Abstract
Dietary nitrate (found in green leafy vegetables, such as rocket, and in beetroot) is now recognized to be an important source of nitric oxide (NO), via the nitrate–nitrite–NO pathway. Dietary nitrate confers several cardiovascular beneficial effects on blood pressure, platelets, endothelial function, mitochondrial efficiency and exercise. While this pathway may now seem obvious, its realization followed a rather tortuous course over two decades. Early steps included the discovery that nitrite was a source of NO in the ischaemic heart but this appeared to have deleterious effects. In addition, nitrate‐derived nitrite provided a gastric source of NO. However, residual nitrite was not thought to be absorbed systemically. Nitrite was also considered to be physiologically inert but potentially carcinogenic, through N‐nitrosamine formation. In Part 1 of a two‐part Review on the nitrate‐nitrite‐NO pathway we describe key twists and turns in the elucidation of the pathway and the underlying mechanisms. This provides the critical foundation for the more recent developments in the nitrate–nitrite–NO pathway which are covered in Part 2.
Keywords: blood pressure, dietary nitrate, endothelial function, ischaemia–reperfusion, nitrite, platelets
Introduction
While the nitrate–nitrite–nitric oxide (NO3 −– NO2 −–NO) pathway has now largely been accepted as an alternative source of NO with an expanding range of beneficial effects, the characterization of the kinetic processes leading to the realization of the pathway was not straightforward. In Part 1 of a two‐part Review on the nitrate‐nitrite‐NO pathway , we describe key twists and turns in the establishment of the pathway over the past two decades.
Background to the established properties of nitrate/nitrite
Inorganic nitrate has a long history as an explosive agent, being a major component of gunpowder, as described in a Chinese manual of war from 1044 AD 1. It was also used in Chinese medicine to treat angina, as described in an 8th century Chinese manuscript from the Dunhuang collection 1. Inorganic nitrate was largely superseded when the organic (i.e. carbon‐containing) nitrate compound, nitroglycerin, synthesized by Sobrero in 1812 2, was stabilized and developed as dynamite by Alfred Nobel. Nitroglycerin was subsequently used medicinally [and renamed glyceryl trinitrate (GTN), to distinguish it from the explosive) to treat angina (including by Nobel). Although prior to the use of GTN, isoamyl nitrite was used, it was subsequently abandoned in favour of organic nitrates owing to its shorter half‐life, and, along with isopropyl and isobutyl nitrite, became drugs of abuse (‘poppers’). As nitrovasodilators, organic nitrates and nitrites were considered to relax vascular smooth muscle via activation of soluble guanylate cyclase (sGC), resulting in the elevation of cGMP either directly or through metabolism to NO, thus increasing coronary blood flow, for example 3. Although potassium nitrate was used as a weak diuretic in the early 20th century 4, inorganic nitrite and nitrate have had a minimal role therapeutically, and more recently as ‘stable end products of NO metabolism’ it was not thought that they could be metabolized back to NO in biological systems. The only current medicinal indication for sodium nitrite is as a treatment for cyanide poisoning.
While they have had minimal therapeutic use, the inorganic nitrate and nitrite anions are common constituents of our diet. Relatively small amounts of nitrite occur naturally in food; however, nitrite (E249, E250) is commonly added in the curing process to preserve meats. By contrast, nitrate is found in large quantities in green leafy vegetables (e.g. spinach and lettuce), as well as in beetroot (red beet), and is also an EU‐approved food additive (E251, E252). In spite of these abundant sources, typically ingested quantities of nitrate do not account for the majority of nitrate exposure. For example, Green et al. (1981) found that urinary nitrate excretion over 24 h exceeded the amount of nitrate ingested (whether small or large quantities) by fourfold 5. This suggested substantial endogenous nitrate production. Following the discovery of endothelium‐derived relaxing factor (EDRF) as NO, the pathway was elucidated by the same group, whereby Leaf et al. 6 showed that the consumption of [15N] L‐arginine led to 15NO3 − excretion in the urine, confirming L‐arginine as the ultimate precursor for nitrate 6. Thus, L‐arginine is the substrate for the NO synthases (NOS), resulting in NO production. NO has multiple biological functions, including being one of the most potent vasodilators in man 7. In addition to nitrate, spinach also contains high concentrations of L‐arginine. However, L‐arginine has rather poor oral bioavailability compared with L‐citrulline, which is found in foods such as watermelon 8 and is metabolized to L‐arginine 9.
As a reactive free radical, NO possesses a short half‐life of milliseconds in the circulation, being oxidized to nitrite and nitrate by oxygen and oxyhaemoglobin 10. In contrast to NO, nitrite and nitrate were thought to lack any useful physiological effect, being considered merely as ‘inert oxidative end products of endogenous NO metabolism’ 10, 11, and, as such, were useful for measuring the activity of vasoactive NO 10. Therefore, the metabolic sequence of L‐arginine–NO–nitrite–nitrate became firmly established (although never described as a distinct L‐arginine–NO–nitrite–nitrate pathway) with the focus on the activity of NO.
Besides being considered to lack any useful physiological effect, nitrite had been implicated as a potential carcinogen through the formation of N‐nitrosamines 12, with concerns that it might lead to the development of oesophago‐gastric cancers 13. Coupled with reports of infantile methaemoglobinaemia, this led to the imposition of the maximum concentration of nitrate in tap water by authorities 14.
However, such concerns over nitrate are inconsistent with ‘healthy’ diets such as vegetarian diets and the Mediterranean diet as these diets typically consist of fresh fruit and vegetables, particularly green leafy varieties, which are high in dietary nitrate, along with high amounts of unsaturated fats and low amounts of red meat. Vegetarian diets have been shown to reduce blood pressure when compared with omnivorous diets 15, 16. The Dietary Approaches to Stop Hypertension (DASH) study showed that a ‘combination diet’ high in fruit, vegetables and low‐fat dairy produce, and low in saturated fat, reduced blood pressure compared with a control diet (low in fruit, vegetables and dairy products, and high in saturated fat) 17. While the mechanisms for this may be multifactorial, the daily nitrate content has been estimated to be ~1200 mg (i.e. ~19 mmol) in the DASH diet 18. Epidemiological evidence from the studies by Joshipura et al. suggested the importance of green leafy vegetables (high in dietary nitrate) in protecting against coronary heart disease and ischaemic stroke 19, 20.
Recent prospective interventional trial evidence supporting the Mediterranean diet was provided by a large multicentre, parallel, randomized, controlled trial performed by Estruch et al. 21. This was conducted in participants with high cardiovascular risk (without established cardiovascular disease), who were allocated to one of three groups: two groups followed a Mediterranean diet with an additional supplement (either a litre of olive oil per week or a selection of mixed nuts), and one followed a control diet (advice to reduce dietary fat) 21. Over a median follow up of 4.8 years, the study demonstrated a ~ 30% reduction in major cardiovascular events (myocardial infarction, stroke, death from cardiovascular causes) with the Mediterranean diets compared with the control diet. The potential for dietary nitrate to be an important factor in such healthy diets will be addressed by considering the following questions.
Is nitrite a source of NO?
Although it had long been acknowledged that nitrite is reduced to NO under acidic conditions, the potential physiological relevance had not been appreciated. NO production from nitrite was originally demonstrated in tissues under hypoxia/ischaemia, conditions associated with an acidic reducing environment. Zweier et al. measured NO production from nitrite using electron paramagnetic resonance (EPR) spectroscopy in isolated rat hearts 22. Under normal perfusion (control), NO production was absent. When subjected to 30 min of ischaemia, a strong signal was detected on EPR, indicating NO production. The intensity of this signal was 100‐fold higher in the presence of added nitrite. Using 1 5⁵NO₂⁻ and observing the generation of 1 5⁵NO, it was confirmed that the NO was derived from nitrite. These effects were seen with a background of NOS inhibition – i.e. this was a NOS‐independent mechanism of NO generation. The quantity of NO produced was inversely associated with the pH of the heart: the lower the pH, the greater the NO production. From this, the authors concluded that NO is produced from nitrite under the reducing environment found in hypoxic/ischaemic/acidic conditions, in contrast to the conventional oxygen‐dependent production of NO from NOS, which is inhibited by hypoxia and ischaemia 23.
What effect does nitrite have in ischaemia‐reperfusion injury (IRI)?
Twenty years ago, nitrite appeared to be predominantly harmful. Zweier et al. 22 found that increasing NO production by adding 10 μM nitrite (enzyme independent) increased postischaemic myocardial injury in a model of IRI in the isolated perfused Langendorff rat heart 22. However, the percentage recovery of the rate pressure product was <10% in controls. Administration of the NOS inhibitor, N‐nitro L‐arginine methyl ester (L‐NAME), decreased NO production by approximately 65% and led to a twofold increase in recovery of contractility, suggesting that NO produced from nitrite could be damaging to myocardial tissue. Understandably, as a result of these findings of a deleterious effect of nitrite/NO in IRI, along with the notion that nitrite produced carcinogenic nitrosamines, and that NO was a pollutant in the atmosphere, interest in nitrite waned.
Almost a decade later, Webb et al. found that, in a similar IRI model, nitrite (10 μM and 100 μM) substantially reduced infarct size and preserved left ventricular pressure following reperfusion 24. This protective effect was lost in the presence of the NO scavenger 2‐(4‐carboxyphenyl)‐4,4,5,5‐tetramethylimidazole‐1‐oxyl 3‐oxide (carboxy‐PTIO), suggesting that nitrite‐derived NO was beneficial. These findings were consistent with an emerging protective rather than damaging function of NO from NOS, as reviewed by Bolli et al. 25. For example, infarct size had been found to be larger in the hearts of endothelial NOS (eNOS) knockout mice compared with wild type 26.
The majority of studies subsequently performed with nitrite supported a protective effect in IRI (Table 1). For example, Duranski et al. demonstrated protective effects of nitrite in vivo during IRI of both the liver and the heart in rats 27. Nitrite limited the rise in liver enzymes during hepatocellular injury, and diminished hepatocellular necrosis, while intraventricular nitrite (48 nmol) prior to reperfusion of the ischaemic heart reduced infarct size by 67%. Administration of nitrite at the time of reperfusion was also effective in limiting infarct size in the brain in a rat model of stroke 28. Table 1 summarizes some of the early studies (1995–2008) which helped to establish the role of nitrite IRI, up to the study in dogs by Gonzalez et al. 29.
Table 1.
The early ischaemia–reperfusion injury (IRI) studies with nitrite from 1995 until the first study in the dog heart (2008)
| Study | Organ/Route | Measures of cytoprotection | Outcome |
|---|---|---|---|
| Zweier et al. 22 | Rat heart IRI (Langendorff) | Recovery of RPP, LVDP, HR | Harmful |
| Webb et al. 24 | Rat heart IRI (Langendorff) | Infarct size, recovery of LVDP | Beneficial |
| Duranski et al. 27 | Rat liver (intraperitoneal) and heart IRI (intraventricular) | Liver – AST/ALT; H&E/TUNEL heart – infarct size | Beneficial |
| Lu et al. 30 | Rat liver IRI | ALT, MPO, ATP, 7‐d mortality, ATP | Beneficial |
| Jung et al. 28 | Rat brain IRI | Infarct volume, Doppler flowmetry; neurological tests | Beneficial |
| Basireddy et al. 31 | Rat kidney IRI | Serum creatinine and blood urea nitrogen, histological scoring for loss of brush border, tubular necrosis, and red blood cell extravasation | Neutral |
| Tripatara et al. 32 | Rat kidney IRI | SCr, AST, CCL, urine flow, FENA +, H&E | Beneficial |
| Bryan et al. 33 | Mouse heart 7 days oral | Infarct size | Beneficial |
| Shiva et al. 34 | Mouse IRI | Infarct size | Beneficial |
| Gonzalez et al. 29 | Dog heart IRI | Infarct size, LVEF, MRI, microsphere blood flow, TUNEL | Beneficial |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CCL, creatinine clearance; FENa+, fractional excretion of Na+; H&E, haematoxylin and eosin; HR, heart rate; LVDP, left ventricular developed pressure; LVEF, left ventricular ejection fraction; MPO, myeloperoxidase; MRI, magnetic resonance imaging; RPP, rate pressure product; SCr, serum creatinine; TUNEL, terminal deoxynucleotidyl transferase‐mediated dUTP‐biotin nick end labeling.
Within a decade, the original discovery of cardioprotection with nitrite in IRI had been translated into two clinical trials in patients with acute ST‐elevation myocardial infarction (STEMI). In a double‐blind, placebo‐controlled, randomized, parallel‐group trial, Siddiqi et al. 35 investigated the effects of intravenous sodium nitrite immediately prior to reperfusion in 229 patients with acute STEMI. Myocardial infarct size [the primary endpoint, measured in terms of the extent of gadolinium enhancement by cardiac magnetic resonance imaging (MRI) 6–8 days postinfarct] did not differ significantly between the groups 35. It is possible that the dose given (70 μmol) was not sufficient to achieve a significant effect. Jones et al. used the intracoronary, rather than the intravenous, route, delivering a high local dose of nitrite (1.8 μmol) prior to balloon dilatation during percutaneous coronary intervention (PCI) in 80 patients with acute STEMI 36. There was no overall difference in the primary outcome – infarct size (vs. placebo), measured by the area under the curve of creatine kinase (AUC CK) profile over 48 h. However, there was an overall improvement (P = 0.05) in myocardial salvage index (measured by cardiac MRI in a subgroup of 68 patients) and a reduction in major adverse cardiac events at 1 year. In a subgroup analysis (n = 66) in patients who had a Thrombolysis In Myocardial Infarction (TIMI) flow grade score ≤1, there was a significant 19% reduction in AUC CK profile and cardiac MRI‐determined infarct size. These findings are entirely compatible with the mechanism of action of nitrite – which requires an ischaemic environment, rather than one in which reperfusion has already partially occurred, and this deserves to be followed up in further trials.
Does nitrite dilate blood vessels?
Furchgott and Bhadrakom had shown in 1953 that high concentrations of sodium nitrite (100 μM and 1000 μM) relaxed strips of rabbit thoracic aorta 37. However, these concentrations are >100‐fold greater than physiological concentrations of nitrite in the circulation, which are usually ~0.1–0.4 μM. In 1999, Cicinelli et al. postulated the existence of an arterial‐to‐venous (AV) gradient of NO, after measuring higher quantities of the NO metabolites nitrite and nitrate in arterial samples than in paired venous samples in healthy volunteers 38. A year later, Gladwin et al. clearly demonstrated the presence of an AV nitrite gradient 39, which further suggested that nitrite is reduced to NO across the vascular bed, with a greater difference shown during handgrip exercise associated with oxygen extraction by the muscles from the vessels, and consistent with enhanced nitrite reduction to NO under conditions of lower oxygen tension.
However, whether nitrite could actually dilate vessels was not clear. In 2001, Lauer et al. showed that, while regional nitrite concentration rose acutely following endothelial NOS stimulation with acetylcholine, reflecting changes in NO generation, the intrabrachial infusion of nitrite failed to produce any vasodilation 40. In the same year, Modin et al. analysed the effects of nitrite in rat aortic rings at concentrations which resembled physiological states and its effect on aortic contractility upon reaching a ‘physiologically acidic milieu’ (pH 6.6). The concentration giving half‐maximal response (EC50) for nitrite‐induced relaxation was decreased at low pH (6.66) relative to neutral pH (7.45), suggesting that nitrite may have an important vasodilatory role in acidic conditions. This effect was inhibited by the coadministration of the sGC inhibitor 1H‐[1,2,4]‐oxadiazolol‐[4,3,‐a]‐quinoxalin‐1‐one (ODQ), suggesting that the vasorelaxatory effect of nitrite was dependent on its reduction to NO 41. In further support of this requirement for reduction of nitrite, the reducing agent ascorbic acid potentiated nitrite's vasorelaxatory effect. Overall, nitrite appeared to have vasodilatory effects on blood vessels via reduction to NO.
Therefore, a hypoxic/acidic/ischaemic environment appeared to be necessary for nitrite reduction to bioactive NO. However, Cosby et al. 42 found that forearm blood flow was increased by intrabrachial infusion of nitrite (36 μmol min−1 and 0.36 μmol min−1), resulting in supra‐ and near‐physiological nitrite concentrations, respectively 42. This vasodilatory effect was enhanced by handgrip exercise, which is associated with greater oxygen extraction from the circulation by the exercising forearm musculature, with a resultant greater formation of deoxyhaemoglobin (deoxyHb) possessing greater capacity for nitrite reduction, as evidenced by the formation of iron‐nitrosylated haemoglobin (Hb). This requirement for hypoxia was confirmed by Maher et al., who demonstrated that nitrite‐induced vasodilatation with intrabrachial nitrite infusions (40 nmol min−1 to 7.84 μmol min−1) was enhanced in hypoxic environments compared with normoxia in humans, as assessed by forearm blood flow 43.
How is nitrite reduced to NO?
The description of enzyme‐independent NO formation from nitrite by Zweier et al. referred to the lack of involvement of NOS 22. It was not clear whether NO production was solely a result of the acidic disproportionation of nitrite, or other enzymes were involved in nitrite metabolism. However, identifying the mechanisms involved in controlling the reduction of nitrite to NO is crucial for understanding the physiological effects of nitrite. As described above, Cosby et al. showed that NO production from nitrite, considered to be responsible for the vasodilatory effect, was confirmed by the rate of formation of iron‐nitrosylated Hb during artery to vein transit 42. The rate of NO formation increased as oxygen saturations fell, suggesting a hypoxia‐regulated mechanism of nitrite bioactivation 11. Maximal reduction of nitrite to NO occurred at an Hb oxygen saturation of ~50% (around the p50 of Hb, the partial pressure of oxygen resulting in 50% Hb oxygen saturation), representing the optimum balance point for maximal availability of Hb in the deoxy‐T state for nitrite to bind to, coupled with maximal availability of Hb in the oxy‐R state for reduction to NO 44, 45, 46, 47. This mechanism of NO formation is therefore dependent on three factors – circulating nitrite as a substrate, the presence of hypoxia (as determined by the level of deoxyHb) and the presence of an acidic environment as a source of H+ – as highlighted by equation (1).
| (1) |
The other ubiquitous globin, deoxymyoglobin (deoxyMb) reduces nitrite approximately 36 times faster than deoxyHb because of its lower redox potential, and is therefore likely to be an important nitrite reductase. The rate of reduction by deoxyMb is increased in lower pH environments. Myoglobin (Mb)‐dependent NO generation occurs as a large burst, whereas Hb‐dependent NO generation is much more prolonged by comparison 48. This may reflect different functions of nitrite reduction in these two molecules. Mb possesses a greater affinity for oxygen, and hence nitrite reduction occurs more readily at low oxygen tensions. Indeed, deoxyMb has been shown to be an effective nitrite reductase 46, 48, catalysing the production of NO ~36‐fold faster than deoxyHb 48. Therefore, the burst of nitrite reduction allows for better regulation of oxygen gradients under hypoxia, relating to the level of NO generation. In addition to Hb and Mb, deoxygenated neuroglobin (Ngb) and cytoglobin (Cgb) have been shown to reduce nitrite to NO 49, 50; however, their low abundance questions their significance as nitrite reductases under physiological conditions 51, 52.
Besides the haem‐containing globins, several studies have highlighted the nitrite reductive capacity of the molybdo‐flavoproteins, in particular xanthine oxidoreductase (XOR) 24, 53. Webb et al. showed that the production of NO from nitrite in rat and human heart homogenates was diminished in the presence of XOR inhibitors by ~50%, suggesting a role for XOR as a cardiac nitrite reductase 24. Four years later, Webb et al. also showed XOR to be present on red blood cell membranes in addition to blood vessels (left internal mammary artery), offering readily accessible sites for nitrite reduction within the circulation 54. The activity of this XOR was upregulated with increasing acidosis and during hypoxia. 55, 56. This provides a regulatory mechanism for the increased activity of nitrite in ischaemic tissue. In addition to XOR, the related molybdo‐flavoprotein, aldehyde oxidase appears to function as a nitrite reductase 57. Aldehyde oxidase is unable to function as a dehydrogenase 57, 58, and is not to be confused with the aldehyde dehydrogenases, although aldehyde dehydrogenase 2, which, when deficient, is associated with oriental (alcohol) flush, may also have nitrite reductase properties 59.
The zinc‐containing enzyme carbonic anhydrase (CA) has also been shown to catalyse the conversion of nitrite to NO 60. However, as zinc in CA lacks redox activity, the enzyme is thought to function as a nitrous anhydrase, rather than a nitrite reductase, forming dinotrogen trioxide (N2O3), which then rapidly breaks down to form nitrogen dioxide (NO2) and NO, or forming nitrosothiols in the presence of thiols (e.g. glutathione), via zinc thiolate and nitrous acid (HNO2) 61. Definitive production of CA‐catalysed NO from nitrite has been difficult to demonstrate, with some studies showing no evidence for this 62. Finally, while, initially, NOS was thought to lack capacity to function as a nitrite reductase, work by Gautier et al. identified such activity for eNOS, located within the oxygenase domain 63. Subsequently, biological relevance was shown in human erythrocytes, whereby inhibition with L‐NAME attenuated the production of NO from nitrite 54.
Can nitrate generate nitrite and NO?
The potential of dietary nitrate as a source of gastric NO was first demonstrated by two independent groups in 1994 64, 66. However, this was viewed as local process specifically related to the acidic environment of the stomach, with relevance strictly restricted to local biological processes (and not as a systemic source of NO). Benjamin et al. demonstrated a tenfold increase in salivary nitrite concentration 45 min after ingestion of potassium nitrate [200 mg (2 mmol nitrate)] in fasting individuals 64. The enterosalivary circulation of nitrate to nitrite had been described previously, whereby ingestion of nitrate‐containing vegetable juices of varying concentration correlated with salivary nitrite concentrations, owing to a concentration of ~25% of the nitrate load in the salivary glands, with subsequent secretion and reduction to nitrite 65. However, the possibility that such nitrate‐derived nitrite might be a source of NO had not been reported. While stomach NO was not measured by Benjamin et al. in that study, concentrations of nitrite such as those found in saliva (e.g. 250 μM) were found to kill Candida albicans and Escherichia coli in a pH‐dependent manner, an effect that was likely to have been due to the production of NO.
Lundberg et al. did measure gastric NO production (in healthy and atopic individuals), with samples obtained through carbonated water‐induced eructation and measured by ozone chemiluminescence 66. Gastric NO production was found to be 100 times higher than exhaled NO. Ingestion of lettuce (50 g, ~1 mmol nitrate) just 5 min prior to the measurement of gastric NO increased the concentration fivefold. Moreover, gastric NO production was inhibited ~95% following ingestion of a high dose of the proton pump inhibitor (PPI) omeprazole (three doses of 80 mg taken over the preceding 24‐h period), with or without lettuce ingestion. Therefore, nitrate was confirmed as a source of NO in the stomach, which was dependent on low pH (typical stomach pH 1–3, without a PPI).
Duncan. et al. found that the salivary production of nitrite was due to bacteria on the posterior third of the tongue with nitrate reductase activity, which was absent in germ‐free rats 67. Following this, McKnight et al. found that ingestion of potassium nitrate (2 mmol) led to a sustained rise in salivary, gastric and plasma nitrate, salivary nitrite and gastric NO concentration for the duration of the study 68. However, there was a small, nonsignificant rise in gastric nitrite, which suggested that the majority of the nitrite was rapidly converted to NO.
Therefore, the understanding of the enterosalivary circulation was that nitrate is readily taken up in the upper gastrointestinal tract, and plasma levels rise and remain elevated. Once in the circulation, ~25% of the nitrate load is actively absorbed and concentrated by the salivary glands. Following secretion of nitrate‐containing saliva into the oral cavity, commensal facultative anaerobic bacteria reduce nitrate to nitrite via nitrate reductases – an important step as mammalian cells cannot effectively metabolize this nitrate 69. Nitrite is then swallowed in the saliva to enter the low pH stomach milieu, where it is rapidly protonated to form nitrous acid, which then decomposes, via the reactions shown in Equations (2)–(5), to form NO 11.
| (2) |
| (3) |
| (4) |
| (5) |
Does nitrate inhibit platelets?
Platelet adhesion and aggregation are pivotal mechanisms in the pathogenesis of acute coronary syndrome and stroke 70. McKnight et al. showed that an oral nitrate load in healthy volunteers resulted in significant inhibition of platelet activity when compared with the control (chloride solution) 14. However, at that time, the mechanism by which nitrate resulted in platelet inhibition was not known. One possibility was the formation of S‐nitrosothiols (RSNOs), from the S‐nitrosylation of thiol‐containing compounds in the presence of nitrosating species derived from nitrite in the acid milieu of the stomach: HNO2, N2O3 and NO+. S‐nitrosothiols were known to be potent inhibitors of platelet function in vitro and in vivo via cGMP‐dependent and ‐independent mechanisms 71.
Does nitrate inhibit platelets via RSNOs?
Richardson et al. 72 found that the ingestion of potassium nitrate (0.5 mmol and 2 mmol) by healthy volunteers increased the gastric RSNO concentration and inhibited the platelet response to collagen. However, there was no increase in plasma or portal blood RSNO, the latter measured in patients with transjugular intrahepatic portosystemic shunts 72. Although there was an association between a rise in gastric RSNO and inhibition of platelet function, it was unclear whether S‐nitrosothiols exerted a direct effect on platelet function. There was also no increase in platelet cGMP levels. Additional studies, using electron paramagnetic spectrometry as well as ozone chemiluminescence, obtained similar findings – an increase in gastric RSNO concentration following ingestion of nitrate, with no significant change in plasma RSNO concentration 73. As plasma nitrite was not thought to increase following a nitrate load, plasma nitrite was not measured in these studies.
Does nitrate increase plasma nitrite?
While ingestion of nitrate led to a large rise in plasma nitrate concentration, it was unclear whether nitrite levels increased in a similar way. Pannala et al. analysed the pharmacokinetic profile of nitrate and nitrite in plasma, urine and saliva following a high nitrate meal (120 g of lettuce – equivalent to 250 mg of nitrate, once daily for three days) 74. They found that, while salivary nitrite increased significantly, there was no significant increase in plasma nitrite (78 ± 4 nmol l−1 and 90 ± 3 nmol l−1 pre‐ and postmeal, respectively); on the other hand, plasma nitrate rose almost sevenfold. Therefore, the explanation for the effects of dietary nitrate on platelet aggregation was not at all clear. As Figure 1 shows, nitrite did not appear to be absorbed from the gut into the circulation, and it was unclear whether RSNOs were absorbed. Besides this, there was major doubt about whether nitrite had physiologically relevant effects; even if it did, it seemed clear at the time that the effects of nitrate were not mediated by nitrite.
Figure 1.
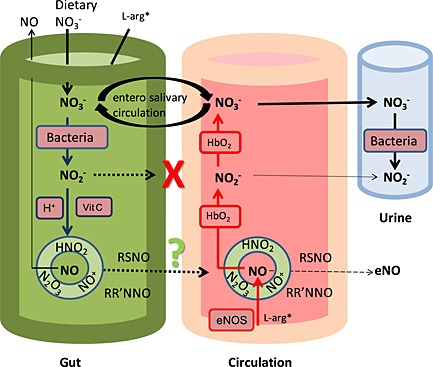
Compartmentalized model (involving the gut, circulation and urinary tract) of the kinetic processes previously (up to a decade ago) understood to occur in the handling of dietary nitrate (NO3 −), with the focus on S‐nitrosothiol production from the nitrosating species (HNO2, N2O3, NO+) generated in the stomach from acidic disproportionation (in addition to the liberation of free NO gas), but with uncertainty over systemic S‐nitrosothiol absorption. The small amounts of residual nitrite (NO2 −) were not thought to be absorbed systemically. L‐arg* (L‐arginine), *non‐essential amino acid, but also derived from the diet, eNOS, endothelial NO synthases; HbO2, oxyhaemoglobin; RR′NNO, (N‐nitrosamine); RSNO, S‐nitrosothiols; Vit, vitamin
In 2004, Lundberg and Govoni found that plasma nitrite concentration increased fourfold in healthy participants following consumption of sodium nitrate (10 mg kg−1), an effect that was prevented when volunteers avoided swallowing after the nitrate load 69. The importance of lingual bacterial nitrate reduction was also shown in the same year in a study in germ‐free sterile rats which showed that gastric NO production was negligible, even following a nitrate load 75. Webb et al. also measured nitrite concentrations after ingestion of nitrate (500 ml beetroot juice) in healthy volunteers 76. They found that plasma nitrite concentrations increased significantly compared with control, peaking at ~3 h after beetroot juice ingestion and returning to baseline levels by 24 h. A limitation in the study by Pannala et al. 74 was that participant blood samples were measured only after 1 h, whereas in the study by Webb et al. 76, a full kinetic profile of plasma nitrite levels was performed, which identified that a significant increase occurred around 2.5–3 h postingestion. The delay in the peak of plasma nitrite, relative to plasma nitrate, is due to the time taken for nitrate to be absorbed through the enterosalivary circuit before reduction to nitrite, followed by accumulation in the circulation 77.
Does nitrate lower blood pressure?
Given the finding of an increase in plasma nitrite following a nitrate load, coupled with the vasodilatory potential of nitrite as demonstrated by Cosby et al. 42, it was possible that dietary nitrate might lower blood pressure. Larsen et al. conducted a randomized crossover study in which healthy, nonsmoking subjects were given sodium nitrate or sodium chloride (both 0.1 mmol kg−1 day−1 for 3 days). While there was no change in systolic blood pressure, diastolic blood pressure was reduced by 3.7 mmHg by sodium nitrate, associated with almost a doubling in the plasma nitrite concentration 77.
In the study by Webb et al. 76, systolic blood pressure was significantly lower following consumption of 500 ml beetroot juice containing ~22 mmol dietary nitrate (and undetectable amounts of nitrite) compared with controls (who consumed 500 ml water), with a peak change at 2.5 h of 10.4 ± 3.0 mmHg (P < 0.01). Peak decreases in diastolic blood pressure and mean arterial pressure were seen at 3 h after ingestion, with changes of 8.1 ± 2.1 mmHg and 8.0 ± 2.1 mmHg, respectively (both P < 0.01) 76. Since then, several studies have explored the effects of dietary nitrate on blood pressure, with 16 included in the meta‐analysis of Siervo et al. 78, which showed a mean difference in systolic blood pressure of −4.4 mmHg (95% confidence interval –5.9, 2.8) 78.
Does nitrate′s systolic blood pressure‐lowering effect occur via nitrite?
In the first study by Webb et al. 76, the peak reduction in blood pressure occurred at 2.5–3 h, corresponding to the peak rise in plasma nitrite concentration, suggesting that nitrite, rather than nitrate, accounted for the change in blood pressure. To provide more evidence that blood pressure reduction with nitrate was related to plasma nitrite concentration, the enterosalivary circulation was interrupted by asking subjects to spit out all of their saliva for 3 h following beetroot juice consumption or to swallow their saliva normally, in a further crossover study. Spitting saliva prevented the increase in plasma nitrite (plasma nitrate was unaffected) and prevented the decrease in systolic blood pressure seen with swallowing saliva normally.
Kapil et al. established that a dose–response relationship existed between nitrate consumption and change in plasma nitrate, nitrite and blood pressure, using capsules containing 4 mmol, 12 mmol and 24 mmol of potassium nitrate 79. Using potassium chloride capsules as control, it was confirmed that the blood pressure‐lowering effect was independent of potassium. Furthermore, the peak plasma nitrite concentration was associated with significantly higher levels of circulating cGMP, a sensitive marker of NO bioactivity 80. This study also gave some insight into individual differences in blood pressure‐lowering responses to nitrate. Baseline blood pressure was inversely correlated with the reduction in blood pressure – i.e. the higher the baseline, the larger the reduction in blood pressure. This suggested the possibility of better blood pressure‐lowering responses in hypertensive individuals. A post hoc analysis also showed that women had a higher baseline concentration of nitrite than men, associated with the lack of a significant blood pressure reduction in response to nitrate. Therefore, baseline nitrite may predict the response of dietary nitrate‐induced blood pressure reduction.
It was now clear that the blood pressure‐lowering effects of dietary nitrate could be explained through the nitrate–nitrite–NO pathway, with nitrite being absorbed systemically. Therefore, nitrite held the key to the puzzle, as summarized in Figure 2.
Figure 2.
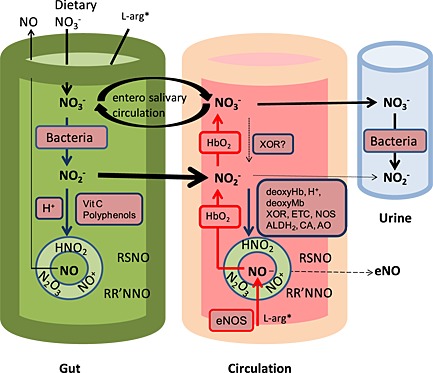
Compartmentalized model (involving the gut, circulation and urinary tract) of the fully realized nitrate–nitrite–nitric acid (NO) pathway. Red and blue arrows represent pathways that are favoured under oxygenated and deoxygenated conditions, respectively 81. ALDH2, aldehyde dehydrogenase type 2; AO, aldehyde oxidase; CA, carbonic anhydrase; DeoxyHb, deoxyhaemoglobin; L‐arg* (L‐arginine), *non‐essential amino acid, but also derived from the diet, eNOS, endothelial NOS; ETC, mitochondrial electron transport chain; HbO2, oxyhaemoglobin; NOS, NO synthases; RR′NNO, (N‐nitrosamine); RSNO, S‐nitrosothiols; Vit, vitamin; XOR, xanthine oxidoreductase
Thus, nitrate is reduced to nitrite on the posterior surface of the tongue, which is then absorbed in the upper gastrointestinal tract into the circulation. Nitrite then undergoes further reduction to produce the biologically active NO in the circulation.
Conclusion
We have described key twists and turns in the realization of the nitrate–nitrite–NO pathway, highlighting some important physiological and therapeutic effects. These are summarized in Table 2. In Part 2, we explore more recently discovered properties and effects of nitrate and nitrite.
Table 2.
Twists and turns in the realization of the nitrate–nitrite–nitric oxide (NO) pathway
| Negative/Neutral/No | Positive/Yes | |
|---|---|---|
| Background to nitrate's (NO 3 − ) and nitrite's (NO 2 − ) properties? | Physiologically inert metabolites of NO 10 | |
| 1. Is nitrite a source of NO? | Nitrite is a physiologically inert metabolite of NO 10 | In the ischaemic heart: Zweier et al. 22 |
| 2. What effect does nitrite have in IRI? | Deleterious – Zweier et al. 22 | Protective – Webb et al. 24 |
| 3. Does nitrite dilate blood vessels? | Lauer et al. 40 | Modin et al. 41, Cosby et al. 42 |
| 4. Are there nitrite reductases? | Non‐enzymatic/non‐NOS Zweier et al. 22 | Hb 42, Mb 82, XOR 24, AO 57, eNOS 63 |
| 5. Is nitrate a source of NO? | Benjamin et al. 64, Lundberg et al. 66 | |
| 6. Does nitrate inhibit platelets? | McKnight et al. 14 | |
| 7. Does nitrate inhibit platelets via RSNOs? | Richardson et al. 72 | |
| 8. Does nitrate increase plasma nitrite? | Pannala et al. 74 | Lundberg et al. 69, Webb et al. 76 |
| 9. Does nitrate lower BP? | Larsen et al. 77, Webb et al. 76 | |
| 10. Does nitrate lower BP via nitrite? | Webb et al. 76 |
AO, aldehyde oxidase; BP, blood pressure; eNOS, endothelial NOS; Hb, haemoglobin; IRI, ischaemia–reperfusion injury; Mb, myoglobin; NOS, NO synthases; RSNOs, S‐nitrosothiols; XOR, xanthine oxidoreductase
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; AW is a share‐holder of HeartBeet Ltd; no other relationships or activities that could appear to have influenced the submitted work.
Khatri, J. , Mills, C. E. , Maskell, P. , Odongerel, C. , and Webb, A. J. (2017) It is rocket science – why dietary nitrate is hard to ‘beet’! Part I: twists and turns in the realization of the nitrate–nitrite–NO pathway . Br J Clin Pharmacol, 83: 129–139. doi: 10.1111/bcp.12913.
References
- 1. Butler AR, Feelisch M. Therapeutic uses of inorganic nitrite and nitrate: from the past to the future. Circulation 2008; 117: 2151–9. [DOI] [PubMed] [Google Scholar]
- 2. Bellamy A. The development of nitroglycerine as an explosive. Atti del convegno in celerazione del centenario della morte di Ascanio Sobrero. Turin, Italy: University of Turin, 1989. [Google Scholar]
- 3. Fung H‐L. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annu Rev Pharmacol Toxicol 2004; 44: 67–85. [DOI] [PubMed] [Google Scholar]
- 4. Keith N, Whelan M, BAnnick EG. The action and excretion of nitrates. Arch Intern Med 1930; 46: 797–832. [Google Scholar]
- 5. Green LC, Ruiz de Luzuriaga K, Wagner DA, Rand W, Istfan N, Young VR, et al Nitrate biosynthesis in man. Proc Natl Acad Sci USA 1981; 78: 7764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leaf CD, Wishnok JS, Tannenbaum SR. L‐Arginine is a precursor for nitrate biosynthesis in humans. Biochem Biophys Res Commun 1989; 163: 1032–7. [DOI] [PubMed] [Google Scholar]
- 7. Moncada S, Palmer RM, Higgs EA. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension 1988; 12: 365–72. [DOI] [PubMed] [Google Scholar]
- 8. Figueroa A, Sanchez‐Gonzalez MA, Wong A, Arjmandi BH. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am J Hypertens 2012; 25: 640–3. [DOI] [PubMed] [Google Scholar]
- 9. Collins JK, Wu G, Perkins‐Veazie P, Spears K, Claypool PL, Baker RA, et al Watermelon consumption increases plasma arginine concentrations in adults. Nutrition 2007; 23: 261–6. [DOI] [PubMed] [Google Scholar]
- 10. Moncada S, Higgs A. The L‐arginine–nitric oxide pathway. N Engl J Med 1993; 329: 2002–12. [DOI] [PubMed] [Google Scholar]
- 11. Lundberg JO, Weitzberg E, Gladwin MT. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 2008; 7: 156–67. [DOI] [PubMed] [Google Scholar]
- 12. Lijinsky W. Health problems associated with nitrites and nitrosamines. Ambio 1976; 5: 67–72. [Google Scholar]
- 13. Tannenbaum SR, Correa P. Nitrate and gastric cancer risks. Nature 1985; 317: 675–6. [DOI] [PubMed] [Google Scholar]
- 14. McKnight GM, Duncan CW, Leifert C, Golden MH. Dietary nitrate in man: friend or foe? Br J Nutr 1999; 81: 349–58. [DOI] [PubMed] [Google Scholar]
- 15. Rouse I, Armstrong B, Beilin L, Vandongen R. Blood‐pressure‐lowering effect of a vegetarian diet: controlled trial in normotensive subjects. Lancet 1983; 321: 5–10. [DOI] [PubMed] [Google Scholar]
- 16. Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A, et al Vegetarian diets and blood pressure: a meta‐analysis. JAMA Intern Med 2014; 174: 577–87. [DOI] [PubMed] [Google Scholar]
- 17. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997; 336: 1117–24. [DOI] [PubMed] [Google Scholar]
- 18. Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 2009; 90: 1–10. [DOI] [PubMed] [Google Scholar]
- 19. Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 1999; 282: 1233–9. [DOI] [PubMed] [Google Scholar]
- 20. Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med 2001; 134: 1106–14. [DOI] [PubMed] [Google Scholar]
- 21. Estruch R, Ros E, Salas‐Salvadó J, Covas M‐I, Corella D, Arós F, et al Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–90. [DOI] [PubMed] [Google Scholar]
- 22. Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme‐independent formation of nitric oxide in biological tissues. Nat Med 1995; 1: 804–9. [DOI] [PubMed] [Google Scholar]
- 23. Giraldez RR, Panda A, Xia Y, Sanders SP, Zweier JL. Decreased nitric‐oxide synthase activity causes impaired endothelium‐dependent relaxation in the postischemic heart. J Biol Chem 1997; 272: 21420–6. [DOI] [PubMed] [Google Scholar]
- 24. Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia–reperfusion damage. Proc Natl Acad Sci USA 2004; 101: 13683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol 2001; 33: 1897–918. [DOI] [PubMed] [Google Scholar]
- 26. Sumeray MS, Rees DD, Yellon DM. Infarct size and nitric oxide synthase in murine myocardium. J Mol Cell Cardiol 2000; 32: 35–42. [DOI] [PubMed] [Google Scholar]
- 27. Duranski MR, Greer JJM, Dejam A, Jaganmohan S, Hogg N, Langston W, et al Cytoprotective effects of nitrite during in vivo ischemia–reperfusion of the heart and liver. J Clin Invest 2005; 115: 1232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jung K‐H, Chu K, Ko S‐Y, Lee S‐T, Sinn D‐I, Park D‐K, et al Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia–reperfusion injury. Stroke 2006; 37: 2744–50. [DOI] [PubMed] [Google Scholar]
- 29. Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu L‐Y, Hon YY, et al Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation 2008; 117: 2986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu P, Liu F, Yao Z, Wang CY, Chen DD, Tian Y, et al Nitrite‐derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia–reperfusion injury. Hepatobiliary Pancreat Dis Int 2005; 4: 350–5. [PubMed] [Google Scholar]
- 31. Basireddy M, Isbell TS, Teng X, Patel RP, Agarwal A. Effects of sodium nitrite on ischemia–reperfusion injury in the rat kidney. Am J Physiol Renal Physiol 2006; 290: F779–86. [DOI] [PubMed] [Google Scholar]
- 32. Tripatara P, Patel NSA, Webb A, Rathod K, Lecomte FMJ, Mazzon E, et al Nitrite‐derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol 2007; 18: 570–80. [DOI] [PubMed] [Google Scholar]
- 33. Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia–reperfusion injury. Proc Natl Acad Sci USA 2007; 104: 19144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, et al Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 2007; 204: 2089–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siddiqi N, Neil C, Bruce M, MacLennan G, Cotton S, Papadopoulou S, et al Intravenous sodium nitrite in acute ST‐elevation myocardial infarction: a randomized controlled trial (NIAMI). Eur Heart J 2014; 35: 1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones DA, Pellaton C, Velmurugan S, Rathod KS, Andiapen M, Antoniou S, et al Randomised phase 2 trial of intra‐coronary nitrite during acute myocardial infarction. Circ Res 2015; 116: 437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Furchgott RF, Bhadrakom S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. J Pharmacol Exp Ther 1953; 108: 129–43. [PubMed] [Google Scholar]
- 38. Cicinelli E, Ignarro LJ, Schonauer LM, Matteo MG, Galantino P, Falco N. Different plasma levels of nitric oxide in arterial and venous blood. Clin Physiol 1999; 19: 440–2. [DOI] [PubMed] [Google Scholar]
- 39. Gladwin MT, Shelhamer JH, Schechter AN, Pease‐Fye ME, Waclawiw MA, Panza JA, et al Role of circulating nitrite and S‐nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci 2000; 97: 11482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, et al Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 2001; 98: 12814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Modin A, Björne H, Herulf M, Alving K, Weitzberg E, Lundberg JON. Nitrite‐derived nitric oxide: a possible mediator of ‘acidic–metabolic' vasodilation. Acta Physiol Scand 2001; 171: 9–16. [DOI] [PubMed] [Google Scholar]
- 42. Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, et al Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 2003; 9: 1498–505. [DOI] [PubMed] [Google Scholar]
- 43. Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, et al Hypoxic modulation of exogenous nitrite‐induced vasodilation in humans. Circulation 2008; 117: 670–7. [DOI] [PubMed] [Google Scholar]
- 44. Gladwin MT, Huang Z, Shiva S, Kim‐Shapiro DB, Patel RP, Ringwood LA, et al Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest 2005; 115: 2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, et al Hypoxia, red blood cells, and nitrite regulate NO‐dependent hypoxic vasodilation. Blood 2006; 107: 566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim‐Shapiro DB, et al The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem 2005; 280: 31126–31. [DOI] [PubMed] [Google Scholar]
- 47. Hub JS, Kubitzki MB, de Groot BL. Spontaneous quaternary and tertiary T‐R transitions of human hemoglobin in molecular dynamics simulation. PLoS Comput Biol 2010; 6: : e1000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, et al Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 2007; 100: 654–61. [DOI] [PubMed] [Google Scholar]
- 49. Tiso M, Tejero J, Basu S, Azarov I, Wang X, Simplaceanu V, et al Human neuroglobin functions as a redox‐regulated nitrite reductase. J Biol Chem 2011; 286: 18277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li H, Hemann C, Abdelghany TM, El‐Mahdy MA, Zweier JL. Characterization of the mechanism and magnitude of cytoglobin‐mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J Biol Chem 2012; 287: 36623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Piknova B, Schechter AN. Comments on 'vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate‐nitrite‐nitric oxide pathway'. Br J Clin Pharmacol 2013; 75: 1541–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Webb AJ, Lidder S. Reply to comments on 'vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate‐nitrite‐nitric oxide pathway'. Br J Clin Pharmacol 2013; 75: 1543–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett 1998; 427: 225–8. [DOI] [PubMed] [Google Scholar]
- 54. Webb AJ, Milsom AB, Rathod KS, Chu WL, Qureshi S, Lovell MJ, et al Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ Res 2008; 103: 957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Godber BLJ, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, et al Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 2000; 275: 7757–63. [DOI] [PubMed] [Google Scholar]
- 56. Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM. Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J Biol Chem 2001; 276: 14359–65. [DOI] [PubMed] [Google Scholar]
- 57. Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem 2008; 283: 17855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cantu‐Medellin N, Kelley EE. Xanthine oxidoreductase‐catalyzed reduction of nitrite to nitric oxide: insights regarding where, when and how. Nitric Oxide 2013; 34: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arif S, Borgognone A, Lin EL‐S, O'Sullivan AG, Sharma V, Drury NE, et al Role of aldehyde dehydrogenase in hypoxic vasodilator effects of nitrite in rats and humans. Br J Pharmacol 2015; 172: 3341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol 2009; 297: H2068–74. [DOI] [PubMed] [Google Scholar]
- 61. Cardenas AJ, Abelman R, Warren TH. Conversion of nitrite to nitric oxide at zinc via S‐nitrosothiols. Chem Commun (Camb) 2014; 50: 168–70. [DOI] [PubMed] [Google Scholar]
- 62. Liu C, Wajih N, Liu X, Basu S, Janes J, Marvel M, et al Mechanisms of human erythrocytic bioactivation of nitrite. J Biol Chem 2014; 290: 1281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gautier C, van Faassen E, Mikula I, Martasek P, Slama‐Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochem Biophys Res Commun 2006; 341: 816–21. [DOI] [PubMed] [Google Scholar]
- 64. Benjamin N, O'Driscoll F, Dougall H, Duncan C, Smith L, Golden M, et al Stomach NO synthesis. Nature 1994; 368: 502. [DOI] [PubMed] [Google Scholar]
- 65. Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N‐nitroso compounds. Food Cosmet Toxicol 1976; 14: 545–8. [DOI] [PubMed] [Google Scholar]
- 66. Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut 1994; 35: 1543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, et al Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med 1995; 1: 546–51. [DOI] [PubMed] [Google Scholar]
- 68. McKnight GM, Smith LM, Drummond RS, Duncan CW, Golden M, Benjamin N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut 1997; 40: 211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 2004; 37: 395–400. [DOI] [PubMed] [Google Scholar]
- 70. Fitzgerald DJ, Roy L, Catella F, FitzGerald GA. Platelet activation in unstable coronary disease. N Engl J Med 1986; 315: 983–9. [DOI] [PubMed] [Google Scholar]
- 71. Radomski MW, Rees DD, Dutra A, Moncada S. S‐nitroso‐glutathione inhibits platelet activation in vitro and in vivo . Br J Pharmacol 1992; 107: 745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Richardson G, Hicks SL, O'Byrne S, Frost MT, Moore K, Benjamin N, et al The ingestion of inorganic nitrate increases gastric S‐nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide 2002; 7: 24–9. [DOI] [PubMed] [Google Scholar]
- 73. Rocks SA, Davies CA, Hicks SL, Webb AJ, Klocke R, Timmins GS, et al Measurement of S‐nitrosothiols in extracellular fluids from healthy human volunteers and rheumatoid arthritis patients, using electron paramagnetic resonance spectrometry. Free Radic Biol Med 2005; 39: 937–48. [DOI] [PubMed] [Google Scholar]
- 74. Pannala AS, Mani AR, Spencer JPE, Skinner V, Bruckdorfer KR, Moore KP, et al The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med 2003; 34: 576–84. [DOI] [PubMed] [Google Scholar]
- 75. Sobko T, Reinders C, Norin E, Midtvedt T, Gustafsson LE, Lundberg JO. Gastrointestinal nitric oxide generation in germ‐free and conventional rats. Am J Physiol Gastrointest Liver Physiol 2004; 287: G993–7. [DOI] [PubMed] [Google Scholar]
- 76. Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008; 51: 784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med 2006; 355: 2792–3. [DOI] [PubMed] [Google Scholar]
- 78. Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta‐analysis. J Nutr 2013; 143: 818–26. [DOI] [PubMed] [Google Scholar]
- 79. Kapil V, Milsom AB, Okorie M, Maleki‐Toyserkani S, Akram F, Rehman F, et al Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite‐derived NO. Hypertension 2010; 56: 274–81. [DOI] [PubMed] [Google Scholar]
- 80. Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta 1999; 1411: 334–50. [DOI] [PubMed] [Google Scholar]
- 81. Lidder S, Webb AJ. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate–nitrite–nitric oxide pathway. Br J Clin Pharmacol 2013; 75: 677–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huang Z, Shiva S, Kim‐Shapiro DB, Patel RP, Ringwood LA, Irby CE, et al Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest 2005; 115: 2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]


