Abstract
Non‐alcoholic fatty liver disease (NAFLD) represents the most common chronic liver disease. It is characterized by a wide spectrum of hepatic changes, which may progress to liver fibrosis and to cirrhosis. NAFLD is considered as the hepatic component of the metabolic syndrome but mechanisms underlying the onset and progression of NAFLD are still under investigation. The traditional ‘two hit hypothesis’ has been developed within a more complex ‘multiple parallel hit hypothesis’ which comprises a wide spectrum of parallel hits. Many therapeutic approaches have been proposed so far and several types of nutraceuticals have been suggested for the treatment of NAFLD and non‐alcoholic steatohepatitis (NASH), the most promising of which are those with antioxidant effects. In particular, vitamin E appears to be effective for the treatment of nondiabetic subjects with more advanced NASH, although the high suggested daily dosages are a matter of concern. Moreover, polyphenols reduce liver fat accumulation, mainly by inhibiting lipogenesis. At present, there are insufficient data to support the use of vitamin C supplements in patients with NAFLD. Data on polyunsaturated fatty acid (PUFA) supplementation are heterogeneous, and no well‐designed randomized controlled studies (RCTs) of adequate size, with histological assessment of steatosis, have been conducted. Based on the available data, silymarin supplementation for the treatment of NAFLD seems to have a favourable effect. The results with anti‐inflammatory agents, such as vitamin D and carnitine are uncertain. In conclusion, there are insufficient data either to support or refute the use of nutraceuticals for subjects with NAFLD. Further RTCs, with histological changes as an outcome measure, are needed.
Keywords: antioxidants, non‐alcoholic fatty liver disease, nutraceuticals, omega‐3 fatty acids, polyphenols
Introduction
Non‐alcoholic fatty liver disease (NAFLD) represents the most common chronic liver disease. It is characterized by a wide spectrum of hepatic changes, which may progress to liver fibrosis and to cirrhosis. NAFLD is considered as the hepatic component of the metabolic syndrome (MetS) 1, but mechanisms underlying its onset and progression are still under investigation. Many studies have demonstrated the presence of insulin resistance 2 and increased oxidative stress 3 in patients with NAFLD/non‐alcoholic steatohepatitis (NASH). Thus, according to the ‘two‐hit hypothesis’, the first hit is represented by lipid accumulation in the hepatocytes, after which oxidative stress may lead to NASH [4]. This traditional view has been developed within a more complex ‘multiple parallel‐hit hypothesis’ which comprises a wide spectrum of parallel hits, such as insulin resistance, oxidative stress, genetic and epigenetic mechanisms, environmental elements, cytokines and microbiota changes 5. These factors may be variously associated, both in presence and absence of MetS.
Several therapeutic approaches have been proposed so far. However, the only proven effective strategy for NAFLD treatment is weight loss and vitamin E administration, the latter only for nondiabetic NASH patients. Notably, there is currently no medically approved treatment for NAFLD 6.
In recent years, the effects of nutraceuticals on NAFLD have received increasing attention and several types of these agents have been suggested for the treatment of NAFLD/NASH (Figure 1). For some of them, a number of clinical trials highlighted an improvement in liver function tests (LFTs) and a possible positive influence on liver histology.
Figure 1.
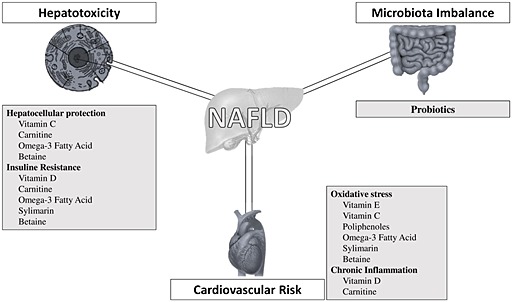
Targets for nutraceutical interventions in non‐alcoholic fatty liver disease
The aim of the present review is to summarize the current evidence on the nutraceuticals proposed for the treatment of patients with NAFLD Table 1. For this purpose, we have divided nutraceuticals according to their anti‐inflammatory or antioxidant properties.
Table 1.
Principal nutraceuticals for the treatment of non‐alcoholic fatty liver disease and possible targets
| Nutraceuticals | Target/mechanism of action | References |
|---|---|---|
| Vitamin D | • Chronic inflammation | Roth et al. 7 |
| • Vitamin D deficiency | Barchetta et al. 11 | |
| • Insulin resistance | Targher er al. 10. | |
| Vitamin E | • Oxidative stress | Chalasani et al. 29 |
| • Hepatocellular protection | ||
| Carnitine | • Insulin resistance | Flanagan et al. 15 |
| • Chronic inflammation | Malaguarnera et al. 16 | |
| • Hepatocellular protection | Somi et al. 17 | |
| • Atherogenic dyslipidaemia | ||
| Vitamin C | • Oxidative stress | Duarte et al. 36 |
| • Hepatocellular protection | Harrison et al. 40 | |
| Omega‐3 fatty acids | • Atherogenic dyslipidaemia | Hedengran et al. 66 |
| • Cardiovascular risk | Endo et al. 42 | |
| • Oxidative stress | Jump et al. 67 | |
| • Hepatocellular protection | Parker et al. 48 | |
| Silymarin | • Oxidative stress | Trappoliere et al. 51 |
| • Chronic inflammation | Sorrentino et al. 54 | |
| • Insulin resistance | Feng et al. 68 | |
| • Hepatocellular protection | ||
| Resveratrol | • Oxidative stress | Gambini et al. 57 |
| • Insulin resistance | Faghihzadeh et al. 58 | |
| • Cardiovascular risk | Poulsen et al. 59 | |
| • Hepatocellular protection |
Anti‐inflammatory agents
Vitamin D
25‐hydroxyvitamin D3 (vitamin D) is a fat‐soluble vitamin which originates in the skin after irradiation of 7‐dehydrocholesterol with ultraviolet light, generating active vitamin D. Its main biological effects include the regulation of mineral metabolism, modulation of the immune system, control of insulin secretion and differentiation of many cellular lines. Vitamin D may also have several favourable metabolic effects acting as an anti‐inflammatory 10 and anti‐fibrotic agent. These properties may be particularly useful in patients with NAFLD 8, 9. It has been suggested that vitamin D deficiency could favour the development of insulin resistance and increase the risk of diabetes mellitus and MetS. Thus, many studies have reported an association between vitamin D deficiency and the presence of NAFLD, with a prevalence of vitamin D deficiency in up to 75% of patients with MetS.
An association between low vitamin D levels and the histological severity of NASH was suggested in patients with chronic elevation of LFTs and hepatic steatosis detected by ultrasound (US) who underwent liver biopsy for suspected NASH [10]. Similar findings were obtained by Barchetta et al. 11, who found a strong association between NAFLD and low vitamin D levels in an adult population with normal LFTs. The same authors showed that the vitamin D receptor (VDR) is widely expressed in the liver, and that the vitamin D/VDR system may play a role in the progression of NASH [12]. Sharifi et al. 13 performed a double‐blind, placebo‐controlled study of 50 000 IU vitamin D3 supplementation vs. placebo, every 14 days for 4 months, in 53 patients. No significant differences in LFTs, the homeostatic model of insulin resistance (HOMA‐IR) or grades of hepatic steatosis between the two groups were observed. Recently, high‐dose oral vitamin D3 supplementation (25 000 IU week–1) over 24 weeks was found to have no impact on liver histology, liver biochemistry, insulin resistance or adipocytokine profile in 12 patients with biopsy‐proven NASH [14]. These negative results should be interpreted with caution, given the limited number of participants and the relatively short‐term follow‐up. Large interventional randomized controlled trials (RCTs) which evaluate directly the effect of vitamin D supplementation on disease progression in NAFLD patients are needed.
Carnitine
L‐carnitine plays a critical role in a number of intracellular and metabolic functions, such as fatty acid transport into the mitochondria, stabilization of cell membranes and reduction of serum lipid levels 15. Moreover, it is also important in the regulation of energy balance in tissues that derive much of their energy from fatty acid oxidation, and is able to modulate the inflammatory response. L‐carnitine supplementation appears to be beneficial in several conditions, such as obesity, type 2 diabetes and liver cirrhosis.
Malaguarnera et al. randomized 74 patients with NASH to receive either oral L‐carnitine (2 g day–1) or placebo for 24 weeks [16]. At the end of the study, subjects treated with L‐carnitine showed significant improvements in LFTs, insulin resistance and inflammatory biomarkers. Repeated liver biopsies showed a reduction in the NASH activity index (defined by steatosis, parenchymal inflammation and hepatocellular injury), and improvement in fibrosis scores. The authors concluded that L‐carnitine supplementation, together with lifestyle changes, can have a positive effect in the treatment of NASH. More recently, data from an RCT of supplementation with L‐carnitine (500 mg twice daily) for1 year for the treatment for NAFLD showed no significant changes in LFTs and US grade 17.
Antioxidant agents
Vitamin E
Oxidative stress is one of key mediators of hepatic damage and, according to the two‐hit hypothesis, is a major contributor to the progression from simple steatosis to steatohepatitis 18. Thus, an increased generation of reactive oxygen species (ROS) can induce lipid peroxidation, leading to inflammation and fibrogenesis through the activation of stellate cells. Lipid peroxidation is increased in NAFLD and can promote inflammation and tissue damage 2.
Several studies have evaluated the pro‐oxidant/antioxidant status in NAFLD 19, 20. We found enhanced systemic oxidative stress in subjects with NAFLD, as assessed by increased levels of urinary 8‐iso‐prostaglandin F2α (8‐iso PGF2α), a valid marker of in vivo oxidative stress, and of serum soluble NADPH oxidase 2 (NOX2)‐derived peptide (sNOX2‐dp), a marker of NOX2 activation which plays an important role in ROS production 21. In our study, we also found a correlation between HOMA‐IR, urinary 8‐iso PGF2α and sNOX2‐dp, confirming the interdependency of insulin resistance and oxidative stress in the pathogenesis of NAFLD. Moreover, both oxidative stress markers were also highly correlated with serum cytokeratin‐18 (CK‐18), a validated marker of liver damage, thus suggesting a possible role of oxidative stress in liver damage. More recently, we found reduced serum vitamin E levels in a cohort of 253 patients with steatosis 22. Subjects with simple steatosis and those with biopsy‐proven NASH had a similar reduction in cholesterol‐adjusted serum levels of vitamin E, suggesting that oxidative stress imbalance could occur in the early stages of fatty liver. Increased oxidative stress is implicated in cardiovascular diseases 23, 24, and may well represent a possible link between NAFLD and cardiovascular events, and constitute an attractive target for antioxidant therapy.
Many clinical trials have evaluated the effects of vitamin E supplementation in NAFLD. In the Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) trial, vitamin E therapy, as compared with placebo, was associated with a significant improvement in steatosis, inflammation, ballooning and resolution of steatohepatitis in adult patients with aggressive NASH who did not have diabetes or cirrhosis 25. However, these findings could not be confirmed in 58 children and adolescents in whom vitamin E, supplied at 800 IU daily for 96 weeks, was found to be no better than placebo at either attaining a sustained reduction in alanine aminotransferase (ALT) levels or improving liver histology 26. Similar negative results were reported by Nobili et al., who showed that vitamin E supplementation provided no benefit in a cohort of 53 children and adolescents with NAFLD 27. Finally, in a recent meta‐analysis conducted by Sato et al. 28, vitamin E treatment improved serum aspartate aminotransferase and ALT levels both in children and adults with NAFLD.
Based only on the results of the PIVENS trial 25, current guidelines recommend vitamin E, at a dose of 800 IU day–1, as the first‐line pharmacotherapy for nondiabetic and noncirrhotic adults with biopsy‐proven NASH [29]. However, some meta‐analyses have reported an increase in all‐cause mortality with high‐dose vitamin E 30, whereas others have failed to confirm such an association 31. Moreover, vitamin E, given at a dose of 400 IU day–1, increased the risk of prostate cancer in relatively healthy men 32.
Other homologues of vitamin E – namely, tocotrienols – exist as isomers designated as alpha, beta, gamma and delta. They are similar to tocopherols, except for the presence of an unsaturated phytyl chain with three unsaturation points compared with a saturated one in tocopherols 33. Tocotrienols have potent anticancer activity and a protective effect in cardiovascular, cerebrovascular and neurodegenerative diseases. They distribute preferentially to the liver and are 40–60 times more potent than alpha‐tocopherol against lipid peroxidation in rat liver microsomes. They attenuate triglyceride accumulation, leading to a reduction in hepatic inflammation and endoplasmic reticulum stress 34.
So far, the effect of tocotrienol supplementation in patients with NAFLD has been evaluated by only one RCT 35, which included hypercholesterolaemic adults with mild US‐proven NAFLD who were randomized to receive 1 year of treatment with either mixed tocotrienols 200 mg twice daily (from palm oil) or placebo. A statistically significant advantage was seen for tocotrienols vs. placebo for normalization of the hepatic echogenic response. However, tocotrienols did not significantly affect lipid profile or liver function when compared with placebo.
Vitamin C
Vitamin C is a water‐soluble antioxidant which acts as free radical scavenger 36. It plays a role in the control of infections and is necessary for normal growth and development. Humans are unable to synthesize vitamin C, and must acquire it through diet, mostly from fruit and vegetables. Vitamin C deficiency affects around 5–10% of the population, with higher prevalence in subjects with an unhealthy lifestyle 37. Low levels of vitamin C have been associated with a number of diseases, such as arterial hypertension, gallbladder disease, cancer, arteriosclerosis and an increased risk of stroke 38.
Some cross‐sectional studies have investigated vitamin C levels in patients with NAFLD 39. Some reported a low daily intake of vitamin C in NAFLD patients, while others found that dietary and/or plasma vitamin C levels were not different between patients with or without NAFLD. These conflicting results may be attributable to the fact that most of the studies evaluated only the estimated dietary intake of vitamin C and did not actually measure plasma concentrations. So far, the effect of vitamin C in patients with NAFLD has been evaluated in only two RCTs. After 6 months of treatment, vitamins C and E (1000 IU and 1 g daily, respectively) improved liver histology, but not LFTs, in 45 subjects with NASH 40. In the St Francis Heart Study 41, atorvastatin 20 mg combined with vitamin C 1 g and vitamin E 1000 IU was effective in reducing the odds of having hepatic steatosis, assessed by computed tomography scans, in individuals with NAFLD at baseline and after 4 years of therapy. However, in both trials 40, 41 it was not possible to detect whether this improvement was due to the combination treatment or to a single compound alone. Therefore, at present, there are insufficient data to support or refute the use of vitamin C supplements in patients with NAFLD.
Omega‐3 fatty acids
The omega‐3 fatty acids are essential polyunsaturated fatty acids (PUFAs) that cannot be synthesized in vivo and derive from alpha‐linolenic acid. Docosahexaenoic acid (DHA) and eicosapentaenoic acid are the most represented omega‐3 PUFAs. Fish oil is a convenient source of omega‐3. Increased dietary intake of PUFAs from marine sources, such as two servings a week of fish, has been proposed to reduce the risk of heart disease 42. The most important mechanism of action is the switching of cellular metabolism from lipogenesis and triacylglycerol accumulation to fatty acid oxidation. PUFAs modulate lipid metabolism in hepatocytes by promoting the restoration of peroxisome proliferator‐activated receptor alpha (PPAR‐α) and decreasing the expression of lipogenesis enzymes by depleting the sterol response element binding protein‐1c (SREBP‐1c), thus favouring fatty acid oxidation and reducing fatty liver. PUFAs may also be anti‐inflammatory and improve insulin sensitivity.
Several clinical trials have assessed the efficacy of PUFAs in the treatment of NAFLD. In the first study, supplementation with PUFAs for 12 months reduced serum triglycerides and ALT, and improved US features of liver steatosis in 56 patients with NAFLD 43. Similar results were found in an RCT performed in 40 subjects with NAFLD supplemented with PUFAs 2 g day–1 for 6 months [44] and in a small study on 11 NAFLD subjects randomized to 6.5 ml olive oil enriched with PUFAs for 12 months or control diet 45. Vega et al. 46 found that in 11 subjects with NAFLD, PUFAs (9 g d–1 of fish oil) significantly reduced levels of plasma triglyceride by 46%, without a significant reduction in hepatic triglyceride content. Finally, Nobili et al. 47 performed an RCT with DHA supplementation (250 mg day–1 and 500 mg day–1) vs. placebo in 60 children with biopsy‐proven NAFLD; children randomized to DHA showed less severe liver steatosis after treatment, a decrease in serum triglycerides and an improvement in insulin sensitivity, with no effect on ALT or body mass index. More recently, in a systematic meta‐analysis including nine studies (five RCTs), involving 355 individuals, the pooled data suggested that PUFA supplementation may decrease LFTs and hepatic fat content, although the effect is generally small 48. In conclusion, present data are heterogeneous, and there are no data available from well‐designed RCTs of adequate size with a histological assessment of steatosis. Moreover, no information is available on the optimal dose of PUFA supplementation, the duration of therapy, and the long‐term efficacy and safety.
Polyphenols
Polyphenols are members of a large family of plant‐derived compounds 49 classified as flavonoids and non‐flavonoids. Non‐flavonoids include stilbenes and phenolic acids. Polyphenols act as antioxidants, reducing liver fat accumulation, mainly by inhibiting lipogenesis. Epidemiological studies and meta‐analyses have suggested that diets rich in plant polyphenols offer protection against cardiovascular and neurodegenerative diseases, diabetes, osteoporosis and cancer 50. Polyphenols may present hepatoprotective effects because they increase fatty acid oxidation, and decrease oxidative stress, insulin resistance and inflammation, the main factors responsible for the progression from NAFLD to NASH.
Silymarin
Silymarin is a flavonoid polyphenol derived from the milk thistle plant which consists of silybin, isosilibinin, silicristin and other compounds. At the liver site, silymarin reduces inflammation and fibrogenesis, stimulates liver regeneration and interferes with leukotriene formation in Kupffer cell cultures, thus inhibiting hepatic stellate cell activation 51. Silymarin exerts membrane‐stabilizing and antioxidant activity. Hence, it has been proposed as complementary treatment for inflammatory liver conditions such as cirrhosis, hepatitis, alcoholic fatty liver disease and NAFLD.
Treatment with silybin plus phosphatidylcholine coformulated with vitamin E for 12 months was associated with an improvement in LFTs, insulin resistance and liver histology in 138 patients with histological NAFLD 52. In a further study, food supplement containing vitamin E, L‐glutathione, L‐cysteine, L‐methionine and Silybum marianum, given twice a day for 3 months in 72 subjects with NAFLD, reduced LFTs and hepatorenal brightness 53. Similar results were obtained in a placebo‐controlled trial of 180 mg day–1 silymarin, in which treatment was associated with significant improvement of biometric parameters (reduction of abdominal circumference, body mass index, ultrasound measurement of right liver lobe), but not with a reduction of LFTs 54. Recently, subjects with MetS and NAFLD were randomized to treatment with diet plus exercise, to silymarin plus vitamin E or to placebo for 3 months. The study suggested that, in subjects with NAFLD and MetS treated with diet and exercise, the addition of silymarin improved the efficacy of the lifestyle intervention 55. We can conclude that silymarin supplementation for the treatment of NAFLD seems to have a favourable effect.
Resveratrol
Resveratrol belongs to stilbene family, which includes a series of compounds occurring in several plants, and is provided in the diet by various foodstuffs, such as grapes, berries, red wine and nuts 56. Resveratrol improves insulin sensitivity and glucose tolerance, and reduces plasma lipids, inflammation and oxidative stress 57.
In an RCT including 50 NAFLD patients, 12 weeks' supplementation with 500 mg resveratrol reduced ALT and hepatic steatosis 58. In a further RCT in 60 subjects with NAFLD, 600 mg resveratrol supplementation for 3 months reduced LFTs, HOMA‐IR, and total and low‐density lipoprotein cholesterol, with no improvement in US‐diagnosed fatty liver 59. By contrast, 8 weeks' administration of resveratrol did not significantly improve any features of NAFLD, compared with placebo, but increased LFTs 60. Moreover, in obese men supplemented with high doses of resveratrol, there was no effect on insulin sensitivity, lipid oxidation, ectopic or visceral fat content, or inflammatory and metabolic biomarkers 61. These conflicting results raise doubt about the role of resveratrol in NAFLD, and future clinical studies will determine its safety and effectiveness in this setting.
Anthocyanins
Anthocyanins are water‐soluble bioactive compounds which belong to the large family of flavonoids. Anthocyanin‐rich foods improve hyperlipidaemia, counteract oxidative stress and ameliorate liver steatosis in experimental NASH. In a study carried out in subjects with borderline nonviral hepatitis, those randomized to purple sweet potato beverage rich in acylated anthocyanins had significantly decreased LFTs, particularly gamma‐glutamyl transpeptidase 62. Recently, improved insulin resistance and decreased plasma ALT, CK‐18 M30 fragment and myeloperoxidase were found in 74 NAFLD patients randomized to a supplement of purified anthocyanin (320 mg day–1) derived from bilberry and black currant (vs. placebo) for 12 weeks [63]. These findings suggest possible benefits of anthocyanin supplementation in subjects with NAFLD, although further studies using liver biopsy are clearly needed 64.
Betaine
Betaine is an important human nutrient obtained from a variety of foods. In the liver, betaine serves as a methyl donor to homocysteine to form methionine, resulting in decreased concentrations of homocysteine and increased concentrations of methionine.
The effect of betaine supplementation in subjects with NAFLD has been assessed in only one RCT, in which betaine did not improve LFTs or histology when compared with placebo 65.
Conclusions
NAFLD is a major challenge to healthcare systems worldwide. Most people with fatty liver do not develop severe liver disease but have an increased chance of developing cardiovascular diseases. Therefore, the treatment of NAFLD patients should be based on a global approach, not only addressing the treatment of insulin resistance and metabolic syndrome, but also including strategies focused on reducing oxidative stress, dyslipidaemia and cardiovascular risk. In this context, nutraceuticals may have an important role in the treatment of NAFLD, in combination with conventional treatments for cardio‐metabolic risk factors.
Several nutraceutical supplements have shown promising results, especially those containing antioxidants and polyphenols. The results with vitamin D and carnitine supplementation are more uncertain. In particular, vitamin E appears to be effective for the treatment of nondiabetic subjects with more advanced NASH, although the high suggested daily dosages are a matter of concern. However, present information derives mostly from small trials with considerable heterogeneity with respect to inclusion criteria, sample size, type of experimental interventions and duration. At present, there are insufficient data either to support or refute the use of nutraceuticals for subjects with NAFLD. Further RTCs, with histological changes as an outcome measure, are needed.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Del Ben, M. , Polimeni, L. , Baratta, F. , Pastori, D. , and Angelico, F. (2017) The role of nutraceuticals for the treatment of non‐alcoholic fatty liver disease. Br J Clin Pharmacol, 83: 88–95. doi: 10.1111/bcp.12899.
References
- 1. Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Maccioni D, Antonini TM, Alessandri C. Non‐alcoholic fatty liver syndrome: a hepatic consequence of common metabolic diseases. J Gastroenterol Hepatol 2003; 18: 588–94. [DOI] [PubMed] [Google Scholar]
- 2. Polimeni L, Del Ben M, Baratta F, Perri L, Albanese F, Pastori D, Violi F, Angelico F. Oxidative stress: new insights on the association of non‐alcoholic fatty liver disease and atherosclerosis. World J Hepatol 2015; 7: 1325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Fiorello S, Cavallo MG, Zalunardo B, Lirussi F, Alessandri C, Violi F. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2005; 90: 1578–82. [DOI] [PubMed] [Google Scholar]
- 4. Angulo P Nonalcoholic fatty liver disease. N Engl J Med 2002; 346: 1221–31. [DOI] [PubMed] [Google Scholar]
- 5. Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non‐alcoholic steatohepatitis (NASH). Int J Mol Sci 2013; 14: 20704–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Ben M, Polimeni L, Baratta F, Pastori D, Loffredo L, Angelico F. Modern approach to the clinical management of non‐alcoholic fatty liver disease. World J Gastroenterol 2014; 20: 8341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, Yeh MM, Nelson JE, Kowdley KV. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll‐like receptor activation. Hepatology 2012; 55: 1103–11. [DOI] [PubMed] [Google Scholar]
- 8. Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25‐hydroxy vitamin d and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans . Diabetes Care 2010; 33: 1373–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eliades M, Spyrou E. Vitamin D: a new player in non‐alcoholic fatty liver disease? World J Gastroenterol 2015; 21: 1718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25‐hydroxyvitamin D3 concentrations and liver histology in patients with non‐alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2007; 17: 517–24. [DOI] [PubMed] [Google Scholar]
- 11. Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med 2011; 9: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, Silecchia G, Leonetti F, Fraioli A, Picardi A, Morini S, Cavallo MG. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology 2012; 56: 2180–7. [DOI] [PubMed] [Google Scholar]
- 13. Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non‐alcoholic fatty liver disease? A randomized clinical trial. Endocrine 2014; 47: 70–80. [DOI] [PubMed] [Google Scholar]
- 14. Kitson MT, Pham A, Gordon A, Kemp W, Roberts SK. High‐dose vitamin D supplementation and liver histology in NASH. Gut 2015. doi:10.1136/gutjnl-2015-310417. [DOI] [PubMed] [Google Scholar]
- 15. Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond). 2010; 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Malaguarnera M, Avitabile T, Li Volti G, Galvano F. L‐carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis – a randomized and controlled clinical trial. Am J Gastroenterol 2010; 105: 1338–45. [DOI] [PubMed] [Google Scholar]
- 17. Somi MH, Fatahi E, Panahi J, Havasian MR, Judaki A. Data from a randomized and controlled trial of L carnitine prescription for the treatment for non‐alcoholic fatty liver disease. Bioinformation 2014; 10: 575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sumida Y, Niki E, Naito Y, Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic Res 2013; 47: 869–80. [DOI] [PubMed] [Google Scholar]
- 19. Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, Sanisoglu SY, Erdil A, Ates Y, Aslan M, Musabak U, Erbil MK, Karaeren N, Dagalp K. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2005; 100: 850–5. [DOI] [PubMed] [Google Scholar]
- 20. Lirussi F, Azzalini L, Orando S, Orlando R, Angelico F. Antioxidant supplements for non‐alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev 2007; 1: CD004996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Del Ben M, Polimeni L, Carnevale R, Bartimoccia S, Nocella C, Baratta F, Loffredo L, Pignatelli P, Violi F, Angelico F. NOX2‐generated oxidative stress is associated with severity of ultrasound liver steatosis in patients with non‐alcoholic fatty liver disease. BMC Gastroenterol 2014; 14: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pastori D, Baratta F, Carnevale R, Cangemi R, Del Ben M, Bucci T, Polimeni L, Labbadia G, Nocella C, Scardella L, Pani A, Pignatelli P, Violi F, Angelico F. Similar reduction of cholesterol‐adjusted vitamin E serum levels in simple steatosis and non‐alcoholic steatohepatitis. Clin Transl Gastroenterol 2015; 6: e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pignatelli P, Pastori D, Carnevale R, Farcomeni A, Cangemi R, Nocella C, Bartimoccia S, Vicario T, Saliola M, Lip GY, Violi F. Serum NOX2 and urinary isoprostanes predict vascular events in patients with atrial fibrillation. Thromb Haemost 2015; 113: 617–24. [DOI] [PubMed] [Google Scholar]
- 24. Cangemi R, Pignatelli P, Carnevale R, Corazza GR, Pastori D, Farcomeni A, Basili S, Davi G, Ferro D, Hiatt WR, Licata G, Lip GY, Loffredo L, Mannucci PM, Vestri A, Violi F; ARA PACIS study group . Cholesterol‐adjusted vitamin E serum levels are associated with cardiovascular events in patients with non‐valvular atrial fibrillation. Int J Cardiol 2013; 168: 3241–7. [DOI] [PubMed] [Google Scholar]
- 25. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander‐Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, Nash CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362: 1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Unalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; Nonalcoholic Steatohepatitis Clinical Research Network . Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011; 305: 1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, Piemonte F, Marcellini M, Angulo P. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology 2008; 48: 119–28. [DOI] [PubMed] [Google Scholar]
- 28. Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, Nakade Y, Ito K, Fukuzawa Y, Yoneda M. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta‐analysis of randomized controlled trials. Nutrition 2015; 31: 923–30. [DOI] [PubMed] [Google Scholar]
- 29. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non‐alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55: 2005–23. [DOI] [PubMed] [Google Scholar]
- 30. Miller ER III, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Ann Intern Med 2005; 142: 37–46. [DOI] [PubMed] [Google Scholar]
- 31. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta‐analysis. JAMA 2007; 297: 842–57. [DOI] [PubMed] [Google Scholar]
- 32. Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306: 1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahsan H, Ahad A, Iqbal J, Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab (Lond). 2014; 11: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel V, Rink C, Gordillo GM, Khanna S, Gnyawali U, Roy S, Shneker B, Ganesh K, Phillips G, More JL, Sarkar A, Kirkpatrick R, Elkhammas EA, Klatte E, Miller M, Firstenberg MS, Chiocca EA, Nesaretnam K, Sen CK. Oral tocotrienols are transported to human tissues and delay the progression of the model for end‐stage liver disease score in patients. J Nutr 2012; 142: 513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magosso E, Ansari MA, Gopalan Y, Shuaib IL, Wong JW, Khan NA, Abu Bakar MR, Ng BH, Yuen KH. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo‐controlled clinical trial. Nutr J 2013; 12: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duarte TL, Lunec J. Review: when is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res 2005; 39: 671–86. [DOI] [PubMed] [Google Scholar]
- 37. Lindblad M, Tveden‐Nyborg P, Lykkesfeldt J. Regulation of vitamin C homeostasis during deficiency. Nutrients 2013; 5: 2860–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen GC, Lu DB, Pang Z, Liu QF. Vitamin C intake, circulating vitamin C and risk of stroke: a meta‐analysis of prospective studies. J Am Heart Assoc 2013; 2: e000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ipsen DH, Tveden‐Nyborg P, Lykkesfeldt J. Does vitamin C deficiency promote fatty liver disease development? Nutrients 2014; 6: 5473–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2003; 98: 2485–90. [DOI] [PubMed] [Google Scholar]
- 41. Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005; 46: 166–72. [DOI] [PubMed] [Google Scholar]
- 42. Endo J, Arita M. Cardioprotective mechanism of omega‐3 polyunsaturated fatty acids. J Cardiol 2016; 67: 22–7. [DOI] [PubMed] [Google Scholar]
- 43. Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G, Svegliati‐Baroni G, Sofi F, Milani S, Abbate R, Surrenti C, Casini A. Prolonged n‐3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non‐alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther 2006; 23: 1143–51. [DOI] [PubMed] [Google Scholar]
- 44. Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, Papa G, Rabuazzo AM, Purrello F. Effects of n‐3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis 2008; 40: 194–9. [DOI] [PubMed] [Google Scholar]
- 45. Sofi F, Giangrandi I, Cesari F, Corsani I, Abbate R, Gensini GF, Casini A. Effects of a 1‐year dietary intervention with n‐3 polyunsaturated fatty acid‐enriched olive oil on non‐alcoholic fatty liver disease patients: a preliminary study. Int J Food Sci Nutr 2010; 61: 792–802. [DOI] [PubMed] [Google Scholar]
- 46. Vega GL, Chandalia M, Szczepaniak LS, Grundy SM. Effects of N‐3 fatty acids on hepatic triglyceride content in humans. J Invest Med 2008; 56: 780–5. [DOI] [PubMed] [Google Scholar]
- 47. Nobili V, Bedogni G, Alisi A, Pietrobattista A, Rise P, Galli C, Agostoni C. Docosahexaenoic acid supplementation decreases liver fat content in children with non‐alcoholic fatty liver disease: double‐blind randomised controlled clinical trial. Arch Dis Child 2011; 96: 350–3. [DOI] [PubMed] [Google Scholar]
- 48. Parker HM, Johnson NA, Burdon CA, Cohn JS, O'Connor HT, George J. Omega‐3 supplementation and non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. J Hepatol 2012; 56: 944–51. [DOI] [PubMed] [Google Scholar]
- 49. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009; 2: 270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salomone F, Godos J, Zelber‐Sagi S. Natural anti‐oxidants for non‐alcoholic fatty liver disease: molecular targets and clinical perspectives. Liver Int 2016; 36: 5–20. [DOI] [PubMed] [Google Scholar]
- 51. Trappoliere M, Caligiuri A, Schmid M, Bertolani C, Failli P, Vizzutti F, Novo E, Di Manzano C, Marra F, Loguercio C, Pinzani M. Silybin, a component of silymarin, exerts anti‐inflammatory and anti‐fibrogenic effects on human hepatic stellate cells. J Hepatol 2009; 50: 1102–11. [DOI] [PubMed] [Google Scholar]
- 52. Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, Chiaramonte M, Cursaro C, Danila M, De Sio I, Floreani A, Freni MA, Grieco A, Groppo M, Lazzari R, Lobello S, Lorefice E, Margotti M, Miele L, Milani S, Okolicsanyi L, Palasciano G, Portincasa P, Saltarelli P, Smedile A, Somalvico F, Spadaro A, Sporea I, Sorrentino P, Vecchione R, Tuccillo C, Del Vecchio Blanco C, Federico A. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med 2012; 52: 1658–65. [DOI] [PubMed] [Google Scholar]
- 53. Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol 2013; 5: 109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sorrentino G, Crispino P, Coppola D, De Stefano G. Efficacy of lifestyle changes in subjects with non‐alcoholic liver steatosis and metabolic syndrome may be improved with an antioxidant nutraceutical: a controlled clinical study. Drugs R&D 2015; 15: 21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hashemi SJ, Hajiani E, Sardabi EH. A placebo‐controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepat Mon 2009; 9: 265–70. [Google Scholar]
- 56. Bavaresco L, Fregoni C, Cantu E, Trevisan M. Stilbene compounds: from the grapevine to wine. Drugs Exp Clin Res 1999; 25: 57–63. [PubMed] [Google Scholar]
- 57. Gambini J, Ingles M, Olaso G, Lopez‐Grueso R, Bonet‐Costa V, Gimeno‐Mallench L, Mas‐Bargues C, Abdelaziz KM, Gomez‐Cabrera MC, Vina J, Borras C. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev 2015; 2015: 837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Faghihzadeh F, Adibi P, Hekmatdoost A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non‐alcoholic fatty liver disease: a randomised, double‐blind, placebo‐controlled study. Br J Nutr 2015; 114: 796–803. [DOI] [PubMed] [Google Scholar]
- 59. Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde‐Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High‐dose resveratrol supplementation in obese men: an investigator‐initiated, randomized, placebo‐controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013; 62: 1186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O'Moore‐Sullivan TM, Lee P, Franklin M, Klein K, Taylor PJ, Ferguson M, Coombes JS, Thomas GP, Cowin GJ, Kirkpatrick CM, Prins JB, Hickman IJ. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2014; 12: 2092–103. [DOI] [PubMed] [Google Scholar]
- 61. Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y, Shu F, Gao Y, Yuan L, Zhang Q, Mi M. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non‐alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis 2015; 47: 226–32. [DOI] [PubMed] [Google Scholar]
- 62. Seymour EM, Singer AA, Kirakosyan A, Urcuyo‐Llanes DE, Kaufman PB, Bolling SF. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator‐activated receptors in rats with intake of tart cherry. J Med Food 2008; 11: 252–9. [DOI] [PubMed] [Google Scholar]
- 63. Suda I, Ishikawa F, Hatakeyama M, Miyawaki M, Kudo T, Hirano K, Ito A, Yamakawa O, Horiuchi S. Intake of purple sweet potato beverage affects on serum hepatic biomarker levels of healthy adult men with borderline hepatitis. Eur J Clin Nutr 2008; 62: 60–7. [DOI] [PubMed] [Google Scholar]
- 64. Zhang PW, Chen FX, Li D, Ling WH, Guo HH. A CONSORT‐compliant, randomized, double‐blind, placebo‐controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine (Baltimore). 2015; 94: e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abdelmalek MF, Sanderson SO, Angulo P, Soldevila‐Pico C, Liu C, Peter J, Keach J, Cave M, Chen T, McClain CJ, Lindor KD. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo‐controlled trial. Hepatology 2009; 50: 1818–26. [DOI] [PubMed] [Google Scholar]
- 66. Hedengran A, Szecsi PB, Dyerberg J, Harris WS, Stender S. n‐3 PUFA esterified to glycerol or as ethyl esters reduce non‐fasting plasma triacylglycerol in subjects with hypertriglyceridemia: a randomized trial. Lipids 2015; 50: 165–75. [DOI] [PubMed] [Google Scholar]
- 67. Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr 2005; 135: 2503–6. [DOI] [PubMed] [Google Scholar]
- 68. Feng B, Meng R, Huang B, Shen S, Bi Y, Zhu D. Silymarin alleviates hepatic oxidative stress and protects against metabolic disorders in high‐fat diet‐fed mice. Free Radic Res 2016; 50: 314–27. [DOI] [PubMed] [Google Scholar]


