Abstract
Aims
Anaemia is common in cancer patients, with treatments including epoetins and blood transfusions. Although an increased risk of venous thromboembolism (VTE) has been associated with both therapeutics, studies comparing the risk of VTE between epoetins and transfusions in cancer patients are lacking.
Methods
A nested case–control study investigated this risk using the German Pharmacoepidemiological Research Database. Cohort members were incident cancer patients receiving first time treatment with epoetin or transfusion. A subcohort including only patients receiving chemotherapy was created, since the formally approved indication of epoetins is chemotherapy‐induced anaemia. Cases were defined as patients developing VTE. For each case up to 10 gender‐ and age‐matched controls were selected from the cohort. Multiple confounder adjusted odds ratios (aORs) with 95% confidence intervals (CIs) for VTE and recent treatment with epoetins or transfusions (last 28 days before index date) compared with past anti‐anaemic treatment were calculated by conditional logistic regression.
Results
Among 69 888 patients receiving first time treatment with epoetin or transfusion, 3316 VTE cases were identified. The aOR for VTE was 1.31 (95% CI 1.03, 1.65) for epoetins, 2.33 (95% CI 2.03, 2.66) for transfusions, and 2.24 (95% CI 1.34, 3.77) for epoetins and transfusions. Sensitivity analyses with a stricter VTE definition or an expanded time window yielded similar results. In the chemotherapy only subcohort the risk difference between epoetins and transfusions could not be verified (aOR 1.48, 95% CI 1.10, 1.98 vs. aOR 1.80, 95% CI 1.49, 2.19). Our study confirmed known VTE risk factors including previous VTE (aOR 14.76, 95% CI 12.79, 17.03) or surgery (aOR 1.83, 95% CI 1.67, 2.01). Epoetin‐associated risk decreased after a safety warning by the European Medicines Agency setting maximum haemoglobin target values to 12 g dl–1.
Conclusions
Transfusions could be associated with a higher VTE risk than epoetins in cancer patients. Moreover, current prescribing patterns may have decreased the VTE risk for epoetins.
Keywords: antianaemic treatment, erythropoietin, drug safety, case‐control study
What is Already Known about this Subject
Epoetins decrease the need for blood transfusions among cancer patients with chemotherapy‐associated anaemia.
Studies in recent years have shown an increased thromboembolic risk, leading to risk minimization measures (e.g. safety warnings) by regulatory authorities.
Until today, no studies have compared the thromboembolic risk between epoetins and blood transfusions.
What this Study Adds
The risk of venous thromboembolism (VTE) in cancer patients associated with epoetins could be lower than the respective risk of blood transfusions.
Following the safety warning published by the European Medicines Agency in 2008 which set 12 g dl–1 as the maximum target haemoglobin value, the epoetin‐associated VTE risk decreased considerably.
Introduction
Cancer‐related anaemia occurs in 40% of patients with non‐myeloid and in 30–80% of patients with myeloid malignancies 1, 2. Possible aetiologies include myelosuppression (due to chemotherapy or radiotherapy, bone marrow infiltration in myeloid malignancies or bone metastases of solid tumours), chemotherapy‐induced nephrotoxicity causing reduced erythropoietin production, haemolysis or bleeding 3. Anaemia has a negative impact on the quality of life and shows a negative statistical association with overall survival in most cancer types 1. Since blood transfusions have been associated with several risks including volume or iron overload, transmission of infections, or venous thromboembolism (VTE), and given the limited blood supply 3, alternative anti‐anaemic treatments are needed.
Erythropoietin stimulating agents (ESAs) or epoetins are recombinant human erythropoietins which increase haemoglobin (Hb) levels and reduce the need for transfusions in most cancer patients 2. They were introduced in the United States in 1989 for anaemia treatment in chronic kidney disease (CKD) 4 and in 1993 chemotherapy‐induced anaemia (CIA) was added as an indication 4. In the last years several studies on epoetin safety in cancer patients were published showing that epoetins are associated with an increased risk of thrombosis and mortality 5, 6, leading to the inclusion of epoetin use in predictive models for symptomatic VTE during neoplastic disease 7. Moreover, safety warnings by the European Medicines Agency (EMA) and the marketing authorization holders of all epoetins in June 2008 restricted their use to symptomatic CIA patients with poor prognosis and set 12 g dl–1 as the maximum target Hb value 8, 9, as epoetins' thrombogenic potential could derive from, among other things such as high dose treatment, increased Hb levels 10. Similar measures were implemented by the U.S. Food and Drug Administration (FDA) 11, 12. According to the European Organization for Research and Treatment of Cancer, though, epoetins could also be used in selected asymptomatic, untreated cancer patients 13.
Despite the considerable amount of data on anti‐anaemic treatment and VTE, no studies comparing the risk of transfusions and epoetins exclusively in cancer patients have been published yet. Therefore, we conducted a nested case–control study based on real‐life data from the German Pharmacoepidemiological Research Database (GePaRD) 14, assessing the VTE risk in incident cancer patients receiving epoetins with or without additional transfusions compared with those receiving transfusions alone. Furthermore, we addressed the same question in a subcohort exclusively consisting of chemotherapy treated incident cancer patients, in order to elucidate epoetin safety also in the formally approved indication.
Methods
Data source and study design
Data for this nested case–control study were obtained from GePaRD, which is based on data from four statutory health insurance (SHI) providers covering over 17 million insurants throughout Germany. GePaRD contains individual demographic characteristics, information on hospitalizations and outpatient physician visits and outpatient dispensation data for each insurance member 14, 15. In Germany, the utilization of health insurance data for scientific research is regulated by the Code of Social Law (SGB X). All contributing SHIs and the regulatory authorities approved the use of the data for this study. Since it was based on pseudonymous data, informed consent was not required by law. The study period was from January 2004 to December 2009.
Study cohort
Cohort members had to fulfil all of the following inclusion criteria: (i) at least 12 months of continuous insurance time before the initial outpatient epoetin dispensation or transfusion administration, (ii) no outpatient epoetin dispensation or code indicating transfusion administration within the 12 months before cohort entry, and (iii) at least one outpatient or inpatient diagnosis of cancer other than non‐melanoma skin cancer or a code indicating chemotherapy within 6 months before cohort entry, but no diagnosis of cancer or code indicating chemotherapy between 6 months and 1 year before cohort entry. Each study participant entered the cohort on the date of the first epoetin dispensation or first transfusion, whichever came first, within the study period if inclusion criteria were fulfilled. Cohort exit was defined as the first of the following dates: (i) end of study period, (ii) death from any cause or (iii) interruption of insurance of more than 3 days or end of insurance. Epoetin treatment was assessed via outpatient dispensations and included all epoetins irrespective of the licensed indication. Transfusions were assessed via the in‐ and outpatient operations and procedures coding system (OPS) and outpatient claim codes for outpatient services and procedures (EBM).
Case definition
VTE was defined as a diagnosis of deep vein thrombosis of the leg/hip or pulmonary embolism (International Classification of Diseases 10 German Modification codes: I80.1, I80.2, I80.3, I80.9, I26). For hospital data only the main discharge diagnoses (reflecting the reason for hospitalisation) were taken into account to identify acute VTE events. For patients with a main discharge diagnosis of VTE, the date of hospital admission was defined as the date of the event. For outpatient data only diagnoses coded as ‘certain’ were used. To identify acute VTE events, a prescription of an antithrombotic agent in the same or the following quarter was required. As outpatient diagnoses can only be allocated to the quarter of a year and not to an exact date, the prescription date of the first antithrombotic agent was considered the date of the VTE event in ambulatory patients. Cases were defined as patients included in the cohort who fulfilled the respective outcome definition criteria irrespective whether they were exposed to one of the treatments at index date. For each case up to 10 gender‐, age‐ and SHI‐matched controls were selected by risk set sampling from the cohort. For the case–control analyses including only patients with (i) lung cancer, (ii) lung or pancreatic cancer and (iii) breast or prostate cancer we additionally matched for presence of metastatic disease. Each control was assigned an index date resulting in the same time of follow‐up as for the corresponding case. Cohort members hospitalized at the index date of the case were excluded from the set of potential controls, since they were not at risk of hospitalization because of VTE or of receiving an outpatient VTE diagnosis. Patients might have served as controls for more than one case and were eligible to be selected as controls until they became a case.
Confounder assessment
Demographic information such as age or gender was assessed at cohort entry. Co‐morbidity, including risk factors for VTE (Table S1), was assessed in the 12 months preceding cohort entry for the in‐ and outpatient setting. To measure and adjust for patients' disease burden, the Charlson Co‐morbidity Index (CCI) 16 according to Quan et al. was used (Table S2). Co‐medication, including drugs associated with an increased VTE risk (Table S3) and antithrombotic agents which might have been used for VTE prevention in patients with known risk factors (Table S4) were assessed in the 90 days preceding the index date.
Statistical analyses
A conditional logistic regression was conducted to estimate adjusted odds ratios (aORs) with corresponding 95% confidence intervals (CIs) for VTE and recent treatment with epoetin, transfusion or epoetin and transfusion. The comparator for all three groups was past treatment of any of the therapies. Treatment was defined as ‘recent’ if the respective therapy ended in the 28 days before or at the index date. This definition was based on a meta‐analysis of randomized, controlled studies evaluating epoetin beta associated overall survival, disease progression and thromboembolic events in cancer patients during and up to 28 days after the end of epoetin beta therapy 17. Treatment was defined as ‘past’ if it ended more than 28 days before the index date.
Sensitivity and subcohort analyses
A sensitivity analysis was performed expanding the time window of the exposure definition to 90 days 18. To elucidate the VTE risk of epoetins in their formally approved indication (CIA) 19, we analyzed a subcohort comprising incident cancer patients with a code indicating chemotherapy within 6 months before cohort entry but no code indicating chemotherapy between 6 months and 1 year before cohort entry (‘chemotherapy only subcohort’). Another analysis applied a more restrictive endpoint definition considering only hospitalized patients with a main discharge diagnosis of VTE, given the generally high quality of inpatient coding 20, thereby discarding patients with outpatient VTE diagnoses. In a further investigation we excluded CKD patients, as they might have been treated with epoetins or transfusions because of CKD. For this analysis, a patient was assumed to suffer from CKD if he/she had at least one CKD diagnosis or any code indicating dialysis. The VTE risk for epoetins or transfusions was also identified in patients with lung cancer only, i.e. in a population with higher homogeneity than the main cohort. Separate case–control analyses were performed in patients with lung or pancreatic cancer (high intrinsic VTE risk) and in patients with prostate or breast cancer (low intrinsic VTE risk) 21, in order to elucidate the role of different cancer entities as possible effect modifiers. Separate case–control analyses were also performed for patients entering the main cohort or the chemotherapy only subcohort until and after June 2008, searching for a possible effect of the EMA recommendations on epoetin‐associated VTE risk. Analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC, USA).
Results
Descriptive analysis
During the study period, 69 888 incident cancer patients receiving a first time treatment with epoetin or transfusion were identified (53% female). Median age at cohort entry was 69 years, the median CCI value was 6 and CKD prevalence reached 20% (Table 1A). The chemotherapy only subcohort (n = 21 407, 56% female) had a lower median age at cohort entry (65 years), a higher median CCI value (8) and a lower CKD prevalence (14%). Regarding treatment patterns, every tenth incident cancer patient was treated with epoetins, while the vast majority was treated with transfusions (Table 1A). The epoetin fraction was considerably higher (21%) in the chemotherapy only subcohort. A continuous decline in epoetin use over time was observed in the main and the chemotherapy only subcohort (Figures 1 and 2 ). Women were more likely to receive epoetins than men in both cohorts (12% vs. 8% and 27% vs. 14%). Epoetin patients were younger (median age 60 vs. 70 years), had lower median CCI (5 vs. 6), and lower prevalence of cancer with unfavourable prognosis (14% vs. 25%) than patients receiving only transfusions (Table 1B). Epoetin patients entering the cohort before June 2008, i.e. the publication date of the EMA recommendations, were of comparable age with the respective patients entering the cohort after June 2008 (age median 60 vs. 61 years), had a similar prevalence of cancer with unfavourable prognosis as defined by the Robert Koch Institute and the association of epidemiological cancer registries in Germany (GEKID) 22 (14% vs. 12%), but showed a lower prevalence of CKD (17% vs. 25%), and a lower median value of CCI (5 vs. 7). Table 1C depicts characteristics of incident cancer patients included in the cohort before and after June 2008 and additionally classified based on antianaemic treatment.
Table 1A.
Characteristics of incident cancer patients treated with epoetin or transfusion classified by gender
| Malesn = 33 042 | Femalesn = 36 846 | Totaln = 69 888 | |
|---|---|---|---|
| Median age at cohort entry (Q1, Q3) | 69 (62, 76) | 69 (59, 79) | 69 (60, 77) |
| Assessment of cancer | |||
| Via diagnoses only | 23 634 (71.5%) | 24 847 (67.4%) | 48 481 (69.4%) |
| Via diagnoses and therapy | 9054 (27.4%) | 11 486 (31.2%) | 20 540 (29.4%) |
| Via therapy only | 354 (1.1%) | 513 (1.4%) | 867 (1.2%) |
| Cancer with unfavourable prognosis * | 9864 (29.9%) | 7532 (20.4%) | 17 396 (24.9%) |
| Chronic kidney disease | 7741 (23.4%) | 5720 (15.5%) | 13 461 (19.3%) |
| Median value of CCI ** (Q1, Q3) | 6 (3, 9) | 6 (3, 9) | 6 (3, 9) |
| Sequence of treatment | |||
| Epoetin only | 621 (1.9%) | 2028 (5.5%) | 2649 (3.8%) |
| Transfusion only | 30 476 (92.2%) | 32 377 (87.9%) | 62 853 (89.9%) |
| Epoetin before transfusion | 585 (1.8%) | 903 (2.5%) | 1488 (2.1%) |
| Transfusion before epoetin | 1334 (4.0%) | 1517 (4.1%) | 2851 (4.1%) |
| Transfusion and epoetin at cohort entry | 26 (0.1%) | 21 (0.1%) | 47 (0.1%) |
Figure 1.
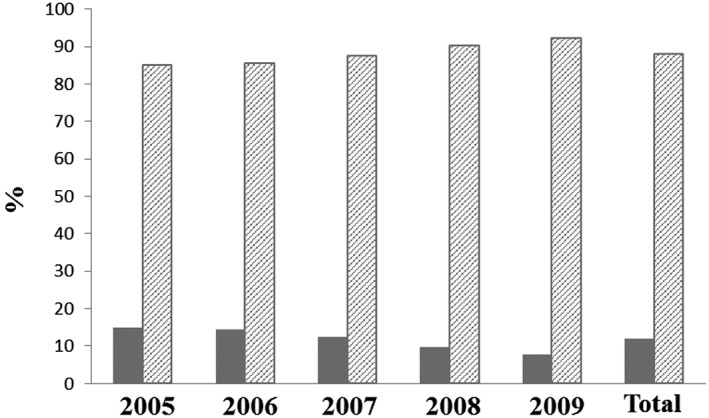
Percentage of incident cancer patients receiving epoetins ( ) or blood (
) or blood ( ) transfusions for each study year
) transfusions for each study year
Figure 2.
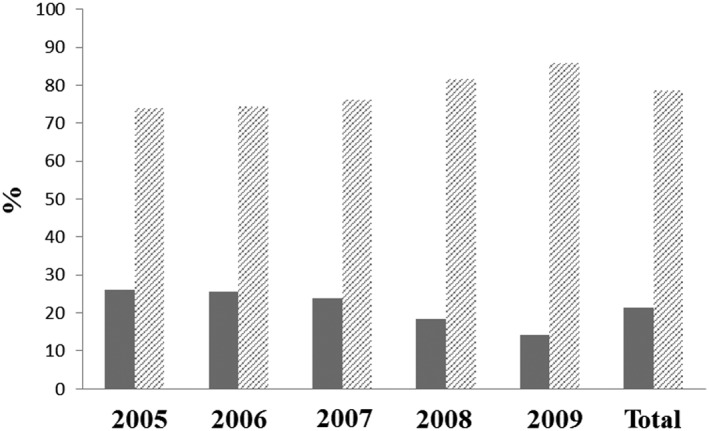
Percentages of incident cancer patients treated with chemotherapy receiving epoetins ( ) or blood (
) or blood ( ) transfusions for each study year
) transfusions for each study year
Table 1B.
Characteristics of incident cancer patients treated with epoetin or transfusion classified by sequence of treatment
| Epoetins onlyn = 2649 | Transfusions onlyn = 62 853 | Epoetins before transfusionsn = 1488 | Transfusions before epoetinsn = 2851 | Transfusions and epoetinsn = 47 | |
|---|---|---|---|---|---|
| Median age at cohort entry (Q1, Q3) | 60 (49, 69) | 70 (59, 79) | 65 (55, 71) | 66 (58, 73) | 64 (54, 69) |
| Assessment of cancer | |||||
| Via diagnoses only | 426 (16.1%) | 46 019 (73.2%) | 305 (20.5%) | 1727 (60.6%) | 4 (8.5%) |
| Via diagnoses and therapy | 2109 (79.6%) | 16 193 (25.8%) | 1144 (76.9%) | 1053 (36.9%) | 41 (87.2%) |
| Via therapy only | 114 (4.3%) | 641 (1.0%) | 39 (2.6%) | 71 (2.5%) | 2 (4.3%) |
| Cancer with unfavourable prognosis * | 357 (13.5%) | 15 850 (25.2%) | 472 (31.7%) | 695 (24.4%) | 22 (46.8%) |
| Chronic kidney disease | 507 (19.1%) | 11 672 (18.6%) | 360 (24.2%) | 918 (32.2%) | 4 (8.5%) |
| Median value of CCI ** (Q1, Q3) | 5 (2, 9) | 6 (3, 9) | 8 (4, 9) | 8 (4, 9) | 8 (4, 10) |
Table 1C.
Characteristics of incident cancer patients treated with epoetin or transfusion before and after June 2008
| Cohort entry before June 2008n = 48 341 | Cohort entry after June 2008n = 21 547 | |||||
|---|---|---|---|---|---|---|
| Epoetins n = 1934 | Transfusions n = 42 848 | Epoetins and transfusions n = 3559 | Epoetins n = 715 | Transfusions n = 20 005 | Epoetins and transfusions n = 827 | |
| Median age at cohort entry (Q1, Q3) | 60 (49, 68) | 69 (61, 78) | 66 (57, 72) | 61 (50, 71) | 70 (61, 78) | 67 (57, 72) |
| Assessment of cancer | ||||||
| Via diagnoses only | 292 (15.1%) | 31 878 (74.4%) | 1690 (47.5%) | 134 (18.7%) | 14 141 (70.7%) | 346 (41.8%) |
| Via diagnoses and therapy | 1568 (81.1%) | 10 533 (24.6%) | 1789 (50.3%) | 541 (75.7%) | 5660 (28.3%) | 449 (54.3%) |
| Via therapy only | 74 (3.8%) | 437 (1.0%) | 80 (2.2%) | 40 (5.6%) | 204 (1.0%) | 32 (3.9%) |
| Cancer with unfavourable prognosis * | 273 (14.1%) | 10 668 (24.9%) | 928 (26.1%) | 84 (11.8%) | 5182 (25.9%) | 261 (31.6%) |
| Chronic kidney disease | 326 (16.9%) | 7606 (17.8%) | 1009 (28.4%) | 181 (25.3%) | 4066 (20.3%) | 273 (33.0%) |
| Median value of CCI ** (Q1, Q3) | 5 (2, 8) | 6 (3, 9) | 8 (4, 9) | 7 (3, 9) | 7 (3, 9) | 8 (4, 9) |
During the time in cohort, 3316 patients (5%) were diagnosed with VTE (median age 69 years, 60% female), about two‐thirds of them in the outpatient setting. The most common diagnoses were ‘thrombosis, phlebitis and thrombophlebitis of other deep vessels of lower extremities’ (49%) and ‘pulmonary embolism’ (28%). In the chemotherapy only subcohort 1241 VTE cases were identified (median age 66 years, 58% female). During the time in cohort 32 345 (46.3%) of the included patients died, making death the reason for cohort exit for almost every second patient. Median time from cohort entry until death was 127 days. Regarding VTE associated death, 9.5% of the patients died within 30 days after VTE diagnosis (21.1% of the patients died within 90 days after VTE diagnosis).
Multivariable analyses
To the 3316 VTE patients 32 617 controls could be matched by year of birth, gender and SHI. The characteristics of cases and controls and the respective crude and aORs are displayed in Table 2. The conditional logistic regression analysis yielded an aOR of 1.31 (95% CI 1.03, 1.65) for epoetin treatment in the 28 days before index date compared with any past treatment, an aOR of 2.33 (95% CI 2.03, 2.66) for transfusions and an aOR of 2.24 (95% CI 1.34, 3.77) for epoetin and transfusions. History of VTE was identified as the major risk factor for VTE (aOR 14.76, 95% CI 12.79, 17.03), followed by previous surgery (aOR 1.83, 95% CI 1.67, 2.01).
Table 2.
Adjusted odds ratios (aOR) and 95% confidence intervals (CI) for venous thromboembolism (VTE)
| Casesn = 3316 | Controlsn = 32 617 | Crude OR (95% CI) | aOR (95% CI)# | |
|---|---|---|---|---|
| Treatment assessed in the 28 days preceding ID * | ||||
| Epoetins (reference group: past treatment with transfusion and/or epoetin) | 96 (2.9%) | 897 (2.8%) | 1.41 (1.13, 1.75) | 1.31 (1.03, 1.65) |
| Transfusions (reference group: past treatment with transfusion and/or epoetin) | 1168 (35.2%) | 9274 (28.4%) | 2.62 (2.30, 2.98) | 2.33 (2.03, 2.66) |
| Epoetins and transfusions (reference group: past treatment with transfusion and/or epoetin) | 22 (0.7%) | 104 (0.3%) | 3.01 (1.88, 4.81) | 2.24 (1.34, 3.77) |
| Co‐morbidities assessed in the year before cohort entry *, † | ||||
| History of VTE | 657 (19.8%) | 482 (1.5%) | 14.76 (12.79, 17.03) | |
| Obesity | 810 (24.4%) | 5588 (17.1%) | 1.53 (1.39, 1.69) | |
| Metastatic solid tumour | 1507 (45.5%) | 11 152 (34.2%) | 1.35 (1.24, 1.46) | |
| Other treatment assessed in the 90 days preceding ID *, † | ||||
| Surgery | 1712 (51.6%) | 14 136 (43.3%) | 1.83 (1.67, 2.01) | |
| Chemotherapy | 1613 (48.6%) | 11 796 (36.2%) | 1.65 (1.50, 1.82) | |
| Other medication associated with increased VTE risk | 1019 (30.7%) | 7770 (23.8%) | 1.22 (1.11, 1.34) | |
| Antithrombotic medication | 1064 (32.1%) | 5962 (18.3%) | 1.30 (1.18, 1.43) |
Matching was performed for age, gender and SHI.
It was adjusted for history of VTE, obesity, metastatic solid tumour, surgery, chemotherapy, other medication associated with increased VTE risk (Table S3), antithrombotic medication (Table S4) coronary artery disease/chronic ischaemic heart disease, arterial hypertension, dyslipidaemia, diabetes mellitus, myocardial infarction and angina pectoris.
Reference group were patients with no such co‐morbidities and patients with no such treatment, respectively.
Past treatment with epoetin and/or transfusion means that the last dispensation of epoetin or administration of transfusion was longer than 28 days before the index date.
ID index date; SHI statutory health insurance.
Only variables with statistically significant increased risks are presented (further variables tested: coronary artery disease/chronic ischaemic heart disease, arterial hypertension, dyslipidaemia, diabetes mellitus, myocardial infarction, angina pectoris).
Sensitivity and subcohort analyses
A sensitivity analysis examined a time window of 90 days preceding the index date and revealed similar risks for epoetin use or transfusions compared to the 28 days window (data not shown). In the chemotherapy only subcohort the risk for epoetins increased (aOR, 1.48, 95% CI 1.10, 1.98), the risk for transfusions decreased (aOR 1.80, 95% CI 1.49, 2.19), and the risk for the combination of the two treatments remained unchanged (aOR 2.20, 95% CI 1.22, 3.95) compared with the main cohort. Applying a more restrictive outcome definition led to a neutralization of the VTE risk associated with epoetins (aOR: 1.05; CI 0.73, 1.52), while the risk for transfusions slightly decreased (aOR 2.14, CI: 1.74, 2.63) and the risk for epoetins and transfusion increased (aOR 2.92, 95% CI 1.49, 5.71). In the subcohort without CKD patients, an increased risk was found for epoetins and transfusions compared to the main cohort (Table 3). In the subcohort comprising only patients additionally suffering from CKD, only transfusions showed an increased risk but the number of exposed cases was, though, very low (Table 3). In the subcohort with only incident lung cancer patients, epoetin use was not significantly associated with VTE in contrast to the respective results for transfusions or epoetins and transfusions (Table 3). In the separate case–control analyses including patients with (i) lung or pancreatic cancer or (ii) prostate or breast cancer, only transfusions were associated with statistically significant VTE risks in both groups and the risk was higher in the latter group (Table 3). Finally, in the separate case–control analyses for patients with cohort entry until and after June 2008, we found a neutralization of epoetin‐associated VTE risk following the EMA recommendations both in the main (Table 3) and the chemotherapy only subcohort (until June 2008: aOR 1.65, 95% CI 1.18, 2.30/after June 2008: aOR 1.07, 95% CI 47, 2.43). All subcohort analyses with June 2008 as cut‐off are shown in Table S5.
Table 3.
Adjusted odds ratios (aOR) and 95% confidence intervals (CI) for venous thromboembolism in subcohorts (numbers in bold indicate significant results)
| Cases n (%) | Controls n (%) | aOR (95% CI)# | |
|---|---|---|---|
| Excluding CKD patients *, † | 2755 (100) | 22 983 (100) | ‐ |
| Epoetins | 71 (2.6) | 549 (2.4) | 1.38 (1.04, 1.84) |
| Transfusions | 962 (34.9) | 6483 (28.2) | 2.46 (2.11, 2.86) |
| Epoetins and transfusions | 19 (0.7) | 79 (0.3) | 2.46 (1.40, 4.34) |
| Only patients additionally suffering from CKD *, † | 556 (100) | 5214 (100 | ‐ |
| Epoetins | 25 (4.5) | 266 (5.1) | 1.56 (0.84, 2.90) |
| Transfusions | 203 (36.5) | 1439 (27.6) | 1.74 (1.06, 2.86) |
| Epoetins and transfusions | 3 (0.5) | 16 (0.3) | 1.72 (0.17, 17.67) |
| Only patients with lung cancer **, † | 333 (100) | 1511 (100) | ‐ |
| Epoetins | 9 (2.7) | 39 (2.6) | 1.65 (0.72, 3.76) |
| Transfusions | 157 (47.2) | 644 (42.6) | 1.84 (1.21, 2.80) |
| Epoetins and transfusions | 5 (1.5) | 12 (0.8) | 3.80 (1.08, 13.42) |
| Only patients with lung or pancreatic cancer **, † | 571 (100) | 2685 (100) | ‐ |
| Epoetins | 16 (2.8) | 83 (3.1) | 1.33 (0.74, 2.41) |
| Transfusions | 266 (46.6) | 1126 (41.9) | 1.85 (1.34, 2.56) |
| Epoetins and transfusions | 6 (1.1) | 16 (0.6) | 2.47 (0.86, 7.06) |
| Only patients with prostate or breast cancer **, † | 411 (100) | 1976 (100) | ‐ |
| Epoetins | 24 (5.8) | 113 (5.7) | 1.05 (0.59, 1.88) |
| Transfusions | 139 (33.8) | 540 (27.3) | 2.60 (1.58, 4.28) |
| Epoetins and transfusions | 2 (0.5) | 11 (0.6) | 0.67 (0.11, 4.06) |
| Start of anaemia treatment until June 2008 *, † | 2500 (100) | 19 402 (100) | ‐ |
| Epoetins | 78 (3.1) | 522 (2.7) | 1.33 (1.02, 1.72) |
| Transfusions | 773 (30.9) | 4229 (21.8) | 2.34 (1.99, 2.76) |
| Epoetins and transfusions | 15 (0.6) | 52 (0.3) | 2.34 (1.23, 4.45) |
| Start of anaemia treatment after June 2008 *, † | 780 (100) | 2280 (100) | ‐ |
| Epoetins | 16 (2.1) | 56 (2.5) | 0.83 (0.40, 1.75) |
| Transfusions | 380 (48.7) | 1025 (45.0) | 2.52 (1.76, 3.60) |
| Epoetins and transfusions | 7 (0.9) | 10 (0.4) | 1.52 (0.47, 4.89) |
Matching was performed for age, gender and SHI.
Matching was performed for age, gender, SHI and metastatic disease.
It was adjusted for history of VTE, obesity, metastatic solid tumour, surgery, chemotherapy, other medication associated with increased VTE risk (Table S3), antithrombotic medication (Table S4) coronary artery disease/chronic ischemic heart disease, arterial hypertension, dyslipidaemia, diabetes mellitus, myocardial infarction and angina pectoris.
Reference group: past users of epoetin and/or transfusion.
Past treatment with epoetin and/or transfusion means that the last dispensation of epoetin or administration of transfusion was longer than 28 days before the index date.
CKD chronic kidney disease; SHI statutory health insurance.
Discussion
The study at hand shows that both use of epoetins or administration of transfusions in cancer patients are associated with an increased risk of VTE, corroborating previously published results 2, 5, 6, 23, 24, 25. Moreover, our data suggest a higher risk for transfusions than for epoetins, with the trend in aOR being consistent after adjusting for numerous risk factors, expanding the time window, narrowing down the analysis on the formally approved epoetin indication (CIA), applying a more restrictive VTE definition or excluding patients with CKD as a potential alternative reason for epoetin or transfusion use.
Every tenth patient in our study used epoetins, while in the chemotherapy only subcohort this percentage doubled reflecting their formally approved indication. Moreover, we saw an annual decrease of epoetin users after 2006. Altogether, our findings illustrate a considerable decline in epoetin utilization compared with the Anaemia Cancer Treatment Study, which was conducted from 2005 to 2007 in several European countries and showed that 62% of CIA patients were treated with epoetins 26. A similar decline in the last years of the previous decade has also been observed in the United States 27 and is probably associated with safety warnings and revised labellings issued in 2007 and 2008 3. The overall VTE incidence in our cohort during the 5 year study period was circa 5%, being in accordance with published data. A study using the California Cancer Registry to assess VTE incidence in cancer patients found a 2 year cumulative incidence of 1.6% with the rate decreasing over time 28.
To the best of our knowledge, this is the first study comparing the VTE risk between the two anti‐anaemic treatments in a cohort exclusively comprising cancer patients. It is also one of few studies examining the thromboembolic risk of transfusions in general. A retrospective cohort study examining hospitalizations of cancer patients for a period of 9 years found transfusions to be a risk factor for VTE (OR 1.60, 95% CI 1.53, 1.67) 24. However, no information on epoetin use was available, and the time relationship between transfusion administration and development of thromboembolic events could not be determined 24. Transfusions were also shown to be a risk factor for VTE in an analysis of patients undergoing colorectal cancer resection, ranging from an OR of 1.39 for 1–2 units to an OR of 3.19 for ≥6 units 25. Epoetin use was not assessed here either. A case–crossover study evaluated triggers of hospitalization for VTE showing increased risks for transfusions (adjusted incidence rate ratio [aIRR] 2.57, 95% CI 1.17, 5.64) and epoetins (aIRR, 9.33, 95% CI 1.19, 73.42). However, only 26% of patients had a cancer diagnosis, making a comparison with our data difficult 18. A possible biological explanation for the higher VTE risk associated with transfusions could involve cytokine‐related inflammation and immunomodulation, since both procedures have been linked to blood transfusions as well as to increased coagulation 25, 29. The fact that increased inflammation does not seem to be of great importance in epoetin‐induced thrombosis 30 further supports this hypothesis.
We confirmed further known risk factors for VTE in cancer patients. History of VTE 21, obesity 31, metastatic disease 21, previous surgery 21 and specific medications such as immunosuppressants, hormonal therapy or chemotherapy 21 showed increased risks in our analysis. The increased risk for antithrombotic medication probably reflects the higher baseline VTE risk of patients receiving such treatment.
As tumour type or tumour stage can affect treatment of anaemia and VTE risk 21, we investigated a possible impact of confounding by disease severity on our results. For this reason, we created a more homogenous subcohort exclusively consisting of lung cancer patients and we additionally matched between cases and controls for presence of metastatic disease. The aOR for transfusions is still higher than the aOR for epoetins. However, given the wide CI due to the lower number of exposed cases in this subcohort a comparable VTE risk between the two treatments cannot be excluded.
Next, we investigated whether the EMA restrictions on epoetin use from June 2008 9 had an effect on VTE risk. Interestingly, the epoetin‐associated risk neutralized after June 2008 in both the main cohort and the chemotherapy only subcohort in contrast to statistically significant increased aORs until June 2008. Assuming physicians did implement the EMA recommendations on reduced target Hb, our data indicate that guideline adherence regarding epoetin treatment can increase the safety of this medication in a real‐life setting.
A further subgroup analysis explored whether the intrinsic VTE risk of different cancer entities has a modifying effect on the respective risks of transfusions or epoetin. Our results indicate that both anti‐anaemic treatments do not augment thrombotic diathesis in cancers with high intrinsic risk (lung or pancreatic cancer), but that transfusions can have a trigger effect in cancers with low intrinsic risk (prostate or breast cancer). However, as the classification in different entities resulted in considerably reduced epoetin‐associated thromboembolic events per group, findings on epoetins should be interpreted with caution.
Some strengths and limitations of our study should be mentioned. The strengths are the size and the representativeness of the data with a complete coverage of all age groups and the lack of non‐response due to the nature of administrative data 14. The study was not restricted to treatment episodes and provided real‐life data for a 5 year study period and an overall observation time of nearly 100 000 person‐years. Moreover, determination of drug therapy based on pharmacy dispensing data is considered the gold standard as recall bias can be ruled out and information is precise in time and dispensed dose 32.
The major limitation of this study is a possible confounding by disease severity. Tumour stage can affect treatment and VTE risk in cancer patients 31, 33, but this variable was not considered in our analysis since it is not included in GePaRD. For example, it seems unlikely that patients with very short life expectancy were started on epoetin therapy, since this treatment requires weeks to reveal its full effect 34. Therefore, a patient's prognosis could also constitute an indication for the treatment with epoetins or transfusions for which we could not control. However, by including metastatic disease in our analyses of the lung cancer only subcohort, we expect to have considerably reduced the effect of this confounder regarding VTE risk. Another potential limitation is the lack of Hb values in GePaRD, as patients with extreme low Hb levels are more likely to be treated with transfusions than with epoetins due to transfusions' faster onset of action. Unfortunately, ICD 10 codes for anaemia do not depict disease severity but possible aetiology, making them unfeasible for such an analysis. However, since CIA is mostly a chronic/subacute form of anaemia with slowly decreasing Hb levels 3, we do not expect the difference in time to response to have played a significant role in treatment selection. Furthermore, the lack of Hb values makes an analysis of exposure intensity difficult, as both transfusions and epoetins are dosed Hb‐dependent. Therefore, a possible dose–risk relationship between epoetins or transfusions and VTE could not be investigated. Finally, due to the low number of events in some subcohort analyses and the resulting wide CI, caution is needed in the interpretation of the respective findings.
In summary, our study shows that transfusions could be associated with a higher VTE risk than epoetins in cancer patients, with confounding for disease severity being the main limitation of our analysis. In cancer patients receiving chemotherapy this risk difference could not be verified. Moreover, we demonstrate that the epoetin risk decreased after the release of EMA recommendations advising against high target Hb values under epoetin treatment. Further studies including more clinical and laboratory details are needed in order to corroborate our results and to evaluate the comparative safety of these two anti‐anaemic treatments in cancer patients.
Funding sources
This study was funded by STADA Arzneimittel AG. The funders had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, in the preparation, review, or approval of the manuscript, and in the decision to submit the manuscript for publication.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work. EG has been a consultant to Bayer, Nycomed, Teva, GSK, Schwabe, and Novartis in the previous 3 years. EG has been running and AD, KJ, BK, and TS are working for a department that occasionally performs studies for pharmaceutical industries (these companies include Bayer, Celgene, GSK, Mundipharma, Novartis, Purdue, Sanofi‐Aventis, Sanofi Pasteur MSD, and STADA).
The authors would like to thank all statutory health insurances for providing data for this study (TK, AOK Bremen/Bremerhaven, DAK‐Gesundheit, HKK). They also want to thank Marieke Niemeyer and Inga Schaffer for contributing to the statistical analyses, Heike Gerds for editing the manuscript and Saskia Konusch for helping with literature research. No compensations were received for these contributions.
Contributions
AD interpreted the results and drafted the article. KJ conceived and designed the study, conducted data analysis, and helped interpreting the results and drafting the article. BK conducted data analysis and helped designing the study. TS helped designing the study, acquiring the data and interpreting the results and revised the article for content. EG supervised the publication, made substantial revisions to the article drafts, helped designing the study, acquiring and interpreting the data. All authors read and approved the final manuscript.
Supporting information
Table S1 Risk factors of venous thromboembolism
Table S2 Diseases and ICD‐10‐GM codes used for the Charlson Co‐morbidity Index according to Quan et al.
Table S3 ATC codes indicating use of medication potentially increasing the risk of venous thromboembolism
Table S4 ATC codes indicating use of antithrombotic medication
Table S5 Adjusted odds ratios (aOR) and 95% confidence intervals (CI) for venous thromboembolism in subcohorts before and after June 2008 (numbers in bold indicate significant results)
Supporting info item
Douros, A. , Jobski, K. , Kollhorst, B. , Schink, T. , and Garbe, E. (2016) Risk of venous thromboembolism in cancer patients treated with epoetins or blood transfusions. Br J Clin Pharmacol, 82: 839–848. doi: 10.1111/bcp.13019.
References
- 1. Schrijvers D, De Samblanx H, Roila F. Erythropoiesis‐stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use. Annals Oncol 2010; 21 (Suppl 5): v244–7. [DOI] [PubMed] [Google Scholar]
- 2. Tonia T, Mettler A, Robert N, Schwarzer G, Seidenfeld J, Weingart O, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012; 12: CD003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer‐related anemia. Am J Hematol 2014; 89: 203–12. [DOI] [PubMed] [Google Scholar]
- 4. Label and Approval History of Epoetin Alfa. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist (last accessed 07 April 2015).
- 5. Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia. JAMA 2008; 299: 914–24. [DOI] [PubMed] [Google Scholar]
- 6. Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta‐analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006; 98: 708–14. [DOI] [PubMed] [Google Scholar]
- 7. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood 2008; 111: 4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ‘Rote Hand Brief’ on safety of erythropoietin‐stimulating agents. In, 2008.
- 9. EMEA . Press release: EMEA recommends a new warning for epoetins for their use in cancer patients. Available at: http://www.emea.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500015069.pdf (last accessed 07 April 2015).
- 10. Glaspy J. Current status of use of erythropoietic agents in cancer patients. Semin Thromb Hemost 2014; 40: 306–12. [DOI] [PubMed] [Google Scholar]
- 11. FDA . FDA Strengthens Boxed Warnings, Approves Other Safety Labeling Changes for Erythropoiesis‐Stimulating Agents (ESAs). Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109024.htm (last accessed 07 April 2015).
- 12. FDA . Erythropoiesis‐Stimulating Agents (ESAs): Procrit, Epogen and Aranesp: Drug Safety Communication. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm200391.htm (last accessed 07 April 2015).
- 13. Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 2007; 43: 258–70. [DOI] [PubMed] [Google Scholar]
- 14. Pigeot I, Ahrens W. Establishment of a pharmacoepidemiological database in Germany: methodological potential, scientific value and practical limitations. Pharmacoepidemiol Drug Saf 2008; 17: 215–23. [DOI] [PubMed] [Google Scholar]
- 15. Jobski K, Enders D, Amann U, Suzart K, Wallander MA, Schink T, et al. Use of rivaroxaban in Germany: a database drug utilization study of a drug started in hospital. Eur J Clin Pharmacol 2014; 70: 975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005; 43: 1130–9. [DOI] [PubMed] [Google Scholar]
- 17. Aapro M, Osterwalder B, Scherhag A, Burger HU. Epoetin‐beta treatment in patients with cancer chemotherapy‐induced anaemia: the impact of initial haemoglobin and target haemoglobin levels on survival, tumour progression and thromboembolic events. Br J Cancer 2009; 101: 1961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rogers MA, Levine DA, Blumberg N, Flanders SA, Chopra V, Langa KM. Triggers of hospitalization for venous thromboembolism. Circulation 2012; 125: 2092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Summary of product characteristics ‐ Epoetin alfa HEXAL®. Available at: http://www.ema.europa.eu/docs/de_DE/document_library/EPAR_-_Product_Information/human/000726/WC500028282.pdf2008 (last accessed 07 April 2015).
- 20. Garbe E, Kloss S, Suling M, Pigeot I, Schneeweiss S. High‐dimensional versus conventional propensity scores in a comparative effectiveness study of coxibs and reduced upper gastrointestinal complications. Eur J Clin Pharmacol 2013; 69: 549–57. [DOI] [PubMed] [Google Scholar]
- 21. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer‐associated venous thrombosis. Blood 2013; 122: 1712–23. [DOI] [PubMed] [Google Scholar]
- 22. Cancer in Germany 2005/2006 . Incidence and Trends. Berlin: Robert Koch‐Institut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V. (Hrsg), 2010. [Google Scholar]
- 23. Chavez‐MacGregor M, Zhao H, Fang S, Srokowski TP, Hortobagyi GN, Giordano SH. Complications associated with erythropoietin‐stimulating agents in patients with metastatic breast cancer: a Surveillance, Epidemiology, and End Results‐Medicare study. Cancer 2011; 117: 3641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med 2008; 168: 2377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res 2012; 129: 568–72. [DOI] [PubMed] [Google Scholar]
- 26. Ludwig H, Aapro M, Bokemeyer C, Macdonald K, Soubeyran P, Turner M, et al. Treatment patterns and outcomes in the management of anaemia in cancer patients in Europe: findings from the Anaemia Cancer Treatment (ACT) study. Eur J Cancer 2009; 45: 1603–15. [DOI] [PubMed] [Google Scholar]
- 27. Hess G, Nordyke RJ, Hill J, Hulnick S. Effect of reimbursement changes on erythropoiesis‐stimulating agent utilization and transfusions. Am J Hematol 2010; 85: 838–43. [DOI] [PubMed] [Google Scholar]
- 28. Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006; 166: 458–64. [DOI] [PubMed] [Google Scholar]
- 29. Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest 2005; 127: 295–307. [DOI] [PubMed] [Google Scholar]
- 30. Glaspy J. Thrombosis during therapy with erythropoiesis stimulating agents in cancer In: Recombinant Human Erythropoietin (rhEPO) in Clinical Oncology, ed Nowrousian M. Vienna: Springer, 2008; 745–57. [Google Scholar]
- 31. Connolly GC, Khorana AA. Risk stratification for cancer‐associated venous thromboembolism. Best Pract Res Clin Haematol 2009; 22: 35–47. [DOI] [PubMed] [Google Scholar]
- 32. Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol 2005; 58: 323–37. [DOI] [PubMed] [Google Scholar]
- 33. Calabrich A, Katz A. Management of anemia in cancer patients. Future Oncol 2011; 7: 507–17. [DOI] [PubMed] [Google Scholar]
- 34. Ludwig H, Fritz E, Leitgeb C, Pecherstorfer M, Samonigg H, Schuster J. Prediction of response to erythropoietin treatment in chronic anemia of cancer. Blood 1994; 84: 1056–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Risk factors of venous thromboembolism
Table S2 Diseases and ICD‐10‐GM codes used for the Charlson Co‐morbidity Index according to Quan et al.
Table S3 ATC codes indicating use of medication potentially increasing the risk of venous thromboembolism
Table S4 ATC codes indicating use of antithrombotic medication
Table S5 Adjusted odds ratios (aOR) and 95% confidence intervals (CI) for venous thromboembolism in subcohorts before and after June 2008 (numbers in bold indicate significant results)
Supporting info item


