Abstract
Aims
To measure the prevalence of beta‐blocker eye drop prescribing and respiratory effect of ocular beta‐blocker administration in people with asthma.
Methods
We measured the prevalence of ocular beta‐blocker prescribing in people with asthma and ocular hypertension, and performed a nested case–control study (NCCS) measuring risk of moderate exacerbations (rescue steroids in primary care) and severe exacerbations (asthma hospitalization) using linked data from the UK Clinical Practice Research Datalink. We then performed a systematic review and meta‐analysis of clinical trials evaluating changes in lung function following ocular beta‐blocker administration in people with asthma.
Results
From 2000 to 2012, the prevalence of non‐selective and selective beta‐blocker eye drop prescribing in people with asthma and ocular hypertension fell from 23.0% to 13.4% and from 10.5% to 0.9% respectively. In the NCCS, the relative incidence (IRR) of moderate exacerbations increased significantly with acute non‐selective beta‐blocker eye drop exposure (IRR 4.83, 95% CI 1.56–14.94) but not with chronic exposure. In the meta‐analysis, acute non‐selective beta‐blocker eye drop exposure caused significant mean falls in FEV1 of −10.9% (95% CI −14.9 to −6.9), and falls in FEV1 of ≥20% affecting one in three. Corresponding values for selective beta‐blockers in people sensitive to ocular non‐selective beta‐blockers was −6.3% (95% CI −11.7 to −0.8), and a non‐significant increase in falls in FEV1 of ≥20%.
Conclusion
Non‐selective beta‐blocker eye drops significantly affect lung function and increase asthma morbidity but are still frequently prescribed to people with asthma and ocular hypertension despite safer agents being available.
Keywords: asthma, beta‐blocker, drug safety, glaucoma, meta‐analysis
What is Already Known about this Subject
Beta‐blocker eye drops may be absorbed into the systemic circulation but the prevalence of beta‐blocker eye drop prescribing and impact on lung function and exacerbations in people with asthma has been poorly quantified.
What this Study Adds
Acute non‐selective beta‐blocker eye drop exposure significantly affects lung function and increases asthma morbidity but are still frequently prescribed to people with asthma and ocular hypertension despite safer agents being available.
Introduction
International guidelines recommend that beta‐blockers are contraindicated in asthma over safety concerns regarding acute bronchoconstriction 1, 2, 3. This effect results from endogenous and exogenous catecholamine antagonism at the pulmonary beta2‐adrenoceptor leading to unopposed cholinergic tone. However, beta‐blockers are not uncommonly prescribed to people with asthma, in part because their risk has been poorly quantified. Although the respiratory effect of oral beta‐blockers in people with asthma appears to vary according to selectivity, dose and individual susceptibility, less is known regarding the effect of beta‐blocker eye drops that may be systemically absorbed 4.
Beta‐blocker eye drops are effective therapy for managing ocular hypertension. They reduce aqueous humour production and intra‐ocular pressure by antagonizing ciliary body beta‐adrenoceptors, thereby preventing complications such as visual loss 5. As with oral agents, beta‐blocker eye drops vary in their degree of beta1:beta2‐adrenoceptor selectivity, with betaxolol being the principal ocular selective beta‐blocker in clinical use 6. Although applied topically, systemic absorption may occur via the nasolacrimal system or the conjunctiva, without undergoing first pass metabolism 7. The respiratory effect of beta‐blocker eye drops in asthma has been poorly quantified despite reports of asthma deaths associated with ocular administration 8. Oral beta‐blockers have been reported to be prescribed to around 2.2% of adults with asthma annually, but the prevalence of beta‐blocker eye drop prescribing in people with asthma, and their subsequent effect on lung function and asthma morbidity remains uncertain 9.
The aims of this study were to: measure beta‐blocker eye drop prescribing in people with asthma and ocular hypertension; quantify the risk of asthma morbidity from ocular beta‐blocker exposure; and meta‐analyse clinical trial data, evaluating changes in lung function following beta‐blocker eye drop administration in people with asthma.
Method
Data source and population for pharmacoepidemiological studies
Data were extracted from the UK Clinical Practice Research Datalink (CPRD) which contains electronic medical record (EMR) data from >5 million UK people (further details of the data source are contained in online supplement 1) 10, 11, 12. People with medically treated asthma and ocular hypertension were identified by Read Codes and prescriptions for asthma and ocular hypertension medicines. The cohort consisted of people ≥18 years of age present in the CPRD between 1 January 2000 and 31 December 2011. Subjects were eligible if they: were permanently registered with a general practice for ≥1 year; were from hospital episode statistics (HES)‐linked practices; were defined by the CPRD as being acceptable for use in research (meaning their data had met quality standards); had a Read Code for asthma and were issued one or more prescriptions for ocular hypertension medicines.
Cohort entry was defined as the first ocular hypertension prescription issued on or after: 1 January 2000; date of the first asthma medicine; date of the patient's 18th birthday; and before the date of the patient's 80th birthday. The cohort was followed until either of the following occurred: an asthma event (defined in the nested case–control section below); deregistration from the general practice; one year following the last asthma medication (thereby censoring people with asthma that had resolved or was inactive); end of ocular hypertension medical treatment; or end of the study period (31 December 2011). Asthma medicines were defined as: inhaled short‐acting beta2‐agonists (SABA); inhaled corticosteroids (ICS); inhaled long‐acting beta2‐agonists (LABA); oral leukotriene antagonists; and oral methylxanthines 13. All patients were issued two or more prescriptions for asthma medication. Ocular hypertension medicines were defined as ocular: beta‐blockers; carbonic anhydrase inhibitors; miotics; sympathomimetics; or prostaglandin analogues 6. End of ocular hypertension medical treatment was defined by the last prescription date for an ocular hypertension medicine (plus a 90‐day grace period) when 180 days had passed without any subsequent prescription of an ocular antihypertensive.
Drug utilization study
The quarterly prevalence of non‐selective and selective beta‐blocker eye drop prescribing was calculated between 1 January 2000 and 31 December 2011. The numerator consisted of the number of people issued at least one non‐selective or selective beta‐blocker eye drop prescription and the denominator the total number of people with active asthma and ocular hypertension in the cohort during the same quarter.
Nested case–control study
Outcomes
A population‐based retrospective cohort using a matched, nested case–control design was used to account for time‐varying confounders and drug exposure 14. Two nested case–control studies were performed evaluating (1) moderate asthma exacerbations and, (2) severe asthma exacerbations. Severe asthma exacerbations were defined as a hospitalization for asthma (defined by ICD codes for asthma as the primary reason for hospitalization). Moderate asthma exacerbations were defined by receipt of rescue oral steroids in primary care, identified as oral prednisolone prescriptions lasting less than 2 weeks in duration using ≥5 mg strength tablets (therefore people taking maintenance corticosteroids were excluded from this analysis). For each outcome, the date of the first asthma event was the index date for case subjects. Please see online supplement 1 for further details.
Controls
Up to four controls were randomly selected from the same population and matched to each case on age decile, gender and calendar year of cohort entry using incidence density sampling. The risk set date was the index date for cases. Four cases of severe asthma exacerbation (3.1%) and 18 moderate exacerbations (3.0%) were initially unmatched, but were later included matched on gender and calendar year of cohort entry only, with sensitivity analysis performed excluding these cases.
Exposure
Exposure was measured by the presence or absence of beta‐blocker eye drop prescriptions issued prior to the index date. Beta‐blocker eye drop exposure was categorized into: current acute exposure (defined as a prescription issued in a 30‐day risk window before the index date and no previous prescriptions issued in days 31–365 before the index date); current chronic exposure (defined as a prescription issued in a 30‐day risk window before the index date and at least one previous prescription issued in days 31–365 before the index date); and no exposure when there was no prescription issued in the risk window prior to the index date.
Confounders
The analyses were adjusted for the following confounders as described in online supplement 1, namely: asthma medicines issued within 90 days of the index date (ICS; LABA; leukotriene antagonists; methylxanthines; oral steroids (for the severe asthma exacerbation analysis), and SABAs); ever hospitalized for asthma; respiratory tract infections (RTI); exact age; smoking status; body mass index (BMI); index of multiple deprivation; nasal polyps; Charlson comorbidity index; and attendance at a primary care asthma review within the previous year. Please see online supplement 1 for further details.
Nested case–control study analysis
Chi‐squared tests and analysis of variance (anova) were used to determine significant differences between patient characteristics. Multiple imputation was used to impute missing data on height, weight and smoking status as described in online supplement 1 15. Conditional logistic regression was used to compute odds ratios for the association between outcomes and ocular beta‐blocker exposure. Using an incidence density sampling approach, odds ratios represented unbiased estimators of incidence rate ratios (IRR). Analysis was carried out using SPSS v21 and STATA v13.
Nested case–control study sensitivity analyses
For the nested case–control study, sensitivity analysis was performed: excluding people hospitalized within the risk period (assessing for potential immeasurable time bias); excluding people over the age of 40 years who smoked (assessing for potential misclassification with COPD where beta‐blockers may be better tolerated and risk underestimated); excluding cases not originally matched on age; adjusting for ICS categorized into high, moderate and low dose; adjusting for a history of animal, drug or food allergy; adjusting for other ocular antihypertensive use; using a 60‐ and 90‐day risk window (to establish whether risk attenuated over time); and a complete case analysis. Finally, a self‐controlled case series controlling for time‐varying confounders was performed to further evaluate the risk of moderate asthma exacerbations with acute non‐selective beta‐blocker eye drop exposure to compare with the nested case–control study 16. Full details of the self‐controlled case series approach are contained in online supplement 2.
Systematic review and meta‐analysis
A systematic review of MEDLINE, EMBASE and CENTRAL was conducted following standard Cochrane methodology identifying controlled clinical trials published through 1 May 2015 evaluating the respiratory effects of acute beta‐blocker eye drop exposure in people with asthma. Data were independently extracted on: mean percentage change in FEV1 (reported as the mean difference, MD); number of people experiencing falls in FEV1 of ≥20% and respiratory symptoms (reported as the risk difference, RD). In studies which included mixed populations (asthma and COPD), only data for patients with asthma were included. For included studies, non‐selective beta‐blocker eye drops were evaluated in people with asthma never exposed to beta‐blocker eye drops (with unknown clinical response), but selective beta‐blocker eye drops (betaxolol) were evaluated only in people with asthma with demonstrated respiratory sensitivity to non‐selective beta‐blockers. A fixed‐effect meta‐analysis was undertaken in Review Manager (RevMan) v5.1 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011). Sensitivity analyses and risk of bias was assessed as described in online supplement 3, which contains further details of the systematic review process including methodology for calculating missing standard deviations 17, 18.
Results
Drug utilization study
The cohort consisted of 4865 people with active asthma and ocular hypertension (mean age 67.8 years, 55.8% women) during which 128 severe asthma exacerbations and 598 moderate asthma exacerbations were identified. During follow‐up, 1128 people (23.2%) were issued 36 300 non‐selective beta‐blocker eye drop prescriptions and 241 people (5.0%) were issued 5544 selective beta‐blocker eye drop prescriptions. The quarterly prevalence of beta‐blocker eye drop prescribing in adults with active asthma and ocular hypertension is presented in Figure 1. The prevalence of non‐selective beta‐blocker eye drop prescribing fell from a high of 23.0% (95% CI 20.0–26.3) in the first quarter of 2000 to 13.4% (95% CI 11.9–15.0) in the last quarter of 2011. The most commonly prescribed non‐selective beta‐blocker eye drops were timolol followed by levobunolol, then carteolol. The prevalence of betaxolol fell from 10.5% (95% CI 8.4–13.0) in the first quarter of 2000 to only 0.9% (95% CI 0.6–1.5) in the last quarter of 2011.
Figure 1.
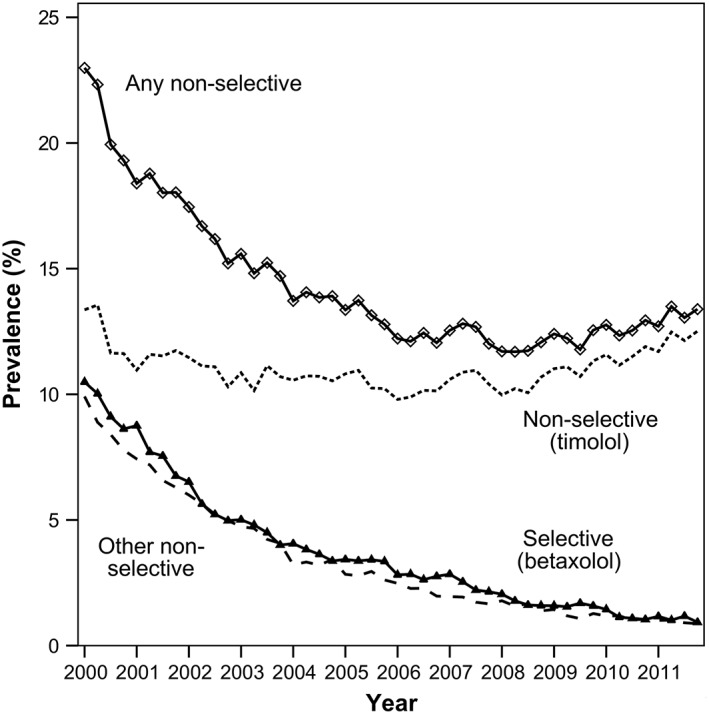
Prevalence of beta‐blocker eye drop prescribing among people with active asthma and ocular hypertension.  Any non‐selective beta‐blocker
Any non‐selective beta‐blocker 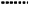 Timolol.
Timolol.  Selective beta blockers (betaxolol).
Selective beta blockers (betaxolol).  Other non‐selective beta‐blocker (levobunolol, carteolol, metipranolol)
Other non‐selective beta‐blocker (levobunolol, carteolol, metipranolol)
Nested case–control study
Characteristics of cases and controls are shown in Table 1. As expected, cases generally had significantly higher use of asthma medication, with a greater proportion having previously been hospitalized for asthma and experiencing an RTI in the 90 days prior to the index date. Crude and adjusted incidence rate ratios for the association between beta‐blocker eye drop exposure and asthma exacerbations are presented in Table 2. Acute non‐selective beta‐blocker eye drop exposure was associated with a 4.8‐fold increased relative incidence of moderate asthma exacerbations (IRR 4.83, 95% CI 1.56–14.94, P = 0.006). Chronic beta‐blocker eye drop exposure was not associated with a significantly increased risk of moderate or severe asthma exacerbations. Risk of severe asthma exacerbations from new non‐selective beta‐blocker eye drop exposure, and risk of both outcomes from new selective beta‐blocker eye drop exposure could not be quantified because of a lack of exposure. Following multivariable adjustment, the strongest risk factors for moderate asthma exacerbations among people with asthma and ocular hypertension included: having had an RTI within the previous 90 days, having previously been hospitalized for asthma, the number of SABA prescriptions issued within the previous 90 days, BMI and smoking status (Table 3).
Table 1.
Characteristics of cases and controls in the nested case control study
| Characteristics | Severe exacerbation Number (%) | Moderate exacerbation Number (%) | ||
|---|---|---|---|---|
| Cases n = 128 | Controls n = 489 | Cases n = 598 | Controls n = 2196 | |
| Age (years, SD) * | 70.0 (12.3) | 73.0 (9.6) | 70.3 (10.5)† | 71.6 (8.9) |
| Female gender | 85 (66.4) | 328 (67.1) | 333 (55.7) | 1231 (56.1) |
| Years of follow‐up (SD) * | 2.4 (2.6) | 2.4 (2.6) | 1.6 (2.2) | 1.5 (2.1) |
| Asthma therapy | ||||
| ICS | 57 (44.5) | 235 (48.1) | 266 (44.5) | 1022 (46.5) |
| LABA | 36 (28.1)† | 66 (13.5) | 72 (12.0) | 213 (9.7) |
| LABAICS | 43 (33.6) | 141 (28.8) | 185 (30.9)† | 560 (25.5) |
| Leukotriene antagonist | 15 (11.7) | 22 (4.5) | 23 (3.8)† | 28 (1.3) |
| Methylxanthine | 24 (18.8) | 51 (10.4) | 28 (4.7) | 90 (4.1) |
| Oral steroid | 72 (56.3)† | 84 (17.2) | n/a | n/a |
| No. of SABA prescriptions (SD) * | 3.0 (2.5)† | 1.7 (1.6) | 1.6 (1.6)† | 1.2 (1.4) |
| Ocular hypertension therapy | ||||
| Non‐selective beta‐blocker | 15 (11.7) | 75 (15.3) | 84 (14.0) | 303 (13.8) |
| Selective beta‐blocker | 5 (3.9) | 10 (2.0) | 15 (2.5) | 77 (3.5) |
| Prostaglandin analogue | 84 (65.6) | 349 (71.4) | 441 (73.7) | 1598 (72.8) |
| Carbonic anhydrase inhibitor | 45 (35.2) | 131 (26.8) | 161 (26.9) | 618 (28.1) |
| Sympathomimetic | 12 (16.4) | 67 (13.7) | 48 (8.0) | 207 (9.4) |
| Miotic | 5 (3.9) | 20 (4.1) | 34 (5.7) | 89 (4.1) |
| Comorbidity | ||||
| Nasal polyps | 9 (7.0) | 24 (4.9) | 23 (3.8) | 99 (4.5) |
| BMI (SD) * | 27.4 (5.7) | 27.3 (5.3) | 28.2 (5.4)† | 27.4 (6.1) |
| Charlson comorbidity index (SD) * | 2.7 (2.0) | 2.5 (1.9) | 2.4 (1.8) | 2.5 (1.9) |
| Smoking status | ||||
| Current smoker | 13 (10.2) | 82 (16.8) | 100 (16.7)† | 229 (10.4) |
| Ex‐smoker | 66 (51.6) | 258 (52.8) | 310 (51.8) | 1131 (51.5) |
| Non‐smoker | 39 (30.5) | 129 (26.4) | 169 (28.3) | 709 (32.3) |
| Missing | 10 (7.8) | 20 (4.1) | 19 (3.2) | 127 (5.8) |
| Primary care asthma review | 51 (39.8) | 151 (30.9) | 253 (42.3) | 882 (40.2) |
| Previous asthma hospitalization | 32 (25.0)† | 20 (4.1) | 30 (5.0)† | 47 (2.1) |
| RTI 90 days prior to index date | 29 (22.7)† | 50 (10.2) | 89 (14.9)† | 129 (5.9) |
Continuous variables analysed with anova, otherwise categorical variable analysed using Chi‐square test.
Characteristics with statistically significant differences between cases and controls (P < 0.05).
BMI, Body mass index; ICS, inhaled corticosteroid; LABA, long‐acting beta2‐agonist; LABAICS, long‐acting beta2‐agonist in combination inhaler with ICS; RTI, respiratory tract infection; SABA, short‐acting beta2‐agonist; SD, standard deviation.
Severe exacerbation = asthma hospitalization. Moderate exacerbation = receipt of rescue oral steroid in primary care.
Table 2.
Crude and adjusted incidence rate ratios (IRR) for the association between beta‐blocker eye drop exposure and asthma exacerbations in the nested case control study
| Any exposure | Acute exposure | Chronic exposure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |||||||
| IRR | IRR | 95% CI | P‐value | IRR | IRR | 95% CI | P‐value | IRR | IRR | 95% CI | P‐value | |
| Non‐selective beta‐blocker | ||||||||||||
| Severe exacerbation | 0.97 | 1.08 | 0.48–2.42 | 0.860 | — | — | — | — | 1.04 | 1.10 | 0.49–2.49 | 0.818 |
| Moderate exacerbation | 1.16 | 1.21 | 0.89–1.66 | 0.224 | 4.01 | 4.83 | 1.56–14.94 | 0.006 | 1.06 | 1.11 | 0.81–1.54 | 0.517 |
| Selective beta‐blocker | ||||||||||||
| Severe exacerbation | 1.85 | 1.85 | 0.32–10.88 | 0.496 | — | — | — | — | 1.85 | 1.85 | 0.32–10.89 | 0.494 |
| Moderate exacerbation | 0.85 | 0.98 | 0.47–2.02 | 0.945 | — | — | — | — | 0.85 | 0.97 | 0.47–2.01 | 0.941 |
Severe exacerbation = asthma hospitalization. Moderate exacerbation = receipt of rescue oral steroid in primary care.
Empty cells = unable to estimate due to lack of exposure in the risk window.
Table 3.
Characteristics associated with risk of moderate asthma exacerbations among people with asthma and ocular hypertension
| Characteristic | Primary care asthma exacerbation | ||
|---|---|---|---|
| Crude IRR (95%CI) | Adjusted IRR (95%CI) | P‐value | |
| RTI within the last 90 days | 2.85 (2.12–3.82) | 2.93 (2.14–4.00) | 0.001 |
| Past asthma hospitalization | 2.93 (1.81–4.70) | 2.10 (1.25–3.53) | 0.001 |
| Smoker | 1.86 (1.37–2.54) | 1.72 (1.22–2.42) | 0.002 |
| SABA prescription * | 1.20 (1.13–1.28) | 1.17 (1.10–1.25) | 0.001 |
| BMI † | 1.02 (1.01–1.04) | 1.03 (1.01–1.05) | 0.002 |
Per additional SABA prescription in the previous 90 days (continuous variable).
Per unit increase in BMI (continuous variable). Other variables are categorical variables.
Sensitivity analyses and self‐controlled case series
Sensitivity analyses were in keeping with the main findings with an increased relative incidence of moderate asthma exacerbations associated with acute non‐selective beta‐blocker eye drop exposure (online supplement 1, supplementary tables S1 and S2). The relative incidence of moderate asthma exacerbations fell with increasing risk window duration in keeping with a short‐lived risk following acute exposure. The self‐controlled case series assessing acute non‐selective beta‐blocker eye drop exposure produced consistent findings, with a 3.7‐fold increased risk of moderate asthma exacerbations within the first 30 days of initiation (IRR 3.69 (95% CI 1.53–8.94), P = 0.004) (please see online supplement 2 for further details).
Systematic review and meta‐analysis of clinical trials
Of 203 references identified, nine controlled clinical trials evaluating single‐dose beta‐blocker eye drop exposure in people with asthma were included (online supplement 3: supplementary Figure S1; supplementary Table S4) 19, 20, 21, 22, 23, 24, 25, 26, 27. Non‐selective beta‐blocker eye drops were evaluated in 55 adults (mean age 47 years, 46% male) and selective beta‐blocker eye drops in 33 adults (mean age 45 years, 66% male). Timolol was the most commonly evaluated non‐selective beta‐blocker and betaxolol the only selective beta‐blocker evaluated.
Non‐selective beta‐blocker eye drops in unselected people with asthma
Compared to control, acute non‐selective beta‐blocker eye drop exposure caused: a mean fall in FEV1 of −10.9% (95% CI −14.9 to −6.9; P < 0.001) (Figure 2); a significant increase in falls in FEV1 of ≥20% (risk difference 0.28, 95% CI 0.14 to 0.42; P < 0.001) (online supplement 3: supplementary Figure S2) with a number needed to harm of approximately one in three; and a non‐significant increase in respiratory symptoms (risk difference 0.40, 95% CI −0.05 to 0.85; P = 0.08).
Figure 2.
Mean percentage change in FEV1 following acute non‐selective and selective beta‐blocker eye drop exposure. For figure 3 ‐ Fall in FEV1 of 15% or greater following acute non‐selective and selective beta‐blocker eye drop exposure
Selective beta‐blocker eye drops in people with asthma with non‐selective beta‐blocker sensitivity
Compared to control, acute selective beta‐blocker eye drop exposure in people with asthma sensitive to non‐selective beta‐blocker eye drops caused: a mean fall in FEV1 from baseline of −6.3% (95% CI −11.7 to −0.8; P = 0.03) (Figure 2); a non‐significant increase in falls in FEV1 of ≥20% (risk difference 0.17, 95% CI −0.05 to 0.40; P = 0.70) (online supplement 3: supplementary Figure S2); and a non‐significant increase in respiratory symptoms (risk difference 0.27, 95% CI −0.06 to 0.61, P = 0.11).
Sensitivity analyses
When FEV1 threshold was varied, non‐selective beta‐blocker eye drops caused a significant increase in falls in FEV1 of ≥15% (risk difference 0.34, 95% CI 0.19 to 0.49; P < 0.001) equating to a number needed to harm of one in three (Figure 3). Betaxolol eye drops in people with asthma sensitive to non‐selective beta‐blockers, caused a significant increase in falls in FEV1 of ≥15% (risk difference 0.35, 95% CI 0.11 to 0.59; P = 0.005) equating to a number needed to harm of one in three in people with prior sensitivity or a number needed to harm of one in nine people unselected on the basis of prior response (Figure 3). The risk difference for falls in FEV1 of ≥15% for betaxolol 1% was 0.41 (95% CI 0.15 to 0.67; P = 0.002) compared to 0.15 (95% CI −0.22 to 0.53) for betaxolol 0.5%. Results from other sensitivity analyses were consistent with the main findings.
Figure 3.
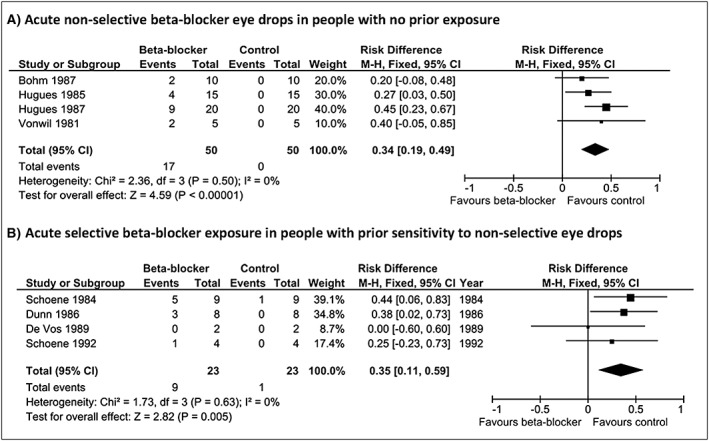
Falls in FEV1 of 15% or greater following acute beta‐blocker eye drop exposure
Risk of bias
No significant statistical heterogeneity was detected. Of the nine studies, five were non‐randomized and two were unblinded at high risk of bias (online supplement 3: supplementary Figure S3). No funnel plot asymmetry was found to suggest publication bias.
Discussion
This study measured the prevalence of beta‐blocker eye drop prescribing and the respiratory effect of beta‐blocker eye drop exposure in people with asthma and ocular hypertension. Although beta‐blocker eye drop prescribing fell over the study period, 14% of people with asthma and ocular hypertension were still being prescribed a non‐selective beta‐blocker eye drop at the end of follow‐up demonstrating a population at risk. Betaxolol prescribing also fell during the study period and now appears to be infrequently prescribed in the UK despite its potentially better safety profile, perhaps related to the increased availability of compound eye drop preparations containing non‐selective beta‐blockers that may be preferentially prescribed to reduce treatment burden 6.
The relative incidence of moderate asthma exacerbations was significantly increased within 30 days of new ocular non‐selective beta‐blocker use with similar results observed using the nested case–control study and the self‐controlled case series designs. In contrast, no significant increase in asthma morbidity was observed with chronic exposure in the nested case–control study. The lack of effect with chronic exposure may be due to attenuation of risk from beta2‐adrenoceptor upregulation with chronic dosing (as suggested by studies evaluating chronic oral beta‐blocker exposure in asthma) or possibly survival bias, whereby longer‐term treatment is more likely to occur in people tolerating acute exposure 28. Several readily identifiable risk factors significantly associated with increased asthma severity or transient airway hyper‐responsiveness were identified for moderate exacerbations which clinicians could use to better judge risk from non‐selective beta‐blocker eye drop exposure. These included having had a recent or concomitant respiratory tract infection, a prior history of asthma hospitalization, being a current smoker, increasing BMI and short‐acting beta2‐agonist use.
The meta‐analysis of controlled clinical trials demonstrated that acute exposure to non‐selective beta‐blocker eye drops caused significant mean falls in FEV1 of 10.9%, falls in FEV1 of ≥20% affecting approximately one in three and a non‐significant increase in respiratory symptoms. For mean falls in FEV1, findings were similar to the effects of oral non‐selective beta‐blockers in people with asthma 4. However, the number of people experiencing falls in FEV1 of ≥20% following oral beta‐blocker administration was smaller suggesting that non‐selective beta‐blocker eye drops may carry a greater risk.
Betaxolol administration in people sensitive to ocular non‐selective beta‐blocker eye drops caused only small significant mean falls in FEV1, and non‐significant increases in falls in FEV1 of ≥20% and respiratory symptoms. However, smaller falls in FEV1 may still be clinically significant, and betaxolol caused significant falls in FEV1 of ≥15% with a number needed to harm equivalent to one in nine people with asthma unselected on the basis of prior response. Falls in FEV1 ≥ 15% following acute betaxolol exposure appeared to be significant for betaxolol 1% compared to betaxolol 0.5%, suggesting a possible dose–response relationship, which has also been demonstrated with acute oral selective beta‐blocker exposure 4.
The most commonly evaluated non‐selective beta‐blocker eye drop in our cohort was timolol, which has greater selectivity for the beta2‐adrenoceptor than other commonly used non‐selective beta‐blockers. In this regard, the absolute degree of beta2‐adrenoceptor binding affinity (i.e. the equilibrium dissociation constant, K d) shows a rank order of timolol > carvedilol > propranolol > nadolol > sotalol, potentially explaining the apparent greater risk following ocular administration potentiated by the lack of first pass liver metabolism and rapid systemic absorption. This rapid systemic absorption has been compared to that of systemic exposure following intravenous beta‐blocker administration in terms of beta2‐adrenoceptor occupancy and cardiopulmonary effects 7. It is uncertain whether any patients in our cohort routinely performed lacrimal duct compression following beta‐blocker eye drop administration which could potentially modify the risk of systemic absorption and therefore risk of exacerbation. Despite this, our findings suggest that acute non‐selective beta‐blocker eye drops cause significant changes in lung function in people with asthma and also increase asthma morbidity in the real world.
Strengths and limitations
A key strength of our study is that it combines pharmaco‐epidemiological analysis of linked routine health data supported by meta‐analysis of clinical trial data, making it the most comprehensive evaluation on the risks of beta‐blocker eye drops in people with asthma. However, our study has several limitations. First, it was not possible to comprehensively evaluate all types of ocular beta‐blocker exposure in our nested case–control study due to limited available data. Residual confounding from unmeasured covariates may also exist because data on lung function was not routinely available. However, the self‐controlled case series is a design in which the person acts as their own control and this produced consistent findings for acute non‐selective beta‐blocker exposure.
Exposure to oral steroids may conceivably induce or worsen ocular hypertension and could act as a potential confounder theoretically increasing the likelihood of treatment with beta‐blocker eye drops. However, this is unlikely to have influenced our results for several reasons. First, the increased risk with non‐selective beta‐blocker eye drops was seen with moderate asthma exacerbations defined by incident rescue oral steroid use. Second, the distribution of ocular hypertensive medication use among cases and controls was similar suggesting no significant difference in the severity of ocular hypertension between groups. Lastly, sensitivity analysis additionally adjusting for ocular antihypertensive use produced consistent results.
The nested case–control study outcomes relied upon accurate electronic prescribing and discharge coding and potentially not all outcomes were identified. Nevertheless, hospital discharges are routinely recorded in the UK and almost all chronic community prescriptions are issued electronically from general practice, including most drugs recommended by specialists. Limitations of our meta‐analysis include the small number of participants, the potential risk of bias from non‐randomized or unblinded studies and the use of FEV1, which may be less sensitive than other methods at measuring airway resistance such as impulse oscillometry 29. Despite these limitations, results were generally consistent between study designs and were similar to a previous meta‐analyses evaluating oral beta‐blockers in asthma 4.
In conclusion, initiating treatment with non‐selective beta‐blocker eye drops causes significant changes in lung function in people with asthma and ocular hypertension, and is associated with increased asthma morbidity in the real world. Nevertheless, people with asthma and ocular hypertension are still frequently prescribed non‐selective beta‐blocker eye drops whilst safer selective agents are infrequently prescribed. These findings support recommendations that non‐selective beta‐blocker eye drops should be avoided in people with asthma and ocular hypertension.
Competing Interests
Professor Lipworth has received research support from Chiesi, Teva, Pharmaxis, and Nycomed; has consultant arrangements with Gurnos, Chiesi, and Hexal; has received payment for lectures from Teva; and has received travel support from GlaxoSmithKline, Chiesi, and Pharmaxis. Professor Donnan has received fees for consulting from the Scottish Medicines Consortium and grant support from GlaxoSmithKline plc, Otsuka America Pharmaceutical, Inc. and Amgen Inc.
The role of DM was funded by a Scottish Government Chief Scientist Office Clinical Academic Fellowship (CAF1107). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributors
DM conceived the idea. DM and TD reviewed the literature and extracted the data for the systematic review and meta‐analysis. All authors contributed to the study design and interpretation of the findings. DM analysed the data and is guarantor of the data. All authors contributed to the drafting of the manuscript and approved the final draft.
Supporting information
Figure S1 PRISMA flow diagram for the systematic review study selection
Figure S2 Fall in FEV1 of 20% or greater following acute beta‐blocker eye drop exposure
Figure S3 Cochrane collaboration tool for assessing risk of bias among included studies
Table S1 Sensitivity analyses for non‐selective beta‐blocker eye drop use and asthma events
Table S2 Sensitivity analyses for selective beta‐blocker eye drop use and asthma events
Table S3 Incidence rate ratios for non‐selective beta‐blocker eye drop exposure and moderate asthma exacerbations in the self‐controlled case series
Table S4 Characteristics of included studies for the systematic review and meta‐analysis
Supporting info item
Supporting info item
Supporting info item
Morales, D. R. , Dreischulte, T. , Lipworth, B. J. , Donnan, P. T. , Jackson, C. , and Guthrie, B. (2016) Respiratory effect of beta‐blocker eye drops in asthma: population‐based study and meta‐analysis of clinical trials. Br J Clin Pharmacol, 82: 814–822. doi: 10.1111/bcp.13006.
References
- 1. British Thoracic Society Scottish Intercollegiate Guidelines Network . British guideline on the management of asthma. Thorax 2008; 63: iv1–iv121. [DOI] [PubMed] [Google Scholar]
- 2. Global Initiative for Asthma . Global strategy for asthma management and prevention. Available at http://wwwginasthmaorg/ (last accessed 31 September 2014).
- 3. American Academy of Ophthalmology Preferred Practice Patterns . Primary Open‐Angle Glaucoma PPP‐2010 [online]. Available at http://one.aao.org/preferred‐practice‐pattern/primary‐openangle‐glaucoma‐ppp‐october‐2010 (last accessed 17 December 2014).
- 4. Morales DR, Jackson C, Lipworth BJ, Donnan PT, Guthrie B. Adverse respiratory effect of acute β‐blocker exposure in asthma: a systematic review and meta‐analysis of randomized controlled trials. Chest 2014; 145: 779–86. [DOI] [PubMed] [Google Scholar]
- 5. Frishman WH, Fuksbrumer MS, Tannenbaum M. Topical ophthalmic beta‐adrenergic blockade for the treatment of glaucoma and ocular hypertension. J Clin Pharmacol 1994; 34: 795–803. [DOI] [PubMed] [Google Scholar]
- 6. Joint Formulary Committee . British National Formulary. London: 2014. Available at http://www.medicinescomplete.com (last accessed 1 September 2014)BMJ Group and Pharmaceutical Press, n.d.Treatment of glaucoma – 11.6 [online] [Google Scholar]
- 7. Korte JM, Kaila T, Saari KM. Systemic bioavailability and cardiopulmonary effects of 0.5% timolol eye drops. Graefes Arch Clin Exp Ophthalmol 2002; 240: 430–5. [DOI] [PubMed] [Google Scholar]
- 8. Goeman DP, Abramson MJ, McCarthy EA, Zubrinich CM, Douglass JA. Asthma mortality in Australia in the 21st century: a case series analysis. BMJ Open 2013; 3: e002539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morales DR, Guthrie B, Lipworth BJ, Donnan PT, Jackson C. Prescribing of β‐adrenoceptor antagonists in asthma: an observational study. Thorax 2011; 66: 502–7. [DOI] [PubMed] [Google Scholar]
- 10. Health and Social Care Information Centre . Read Codes [online]. Available at http://systemshscicgovuk/data/uktc/readcodes (last accessed 1 September 2014).
- 11. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang EM, Pinheiro SP, Hammad TA, Abou‐Ali A. Evaluating the validity of clinical codes to identify cataract and glaucoma in the UK Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf 2015; 24: 38–44. [DOI] [PubMed] [Google Scholar]
- 13. Joint Formulary Committee . British National Formulary. London: 2014. Available at http://www.medicinescomplete.com (last accessed 1 September 2014)BMJ Group and Pharmaceutical Press, n.d.Respiratory system – 3 [online] [Google Scholar]
- 14. Etminan M, Samii A. Pharmacoepidemiology I: a review of pharmacoepidemiologic study designs. Pharmacotherapy 2004; 24: 964–9. [DOI] [PubMed] [Google Scholar]
- 15. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007; 16: 219–42. [DOI] [PubMed] [Google Scholar]
- 16. Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self‐controlled case series method. Stat Med 2006; 25: 1768–97. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 18: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. Int J Epidemiol 2002; 31: 140–9. [DOI] [PubMed] [Google Scholar]
- 19. Bleckmann H, Dorow P. Treatment with betaxolol and placebo eye drops in patients with glaucoma and reactive airway diseases. Klin Monbl Augenheilkd 1987; 191: 199–202. [DOI] [PubMed] [Google Scholar]
- 20. Böhm E, Fabel H. Changes in lung function following administration of eye drops containing timolol, metipranolol, pindolol and pilocarpine in healthy probands and patients with mild bronchial asthma. Klin Wochenschr 1987; 65: 920–4. [DOI] [PubMed] [Google Scholar]
- 21. De Vos M. Double‐masked challenge of timolol‐sensitive patients with topical betaxolol and placebo. Int Ophthalmol Clin 1989; 29: S22. [Google Scholar]
- 22. Dunn TL, Gerber MJ, Shen AS, Fernandez E, Iseman MD, Cherniack RM. The effect of topical ophthalmic instillation of timolol and betaxolol on lung function in asthmatic subjects. Am Rev Respir Dis 1986; 133: 264–8. [DOI] [PubMed] [Google Scholar]
- 23. Hugues FC, Le Jeunne C, Munera Y, Dufier JL. Evaluation of the systemic effects of timolol maleate in eye drops. J Fr Ophtalmol 1985; 8: 389–94. [PubMed] [Google Scholar]
- 24. Hugues FC, Le Jeunne C, Munera Y, Dufier JL. Comparison of the effects of carteolol and metipranolol eye drops on the ventilatory and cardiovascular functions in asthmatics. J Fr Ophtalmol 1987; 10: 485–90. [PubMed] [Google Scholar]
- 25. Schoene RB, Abuan T, Ward RL, Beasley CH. Effects of topical betaxolol, timolol, and placebo on pulmonary function in asthmatic bronchitis. Am J Ophthalmol 1984; 97: 86–92. [DOI] [PubMed] [Google Scholar]
- 26. Schoene RB, Verstappen A, McDonald TO. Betaxolol use is not related to adverse pulmonary reactions reported in patients with reactive airways disease: a report on 12 double‐masked rechallenges. Glaucoma 1992; 14: 39–45. [Google Scholar]
- 27. Vonwil A, Landolt M, Flammer J, Bachofen H. Bronchoconstrictive side effects of timolol eye drops in patients with obstructive lung disease. Schweiz Med Wochenschr 1981; 111: 665–9. [PubMed] [Google Scholar]
- 28. Brodde OE, Daul A, Stuka N, O'Hara N, Borchard U. Effects of beta‐adrenoceptor antagonist administration on beta 2‐adrenoceptor density in human lymphocytes. The role of the "intrinsic sympathomimetic activity". Naunyn Schmiedebergs Arch Pharmacol 1985; 328: 417–22. [DOI] [PubMed] [Google Scholar]
- 29. Short PM, Williamson PA, Lipworth BJ. Sensitivity of impulse oscillometry and spirometry in beta‐blocker induced bronchoconstriction and beta‐agonist bronchodilatation in asthma. Ann Allergy Asthma Immunol 2012; 109: 412–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 PRISMA flow diagram for the systematic review study selection
Figure S2 Fall in FEV1 of 20% or greater following acute beta‐blocker eye drop exposure
Figure S3 Cochrane collaboration tool for assessing risk of bias among included studies
Table S1 Sensitivity analyses for non‐selective beta‐blocker eye drop use and asthma events
Table S2 Sensitivity analyses for selective beta‐blocker eye drop use and asthma events
Table S3 Incidence rate ratios for non‐selective beta‐blocker eye drop exposure and moderate asthma exacerbations in the self‐controlled case series
Table S4 Characteristics of included studies for the systematic review and meta‐analysis
Supporting info item
Supporting info item
Supporting info item


