Abstract
Several studies have been conducted with mixed results since our initial report of increased Parkinson's disease risk in individuals with red hair and/or red hair‐associated p.R151C variant of the MC1R gene, both of which confer high melanoma risk. We performed a meta‐analysis of six publications on red hair, MC1R, and Parkinson's disease. We found that red hair (pooled odds ratios = 1.68, 95% confidence intervals: 1.07, 2.64) and p.R151C (pooled odds ratios = 1.10, 95% confidence intervals: 1.00, 1.21), but not p.R160W, were associated with greater risk for Parkinson's disease. Our results support potential roles of pigmentation and its key regulator MC1R in the pathogenesis of Parkinson's disease.
Introduction
Although there is a general inverse association between cancer and Parkinson's disease (PD),1, 2 the one exception of melanoma has been well‐documented, not only in patients themselves but also in their relatives.3, 4 Little is known regarding mechanisms underlying the reciprocally increased risk of the two disparate diseases. However, efforts have been made in recent years to investigate potential genetic intersections and common pathological pathways.2, 5 Melanoma is strongly tied to red hair/fair skin, a phenotype of loss‐of‐function of the melanocortin‐1 receptor gene (MC1R), the key pigmentation gene.6 Our initial investigation based on more than 120,000 US men and women demonstrated that red hair color and red hair‐associated MC1R p.R151C polymorphism (rs1805007) were associated with higher risk of PD.5 MC1R variants have since garnered considerable interest and debate over their significance in the PD and melanoma association.7, 8, 9, 10, 11 In a case–control study based on 870 PD cases in Spain, another red hair‐associated MC1R variant p.R160W (rs1805008) was found to be associated with higher PD risk.8 However, other epidemiological studies on this topic did not support an MC1R‐PD link.9, 10, 11 In this report, we searched all literature indexed in PubMed and performed meta‐analyses of studies that examined the association between red hair color, MC1R p.R151C or p.R160W, two most studied MC1R loss‐of‐function polymorphisms, and PD risk.
Methods
Literature search and data extraction
We searched all published literature in MEDLINE via PubMed up to July 2016 that reported PD associations with hair color, MC1R variants, and amino acid changes. For inclusion in MC1R variant analyses, all studies must have reported the specific variants or amino acid changes of interest, and the relative risks (RRs) or odds ratios (ORs). We extracted information on year of study, study type and population, cases and controls population size, country origin, focus of study, PD ascertainment method, variant of interest, lower and upper confidence intervals (CIs), minor allele frequency (MAF) in PD patients and controls, model or test type, and adjustment covariates.
Statistical analyses
We used Q statistic to examine heterogeneity among the studies and the significance level was set at 0.1. We used fixed‐effects models to calculate the summary ORs as no significant heterogeneity was identified (P‐heterogeneity ≥0.2 for all analyses). We did not adjust for multiple comparison because the current analyses were hypothesis‐driven. Publication bias was examined with the Begg and Egger tests.
Results
We identified six publications based on eight study cohorts in total from 2009 to 2016, all with study focus on hair color or MC1R p.R151C or p.R160W polymorphisms (Table 1).5, 7, 8, 9, 10, 11 PD cases were of US, French/French‐Canadian, UK, German, Greek, Dutch, and Spanish origins, overall representing white populations.5, 7, 8, 9, 10, 11 PD diagnosis was generally ascertained by a neurologist or clinician according to the United Kingdom Parkinson's Disease Society Brain Bank Criteria.
Table 1.
Characteristics of publications included in meta‐analysis of hair color, MC1R p.R151C, and p.R160W polymorphisms and risk for PD
| Publication (reference#) | Study type | Study/population | Study size | Exposures | Effect estimate RR or OR (95% CI) | Adjustment |
|---|---|---|---|---|---|---|
| Gao5 |
Cohort nested case– control |
HPFS NHS |
Cohort study: 132,302 participants and 539 PD cases Case–control study: PD cases: 272 Controls: 1185 |
Red versus black hair color p.R151C |
RR = 1.93 (1.08, 3.43) OR = 1.37 (0.99, 1.89) |
smoking, ethnicity, BMI, nonsteroid antiinflammatory drug, alcohol intake, caffeine intake, lactose |
| Dong7 | Nested case– control | PAGE within NIH‐AARP Diet and Health Cohort | PD cases: 808 Controls: 1623 |
Red versus black hair color p.R160W |
OR = 1.36 (0.66, 2.79) OR = 1.14 (0.89, 1.44) |
age, sex, smoking status, and caffeine intake |
| Case–control | IPDGC | PD cases: 5333 Controls: 12019 | p.R151C | OR = 1.06 (0.94, 1.09) | ||
| p.R160W | OR = 0.98 (0.89, 1.07) | |||||
| Tell‐Marti8 | Case–control |
Parkinson's Disease and Movement Disorders Unit of Hospital Clinic of Barcelona Spanish Mediterranean Caucasians |
PD cases: 870 Controls: 736 | p.R151C | OR = 1.25 (0.80, 1.95) | age, sex |
| p.R160W | OR = 2.10 (1.18, 3.73) | |||||
| Lubbe9 | Cohort | “An additional large cohort collected through IPDGC”9 |
PD cases: 5944 Controls: 4642 |
p.R160W | OR = 1.01 (0.90, 1.13) | sex, population stratification |
| Gan‐Or10 | Case–control | Columbia University Medical Center, New York | PD cases: 539 Controls: 265 | p.R151C | OR = 0.77 (0.48, 1.23) | age, sex |
| p.R160W | OR = 0.92 (0.53, 1.57) | |||||
| Case–control |
Montreal Neurological Institute European ancestry |
PD cases: 551 Controls: 956 | p.R151C | OR = 0.92 (0.57, 1.47) | ||
| p.R160W | OR = 1.13 (0.68, 1.90) | |||||
| Lorenzo‐Betancor11 | Case–control |
Mayo Clinic Non‐Hispanic Caucasian |
PD cases: 889 Controls: 940 | p.R151C | OR = 1.27 (P = 0.068) | |
| p.R160W | OR = 1.06 (P = 0.62) |
RR, the relative risks; OR, odds ratios; CI, confidence intervals; HPFS, the health professionals follow‐up study; NHS, the nurses’ health study; PD, Parkinson's disease; BMI, body mass index; PAGE, Parkinson's genes and environment; AARP, the American Association of Retired Persons; IPDGC, International Parkinson's Disease Genomics Consortium.
Red hair was associated with significantly higher risk of PD, relative to black hair (pooled OR = 1.68; 95% CI: 1.07, 2.64; P = 0.02; PD case number = 13475, 7] (Fig. 1A). When we further examined two MC1R red hair color alleles, we found that p.R151C variant was associated with marginally increased risk of PD (pooled OR = 1.101; 95% CI: 1.002, 1.210; P = 0.046; PD case number = 84545, 7, 8, 9, 10] (Fig. 1B). However, we did not find significant association between p.R160W and PD risk (pooled OR = 1.019; 95% CI: 0.956, 1.807; P = 0.57; PD case number = 14,934)7, 8, 9, 10, 11 (Fig. 1C). There was no strong evidence of publication bias based on the Begg and Egger tests (P > 0.05 for all).
Figure 1.
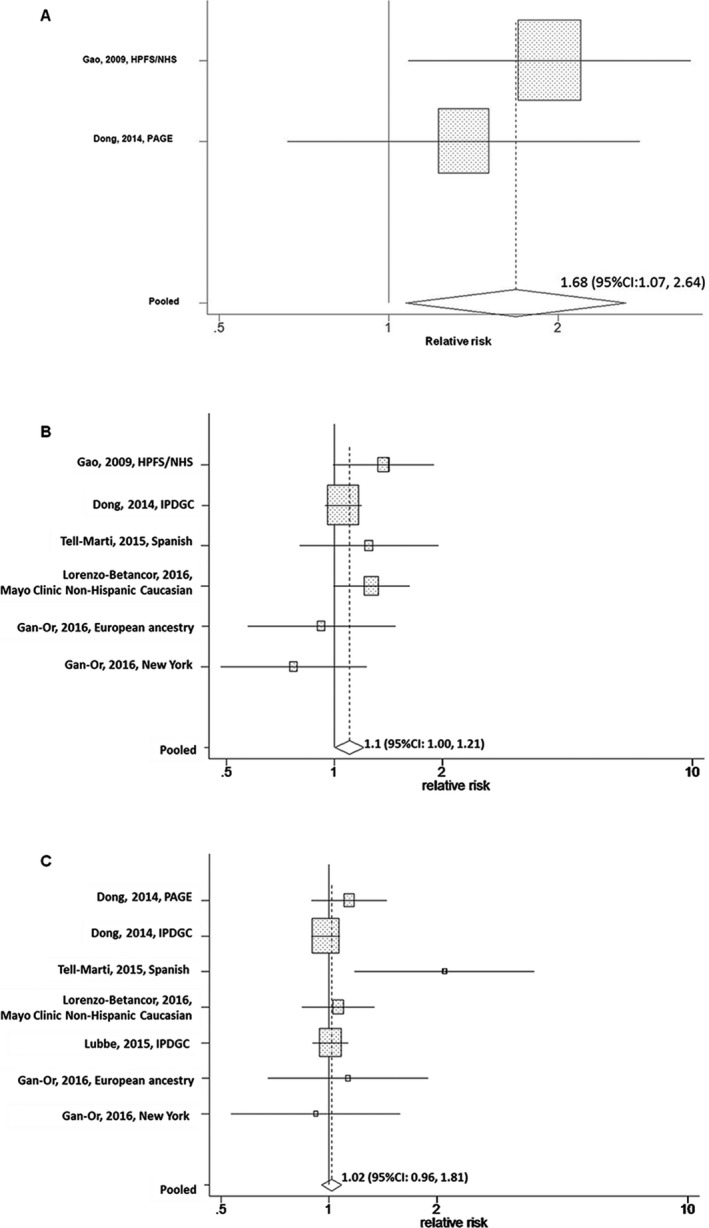
Pooled relative risks and their 95% confidence intervals (95% CIs) for Parkinson's disease (PD) according to red hair color status (A; 1347 PD cases), MC1R p.R151C (rs1805007) (B; 8454 PD cases), and p.R160W (rs1805008) (C; 14934 PD cases) polymorphisms. HPFS = the health professionals follow‐up study; NHS = the nurses’ health study; PAGE = Parkinson's genes and environment; IPDGC = International Parkinson's Disease Genomics Consortium.
Discussion
In this meta‐analysis of six publications, totaling eight study cohorts,5, 7, 8, 9, 10, 11 we found that red hair color was significantly associated with higher risk for PD. Red hair‐associated MC1R variant p.R151C had a significant association with PD risk. Another red hair color variant, p.R160W, was not associated with PD risk.
The associations of red hair color and MC1R p.R151C with PD risk are consistent with our initial report.5 Skin/hair color is determined by relative production of brown–black eumelanin and yellow–red pheomelanin. Binding to its ligand α‐melanocyte‐stimulating hormone, the G protein‐coupled MC1R induces synthesis of eumelanin through cAMP cascade.6, 12 Loss‐of‐function MC1R variants including p.R151C facilitate pheomelanin formation and are associated with red hair/fair skin and increased risk of melanoma.6, 12, 13 MC1R is also involved in the regulation of other cellular functions independent of pigmentation.12 Evidence supports a critical role of pheomelanin as a prooxidant in MC1R melanomagenesis,6 and involvement of red hair pigmentation in PD is supported by our original report5 and the present meta‐analysis. Other studies have also implicated a potential role of general pigmentation in PD. For example, primary skin cultures from individuals with red hair color showed deregulation of genes involved in neurodegenerative diseases such as PD.14 A clinical study reported a correlation between light pigmentation phenotype and increased echogenicity of the substantia nigra.15 Furthermore, a GWA study identified melanogenesis as significant pathways for PD.16 However, it is not clear whether skin/hair pigmentation accounts all or in part for MC1R p.R151C‐PD association.
Interestingly, despite limited knowledge about its biosynthetic pathway and its exact role in dopaminergic neuron degeneration, neuromelanin, the third melanin in humans in fact has a pheomelanin core and a eumelanin surface.17 This finding has led to a hypothesis that thinning eumelanin surface and exposing pheomelanin core may be responsible for the selective vulnerability of pigmented dopaminergic neurons in PD.18 Although early evidence supports expression of MC1R in brain,19 and MC1R has been proposed to be neuroprotective,8 it remains to be determined whether MC1R signaling plays any role in the synthesis and functions of neuromelanin and how neuromelanin is related to peripheral pigmentation, that is, skin/hair color.
MC1R p.R160W, another red hair‐associated polymorphism that was associated with higher PD risk in one study with a Spanish population,8 did not show a significant positive association in our meta‐analysis. One of the difficulties in ascertaining the association stems from the relatively low MAF and penetrance of MC1R in both PD and non‐PD patients5, 7, 8, 9, 10, 11 compared to other PD predisposing genes such as those of the family of PARK genes, though the frequency of MC1R mutations is comparable to those of melanoma cases.6, 12 Furthermore, the frequency of minor alleles differs between populations,20 and possible differential biological mechanisms and environmental interactions contributing to the mixed evidence for the association with PD should be clarified.
Although our original findings5 are generally supported by this meta‐analysis, larger, prospective cohorts with different ethnic backgrounds are needed to verify the associations between hair color, p.R151C, and possibly other MC1R red hair variants and PD risk. In addition, laboratory studies would provide critical insight into the mechanistic roles of MC1R and pigmentation in dopaminergic neuron degeneration in PD.
Author Contributions
X.C., D.F., and G.X. designed the study, acquired and analyzed data, and wrote the paper. M.A.S. provided critical comments on the study design and revised the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors are supported by the Michael J. Fox Foundation (9908 to X. C.), National Institute of Health (1R21NS090246‐01A1 to X. C., 5R21NS087235‐02 to X. G.), and National Natural Science Foundation of China (81471293 to X.C.).
Contributor Information
Xiqun Chen, Email: xchen17@mgh.harvard.edu.
Xiang Gao, Email: xxg14@psu.edu.
References
- 1. Liu R, Gao X, Lu Y, Chen H. Meta‐analysis of the relationship between Parkinson disease and melanoma. Neurology 2011;76:2002–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang P, Yang XD, Chen SD, et al. The association between Parkinson's disease and melanoma: a systematic review and meta‐analysis. Transl Neurodegener 2015;. doi:10.1186/s40035‐015‐0044‐y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao X, Simon KC, Han J, et al. Family history of melanoma and Parkinson disease risk. Neurology 2009;73:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kareus SA, Figueroa KP, Cannon‐Albright LA, et al. Shared Predispositions of Parkinsonism and Cancer: a Population‐Based Pedigree‐Linked Study. Arch Neurol 2012;69:1572–1577. [DOI] [PubMed] [Google Scholar]
- 5. Gao X, Simon KC, Han J, et al. Genetic determinants of hair color and Parkinson's disease risk. Ann Neurol 2009;65:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roider EM, Fisher DE. Red Hair, Light Skin, and UV‐Independent Risk for Melanoma Development in Humans. JAMA Dermatol 2016;152:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong J, Gao J, Nalls M, et al. Susceptibility loci for pigmentation and melanoma in relation to Parkinson's disease. Neurobiol Aging 2014;35:1512.e5–1512.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tell‐Marti G, Puig‐Butille JA, Potrony M, et al. The MC1R melanoma risk variant p.R160W is associated with Parkinson disease. Ann Neurol 2015;77:889–894. [DOI] [PubMed] [Google Scholar]
- 9. Lubbe SJ, Escott‐Price V, Brice A, et al. Is the MC1R variant p.R160W associated with Parkinson's? Ann Neurol 2016;79:159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gan‐Or Z, Mohsin N, Girard SL, et al. The role of the melanoma gene MC1R in Parkinson disease and REM sleep behavior disorder. Neurobiol Aging 2016;43:180.e7–180.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lorenzo‐Betancor O, Wszolek ZK, Ross OA. Rare variants in MC1R/TUBB3 exon 1 are not associated with Parkinson's disease. Ann Neurol 2016;79:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolf Horrell EM, Boulanger MC, D'Orazio JA. Melanocortin 1 Receptor: structure, Function, and Regulation. Front Genet 2016;7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beaumont KA, Newton RA, Smit DJ, et al. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet 2005;14:2145–2154. [DOI] [PubMed] [Google Scholar]
- 14. Puig‐Butille JA, Escámez MJ, Garcia‐Garcia F, et al. Capturing the biological impact of CDKN2A and MC1R genes as an early predisposing event in melanoma and non melanoma skin cancer. Oncotarget 2014;5:1439–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rumpf JJ, Schirmer M, Fricke C, et al. Light pigmentation phenotype is correlated with increased substantia nigra echogenicity. Mov Disord 2015;30:1848–1852. [DOI] [PubMed] [Google Scholar]
- 16. Edwards YJ, Beecham GW, Scott WK, et al. Identifying consensus disease pathways in Parkinson's disease using an integrative systems biology approach. PLoS ONE 2011;6:e16917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bush WD, Garguilo J, Zucca FA, et al. The surface oxidation potential of human neuromelanin reveals a spherical architecture with a pheomelanin core and a eumelanin surface. Proc Natl Acad Sci U S A 2006;103:14785–14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ito S. Encapsulation of a reactive core in neuromelanin. Proc Natl Acad Sci U S A 2006;103:14647–14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xia Y, Wikberg JE, Chhajlani V. Expression of melanocortin 1 receptor in periaqueductal gray matter. NeuroReport 1995;6:2193–2196. [DOI] [PubMed] [Google Scholar]
- 20. Gerstenblith MR, Goldstein AM, Fargnoli MC, et al. Comprehensive evaluation of allele frequency differences of MC1R variants across populations. Hum Mutat 2007;28:495–505. [DOI] [PubMed] [Google Scholar]


