for postoperative nausea and vomiting: are they all the same? CNS Drugs 2005; 19: 225–38. [DOI] [PubMed] [Google Scholar]
- 33. Gan TJ. Postoperative nausea and vomiting—can it be eliminated? JAMA 2002; 287: 1233–6. [DOI] [PubMed] [Google Scholar]
- 34. Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 1992; 77: 162–84. [DOI] [PubMed] [Google Scholar]
- 35. Ogata A, Yamada Y, Sugiura M, Takayanagi R, Sawada Y, Iga T. Analysis of 5‐HT3 receptor antagonist, ramosetron hydrochloride, based on receptor occupancy considering its active metabolite. Yakugaku Zasshi 2001; 121: 793–8. [DOI] [PubMed] [Google Scholar]
- 36. Rabasseda X. Ramosetron, a 5‐HT3 receptor antagonist for the control of nausea and vomiting. Drugs Today (Barc) 2002; 38: 75–89. [DOI] [PubMed] [Google Scholar]
- 37. de Alwis DP, Aarons L, Palmer JL. Population pharmacokinetics of ondansetron: a covariate analysis. Br J Clin Pharmacol 1998; 46: 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mihara T, Tojo K, Uchimoto K, Morita S, Goto T. Reevaluation of the effectiveness of ramosetron for preventing postoperative nausea and vomiting: a systematic review and meta‐analysis. Anesth Analg 2013; 117: 329–39. [DOI] [PubMed] [Google Scholar]
- 39. Janicki PK, Schuler HG, Jarzembowski TM, Rossi M. Prevention of postoperative nausea and vomiting with granisetron and dolasetron in relation to CYP2D6 genotype. Anesth Analg 2006; 102: 1127–33. [DOI] [PubMed] [Google Scholar]
- 40. Sistonen J, Sajantila A, Lao O, Corander J, Barbujani G. Fuselli SCYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics 2007; 17: 93–101. [DOI] [PubMed] [Google Scholar]


