Abstract
Aims
To analyse clinical outcomes with direct oral anticoagulants in patients with atrial fibrillation according to geographic region.
Methods
We systematically searched MEDLINE, CENTRAL, websites of regulatory agencies, clinical trials registers and conference proceedings for randomized controlled trials of direct oral anticoagulants (DOAC: dabigatran, rivaroxaban, apixaban or edoxaban) against warfarin for prophylaxis of stroke and systemic embolic events (SEE) in patients with atrial fibrillation (AF). Two investigators independently extracted data. Relative risks of stroke and SEE as well as major bleeding depending on geographic region were estimated using a random effect meta‐analysis.
Results
Five trials in 72 963 patients were analysed; 32 089 (44%) patients were recruited in Europe (Western Europe: 13 676; Eastern Europe: 18 413). We found significant subgroup differences for stroke/SEE depending on the geographic region (interaction P = 0.003; I 2 88.5%), with a neutral effect of the DOAC vs. warfarin in Europe [relative risk (RR) 0.97, 95% confidence interval (CI) 0.85–1.11, I 2 0%] and a significant reduction of stroke/SEE in other regions including North America, Latin America and Asia‐Pacific/other (RR 0.72, 95% CI 0.63–0.83, I 2 33%). There was a similar reduction in risk of major bleeding in Europe (RR 0.82, 95% CI 0.73–0.92, I 2 0%) and in other regions (RR 0.86, 95% CI 0.72–1.02, I 2 78%).
Conclusion
The DOAC did not provide additional benefit in reducing the risk of stroke/SEE compared with warfarin in European patients with AF, but were generally associated with a lower bleeding tendency than warfarin regardless of geographic region.
Keywords: anticoagulants, arrhythmia, meta‐analysis, stroke, warfarin
What is Already Known about this Subject
Dabigatran, rivaroxaban, apixaban and edoxaban are direct‐acting oral anticoagulants (DOAC) licensed for stroke prevention in atrial fibrillation.
Pivotal trials in atrial fibrillation included a heterogeneous population from worldwide regions with different standards of care.
It is uncertain whether there are regional differences in the effect of the DOAC on stroke and major bleeding in comparison with warfarin.
What this Study Adds
Our meta‐analysis shows significant differences in the relative efficacy of the DOAC versus warfarin depending on geographic region.
Compared with warfarin, the novel compounds did not reduce the risk of stroke and systemic embolism in European patients, but were generally associated with a lower bleeding tendency than warfarin in Europe and other regions.
Geographic differences found in our meta‐analysis appear mainly related to regional variations in stroke rates with warfarin.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in the developed world, being associated with a five‐fold risk of stroke and higher mortality 1. Warfarin and other vitamin K antagonists (VKA) are highly effective treatments in reducing the risk of stroke, but their management remains problematic due to their narrow therapeutic index and variability in drug exposure, necessitating routine coagulation monitoring [international normalized ratio (INR)], clinical surveillance and continuous patient education.
In recent years, several direct‐acting oral anticoagulants (DOAC) (dabigatran, rivaroxaban, apixaban and edoxaban) 2, also referred to in the literature as “novel anticoagulants” or “non‐vitamin K antagonist oral anticoagulants” (NOAC) and “target‐specific oral anticoagulants” (TSOAC), have been developed to overcome some of these limitations. The pivotal studies that support the use of DOAC for prevention of stroke and systemic embolic events (SEE) in AF have encompassed a heterogeneous population from worldwide regions with different standards of medical care. Globally, these studies have shown a benefit of DOAC compared with warfarin in reducing stroke/SEE, as well as intracranial bleeding (ICB) and mortality 3. The assessment of the “transferability” of multinational trials to specific countries or regions 4 is a hot issue that may have important consequences for the cost‐effectiveness analyses and recommendations for use that are usually based on the global results of these pivotal studies. Several subanalyses and reviews of results in Asian patients vs. non‐Asians have been published recently 5. In Asia, the DOAC seems to provide the highest clinical benefit compared with warfarin. However, no comprehensive review of disaggregated results in other geographic regions is currently available.
We systematically reviewed and meta‐analysed data from randomized controlled trials of the DOAC for prophylaxis against stroke/SEE in patients with AF according to geographic region to assess if the overall study results can be transferred to the European population. We made direct comparisons between the DOAC and warfarin on the clinical outcomes of stroke/SEE and major bleeding depending on geographic region.
Methods
Eligibility criteria
We considered randomized controlled trials comparing any of the approved new oral anticoagulants (rivaroxaban, dabigatran, apixaban and edoxaban) with warfarin in patients with AF at risk of stroke/SEE and at least one year follow‐up.
The doses tested in the experimental arms had to correspond to the doses approved for the DOAC in this indication 2. We included the dabigatran low dose (110 mg twice daily) in addition to the dabigatran high dose (150 mg twice daily), because the low dose is recommended in a significant proportion of the target population (patients ≥80 yr, concomitant verapamil or high risk of bleeding) 2, and no differences in efficacy between the high and low dabigatran dose have been observed in the long term 6. However, we excluded the edoxaban low dose in the base case analysis (30 mg once daily, reduced to 15 mg in patients with presumed increased exposure), because it has not been approved for use in Europe and North America in this indication.
Trial identification and data collection
We searched Medline and CENTRAL (up to July 2015), websites of regulatory agencies, clinical trial registries and relevant conference proceedings (Appendix S1). No language restrictions were applied. Two investigators (AG‐O and AIT‐F) independently and separately assessed trials for eligibility and extracted data. If a trial was covered in more than one report, we used the following hierarchy of data sources: public reports from regulatory authorities, peer reviewed articles, reports from the web‐based repository for results of clinical studies and other sources. Finally, we contacted the main investigators to retrieve unpublished data from clinical trials (demographic characteristics and data on ICB and deaths by region). In case of no response, we sent a reminder to the main investigator after one week, with a copy to a Sponsor's representative (e.g., co‐author(s) being employee(s) of the Sponsor).
Study characteristics and quality assessment
We collected data on patients' characteristics, numbers of patients evaluable for efficacy and safety, dosage used in the experimental and control groups, duration of treatment and follow‐up, inclusion and exclusion criteria. We assessed study quality using the Cochrane Collaboration's tool for assessing risk of bias in randomized studies 7. Additionally, we used the Jadad scale to assess study quality 8.
Outcome measures
The pre‐specified primary outcome was stroke/SEE. The pre‐specified primary safety outcome was major bleeding. We also aimed to retrieve data on ICB and all‐cause death as secondary outcomes whenever possible.
Quantitative data synthesis
We conducted this meta‐analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses recommendations 9. We used the intention‐to‐treat population for efficacy and safety whenever available. We calculated relative risks (RR) and their respective 95% confidence intervals (CI) for each study and for the pooled studies. Heterogeneity within subgroups and interaction between subgroups was assessed using the Cochran Q test and the Higgins I 2 test. A Cochran's Q P < 0.05 and I 2 > 50% within subgroups indicates significant heterogeneity, and between subgroups indicates statistically significant interaction (subgroup differences). We used the random effects model described by Der‐Simonian and Laird 10 for the main analysis.
The base case was focused on the dichotomized comparison of RR of events in Europe vs. other regions. We also conducted a secondary analysis of the data in each of the regions defined within the trials (Western Europe, Eastern Europe, North America, Latin America and Asia‐Pacific). We conducted sensitivity analyses taking into account different methodological issues that could influence the results of the meta‐analysis: (a) geographic region definition (Western Europe instead of all Europe vs. other regions); (b) DOAC doses tested (not excluding the 30/15 mg edoxaban low dosing; excluding the 110 mg dabigatran dose); (c) statistical model (fixed effects instead of the random effects model); (d) type of measure (absolute risk or odds ratio instead of RR); (e) adjustment by exposure (events by patient‐years instead of events by patients); (f) study location (only multinational studies instead of all studies); and (g) study quality (studies at low risk of bias instead of all studies). We also conducted a proportion meta‐analysis within treatment groups to describe the average rate of events in each treatment group by trial and geographic region adjusted by exposure (per patient‐year of follow‐up on the basis of the mean reported follow‐up). Rates of events were expressed per 100 patient‐years (%/yr) to standardize different follow‐up durations across studies.
Direct comparisons were carried out using the RevMan statistical software, version 5.1 (Nordic Cochrane Center). The descriptive analysis of event rates by treatment group and region was performed using StatsDirect software, version 2.8.0 (StatsDirect Ltd, Cheshire, United Kingdom).
Results
Study selection, design and methodology
The literature search identified 3784 articles, 356 of which related to clinical trials or protocols with rivaroxaban, dabigatran, apixaban or edoxaban (Figure 1). Of these, 11 were clinical trials in AF, and were selected for checking as full text. Five of the 11 studies were eligible for inclusion 11, 12, 13, 14, 15 and the remaining six were excluded because they were Phase II studies with insufficient follow‐up or used aspirin as control rather than warfarin 16, 17, 18, 19, 20, 21. Additional data from included trials were obtained from Food and Drug Administration (FDA) reviews and in a subanalysis of dabigatran in AF 22, 23, 24, 25.
Figure 1.
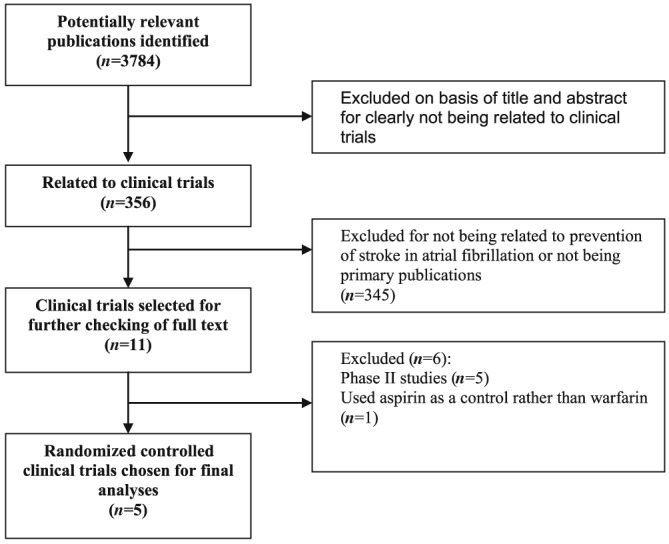
Study identification, selection and exclusions
The five studies comprised 72 963 patients (Table 1). Four of them were multinational studies that compared dabigatran 11, rivaroxaban 12, apixaban 13 and edoxaban 14 with warfarin in AF. The remaining trial corresponded to a Phase III study with rivaroxaban conducted in Japan only 15. The risk of bias was low in three studies 12, 13, 14 and unclear in RE‐LY due to lack of double‐blinding 11, and J ROCKET due to no reporting of allocation concealment 15 (Appendix S2). For the same reasons, three studies scored five points in the Jadad scale 12, 13, 14 and the remaining two studies 11, 15 scored four points (Table 1).
Table 1.
Characteristics of trials included in the systematic review
| Characteristic | RE‐LY | ROCKET AF | ARISTOTLE | ENGAGE AF | J ROCKET |
|---|---|---|---|---|---|
| N Randomized * | 18 113 | 14 264 | 18 201 | 21 105 | 1280 |
| ITT patients * | 18 113 | 14 171 | 18 201 | 21 026 | 1280 |
| Safety patients | 18 113 | 14 236 | 18 201 | 21 026 | 1278 |
| Patient‐years | 31 273 | 22 493 | 30 943 | 46 888 | 1718 |
| Experimental drug | Dabigatran 150 mg and 110 mg twice daily | Rivaroxaban 20 mg once daily† | Apixaban 5 mg twice daily† | Edoxaban 60 mg and 30 mg once daily† | Rivaroxaban 15 mg once daily† |
| Exposure, mean (yrs) | 1.69–1.71 | 1.57 | 1.72 | 2.21–2.26 | 1.37 |
| Control drug | Warfarin‡ | Warfarin‡ | Warfarin‡ | Warfarin‡ | Warfarin‡ |
| Exposure, mean (yrs) | 1.78 | 1.59 | 1.68 | 2–22 | 1.32 |
| TTR (%), mean | 64.4 | 55.2 | 62.2 | 65 | 65 |
| Median follow‐up (yrs) | 2 | 1.9 | 1.8 | 2.8 | 1.3 |
| Trial phase | III | III | III | III | III |
| Design | Open‐label PROBE | Double‐blind | Double‐blind | Double‐blind | Double‐blind |
| Non‐inferiority margin | 1.46 | 1.46 | 1.44 | 1.38 | 2.00§ |
| Main efficacy outcome | Stroke or SEE | Stroke or SEE | Stroke or SEE | Stroke or SEE | Stroke or SEE |
| Main safety outcome | Major bleeding | Clinically relevant bleeding | Major bleeding | Major bleeding | Clinically relevant bleeding |
| Pre‐specified subgroup analysis by region? | Yes | Yes | Yes | Yes | No |
| Randomization stratified by centre/geographic region? | NA | Yes | Yes | No | No |
| Risk of bias (Cochrane) | Unclear | Low | Low | Low | Unclear |
| Jadad Score | 4 | 5 | 5 | 5 | 4 |
NA, not available; PROBE, Prospective, randomized, open‐label, blinded‐endpoint.
Randomized patients in RE‐LY, J ROCKET and ARISTOTLE; ITT to site notification in ROCKET AF; mITT on‐treatment in ENGAGE.
Dose‐reduction was applied in patients with CrCl 15–50 ml min−1 (rivaroxaban: from 20 mg to 15 mg in ROCKET AF; from 15 mg to 10 mg in J ROCKET), in patients with at least two of the following characteristics: age ≥ 80 years, body weight ≤ 60 kg or serum creatinine ≥ 1.5 mg dl−1 (apixaban: from 5 mg to 2.5 mg twice daily) and in patients with a CrCl 30–50 ml min−1, a body weight ≤ 60 kg or concomitant potent P‐gp inhibitors (edoxaban: from 60 mg to 30 mg once daily in the high dosing group; from 30 mg to 15 mg once daily in the low dosing group).
Dose adjusted to an international normalized ratio (INR) between 2 and 3, once daily.
Non‐inferiority was focused on efficacy in all studies with the exception of J ROCKET, in which non‐inferiority was focused on clinically relevant bleeding.
Mean or median age ranged between 70 and 73 years (Table 2). There was predominance of men (range: 60–81%) and permanent/persistent AF (range: 67–83%). Mean thromboembolic risk (CHADS2) ranged between 2.1 (RE‐LY and ARISTOTLE) to 3.5 (ROCKET AF). History of prior stroke or transient ischemic attack ranged between 19% and 64%, and rate of VKA naive patients ranged from 10% to 50%.
Table 2.
Characteristics of patients
| Characteristic | Drug, trial | ||||
|---|---|---|---|---|---|
| Dabigatran RE‐LY | Rivaroxaban ROCKET AF | Apixaban ARISTOTLE | Edoxaban ENGAGE AF | Rivaroxaban J ROCKET | |
| Randomized | 18 113 | 14 264 | 18 201 | 21 105 | 1280 |
| Age (years) | 72 (mean) | 73 (median) | 70 (median) | 72 (median) | 71 (mean) |
| Male gender (%) | 64 | 60 | 65 | 62 | 81 |
| Atrial fibrillation type | |||||
| Permanent/persistent (%) | 67 | 81 | 83 | 75 | NA |
| Paroxysmal (%) | 33 | 18 | 17 | 25 | NA |
| CHADS 2 score (mean) | 2.1 | 3.5 | 2.1 | 2.8 | 3.3 |
| Prior stroke/TIA (%) | 20 | 55 | 19 | 28 | 64 |
| VKA naive (%) | 50 | 37 | 43 | 41 | 10 |
| Region * | |||||
| Europe, n (%) | 6770 (38) | 7596 (53) | 7343 (40) | 10 380 (49) | 0 (0) |
| Western Europe | 4651 (26) | 2096 (15) | 3693 (20) | 3236 (15) | — |
| Eastern Europe | 2119 (12) | 5500 (38) | 3650 (20) | 7144 (34) | — |
| Other regions, n (%) | 11 343 (62) | 6668 (47) | 10 858 (60) | 10 725 (51) | 1280 (100) |
| North America | 6533 (36) | 2681 (19) | 4474 (25) | 4681 (22) | — |
| Latin America | 956 (5) | 1878 (13) | 3468 (19) | 2661 (13) | — |
| Asia Pacific, other | 3854 (21) | 2109 (15) | 2916 (16) | 3383 (16) | 1280 (100) |
NA, not available.
The distribution by region corresponds to randomized patients. Calculation of numbers of patients enrolled in Western and Eastern Europe in ARISTOTLE was made based on patients enrolled by countries (see Supplementary Appendix).
A total of 32 089 patients (44%) were recruited in Europe, while 40 874 (56%) were recruited in other regions. For detailed information on the definitions of geographic regions and countries by trial as well as the pooling strategy by region, please see Appendix S3.
Demographic characteristics by region were available from the RE‐LY study (kindly provided by the Sponsor after request) and collected for the ROCKET‐AF study from a secondary publication of the study 26 (Appendix S4). Patients recruited in Europe and North America had a much higher mean body weight than patients recruited in Latin America and Asia. History of prior stroke or transient ischemic attack (TIA) at study enrolment was more frequent among Asian patients, while VKA‐experienced patients were more frequent in Western Europe and North America. Highest mean percentage time in therapeutic INR range (TTR) was reported in Western Europe (69%) and lowest in Asia (53–55%) (Appendix S5).
Descriptive analysis of event rates
Pooled stroke/SEE rates with the DOAC were the lowest in North America (1.3%/yr) and the highest in Asia (2.1%/yr) (Table 3). Pooled stroke/SEE with warfarin ranged from 1.4%/yr in Western Europe to 2.9%/yr in Asia. There was also variability across trials, with the highest stroke rates in both treatment arms observed in ROCKET AF.
Table 3.
Descriptive analysis of events by trial and region and adjusted event rates per 100 patients per year
| Characteristic | Type of direct oral anticoagulant, trial events (%/year) | Total* %/year | ||||
|---|---|---|---|---|---|---|
| Dabigatran RE‐LY | Rivaroxaban ROCKET AF | Apixaban ARISTOTLE | Edoxaban ENGAGE AF | Rivaroxaban J ROCKET | ||
| Stroke/SEE | ||||||
| Direct oral anticoagulants | ||||||
| Europe pooled, n (%) | 111 (1.4) | 140 (2.4) | 75 (1.2) | 104 (1.4) | — | 1.6 (1.1–2.1) |
| Western Europe | 80 (1.5) | 40 (2.4) | 30 (0.9) | 37 (1.6) | — | 1.6 (1.1–2.1) |
| Eastern Europe | 31 (1.3) | 100 (2.4) | 45 (1.4) | 67 (1.3) | — | 1.6 (1.1–2.1) |
| Other regions pooled, n (%) | 206 (1.6) | 129 (2.5) | 137 (1.5) | 78 (1.0) | 22 (2.5) | 1.7 (1.2–2.2) |
| North America | 103 (1.4) | 47 (2.2) | 42 (1.1) | 23 (0.7) | — | 1.3 (0.8–1.9) |
| Latin America | 15 (1.4) | 37 (2.5) | 43 (1.4) | 20 (1.0) | — | 1.6 (1.0–2.2) |
| Asia Pacific, other | 88 (2.0) | 45 (2.7) | 52 (2.1) | 35 (1.4) | 22 (2.5) | 2.1 (1.7–2.5) |
| Warfarin | ||||||
| Europe pooled, n (%) | 58 (1.4) | 157 (2.6) | 77 (1.2) | 93 (1.2) | — | 1.6 (1.0–2.3) |
| Western Europe | 45 (1.6) | 43 (2.6) | 25 (0.8) | 25 (1.1) | — | 1.4 (0.8–2.2) |
| Eastern Europe | 13 (1.0) | 114 (2.6) | 52 (1.7) | 68 (1.3) | — | 1.7 (1.0–2.4) |
| Other regions pooled, n (%) | 144 (2.1) | 149 (2.8) | 188 (2.1) | 139 (1.8) | 26 (3.1) | 2.3 (1.9–2.7) |
| North America | 67 (1.7) | 50 (2.3) | 56 (1.5) | 42 (1.2) | — | 1.7 (1.3–2.1) |
| Latin America | 9 (1.6) | 45 (3.0) | 52 (1.8) | 42 (2.1) | — | 2.2 (1.6–2.8) |
| Asia Pacific, other | 68 (3.0) | 54 (3.2) | 80 (3.2) | 55 (2.2) | 26 (3.1) | 2.9 (2.5–3.4) |
| Major bleeding | ||||||
| Direct oral anticoagulants | ||||||
| Europe pooled, n (%) | 180 (2.3) | 137 (2.3) | 110 (1.7) | 150 (2.0) | — | 2.1 (1.8–2.4) |
| Western Europe | 131 (2.5) | 49 (3.0) | 74 (2.3) | 71 (3.0) | — | 2.6 (2.3–3.0) |
| Eastern Europe | 49 (2.0) | 88 (2.0) | 36 (1.1) | 79 (1.5) | — | 1.7 (1.3–2.1) |
| Other regions pooled, n (%) | 561 (4.4) | 258 (4.9) | 217 (2.3) | 268 (3.4) | 23 (2.6) | 3.5 (2.6–4.6) |
| North America | 403 (5.4) | 149 (7.1) | 106 (2.7) | 135 (3.9) | — | 4.7 (3.1–6.5) |
| Latin America | 26 (2.4) | 46 (3.1) | 60 (2.0) | 48 (2.5) | — | 2.5 (2.0–3.0) |
| Asia Pacific, other | 132 (3.0) | 63 (2.9) | 51 (2.0) | 85 (3.4) | 23 (2.6) | 3.0 (2.4–3.6) |
| Warfarin | ||||||
| Europe pooled, n (%) | 104 (2.6) | 153 (2.5) | 135 (2.2) | 206 (2.7) | — | 2.5 (2.3–2.7) |
| Western Europe | 80 (2.9) | 69 (4.1) | 77 (2.5) | 86 (3.6) | — | 3.2 (2.6–4.0) |
| Eastern Europe | 24 (1.9) | 84 (1.9) | 58 (1.9) | 120 (2.3) | — | 2.1 (1.8–2.3) |
| Other regions pooled, n (%) | 317 (4.7) | 233 (4.4) | 327 (3.6) | 318 (4.0) | 27 (3.2) | 4.1 (3.6–4.6) |
| North America | 209 (5.4) | 111 (5.2) | 137 (3.7) | 152 (4.4) | — | 4.6 (3.9–5.5) |
| Latin America | 17 (3.0) | 41 (2.7) | 94 (3.2) | 67 (3.4) | — | 3.2 (2.8–3.6) |
| Asia Pacific, other | 91 (4.0) | 81 (4.8) | 96 (3.9) | 99 (4.0) | 27 (3.2) | 4.1 (3.7–4.5) |
SEE, systemic embolic events.
Proportion meta‐analysis, random effects model, StatsDirect software.
Pooled major bleeding rates with the DOACs showed variability across regions, ranging from 1.7%/yr in Eastern Europe to 4.7%/yr in North America. The same trend, though less pronounced, was apparent in the warfarin arm, with major bleeding rates ranging from 2.1%/yr in Eastern Europe to 4.6%/yr in North America. There was variability in major bleeding rates across trials, being more pronounced within the DOAC groups than within the warfarin groups (Table 3).
Rates of ICB and deaths by region were not available from the literature search and were requested from the main investigators of the studies. A positive response was obtained from the main investigator of the RE‐LY trial (Dr. Connolly) who delegated to the Sponsor who kindly provided the data (Appendix S5). Asian patients had the highest rate of ICB (1.1%/yr) and patients from Latin America had the highest mortality rates (6.2%/yr) with warfarin, while European patients had the lowest rates of both ICB (0.6% yr) and mortality (3.8%/yr) with warfarin.
Primary efficacy outcome: stroke and systemic embolism
We found significant subgroup differences for stroke/SEE depending on the geographic region (P for interaction = 0.003; I 2 = 88.5%) with a neutral effect of the DOAC vs. warfarin in Europe (RR 0.97; 95% CI 0.85–1.11; I 2 0%) and a significant reduction of stroke/SEE in other regions (RR 0.72; 95% CI 0.63–0.83; I 2 33%) (Figure 2).
Figure 2.
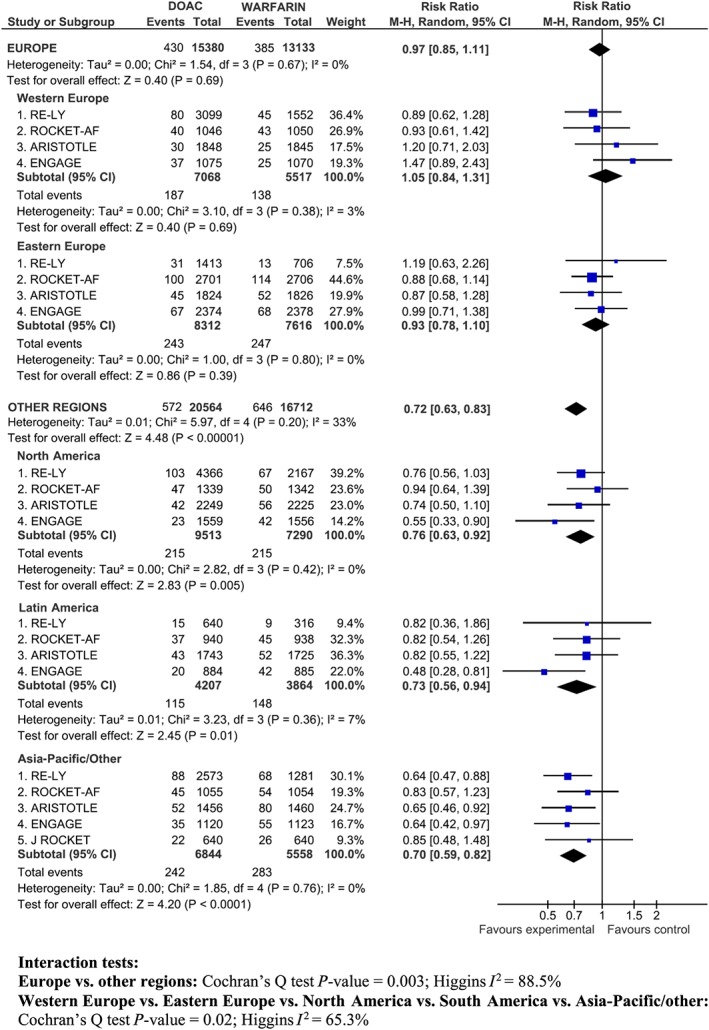
Stroke/SEE in Europe (Western and Eastern Europe) and other regions (North America, South America, Asia‐Pacific/Other)
The analysis was repeated for the five disaggregated regions (Western Europe, Eastern Europe, North America, Latin America, Asia‐Pacific), and subgroup differences still remained statistically significant (P for interaction = 0.02; I 2 65.3%) (Figure 2). Across European sub‐regions, the point estimate for the RR of stroke tended to favour warfarin in Western Europe, particularly in ARISTOTLE and ENGAGE, and to slightly favour the DOAC in Eastern Europe, without statistically significant differences between the DOAC and warfarin. DOAC significantly reduced the RR of stroke/SEE in other regions, with a more pronounced effect in Asia and Latin America.
Primary safety outcome: major bleeding
There was a similar reduction in risk of major bleeding in Europe (RR 0.82; 95% CI 0.73–0.92) and in other regions (RR 0.86, 95% CI 0.72–1.02) (P for interaction = 0.66; I 2 0%) (Figure 3). However, there was evidence of statistical heterogeneity within other regions (P = 0.001; I 2 78%) (Figure 3).
Figure 3.
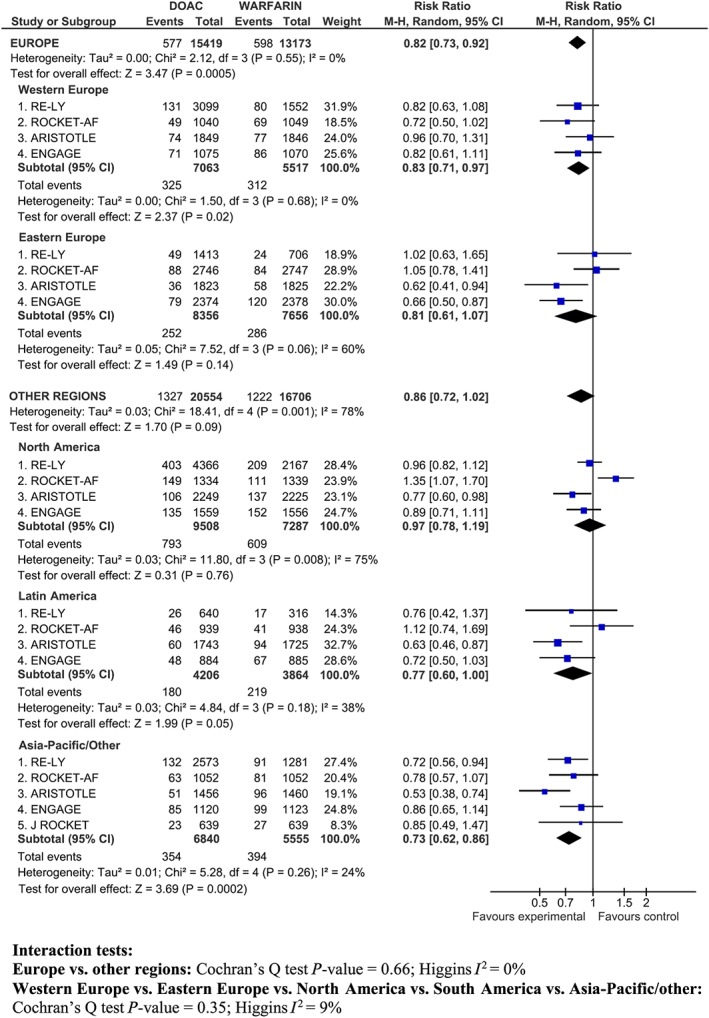
Major bleeding in Europe (Western and Eastern Europe) and other regions (North America, South America, Asia‐Pacific/Other)
In Western Europe, the RR of bleeding was lower in ARISTOTLE and in ENGAGE than in the other studies. In other regions, the heterogeneity was mainly due to the increase in major bleeding observed in North America in the ROCKET‐AF study (see also the discussion for potential explanations) and, to a lesser extent, by the high relative reduction in risk of major bleeding (47%) reported in Asian patients in ARISTOTLE (Figure 3).
Sensitivity analyses
All the ten sensitivity analyses conducted to explore the geographic differences in the effect on stroke/SEE showed statistically significant results (Appendix S6) that were consistent with the primary analysis. Geographic differences were apparent regardless of included/excluded doses of the DOAC, European region definition, statistical model, adjustment by exposure, effect measure, exclusion of studies conducted in a single region (J ROCKET) and exclusion of studies at uncertain risk of bias (RE‐LY and J ROCKET).
Consistent with the primary analysis of major bleeding, none of the ten sensitivity analyses showed geographic differences in the effect on major bleeding (Appendix S6).
Absolute difference in events per 1000 patients treated per year in the various regional subgroups
There were no significant differences between the DOAC and warfarin in stroke/SEE events per 1000 patient‐years in Europe (Dif.: 0 events; 95% CI −2 to 2) (Table 4). On the contrary, significant reductions in stroke/SEE were found in other regions (Table 4), ranging between four events avoided in North America, six events avoided in Latin America and nine events avoided in Asia per 1000 patient‐years.
Table 4.
Absolute difference in events per 1000 patients treated per year in the various regional subgroups*
| Comparison | Stroke/SEE | Major bleeding |
|---|---|---|
| Risk difference (95%CI) | Risk difference (95%CI) | |
| All Europe (n = 32 089) | ||
| Dabigatran vs. warfarin | 0 (−5 to 5) | −2 (−8 to 4) |
| Rivaroxaban vs. warfarin | −2 (−8 to 3) | −2 (−8 to 3) |
| Apixaban vs. warfarin | −1 (−4 to 3) | −4 (−9 to 0.4) |
| Edoxaban vs. warfarin | 2 (−2 to 5) | −7 (−12 to −2) |
| All DOACs vs. warfarin | 0 (−2 to 2) | −4 (−7 to − 2) |
| Western Europe (n = 13 676) | ||
| Dabigatran vs. warfarin | −2 (−7 to 5) | −4 (−12 to 3) |
| Rivaroxaban vs. warfarin | −1 (−12 to 10) | −11 (−24 to 1) |
| Apixaban vs. warfarin | 1 (−3 to 6) | −2 (−9 to 6) |
| Edoxaban vs. warfarin | 5 (−1 to 12) | −6 (−17 to 4) |
| All DOACs vs. warfarin | 1 (−1 to 4) | −5 (−10 to − 0.1) |
| Eastern Europe (n = 18 413) | ||
| Dabigatran vs. warfarin | 3 (−5 to 10) | 1 (−8 to 11) |
| Rivaroxaban vs. warfarin | −3 (−10 to 4) | 1 (−5 to 7) |
| Apixaban vs. warfarin | −3 (−9 to 4) | −7 (−14 to −1) |
| Edoxaban vs. warfarin | 0 (−4 to 4) | −8 (−13 to −3) |
| All DOACs vs. warfarin | −1 (−4 to 2) | −4 (−9 to 1) |
| North‐America (n = 18 369) | ||
| Dabigatran vs. warfarin | −4 (−8 to 1) | 0 (−9 to 9) |
| Rivaroxaban vs. warfarin | −1 (−10 to 8) | 19 (5 to 34) |
| Apixaban vs. warfarin | −4 (−9 to 1) | −9 (−17 to −1) |
| Edoxaban vs. warfarin | −6 (−10 to −1) | −5 (−14 to 5) |
| All DOACs vs. warfarin | −4 (−7 to − 2) | 0 (−10 to 10) |
| Latin‐America (n = 8963) | ||
| Dabigatran vs. warfarin | −2 (−15 to 10) | −6 (−23 to 11) |
| Rivaroxaban vs. warfarin | −5 (−17 to 7) | 4 (−8 to 16) |
| Apixaban vs. warfarin | −4 (−10 to 3) | −12 (−21 to −4) |
| Edoxaban vs. warfarin | −11 (−19 to −3) | −10 (−20 to 1) |
| All DOACs vs. warfarin | −6 (−10 to − 2) | −7 (−14 to − 0.1) |
| Asia Pacific, other (n = 13 542) | ||
| Dabigatran vs. warfarin | −10 (−18 to −2) | −10 (−20 to −0.2) |
| Rivaroxaban vs. warfarin † | −5 (−15 to 4) | −8 (−19 to 2) |
| Apixaban vs. warfarin | −12 (−21 to −3) | −19 (−28 to −9) |
| Edoxaban vs. warfarin | −8 (−15 to −1) | −5 (−16 to 5) |
| All DOACs vs. warfarin | −9 (−13 to − 5) | −11 (−16 to − 6) |
CI, confidence interval; DOAC, direct oral anticoagulants; SEE, systemic embolic events.
Random effects model. The base case excludes the 30 mg/15 mg edoxaban dose.
Includes pooled data from ROCKET‐AF (subgroup of Asian patients) and J ROCKET.
The DOAC significantly avoided four major bleeding events per 1000 patient‐years in comparison with warfarin in Europe. The reduction in bleeding events was particularly high in Asia and Latin America, in which the DOAC avoided 11 and 7 additional major bleedings per 1000 patient‐years compared with warfarin, respectively (Table 4). Finally, rivaroxaban tended to increase the number of major bleeding events compared with warfarin in North America (potential explanations are included in the Discussion).
Selective outcome reporting, dissemination bias and missing data
Subgroups by geographic region for the main efficacy and safety outcomes were pre‐specified in the protocols and reported in the publications or regulatory reviews of the large multicentre studies included in this meta‐analysis. There were three trials with missing outcome data for secondary outcomes (intracranial bleeding and mortality) where we were unable to obtain the data from the authors.
Discussion
This systematic review and meta‐analysis indicates that, although the DOAC have a positive benefit‐risk balance for prevention of stroke/SEE, the extent of such benefit may differ between geographic regions according to differences in stroke rates with warfarin. Asia and Latin America were the regions in which the effect of the DOAC over warfarin on stroke/SEE was more relevant, while no significant reduction of stroke/SEE was apparent in Europe, which comprised approximately 32 000 patients and 59 000 patient‐years in these trials. The robustness of the results is strongly supported by ten sensitivity analyses.
To the best of our knowledge, this is the first systematic review to explore the efficacy and safety of the DOAC in each of the geographical regions included in the Phase III clinical trials conducted with the new compounds for the prevention of stroke/SEE in patients with AF. A previous relevant meta‐analysis reviewed the pivotal trials of the DOAC in AF 3, but did not analyse the efficacy and safety across geographical regions. A relevant Cochrane review 27 focused only on two direct thrombin inhibitors [dabigatran (Pradaxa) and ximelagatran (Exanta; withdrawn from the market due to liver toxicity)], and did not include an analysis by geographic region. In addition, none of these reviews included a calculation of event rates corrected by exposure (events per 100 patients per year) that are important to ascertain the absolute differences between treatments and, therefore, the clinical relevance of the effect.
While there are benefits from trial globalization in terms of the worldwide evaluation of safety and efficacy, differences in degree of development, medical culture and standard of care raise important questions about the impact of the trial and the comparability of individual national/regional outcomes to the total international population 28. Exploring the causes of heterogeneity across regions is a necessary exercise demanded by healthcare providers and may be informative for healthcare professionals and patients.
The benefit of oral anticoagulation with VKA is largely dependent on the quality of INR control as measured by the TTR 29, 30. The quality of anticoagulation with VKA during pivotal studies with the DOAC greatly differed across trials, with the highest TTR reported in Western Europe, and the lowest TTR in centres in Asia and Latin America 26, 31. These data are consistent with TTR reported across regions in worldwide AF registries 32 and meta‐analyses 33. A recent review of 55 studies on AF shows that patients in Europe have better INR control than those in other regions, as measured by the time spent with the INR in therapeutic range (67% in Europe and between 47% and 61% in other regions) 33, which is broadly consistent with the differences in mean TTR by geographic region reported in our meta‐analysis (69% in Western Europe and between 53% and 66% in other regions; see Appendix S4). Therefore, regional differences in quality and organization of care 33, which could comprise among other factors a longer tradition of anticoagulation clinics with good INR control 34, are likely to explain why the relative efficacy of the DOAC vs. warfarin was substantially lower in Europe than in other regions. Current analysis of efficacy and safety in the European population is also fully consistent with a previous subgroup analysis that did not show a significant reduction in non‐haemorrhagic stroke and SEE with the DOAC at centres that achieved a good quality of anticoagulation, defined as a centre‐based TTR of more than 65% 35. On the other hand, it is reassuring that the DOAC may be considered at least non‐inferior to warfarin under the worst case circumstances (European centres and good control of anticoagulation).
With respect to bleeding, our meta‐analysis showed a consistent overall reduction in risk of major bleeding across regions with most DOACs, with the more impressive risk reductions in major bleeding reported in Asia. A recent genetic substudy of ENGAGE‐AF shows that 62% of Asian patients are sensitive responders to warfarin (defined by combinations of different CYP2C9 and VKORC1 genotype functional bins), compared with only 4% of the European population. Sensitive responders spent greater proportions of time over‐anticoagulated and had a 31% increased risk of bleeding when compared with normal responders 36. On the other hand, despite major bleeding risk being significantly reduced in most regions with the DOAC, there was an increase in major bleeding with rivaroxaban (ROCKET AF study) in North America, which was also responsible for the heterogeneity found in this outcome in other regions as compared to Europe. This could be chance finding, but the combination of a high rate of VKA‐experienced patients, good TTR with warfarin and the higher prevalence of older patients and co‐morbidities associated with higher bleeding rates (e.g., hypertension, anaemia) in North America may also have contributed to these differences 37. This imbalance raises uncertainty about the real risk of bleeding with the DOAC in fragile populations in comparison with well‐managed warfarin.
Finally, data on ICB and all‐cause mortality by region could only be obtained from the RE‐LY study, which showed a relatively low rate of ICB (0.6%/yr) and mortality (3.3 %/yr) with warfarin in Europe and a higher rate of ICB (1.1%/yr) and mortality (6.1%/yr) with warfarin in Asia and Latin America, respectively. A recent review of subanalyses in non‐Asians vs. Asians indicates that the absolute reduction in risk of ICB in comparative pivotal studies with the DOAC and warfarin is much lower in non‐Asians than in Asians 5. As ICB rates with warfarin in Europe are approximately 0.4–0.5%/yr 38, 39, the 52% RR reduction with the DOAC seen in pivotal trials 3 would translate in Europe into approximately 0.2%/yr absolute risk reduction vs. warfarin. Within Europe, as in the overall study populations, there may be subpopulations in which the benefit of the DOACs with respect to ICB may be particularly relevant, like in those with a history of stroke/TIA and high bleeding risk 35. With respect to mortality, considering the lack of differences in stroke rates in Europe between treatments and the relatively modest contribution of stroke to all‐cause mortality in AF (10–13% of all deaths across pivotal studies) 11, 12, 13, 14, it is not possible to conclude that DOACs would reduce stroke‐related death in Europe. Whether some numerical benefit in haemorrhage‐related deaths exists in Europe remains uncertain. This is far from indicating that European patients do not benefit from DOAC therapy, but is rather indicating that the quality of anticoagulation control differs across regions, as discussed previously.
Our review has several limitations. Firstly, it was based on subgroup analyses that have well‐known limitations and are observational in their nature. However, subgroups by geographic region were pre‐specified in all studies because it was clinically plausible to assume that medical practice and quality of anticoagulation with the control drug warfarin would differ between regions. Therefore, these secondary analyses may be used to illustrate applicability across regions. In addition, we choose a conservative threshold of significance (P < 0.05) to limit the risk of false positive results despite the lack of power of the interaction test, and the ten sensitivity analyses conducted were all significant. Secondly, the main comparison on our meta‐analysis (Europe vs. other regions) was defined post‐hoc. However, our secondary analysis was based on the regions that were pre‐specified in the trials, and yielded similar results to the main analysis. An additional limitation is the absence of patient‐level data that precludes investigating or adjusting for patient‐level differences between regions.
In summary, our review shows significant regional differences in the extent of the benefit of the DOAC compared with warfarin for the prevention of stroke/SEE in patients with AF. The DOAC did not provide additional benefit in reducing the risk of stroke/SEE compared with warfarin in European patients. However, they were generally associated with a lower bleeding tendency than warfarin regardless of geographic region. These differences appear to be mainly related to differences in patient care of AF, thus resulting in different quality of anticoagulation with warfarin across regions. These regional differences are to be taken into account when interpreting the results from pivotal trials, as well as in the design and assumptions taken in pharmaco‐economic analyses and indirect comparisons between the new compounds in specific regions and countries.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Contributors
AGO, AITF, GCR, MLSG and EVC conceived and designed the study. AGO and AITF collected the data. AGO carried out the statistical analysis and drafted the manuscript. EVC supervised the study. All authors analysed and interpreted the data and critically revised the manuscript for important intellectual content. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of their institutions or any other party. AGO and EVC are the guarantors.
Supporting information
Appendix S1 Search strategy
Appendix S2 Study quality assessment for included randomized controlled trials
Appendix S3 Definitions of geographic regions
Appendix S4 Demographic characteristics by region
Appendix S5 Intracranial bleeding and all‐cause death by region
Appendix S6 Sensitivity analyses
File S1 PRISMA statement
Supporting info item
Gómez‐Outes, A. , Terleira‐Fernández, A. ‐I. , Calvo‐Rojas, G. , Suárez‐Gea, M. L. , and Vargas‐Castrillón, E. (2016) Direct oral anticoagulants for stroke prevention in patients with atrial fibrillation: meta‐analysis by geographic region with a focus on European patients. Br J Clin Pharmacol, 82: 633–644. doi: 10.1111/bcp.13005.
References
- 1. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J 2012; 33: 2719–47. [DOI] [PubMed] [Google Scholar]
- 2. Gómez‐Outes A, Suárez‐Gea ML, Lecumberri R, Terleira‐Fernández AI, Vargas‐Castrillón E. Direct‐acting oral anticoagulants: pharmacology, indications, management, and future perspectives. Eur J Haematol 2015; 95: 389–404. [DOI] [PubMed] [Google Scholar]
- 3. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014; 383: 955–62. [DOI] [PubMed] [Google Scholar]
- 4. Mullins CD, Onwudiwe NC, Brando de Araújo GT, Chen W, Xuan J, Tichopád A, et al. Guidance document: global pharmacoeconomic model adaption strategies. ViHRI 2014; 5: 7–13. [DOI] [PubMed] [Google Scholar]
- 5. Lip GY, Wang KL, Chiang CE. Non‐vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol 2015; 180: 246–54. [DOI] [PubMed] [Google Scholar]
- 6. Eikelboom JW, Connolly SJ, Hart RG, Wallentin L, Reilly P, Oldgren J, et al. Balancing the benefits and risks of 2 doses of dabigatran compared with warfarin in atrial fibrillation. J Am Coll Cardiol 2013; 62: 900–8. [DOI] [PubMed] [Google Scholar]
- 7. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 11. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran vs. warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–51. [DOI] [PubMed] [Google Scholar]
- 12. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban vs. warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–91. [DOI] [PubMed] [Google Scholar]
- 13. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban vs. warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–92. [DOI] [PubMed] [Google Scholar]
- 14. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban vs. warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–104. [DOI] [PubMed] [Google Scholar]
- 15. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J‐ROCKET AF study. Circ J 2012; 76: 2104–11. [DOI] [PubMed] [Google Scholar]
- 16. Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham‐Azad K, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007; 100: 1419–26. [DOI] [PubMed] [Google Scholar]
- 17. Ogawa S, Shinohara Y, Kanmuri K. Safety and efficacy of the oral direct factor Xa inhibitor apixaban in Japanese patients with non‐valvular atrial fibrillation. The ARISTOTLE‐J study. Circ J 2011; 75: 1852–9. [DOI] [PubMed] [Google Scholar]
- 18. Yamashita T, Koretsune Y, Yasaka M, Inoue H, Kawai Y, Yamaguchi T, et al. Randomized, multicenter, warfarin‐controlled phase II study of edoxaban in Japanese patients with non‐valvular atrial fibrillation. Circ J 2012; 76: 1840–7. [DOI] [PubMed] [Google Scholar]
- 19. Chung N, Jeon HK, Lien LM, Lai WT, Tse HF, Chung WS, et al. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non‐valvular atrial fibrillation. Thromb Haemost 2011; 105: 535–44. [DOI] [PubMed] [Google Scholar]
- 20. Weitz JI, Connolly SJ, Patel I, Salazar D, Rohatagi S, Mendell J, et al. Randomised, parallel‐group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost 2010; 104: 633–41. [DOI] [PubMed] [Google Scholar]
- 21. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364: 806–17. [DOI] [PubMed] [Google Scholar]
- 22. Beasley N, Thompson A. Pradaxa (dabigatran). FDA Draft Briefing Document for the Cardiovascular and Renal Drugs Advisory Committee. Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM247244.pdf (last accessed 5 August 2015).
- 23. Beasley N, Dunnmon P, Rose M. Xarelto (rivaroxaban). FDA Draft Briefing Document for the Cardiovascular and Renal Drugs Advisory Committee (CRDAC). September 8, 2011. Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/drugs/CardiovascularandRenalDrugsAdvisoryCommittee/ucm270796.pdf (last accessed 5 August 2015).
- 24. Rose M, Beasley N. Eliquis (apixaban). Clinical Review Addendum December 17, 2012. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202155Orig1s000MedR.pdf (last accessed 5 August 2015).
- 25. Blank M, McDowell TY, Rose M. Savaysa (edoxaban). FDA Draft Briefing Document for the Cardiovascular and Renal Drugs Advisory Committee (CRDAC). October 30, 2014. Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM420704.pdf (last accessed 5 August 2015).
- 26. Singer DE, Hellkamp AS, Piccini JP, Mahaffey KW, Lokhnygina Y, Pan G, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc 2013; 2: e000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salazar CA, del Aguila D, Cordova EG. Direct thrombin inhibitors vs. vitamin K antagonists for preventing cerebral or systemic embolism in people with non‐valvular atrial fibrillation. Cochrane Database Syst Rev 2014; 3: CD009893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mentz RJ, Kaski JC, Dan GA, Goldstein S, Stockbridge N, Alonso‐Garcia A, et al. Implications of geographical variation on clinical outcomes of cardiovascular trials. Am Heart J 2012; 164: 303–12. [DOI] [PubMed] [Google Scholar]
- 29. Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008; 118: 2029–37. [DOI] [PubMed] [Google Scholar]
- 30. Van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest 2006; 129: 1155–66. [DOI] [PubMed] [Google Scholar]
- 31. Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet 2010; 376: 975–83. [DOI] [PubMed] [Google Scholar]
- 32. Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE‐LY Atrial Fibrillation Registry. Circulation 2014; 129: 1568–76. [DOI] [PubMed] [Google Scholar]
- 33. Mearns ES, White CM, Kohn CG, Hawthorne J, Song JS, Meng J, et al. Quality of vitamin K antagonist control and outcomes in a trial fibrillation patients: a meta‐analysis and meta‐regression. Thromb J 2014; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fauchier L, Taillandier S. Geographic variations in the quality of oral anticoagulation with vitamin K antagonists in the era of new anticoagulants. J Am Heart Assoc 2013; 2: e000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gómez‐Outes A, Terleira‐Fernández AI, Calvo‐Rojas G, Suárez‐Gea ML, Vargas‐Castrillón E. Dabigatran, rivaroxaban, or apixaban vs. warfarin in patients with nonvalvular atrial fibrillation: a systematic review and meta‐analysis of subgroups. Thrombosis 2013; 2013: 640723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mega JL, Walker JR, Ruff CT, Vandell AG, Nordio F, Deenadayalu N, et al. Genetics and the clinical response to warfarin and edoxaban: findings from the randomised, double‐blind ENGAGE AF‐TIMI 48 trial. Lancet 2015; 385: 2280–7. [DOI] [PubMed] [Google Scholar]
- 37. Goodman SG, Wojdyla DM, Piccini JP, White HD, Paolini JF, Nessel CC, et al. Factors associated with major bleeding events: insights from the ROCKET AF trial (rivaroxaban once‐daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol 2014; 63: 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Björck F, Renlund H, Lip GYH, Wester P, Svensson PJ, Själander A. Outcomes in a warfarin‐treated population with atrial fibrillation. JAMA Cardiol 2016; 1: 172–80. [DOI] [PubMed] [Google Scholar]
- 39. Gallagher AM, van Staa TP, Murray‐Thomas T, Schoof N, Clemens A, Ackermann D, et al. Population‐based cohort study of warfarin‐treated patients with atrial fibrillation: incidence of cardiovascular and bleeding outcomes. BMJ Open 2014; 4: e003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy
Appendix S2 Study quality assessment for included randomized controlled trials
Appendix S3 Definitions of geographic regions
Appendix S4 Demographic characteristics by region
Appendix S5 Intracranial bleeding and all‐cause death by region
Appendix S6 Sensitivity analyses
File S1 PRISMA statement
Supporting info item


