Abstract
Within the last years the implementation of systems biology in nutritional research has emerged as a powerful tool to understand the mechanisms by which dietary components promote health and prevent disease as well as to identify the biologically active molecules involved in such effects. Systems biology, by combining several ‘‐omics’ disciplines (mainly genomics/transcriptomics, proteomics and metabolomics), creates large data sets that upon computational integration provide in silico predictive networks that allow a more extensive analysis of the individual response to a nutritional intervention and provide a more global comprehensive understanding of how diet may influence health and disease. Numerous studies have demonstrated that diet and particularly bioactive food components play a pivotal role in helping to counteract environmental‐related oxidative damage. Oxidative stress is considered to be strongly implicated in ageing and the pathophysiology of numerous diseases including neurodegenerative disease, cancers, metabolic disorders and cardiovascular diseases. In the following review we will provide insights into the role of systems biology in nutritional research and focus on transcriptomic, proteomic and metabolomics studies that have demonstrated the ability of functional foods and their bioactive components to fight against oxidative damage and contribute to health benefits.
Keywords: dietary components, health, metabolomics, proteomics, systems biology, transcriptomics
‐OMICS applications as a foundation for systems biology in nutritional research
Nutritional research focuses on understanding the link between diet and health in order to promote natural ways of disease prevention. To this end, nutritional science covers a broad range of research areas that expand from a better understanding of the molecular mechanisms of prevention and protection to the identification of the biologically active food components and the demonstration of their biological efficacy. In the last decade the implementation of systems biology has emerged as a powerful tool to achieve these objectives. While a definitive and agreed‐upon definition of what constitutes systems biology is still missing systems biology can be regarded as the combination of various ‘‐omics’ technologies in different biological systems (tissue, cells and/or biological fluids) in order to create large molecular datasets which, once integrated in computational modelling approaches, provide predictive networks (e.g. signalling networks from the cell membrane to the nucleus) that aim ultimately to understand how the biological system under scrutiny behaves upon a perturbation (e.g. dietary intervention) 1. In this scenario, the evolvement of three main ‘‐omics’ platforms including transcriptomics, proteomics and metabolomics has allowed the study of how food impacts health status, to assist in differentiating dietary responders from non‐responders and to identify the nutritional bioactive compounds responsible for the health outcomes 2, 3. While transcriptomics provides a comprehensive view on all genes active at a given time point in a given sample, the proteomic platform identifies and quantifies protein expression and characterizes post‐translational modifications 4, 5. Although both disciplines may deliver markers of efficacy and targets for intervention, proteomics do so in a more robust and efficacious manner since changes induced in mRNA expression do not linearly correlate with the changes seen at a protein level 6. Finally, metabolomics focus on the analysis of metabolites, their dynamics, composition and interactions and is considered to provide the most functional data 7. Without doubt, the integration in nutritional systems of gene and protein expression profiles with metabolic fingerprints allows a more extensive analysis of the individual response to a nutritional intervention and provides a more global comprehensive understanding of how food components influence health status (Figure 1).
Figure 1.
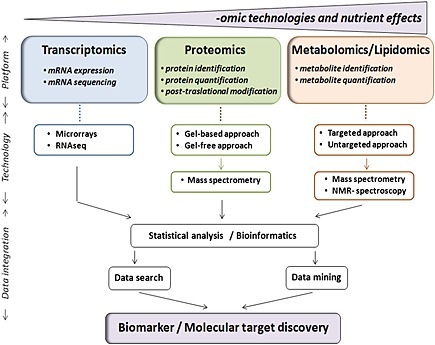
Integration of ‐omic technologies used in nutritional systems biology
Dietary patterns and functional foods with beneficial properties against oxidative stress triggered diseases
Oxidative damage is strongly implicated in the pathophysiology of numerous diseases including neurodegenerative disease, cancers, metabolic disorders and cardiovascular diseases. As per the latter, oxidative injury of endothelial cells and oxidation of low density lipoprotein particles promote and facilitate lipid accumulation within the arterial wall and subsequent atherosclerotic plaque formation, the underlying cause of most cardiovascular events 8, 9, 10.
During the last few years, research in nutritional interventions has focused on elucidating the effect of dietary patterns and bioactive food components on health as well as identifying the mechanisms/contributors behind.
The Mediterranean diet
The Mediterranean nutritional pattern has demonstrated to protect against cardiovascular disease, obesity, diabetes, cancer and neurodegenerative disorders and, therefore, is considered healthy eating 11, 12, 13. The beneficial properties of Mediterranean diet have been mainly attributed to the presence of a large amount of single functional foods with atheroprotective effects and potent antioxidant properties 13, 14. We have reported in a substudy of the Predimed Trial 15 through direct transcriptomic analysis that intake of a Mediterranean diet for 3 months downregulates the expression of pro‐atherogenic genes in circulating inflammatory cells 16. On the other hand, the Mediterranean diet is enriched in phenolic compounds (nuts, olive oil, vegetables and fruits and their derivatives) capable of scavenging free radicals, acting as chelating agents, preserving serum antioxidant activity and/or regulate transcription factors (i.e. Nrf2) involved in the modulation of several genes encoding for antioxidant enzymes and other proteins involved in cellular redox balance 17. A recent work in the setting of atrial fibrillation (AF), a cardiac disease characterized by enhanced oxidative stress, has demonstrated that high adherence to the Mediterranean diet is associated to a significant decrease in cardiovascular events and concomitant reduction in oxidative stress markers. As such, NOX2 (the most important cellular producer of superoxide anion) and consequent F2‐isoprostane formation was found markedly diminished in AF patients strictly following a Mediterranean diet for an average of 3.3 years. Of note, these benefits were observed without any changes in anticoagulation stability 18.
Polyphenols and pomegranate
Mullen et al. 19 have identified in urine by using a differential proteomic approach changes in the protein pattern of seven peptides after a 2 week diet supplementation with a polyphenol‐rich drink. Importantly, these peptides had been previously associated with coronary artery disease and the detected changes point towards a healthier state. A non‐targeted metabolomics analysis in plasma and urine from young healthy adults also clearly indicates that a regular diet supplementation with pomegranate and grape (i.e. antioxidant‐rich) juice during 8 weeks positively affects both lipid and oxidative pathways without modifying anthropometrical or biochemical parameters 20. As described by the authors 20, metabolomic changes in the polyphenol punicalagin (ellagitannin present in pomegranate) and the ascorbic acid sulfate (derived from hepatic conjugation of ascorbic acid) support the contribution of polyphenols and vitamin C as main contributors for the observed modifications in the oxidative status. We have recently demonstrated protective effects against organ damage 16. Thus, in an in vivo dyslipidaemic porcine model, regular intake of a pomegranate extract rich in punicalagins elicits vascular protection and favourably counteracts vascular inflammation and oxidative damage 21. More specifically, the study has provided evidence that diet supplementation with the pomegranate extract prevents hypercholesterolaemia‐induced endothelial dysfunction through beneficial effects involving activation of the vascular Akt/eNOS axis, lower monocyte chemoattractant protein (MCP)‐1 expression and decreased arterial oxidative damage as well as an overall decline in systemic oxidative stress 16. Pomegranate contains high levels of polyphenols, which not only have shown potent antioxidant and anti‐inflammatory properties but also exert anti‐proliferative and pro‐apoptotic effects. Indeed, pomegranate has been shown to modulate the expression and phenotype of proteins involved in cytoskeletal functions, proteasome activity and NF‐κB signalling as demonstrated by differential proteomic analysis in human prostate cancer cells 22. Moreover, extending these findings, DNA microarray analysis of breast cancer cells has revealed that pomegranate extracts downregulate important genes involved in DNA double strand break repair by homologous recombination strengthening its beneficial effects in growth inhibition and apoptosis 23.
Lycopene, tomatoes and tomato‐based foods
Tomatoes and tomato‐based foods are the major known source of dietary lycopene, a lipid soluble antioxidant which acts by protecting cell membranes from lipid peroxidation 24. Clinical trials in healthy individuals or in patients with cardiovascular risk factors have provided consistent evidence that lycopene supplementation protects against oxidative damage and reduces blood pressure exerting cardiovascular protective effects 25. In addition, epidemiological studies have reported potential lycopene benefits in several types of cancer, especially prostate cancer 26. In this regard, Herzog et al. 27 described in rats by using a GeneChip hybridization transcriptomic approach that lycopene reduces gene expression of androgen‐related metabolizing enzymes and targets, IGF‐I expression and basal inflammatory signals in normal prostate tissue, suggesting a chemoprevention function in prostate cancer. Moreover, a high throughput proteomic analysis of prostate cancer cells has identified further lycopene‐related antioxidant beneficial properties. As such, lycopene intake has been found to be associated with the upregulation of proteins predominantly located in the nuclear compartment and involved in detoxification of reactive oxygen species 28.
Whether lycopene as a dietary supplement can deliver similar cardiovascular benefits to tomatoes or tomato‐based products remains unresolved. Of note, besides lycopene, tomato contains a broad range of bioactive compounds that might partly account for the beneficial effects observed upon tomato consumption in cardiovascular health. Moreover, tomato cooking and/or processing (e.g. tomato sauce) converts the natural existing trans‐isomer lycopene into the cis‐lycopene form with higher bioavailability. A 4 week crossover nutritional intervention addressed to determine, by magnetic nuclear resonance (NMR)‐based analysis, the metabolomic status of 24 healthy young subjects receiving two tomato sauces with different lycopene content, found subtle differences in the serum concentrations of some amino acids, lipids, ascorbic acid and energy metabolism related compounds 29. Whereas changes in metabolites such as creatine, creatinine, lactate and pyruvate were associated with the antioxidant and myocardial protective effects of tomato in terms of lycopene content, changes in the concentrations of the essential amino acid methionine and in ascorbic acid were primary related with a different ripening stage of the tomatoes.
Experimental studies and high‐throughput metabolomics analysis have also supported beneficial effects of tomato‐derived products on lipid metabolism. Up to now, however, results from clinical trials are inconsistent with the exception of the benefits of tomato and lycopene intake on high density lipoprotein (HDL) metabolism. To this respect, different studies have provided evidence that tomato‐derived products induce improvements in HDL‐cholesterol levels 25. We have also demonstrated that medium term dietary supplementation with cooked tomato sauce (CTS) Mediterranean style (sofrito), also containing olive oil, improves the functionality (antioxidant potential) of HDL particles and induces changes in the protein profile of apolipoprotein A‐I (ApoA‐I) and apolipoprotein J (ApoJ), two major protein components of the HDL‐lipoprotein micelle 30. As such, differential serum proteomic analysis from hypercholesterolaemic swine supplemented with/without CTS (100 g containing 21.5 mg lycopene) for 10 days revealed that regular CTS intake is associated with a significant increase in one of the most abundant mature forms of Apo A‐I likely contributing to enhance HDL antioxidant potential 30, 31. In line with these findings, lycopene administration for 12 weeks to moderately overweight middle‐aged individuals has been reported to enhance paraoxonase‐1 activity, an antioxidant enzyme that normally associates with HDL's Apo A‐I 32. We have also identified a significant rise in the serum content of ApoJ, also known to protect against oxidation 33, in those hypelipaemic pigs supplemented with CTS suggesting a synergistic antioxidant effect between ApoJ and Apo A‐I in the HDL micelles 34.
In summary, tomato contains a number of key phytochemicals, including lycopene, which support cardiovascular health benefits. However, more studies are needed to unveil better whether phytochemicals other than lycopene exert healthy effects as well as to determine the potential synergistic effects of other components related with tomato processing such as olive oil. In this regard, proteomics and network analysis has revealed that intake of olive oil polyphenols (mainly hydroxityrosol) not only donate hydrogen to reactive free radical species counteracting oxidative stress but also downregulate hepatic mitochondrial aldehyde dehydrogenase (ALDH2; enzyme involved in hepatic lipid peroxidation) protein and activity and increase the expression of superoxide dismutase (SOD; cellular antioxidant enzyme) likely impacting on cardiovascular health 35. Interestingly, both ALDH and SOD seem to be regulated by the Nrf2 transcription factor 35.
Lipid‐lowering foods
Sterol/phytosterols
The health benefits of foods rich in phytosterols have mainly focused in their ability to act as cholesterol lowering agents. Recently, however, the interest of the scientific community has moved towards a better understanding of the metabolomic effects of sterols opening new dietary options in cardiovascular disease prevention.
Phytosterols are normal bioactive components in plants that closely resemble cholesterol in their molecular structure. Phytosterols have been shown to lower LDL cholesterol concentrations by competing with cholesterol absorption 36, 37. A high‐throughput protein analysis addressed towards the characterization of protein expression changes in the intestinal mucosa in response to a phytosterol‐enriched diet in ApoE−/− hypercholesterolaemic mice identified, using a 2D‐ difference gel electrophoresis (2D‐DIGE) and MALDI‐TOF mass‐spectrometry approach, nine differentially expressed proteins. These proteins were mostly associated with the annexin family, a family of proteins mainly involved in the stabilization of the plasma membrane and the assembly of the cytoskeleton network 37. Interestingly, these changes in the protein profile were detectable in normolipaemic animals and did not correlate with the expression levels of the corresponding genes 37. In line with these findings, other animal and human studies have reported that phytosterol or phytostanol intake do not activate the expression of genes involved in intestinal sterol metabolism.
Recent lipidomic and metabolomic studies have pointed to a variety of lipid species, not only to LDL‐cholesterol concentrations, as potential targets to protect against disease development 38 (Figure 2). As such, a lipidomic approach based on liquid chromatograpgy/mass spectrometry (LC/MS) analysis applied to serum samples from a placebo‐controlled, parallel human intervention study of 4 week consumption of two phytosterol‐enriched, yoghurt drinks differing in fat content, reported that intake of a phytosterol‐enriched 0.1% dairy fat yoghurt drink reduces the levels of several sphingomyelins which, in turn, correlate with the reduction in LDL‐cholesterol 39. In addition, this study reported serological changes in two lysophosphatidylcholines metabolites [LPC(16:1), LPC(20:1)] and in cholesteryl arachidonate, suggesting a protective role of phytosterol intake in inflammation 39. More recently, we have used a top‐down lipidomic approach (LC‐ESI‐MS/MS strategy) to gain new insights into the lipid biochemical changes induced in LDL by the dietary intake of phytosterol‐supplemented milk. We performed a double blind, randomized longitudinal crossover exploratory study in overweight and moderately hypercholesterolaemic patients who received milk (250 ml day–1) enriched either in phytosterols or omega‐3 fatty‐acids (EPA + DHA) as comparative control treatment during two sequential 28 day intervention periods 40. We demonstrated that regular intake of phytosterols and omega‐3 milk resulted in differential LDL lipid‐metabolite patterns, mainly of the cholesterol esters and glycerophospholipid families. Interestingly, the intervention with phytosterol‐supplemented milk significantly reduced plasma LDL cholesterol levels without inducing changes in the LDL cholesteryl ester profile. These observations suggest a major effect of dietary phytosterols on the free cholesterol fraction of LDL. In contrast, intake of phytosterol‐enriched milk significantly reduced the content of LDL‐glycerophospholipids, primarily of the phophatidylcholines (PC) and lyso‐phophatidylcholines (LPC) subclasses. The recently published Ludwigshafen Risk and Cardiovascular Health (LURIC) study has reported a positive association between five plasma PC species and cardiovascular mortality 41. Particularly, a strong correlation was found for the PC32:0 subclass. We found a reduction in PC32:0 in those individuals under the phytosterol milk intervention as well as in PC34:1, a highly abundant PC in LDL particles 40. The fact that the strongest coordinated changes after phytosterol‐enriched milk were observed between PC metabolites differing by two carbon atoms in their fatty acid side chain suggests that changes result from products of elongation processes occurring in the LDL after the dietary intervention.
Figure 2.
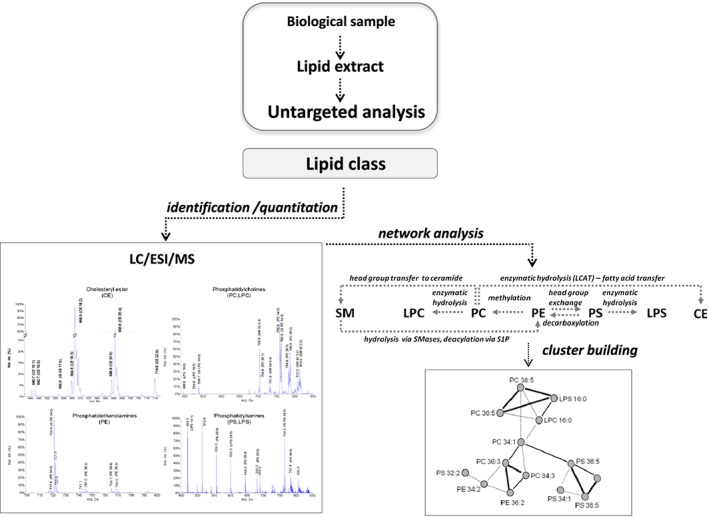
Flow chart illustrating the untargeted lipidomic approach to screen and characterize the effects of nutrients on components of different lipid families. Data partly published in Padro et al. 38
Another important finding of our study was the detected decrease in LDL susceptibility to oxidation after intake of phytosterol‐enriched milk 40. The susceptibility of LDL to oxidation has been linked to their content in sphingomyelines, lipid components reported as physiological inhibitors of lipoprotein oxidation due to their effects in LDL surface fluidity and propagation of the oxidation reaction in the PC monolayer 42. However, our LDL metabolomic analysis suggests that phytosterol protection against LDL‐oxidation was related to a decrease in LPC‐content, particularly in the LPC16:0 metabolite (a reliable marker of oxidative stress) 43 rather than changes in the pattern of spingomyelines.
Extending the effects of phytosterols in atherosclerosis protection, we have provided evidence by direct transcriptomic analysis that the intake of a plant sterol ester‐enriched diet directly interferes with the expression of the canonical Wnt signalling receptor and prevents pro‐atherogenic effects in the aorta through a mechanism mediated by the modulation of the low density lipoprotein receptor‐related protein 5 (LRP5) 44, protein involved in the uptake of lipid by macrophages 45, as well as in their differentiation and migration capacity 45, 46.
Omega (n)‐3 polyunsaturated fatty acids (PUFA)
Differing from sterol rich foods, long chain omega‐3 (ω3) PUFAs have shown to exert health promoting effects particularly on the cardiovascular system mainly by lowering fasting and post‐prandial serological triglycerides 47 In addition, whole genome gene expression analysis of peripheral blood mononuclear cells (PBMC) from 21 male subjects consuming shakes enriched in PUFAs has demonstrated that PUFA intake reduces the expression of genes involved in liver X receptor (LXR) signalling, glutathione metabolism, oxidative stress and inflammation 48. Moreover, Ahmed et al. 49, using a proteomic approach based on gel electrophoresis and liquid chromatography–tandem mass spectrometry, evidenced that high dietary ω3PUFA modulates proteins directly involved in lipid and carbohydrate metabolism, such as regucalcin or hepatic fructose‐1 and ketohexokinase, as well as proteins involved in protein synthesis. From a metabolomic point of view we have demonstrated that intake of ω3‐enriched milk for 4 weeks induces changes in the lipidomic compostion of the LDL‐fraction leading to an increase in long chain polyunsaturated cholesteryl‐esters CE20:5 and CE:22:6 content as well as in the ratio PC 36:5 : LysoPC16:0, thus conferring LDL particles with a lower pro‐inflammatory potential 40. In summary, dietary intake of ω3 long chain fatty acids and plant sterols seem to exert independent but interactive effects on the plasma lipid profile suggesting that their combination as food supplements may represent an improved strategy to prevent cardiovascular risk 50. In support of this hypothesis we have shown that intake of ω3‐enriched‐milk not only reduces triglyceride plasma concentrations but also increases the long chain ω3‐PUFA in LDL, mainly as cholesteryl‐esters 51.
Caloric restriction
Oxidative stress also plays a key role in ageing and ageing‐associated co‐morbidities such heart disease, neurodegeneration and cancer 52. Although there has been intense research during the last years to identify genes that influence longevity the findings have been rather limited and only one gene, the APOE, highly relevant in cardiovascular diseases and dementia, has emerged as being consistently associated with the ageing phenotypes and longevity in genomic approaches 53. Interestingly, caloric restriction has been considered to be the main environmental factor capable of influencing longevity in non‐human experimental models. Implementation of systems biology has revealed that a reduction of 20–40% in habitual daily energy intake (without malnutrition) is associated with the modulation of pathways related to longevity including the insulin‐like signalling (involved in cell division), the sirtuin pathway (enhance mitochondrial antioxidant defense), the target of rapamycin (mTOR) signalling (regulates autophagy) and AMP‐activated protein kinase (AMPK) signalling (energy sensor) 54, 55, 56, 57. Moreover, regulation of these pathways leads to a reduction in oxidative damage, to a modulation of energy‐requiring functions and to a decrease of cell division 57, 58 preventing against the risk of suffering from obesity, insulin resistance, type II diabetes and cardiovascular diseases, ultimately increasing lifespan 59. Although caloric restriction has not been implemented in humans, intermittent fasting has also been shown to decrease oxidative stress levels and improve insulin sensitivity in men likely promoting healthy ageing 60. In line with these observations, a transcriptomic study in obese men has shown a reduction in the expression of oxidative stress‐ and inflammation‐ related genes in response to an 8 week low calorie diet intake which, in turn, was associated with a decrease in body weight 61.
Conclusion
Diet is the most important environmental factor for maintaining health and preventing disease. The tremendous progress in science and technology occurred during the last decade has paved the way for the development and implementation of systems biology in nutritional research. The availability and progress of transcriptomic, proteomic and metabolomic platforms have opened up new opportunities to build up network models with accurate predictive value in nutrition‐related disease, to decipher the health promoting properties of functional foods and to design dietary interventions for healthy ageing. Exploiting systems biology in the nutrition and health arenas may provide new opportunities oriented towards personalized therapy.
Competing Interests
All authors have completed the Unified Competing Interest forms and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by the Spanish Ministry of Economy and Competitiveness [SAF2013‐42962‐R to LB and SAF2015‐71653‐R to GV], Institute of Health Carlos III‐ ISCIII [FIS PI13/02850 to TP/‘Red de Investigación Cardiovascular’ RIC–RD12/0042/0027 to LB and TerCel (Red de Terapia Celular) [RD/12/0019/0026 to LB], FEDER ‘Una Manera de Hacer Europa’ and AGAUR (Agència de Gestió d'Ajuts Universitaris i de Recerca) [2014SGR1303 to LB]. We thank Fundación Jesus Serra, Barcelona, for their continuous support.
Badimon, L. , Vilahur, G. , and Padro, T. (2017) Systems biology approaches to understand the effects of nutrition and promote health. Br J Clin Pharmacol, 83: 38–45. doi: 10.1111/bcp.12965.
References
- 1. Cassman M. Barriers to progress in systems biology. Nature 2005; 438: 1079. [DOI] [PubMed] [Google Scholar]
- 2. Odriozola L, Corrales FJ. Discovery of nutritional biomarkers: future directions based on omics technologies. Int J Food Sci Nutr 2015; 66: S31–40. [DOI] [PubMed] [Google Scholar]
- 3. Kussmann M, Raymond F, Affolter M. OMICS‐driven biomarker discovery in nutrition and health. J Biotechnol 2006; 124: 758–87. [DOI] [PubMed] [Google Scholar]
- 4. Moore JB, Weeks ME. Proteomics and systems biology: current and future applications in the nutritional sciences. Adv Nutr 2011; 2: 355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weston AD, Hood L. Systems biology, proteomics, and the future of health care: toward predictive, preventative, and personalized medicine. J Proteome Res 2004; 3: 179–96. [DOI] [PubMed] [Google Scholar]
- 6. Griffin TJ, Gygi SP, Ideker T, Rist B, Eng J, Hood L, et al. Complementary profiling of gene expression at the transcriptome and proteome levels in Saccharomyces cerevisiae . Mol Cell Proteomics 2002; 1: 323–33. [DOI] [PubMed] [Google Scholar]
- 7. Whitfield PD, German AJ, Noble PJ. Metabolomics: an emerging post‐genomic tool for nutrition. Br J Nutr 2004; 92: 549–55. [DOI] [PubMed] [Google Scholar]
- 8. Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med 2014; 276: 618–32. [DOI] [PubMed] [Google Scholar]
- 9. Badimon L, Padro T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care 2012; 1: 60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ibanez B, Vilahur G, Badimon JJ. Plaque progression and regression in atherothrombosis. J Thromb Haemost 2007; 5: 292–9. [DOI] [PubMed] [Google Scholar]
- 11. Estruch R, Ros E, Salas‐Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–90. [DOI] [PubMed] [Google Scholar]
- 12. de Lorgeril M, Salen P. Dietary prevention of coronary heart disease: the Lyon diet heart study and after. World Rev Nutr Diet 2005; 95: 103–14. [DOI] [PubMed] [Google Scholar]
- 13. Lopez‐Miranda J, Perez‐Jimenez F, Ros E, De Caterina R, Badimon L, Covas MI, et al. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr Metab Cardiovasc Dis 2010; 20: 284–94. [DOI] [PubMed] [Google Scholar]
- 14. Damasceno NR, Sala‐Vila A, Cofan M, Perez‐Heras AM, Fito M, Ruiz‐Gutierrez V, et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis 2013; 230: 347–53. [DOI] [PubMed] [Google Scholar]
- 15. Estruch R, Ros E, Martinez‐Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med 2013; 369: 676–7. [DOI] [PubMed] [Google Scholar]
- 16. Llorente‐Cortes V, Estruch R, Mena MP, Ros E, Gonzalez MA, Fito M, et al. Effect of Mediterranean diet on the expression of pro‐atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010; 208: 442–50. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen T, Nioi P, Pickett CB. The Nrf2‐antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 2009; 284: 13291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pastori D, Carnevale R, Bartimoccia S, Nocella C, Tanzilli G, Cangemi R, et al. Does Mediterranean diet reduce cardiovascular events and oxidative stress in atrial fibrillation? Antioxid Redox Signal 2015; 23: 682–7. [DOI] [PubMed] [Google Scholar]
- 19. Mullen W, Gonzalez J, Siwy J, Franke J, Sattar N, Mullan A, et al. A pilot study on the effect of short‐term consumption of a polyphenol rich drink on biomarkers of coronary artery disease defined by urinary proteomics. J Agric Food Chem 2011; 59: 12850–7. [DOI] [PubMed] [Google Scholar]
- 20. Diaz‐Rubio ME, Perez‐Jimenez J, Martinez‐Bartolome MA, Alvarez I, Saura‐Calixto F. Regular consumption of an antioxidant‐rich juice improves oxidative status and causes metabolome changes in healthy adults. Plant Foods Hum Nutr 2015; 70: 9–14. [DOI] [PubMed] [Google Scholar]
- 21. Vilahur G, Padro T, Casani L, Mendieta G, Lopez JA, Streitenberger S, et al. Polyphenol‐enriched diet prevents coronary endothelial dysfunction by activating the Akt/eNOS pathway. Rev Esp Cardiol (Engl Ed) 2015; 68: 216–25. [DOI] [PubMed] [Google Scholar]
- 22. Lee ST, Wu YL, Chien LH, Chen ST, Tzeng YK, Wu TF. Proteomic exploration of the impacts of pomegranate fruit juice on the global gene expression of prostate cancer cell. Proteomics 2012; 12: 3251–62. [DOI] [PubMed] [Google Scholar]
- 23. Shirode AB, Kovvuru P, Chittur SV, Henning SM, Heber D, Reliene R. Antiproliferative effects of pomegranate extract in MCF‐7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol Carcinog 2014; 53: 458–70. [DOI] [PubMed] [Google Scholar]
- 24. Story EN, Kopec RE, Schwartz SJ, Harris GK. An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol 2010; 1: 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burton‐Freeman B, Sesso HD. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr 2014; 5: 457–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller EC, Giovannucci E, Erdman JW Jr, Bahnson R, Schwartz SJ, Clinton SK. Tomato products, lycopene, and prostate cancer risk. Urol Clin North Am 2002; 29: 83–93. [DOI] [PubMed] [Google Scholar]
- 27. Herzog A, Siler U, Spitzer V, Seifert N, Denelavas A, Hunziker PB, et al. Lycopene reduced gene expression of steroid targets and inflammatory markers in normal rat prostate. FASEB J 2005; 19: 272–4. [DOI] [PubMed] [Google Scholar]
- 28. Goo YA, Li Z, Pajkovic N, Shaffer S, Taylor G, Chen J, et al. Systematic investigation of lycopene effects in LNCaP cells by use of novel large‐scale proteomic analysis software. Proteomics Clin Appl 2007; 1: 513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bondia‐Pons I, Canellas N, Abete I, Rodriguez MA, Perez‐Cornago A, Navas‐Carretero S, et al. Nutri‐metabolomics: subtle serum metabolic differences in healthy subjects by NMR‐based metabolomics after a short‐term nutritional intervention with two tomato sauces. OMICS 2013; 17: 611–8. [DOI] [PubMed] [Google Scholar]
- 30. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001; 104: 2673–8. [DOI] [PubMed] [Google Scholar]
- 31. Cubedo J, Padro T, Badimon L. Glycoproteome of human apolipoprotein A‐I: N‐ and O‐glycosylated forms are increased in patients with acute myocardial infarction. Transl Res 2014; 164: 209–22. [DOI] [PubMed] [Google Scholar]
- 32. McEneny J, Wade L, Young IS, Masson L, Duthie G, McGinty A, et al. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle‐aged individuals. J Nutr Biochem 2013; 24: 163–8. [DOI] [PubMed] [Google Scholar]
- 33. Schwarz M, Spath L, Lux CA, Paprotka K, Torzewski M, Dersch K, et al. Potential protective role of apoprotein J (clusterin) in atherogenesis: binding to enzymatically modified low‐density lipoprotein reduces fatty acid‐mediated cytotoxicity. Thromb Haemost 2008; 100: 110–8. [DOI] [PubMed] [Google Scholar]
- 34. Mackness MI, Durrington PN. HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis 1995; 115: 243–53. [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez‐Gutierrez G, Duthie GG, Wood S, Morrice P, Nicol F, Reid M, et al. Alperujo extract, hydroxytyrosol, and 3,4‐dihydroxyphenylglycol are bioavailable and have antioxidant properties in vitamin E‐deficient rats–a proteomics and network analysis approach. Mol Nutr Food Res 2012; 56: 1137–47. [DOI] [PubMed] [Google Scholar]
- 36. AbuMweis SS, Vanstone CA, Lichtenstein AH, Jones PJ. Plant sterol consumption frequency affects plasma lipid levels and cholesterol kinetics in humans. Eur J Clin Nutr 2009; 63: 747–55. [DOI] [PubMed] [Google Scholar]
- 37. Nissinen M, Gylling H, Vuoristo M, Miettinen TA. Micellar distribution of cholesterol and phytosterols after duodenal plant stanol ester infusion. Am J Physiol Gastrointest Liver Physiol 2002; 282: G1009–15. [DOI] [PubMed] [Google Scholar]
- 38. De Smet E, Mensink RP, Boekschoten MV, de Ridder R, Germeraad WT, Wolfs TG, et al. An acute intake of plant stanol esters alters immune‐related pathways in the jejunum of healthy volunteers. Br J Nutr 2015; 113: 794–802. [DOI] [PubMed] [Google Scholar]
- 39. Szymanska E, van Dorsten FA, Troost J, Paliukhovich I, van Velzen EJ, Hendriks MM, et al. A lipidomic analysis approach to evaluate the response to cholesterol‐lowering food intake. Metabolomics 2012; 8: 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Padro T, Vilahur G, Sanchez‐Hernandez J, Hernandez M, Antonijoan RM, Perez A, et al. Lipidomic changes of LDL in overweight and moderately hypercholesterolemic subjects taking phytosterol‐ and omega‐3‐supplemented milk. J Lipid Res 2015; 56: 1043–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sigruener A, Kleber ME, Heimerl S, Liebisch G, Schmitz G, Maerz W. Glycerophospholipid and sphingolipid species and mortality: the Ludwigshafen risk and cardiovascular health (LURIC) study. PLoS One 2014; 9: e85724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Subbaiah PV, Subramanian VS, Wang K. Novel physiological function of sphingomyelin in plasma. Inhibition of lipid peroxidation in low density lipoproteins. J Biol Chem 1999; 274: 36409–14. [DOI] [PubMed] [Google Scholar]
- 43. Kim JY, Kim OY, Paik JK, Kwon DY, Kim HJ, Lee JH. Association of age‐related changes in circulating intermediary lipid metabolites, inflammatory and oxidative stress markers, and arterial stiffness in middle‐aged men. Age (Dordr) 2013; 35: 1507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Borrell‐Pages M, Romero JC, Badimon L. Cholesterol modulates LRP5 expression in the vessel wall. Atherosclerosis 2014; 235: 363–70. [DOI] [PubMed] [Google Scholar]
- 45. Borrell‐Pages M, Romero JC, Juan‐Babot O, Badimon L. Wnt pathway activation, cell migration, and lipid uptake is regulated by low‐density lipoprotein receptor‐related protein 5 in human macrophages. Eur Heart J 2011; 32: 2841–50. [DOI] [PubMed] [Google Scholar]
- 46. Borrell‐Pages M, Romero JC, Badimon L. LRP5 negatively regulates differentiation of monocytes through abrogation of Wnt signalling. J Cell Mol Med 2014; 18: 314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roche HM, Gibney MJ. Effect of long‐chain n‐3 polyunsaturated fatty acids on fasting and postprandial triacylglycerol metabolism. Am J Clin Nutr 2000; 71: 232S–7S. [DOI] [PubMed] [Google Scholar]
- 48. Bouwens M, Grootte Bromhaar M, Jansen J, Muller M, Afman LA. Postprandial dietary lipid‐specific effects on human peripheral blood mononuclear cell gene expression profiles. Am J Clin Nutr 2010; 91: 208–17. [DOI] [PubMed] [Google Scholar]
- 49. Ahmed AA, Balogun KA, Bykova NV, Cheema SK. Novel regulatory roles of omega‐3 fatty acids in metabolic pathways: a proteomics approach. Nutr Metab (Lond) 2014; 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khandelwal S, Demonty I, Jeemon P, Lakshmy R, Mukherjee R, Gupta R, et al. Independent and interactive effects of plant sterols and fish oil n‐3 long‐chain polyunsaturated fatty acids on the plasma lipid profile of mildly hyperlipidaemic Indian adults. Br J Nutr 2009; 102: 722–32. [DOI] [PubMed] [Google Scholar]
- 51. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti‐inflammatory and pro‐resolution lipid mediators. Nat Rev Immunol 2008; 8: 349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suzman R, Beard JR, Boerma T, Chatterji S. Health in an ageing world–what do we know? Lancet 2015; 385: 484–6. [DOI] [PubMed] [Google Scholar]
- 53. Garatachea N, Emanuele E, Calero M, Fuku N, Arai Y, Abe Y, et al. ApoE gene and exceptional longevity: insights from three independent cohorts. Exp Gerontol 2014; 53: 16–23. [DOI] [PubMed] [Google Scholar]
- 54. Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, et al. Altered proteome turnover and remodeling by short‐term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 2014; 13: 529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee CK, Weindruch R, Prolla TA. Gene‐expression profile of the ageing brain in mice. Nat Genet 2000; 25: 294–7. [DOI] [PubMed] [Google Scholar]
- 56. Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 2011; 146: 682–95. [DOI] [PubMed] [Google Scholar]
- 57. Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med 2011; 32: 159–221. [DOI] [PubMed] [Google Scholar]
- 58. Shinmura K. Effects of caloric restriction on cardiac oxidative stress and mitochondrial bioenergetics: potential role of cardiac sirtuins. Oxid Med Cell Longev 2013; 2013: 528935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lacroix S, Lauria M, Scott‐Boyer MP, Marchetti L, Priami C, Caberlotto L. Systems biology approaches to study the molecular effects of caloric restriction and polyphenols on aging processes. Genes Nutr 2015; 10: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wegman MP, Guo MH, Bennion DM, Shankar MN, Chrzanowski SM, Goldberg LA, et al. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res 2015; 18: 162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crujeiras AB, Parra D, Milagro FI, Goyenechea E, Larrarte E, Margareto J, et al. Differential expression of oxidative stress and inflammation related genes in peripheral blood mononuclear cells in response to a low‐calorie diet: a nutrigenomics study. OMICS 2008; 12: 251–61. [DOI] [PubMed] [Google Scholar]


