Abstract
High dietary polyphenol intake is associated with reduced all‐cause mortality and a lower incidence of cardiovascular events. However, the mechanisms involved are not fully understood. The aim of the present substudy of the PREvención con DIetaMEDiterránea (Prevention with Mediterranean diet; PREDIMED) trial was to analyse the relationship between polyphenol intake measured by total urinary polyphenol excretion (TPE), and circulating inflammatory biomarkers and cardiovascular risk factors in elderly individuals. A substudy of 1139 high‐risk participants was carried out within the PREDIMED trial. The subjects were randomly assigned to a low‐fat control diet or to two Mediterranean diets, supplemented with either extra‐virgin olive oil or nuts. Dietary intake, anthropometric data, clinical and laboratory assessments, including inflammatory biomarkers, and urinary TPE were measured at baseline and after the one‐year intervention. Participants in the highest tertile of changes in urinary TPE (T3) showed significantly lower plasma levels of inflammatory biomarkers [vascular cell adhesion molecule 1 (VCAM‐1) (–9.47 ng ml–1), intercellular adhesion molecule 1 (–14.71 ng ml–1), interleukin 6 (–1.21 pg ml–1), tumour necrosis factor alpha (–7.05 pg ml–1) and monocyte chemotactic protein 1 (–3.36 pg ml–1)] than those inthe lowest tertile (T1, P < 0.02; all). A significant inverse correlation existed between urinary TPE and the plasma concentration of\VCAM‐1 (r = –0.301; P < 0.001). In addition, systolic and diastolic blood pressure (BP) decreased and plasma high‐density lipoprotein cholesterol increased in parallel with increasing urinary TPE (T3 vs. T1) (P < 0.005 and P = 0.004, respectively). Increases in polyphenol intake measured as urinary TPE are associated with decreased inflammatory biomarkers, suggesting a dose‐dependent anti‐inflammatory effect of polyphenols. In addition, high polyphenol intake improves cardiovascular risk factors– mainly BP and the lipid profile.
Keywords: blood pressure, Folin–Ciocalteu, hypertension, inflammatory biomarkers, Mediterranean diet, urinary polyphenol biomarker
Introduction
Atherosclerosis, the leading cause of death worldwide, is considered to be a low‐grade inflammatory disease of the cardiovascular system 1. At the earliest stage, vascular inflammation is activated by proinflammatory stimuli such as saturated fat intake, hypercholesterolaemia, obesity, hyperglycaemia and hypertension, stimulating the secretion of inflammatory cytokines that promote the formation of endothelial adhesion molecules. These molecules are subsequently released into the circulation, where they mediate the adhesion of circulating monocytes and lymphocytes to the vascular endothelium 2, 3.
The first step in the management of hypertension and other cardiovascular risk factors is to follow a healthy diet, such as the traditional Mediterranean diet (Med diet) 4 or the Dietary‐Approaches‐to‐Stop‐Hypertension (DASH) diets 5 and to improve additional lifestyle measures, such as reducing body weight and increasing physical activity 6. The Med and DASH diets are both rich in fruit and vegetables (F&V), which have been considered to be rich sources of phytochemicals, and are inversely associated with a high blood pressure (BP) and incidence of hypercholesterolaemia, among other effects 7, 8.
Consequently, numerous epidemiological studies have suggested an inverse association between adherence to the traditional Med diet and a reduction in coronary heart disease (CHD); this protective effect has been attributed, in part, to the richness of this diet in antioxidants 9. Polyphenol‐rich foods such as cocoa, F&V, tea, olive oil and wine have been inversely associated with the risk of overall mortality and cardiovascular disease in numerous epidemiological studies 7, 10, 11, 12, 13, 14, 15, 16.
At least some of the benefits of the Med diet, and of its main components such as extra‐virgin olive oil (EVOO), nuts, fruit, vegetables and wine, have been attributed to their antioxidant and anti‐inflammatory effects 17. On the other hand, previous clinical and laboratory studies have pointed out that dietary polyphenols may exert anti‐inflammatory effects 18, 19. In this setting, we undertook a substudy of the PREvención con DIetaMEDiterránea (Prevention with Mediterranean diet; PREDIMED) trial, to evaluate the relationship between dietary polyphenol intake measured by urinary total polyphenol excretion (TPE), plasma inflammatory biomarkers related to atherosclerosis, and the main cardiovascular risk factors. To our knowledge, this was the first intervention study to assess these associations using biochemical analyses of total polyphenols (TP) in spot urine samples, and directly measuring classical vascular risk factors and plasma inflammatory biomarkers in a large sample of older individuals at high cardiovascular risk.
Materials and methods
Subjects
The PREDIMED study is a large, parallel‐group, multicentre, randomized, controlled clinical trial of 4.8 years’ duration, aimed at assessing the effects of the Med diet on cardiovascular events (www.predimed.es). The detailed study protocol has been described previously 20, 21.
We randomly selected 1170 participants from primary health centres affiliated with three university hospitals in Spain. Eligible participants were community‐dwelling men aged 55–80 years and women aged 60–80 years, and those included in the current substudy were similar to those in the overall PREDIMED population. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. All participants provided written informed consent and the study protocol was approved by the Institutional Review Boards of the participating centres. This trial has been registered with the International Standard Randomized Controlled Trial Number under registration number 35739639.
Assessments and intervention
At baseline, all participants completed a validated semiquantitative food frequency questionnaire (FFQ) including 137 items, the validated Spanish version of the Minnesota Leisure Time Physical Activity Questionnaire, a validated 14‐point Med diet score and a 47‐item questionnaire about education, lifestyle, history of illnesses and medication use 20. Trained dieticians were responsible for all aspects of the intervention. Participants in both Med diet intervention groups (see below) were given personalized advice for dietary changes directed at achieving a diet as close as possible to the traditional Med diet. The participants from the Med diet supplemented with EVOO group received free EVOO (1 l week–1) for all the family, and those from the Med diet plus nuts group were provided with mixed nuts (30 g day–1, as 15 g walnuts, 7.5 g almonds and 7.5 g hazelnuts). Participants assigned to the control diet received personal dietary advice for following a low‐fat diet according to the American Heart Association guidelines 22. In all three groups, the general guideline recommendation included increasing the intake of fresh fruit (≥3 servings per day); vegetables (≥2 servings per day); legumes; fish or seafood (≥3 servings per week); and white meat; negative recommendations included eliminating and/or reduction of detrimental foods (red and processed meats, fat‐rich dairy products, commercial pastries, snacks and sugar‐sweetened beverages). No specific recommendation was given in relation to wine and beer intake.
Clinical measurements
Trained nurses measured the height and weight of subjects using a wall‐mounted stadiometer and calibrated scales, respectively, as well as their BP in triplicate using a validated semi‐automatic oscillometer (Omron HEM‐705CP 23; Hoofddorp, The Netherlands). Waist circumference was measured midway between the lowest rib and the iliac crest using an anthropometric tape. Urine and blood samples were obtained after an overnight fast; they were coded, shipped to a central laboratory and frozen at –80 °C until analysis. Analyses of the frozen samples of whole serum or plasma, as appropriate, from the participants determined blood glucose level by using the glucose oxidase method; serum insulin level by using radioimmunoassay; cholesterol and triglyceride levels by using enzymatic procedures; and high‐density lipoprotein (HDL) cholesterol levels after precipitation with phosphotungstic acid and magnesium chloride. We performed all the analyses in duplicate. Analysis of TP and creatinine in urine samples was performed following the procedure described by Medina‐Remón et al. 24; TPE was expressed as mg gallic acid equivalent (GAE) per gram of creatinine. Energy and nutrient intake was derived from Spanish food composition tables 25.
The concentrations of soluble adhesion molecules were measured using xMAP technology on the Luminex platform (Luminex Corporation, Austin, TX, USA), according to the manufacturer's instructions, and analysed using the Bio‐Plex Manager TM Software (Bio‐Rad Laboratories, Inc., Hercules, CA, USA). We determined the concentration of five potential biomarkers: plasma‐soluble vascular cell adhesion molecule 1 (VCAM‐1), intercellular adhesion molecule 1 (ICAM‐1), interleukin 6 (IL‐6), tumour necrosis factor alpha (TNF‐α) and monocyte chemotactic protein 1 (MCP‐1), by using a Human Cytokine Plex assay (Bio‐Rad Laboratories, Inc.) which is based on magnetic bead‐based multiplex assays designed to measure multiple cytokines, adhesion molecules and chemokines in plasma matrices. Samples were thawed and analysed at a 1:3 dilution for every molecule except for ICAM‐1 and VCAM‐1, which were analysed at a 1:100 dilution. For all assays, the procedures were performed by a single blinded researcher following the manufacturer's instructions.
We used a sample size of 12.5 μl. The principle behind 96‐well, plate‐formatted, bead‐based assays is similar to that of a capture sandwich immunoassay. An antibody directed against the desired cytokine or chemokine target is covalently bound to internally dyed beads. The bound beads are allowed to react with the sample containing the target biomolecules. After a series of washes to remove unbound protein, a biotinylated detection antibody specific to an epitope different from that of the capture antibody is added to the reaction. This results in the formation of a sandwich of antibodies around the cytokine, chemokine or adhesion molecules. A streptavidin–phycoerythrin reporter complex is then added to bind to the biotinylated detection antibodies on the bead surface. The plate is then analysed on a Luminex 100™ instrument (Luminex Corporation) using Bio‐Plex Manager Software (Bio‐Rad Laboratories, Inc.). A high‐speed digital processor manages the data output, which is further analysed and presented as fluorescence intensity and target concentration on Bio‐Plex Manager Software. Analyte concentrations were obtained using standard calibration curves. The results are shown in pg ml–1 or ng ml–1. All measurements were performed in duplicate.
Statistical analyses
Analyses were performed using SPSS software v19.0 (Chicago, IL, USA). The baseline characteristics of the participants were expressed as means or percentages and standard deviations (SD). Variables were examined for normality and skewness (Kolmogorov and Levene tests). Analysis of variance (ANOVA), one factor was used for continuous variables and the χ2‐test for categorical variables. Changes in all outcomes were assessed using repeated‐measures ANOVA for the two factors: tertiles of changes in urinary TPE [T1 (<–14.03), T2 (–14.04 to 31.56) and T3 (>31.57)] and time (baseline and one‐year), and their interaction, using the Bonferroni post‐hoc test to compare differences in the effects of each tertile within and between groups. Within‐ and between‐group differences are expressed as mean percentage difference [95% confidence interval (CI)].
We used the general linear model (GLM) approach to analysis of covariance to determine the effects of tertiles 2 and 3 of changes in TPE (fixed factors) (compared with tertile 1); and VCAM‐1, ICAM‐1, IL‐6, TNF‐α and MCP‐1 concentrations after 1 year (dependent variables), using the baseline measurements as covariates and others as additional covariates. Model 1 was unadjusted and model 2 was adjusted by baseline inflammatory biomarkers, intervention group, gender, age, body mass index, smoking status, physical activity, medication use (antihypertensive agents, statins or other hypolipidaemic drugs, insulin, oral hypoglycaemic drugs, and aspirin or other antiplatelet drugs), supplements taken in the previous month, and intake of sodium, potassium, total energy, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA) and saturated fatty acids (SFA). P‐values < 0.05 (two‐tailed) were considered statistically significant.
Results
We excluded 31 of 1170 eligible participants before randomization, for different reasons: not meeting inclusion criteria (n = 9), not accepting changes in their dietary habits (n = 6), food allergies (n = 5) or refusing to participate (n = 11). The baseline characteristics of the three groups (comprising 511 men and 628 women) are shown in Table 1; most of them were overweight or obese (>90%), and with a sizeable burden of cardiovascular risk factors (>75% had hypertension; more than 40% were diabetic and/or had family history of cardiovascular disease, and >60% had dyslipidaemia). Most of them were taking antihypertensive drugs, nearly half were taking statins or other hypolipidaemic drugs, and nearly a quarter were taking oral hypoglycaemic drugs, and aspirin or other antiplatelet drugs. Subsequent data refer only to the 1139 participants who completed the study.
Table 1.
Baseline of the study participants, according to tertiles of changes in total urinary polyphenols excreted, expressed as mg GAE per gram of creatinine
| Δ Urine mg GAE per gram creatinine tertiles | ||||
|---|---|---|---|---|
| T1 (<–14.03) | T2 (–14.04 to 31.56) | T3 (>31.57) | P * | |
| No. of subjects | 379 | 380 | 380 | |
| Age (years), mean (SD) | 67.4 (6.2) | 67.5 (5.9) | 68.0 (5.7) | 0.318 |
| Women, n (%) | 217 (57.3) | 186 (48.9) | 225 (59.2) | 0.010 |
| BMI (kg m–2), mean (SD) | 29.3 (3.5) | 29.1 (3.3) | 29.5 (3.4) | 0.281 |
| Overweight or obese (BMI ≥25 kg m–2), n (%) | 329 (86.8) | 348 (91.6) | 353 (92.9) | 0.011 |
| Hypertension, n (%) | 303 (79.9) | 300 (78.9) | 302 (79.5) | 0.943 |
| Diabetes, n (%) | 183 (48.3) | 172 (45.3) | 148 (38.9) | 0.030 |
| Dyslipidaemia, n (%) | 253 (66.8) | 234 (61.6) | 254 (66.8) | 0.304 |
| Current smoker, n (%) | 55 (14.5) | 79 (20.8) | 53 (13.9) | 0.018 |
| Family history of CHD, n (%) | 165 (44.0) | 174 (46.5) | 194 (51.2) | 0.026 |
| Medication, n (%) | ||||
| Antihypertensive drugs | 287 (76.7) | 252 (70.2) | 278 (74.9) | 0.115 |
| Statins (hypolipidaemic drugs) | 179 (47.2) | 149 (39.5) | 153 (40.4) | 0.063 |
| Insulin | 25 (6.6) | 14 (3.7) | 21 (5.5) | 0.192 |
| Oral hypoglycaemic drugs | 118 (31.2) | 104 (27.5) | 96 (25.3) | 0.190 |
| Aspirin or other antiplatelet drugs | 75 (19.9) | 69 (18.3) | 72 (19.0) | 0.678 |
| Vitamins or supplements, n (%) | 40 (10.7) | 27 (7.4) | 47 (12.6) | 0.068 |
| Educational level, n (%) | ||||
| Primary school | 279 (73.6) | 274 (72.1) | 277 (72.9) | 0.896 |
| High school | 61 (16.1) | 49 (12.9) | 64 (16.8) | 0.275 |
| University | 27 (7.1) | 36 (9.5) | 33 (8.7) | 0.495 |
| Energy expenditure in physical activity (kcal day–1), mean (SD) | 284.3 (235.2) | 293.5 (236.2) | 271.2 (196.5) | 0.390 |
BMI, body mass index (calculated as weight in kilograms divided by height in square metres); CHD, coronary heart disease; GAE, gallic acid equivalent; SD, standard deviation.
Analysis of variance–one factor was used for continuous variables and the χ2‐test for categorical variables.
Food, energy and nutrient intake
After 1 year of intervention, the main dietary changes that were observed in the three groups were a substantial increase in the consumption of EVOO, total nuts, fruit, vegetables and legumes in the three groups, and a reduced consumption of meat and meat products, and pastries, cakes and sweets (Table 2). The Med diet score was increased significantly in all groups. Table 3 shows the changes in total energy intake, energy expenditure in physical activities, and daily nutrient intake at baseline and after 1 year in each tertile of urinary TPE. Fibre and total fat intake increased significantly in all groups, the latter as a result of an increased consumption of MUFA, which was attributable, in part, to increased use of olive oil, and PUFA, owing to increased nut consumption. SFA and total cholesterol intake decreased significantly in all groups, as well as sodium intake. In all groups, magnesium and potassium intake increased significantly.
Table 2.
Changes in daily intake of selected foods and Mediterranean diet score after 1 year, according to tertiles of changes in total urinary polyphenols excreted†
| Δ Urine mg GAE per gram creatinine tertiles | Repeated‐measures ANOVA‡ | P value for differences§ | ||||
|---|---|---|---|---|---|---|
| T1 (<–14.03) | T2 (–14.04 to 31.56) | T3 (>31.57) | ||||
| Time x Group | T2 vs. T1 | T3 vs. T1 | ||||
| No. of subjects | 379 | 380 | 380 | |||
| Total nuts (g) | ||||||
| Baseline | 9.6 (12.6) | 10.4 (13.8) | 10.7 (11.6) | 0.651 | 0.217 | 0.877 |
| 1 year | 17.8 18.6 ()** | 17.1 (17.1)** | 18.2 (15.6)** | |||
| EVOO (g) | ||||||
| Baseline | 18.2 (23.0) | 17.7 (22.3) | 22.0 (23.9) | <0.001 | 0.017 | <0.001 |
| 1 year | 29.6 (27.0)**,a | 34.9 (27.6)**,b | 39.5 (27.9)**,b | |||
| Fruit (g) | ||||||
| Baseline | 372.3 (204.0) | 370.0 (194.2) | 374.6 (181.3) | 0.630 | 0.077 | 0.669 |
| 1 year | 450.6 (218.5)** | 429.8 (185.6)** | 445.8 (185.9)** | |||
| Vegetables (g) | ||||||
| Baseline | 301.6 (127.5) | 314.0 (132.7) | 288.0 (104.7) | 0.007 | 0.700 | 0.437 |
| 1 year | 328.6 (139.2)** | 341.2 (149.2)**,a | 313.8 (112.1)**,b | |||
| Legumes (g) | ||||||
| Baseline | 18.7 (8.8) | 18.8 (8.8) | 17.8 (7.8) | 0.084 | 0.289 | 0.878 |
| 1 year | 20.6 (8.9)** | 21.5 (9.6)** | 20.1 (7.5)** | |||
| Fish or seafood (g) | ||||||
| Baseline | 99.8 (41.3) | 96.7 (40.9) | 87.3 (36.2) | <0.001 | 0.661 | 0.565 |
| 1 year | 99.8 (39.1)a | 100.1 (41.0)a | 91.9 (36.1)*,b | |||
| Meat or meat products (g) | ||||||
| Baseline | 142.8 (56.0) | 142.5 (49.3) | 135.8 (51.4) | 0.198 | 0.511 | 0.626 |
| 1 year | 127.1 (45.2)** | 129.9 (43.9)** | 125.8 (44.5)** | |||
| Cereals (g) | ||||||
| Baseline | 240.6 (105.6) | 258.9 (114.7) | 232.6 (102.9) | 0.006 | 0.069 | 0.019 |
| 1 year | 239.3 (98.7) | 239.3 (93.5)** | 223.8 (89.9) | |||
| Milk and dairy products (g) | ||||||
| Baseline | 370.8 (212.7) | 349.5 (185.0) | 393.1 (254.2) | 0.004 | 0.380 | 0.005 |
| 1 year | 355.1 (204.9)a | 356.7 (185.3)a | 404.7 (245.7)b | |||
| Pastries, cakes or sweets (g) | ||||||
| Baseline | 21.9 (27.8) | 26.0 (28.7) | 23.7 (26.9) | 0.048 | 0.168 | 0.882 |
| 1 year | 16.7 (19.5)** | 20.7 (25.9)** | 17.5 (20.7)** | |||
| Alcohol (g) | ||||||
| Baseline | 10.2 (16.7) | 14.2 (19.2) | 11.0 (17.5) | 0.025 | 0.490 | 0.915 |
| 1 year | 9.8 (16.4) | 12.2 (16.4)** | 10.3 (14.8) | |||
| Tea (ml) | ||||||
| Baseline | 3.4 (13.3) | 3.9 (15.2) | 6.4 (20.9) | 0.023 | 0.218 | 0.931 |
| 1 year | 4.0 (14.2) | 2.9 (11.6) | 5.8 (22.3) | |||
| Coffee (ml) | ||||||
| Baseline | 29.3 (48.1) | 30.1 (46.1) | 34.3 (49.8) | 0.406 | 0.386 | 0.970 |
| 1 year | 31.8 (51.5) | 30.8 (47.9) | 34.2 (50.7) | |||
| Med diet score | ||||||
| Baseline | 8.8 (1.9) | 8.8 (1.8) | 9.0 (1.7) | <0.001 | 0.709 | <0.001 |
| 1 year | 10.2 (1.9)**,a | 10.2 (2.0)**,a | 10.8 (1.8)**,b | |||
Data are given as means (standard deviation). Different letters in rows shows significant difference between groups by the Bonferroni post‐hoc test (P < 0.05). Values with asterisks are statistically different from baseline by the Bonferroni post‐hoc test
P < 0.05;
P < 0.01.
Data analysed by repeated‐measures two‐factor analysis of variance (ANOVA) (P < 0.05).
Data analyzed by analysis of covariance (P < 0.05), and adjusted for energy intake and the baseline value of each variable. EVOO, extra‐virgin olive oil; GAE, gallic acid equivalent.
Table 3.
Changes in energy and daily nutrient intake after 1 year, according to tertiles of changes in total urinary polyphenols excreted†
| Δ Urine mg GAE per gram creatinine tertiles | Repeated measures ANOVA‡ | P value for differences§ | ||||
|---|---|---|---|---|---|---|
| T1 (<–14.03) | T2 (–14.04 to 31.56) | T3 (>31.57) | Time x Group | T2 vs. T1 | T3 vs. T1 | |
| No. of subjects | 379 | 380 | 380 | |||
| Total energy, kcal day –1 | ||||||
| Baseline | 2250.7 (536.1) | 2345.5 (588.6) | 2236.8 (584.1) | 0.039 | 0.996 | 0.813 |
| 1 year | 2282.8 (519.6) | 2335.6 (516.1) | 2268.0 (492.9) | |||
| Total protein (g) | ||||||
| Baseline | 92.5 (19.2) | 93.0 (20.1) | 89.1 (22.4) | 0.069 | 0.760 | 0.752 |
| 1 year | 90.8 (19.0) | 91.8 (18.8) | 89.8 (18.3) | |||
| Total carbohydrate (g) | ||||||
| Baseline | 233.5 (71.1) | 245.9 (80.8) | 231.9 (78.0) | 0.056 | 0.041 | 0.030 |
| 1 year | 234.7 (67.3) | 236.4 (68.2)* | 227.4 (63.5) | |||
| Fibre (g) | ||||||
| Baseline | 24.4 (8.4) | 24.8 (7.7) | 23.7 (6.8) | 0.225 | 0.117 | 0.465 |
| 1 year | 26.6 (9.1)** | 26.4 (7.4)** | 25.8 (7.1)** | |||
| Total fat (g) | ||||||
| Baseline | 97.3 (26.5) | 99.0 (27.6) | 97.3 (27.3) | 0.424 | 0.570 | 0.048 |
| 1 year | 101.3 (26.4)** | 104.1 (25.1)** | 102.9 (25.2)** | |||
| SFA (g) | ||||||
| Baseline | 24.7 (8.0) | 25.3 (8.4) | 24.2 (8.7) | 0.048 | 0.443 | 0.374 |
| 1 year | 23.4 (7.2)** | 24.2 (7.0)**,a | 22.8 (7.0)**,b | |||
| MUFA (g) | ||||||
| Baseline | 48.7 (14.5) | 49.1 (14.2) | 49.7 (14.0) | 0.131 | 0.251 | <0.001 |
| 1 year | 51.9 (14.3)**,a | 53.7 (13.5)** | 54.5 (13.6)**,b | |||
| PUFA (g) | ||||||
| Baseline | 15.5 (6.6) | 16.0 (6.9) | 15.0 (6.3) | 0.115 | 0.488 | 0.403 |
| 1 year | 17.2 (6.9)** | 17.5 (6.6)** | 16.7 (5.6)** | |||
| Cholesterol (g) | ||||||
| Baseline | 361.5 (108.9) | 363.9 (114.4) | 343.1 (117.5) | 0.030 | 0.686 | 0.520 |
| 1 year | 339.9 (115.9)** | 342.4 (107.1)** | 328.3 (86.9)* | |||
| Magnesium (mg) | ||||||
| Baseline | 367.8 (105.1) | 374.8 (101.0) | 354.6 (91.7) | 0.040 | 0.112 | 0.822 |
| 1 year | 385.6 (107.9)** | 386.2 (91.3)* | 375.0 (82.8)** | |||
| Potassium (mg) | ||||||
| Baseline | 4289.2 (1053.9) | 4350.0 (1033.9) | 4197.8 (1026.3) | 0.172 | 0.220 | 0.784 |
| 1 year | 4473.3 (1071.8)** | 4480.8 (960.0)** | 4391.4 (855.3)** | |||
| Sodium (mg) | ||||||
| Baseline | 2349.1 (846.0) | 2438.5 (921.6) | 2164.5 (924.2) | <0.001 | 0.223 | 0.003 |
| 1 year | 2251.8 (719.8)*,a | 2273.5 (818.9)**,a | 2072.1 (697.5)*,b | |||
| Energy expenditure in physical activity (kcal day –1 ) | ||||||
| Baseline | 284.3 (235.2) | 293.5 (236.2) | 271.2 (196.5) | 0.527 | 0.310 | 0.871 |
| 1 year | 303.1 (240.9) | 299.6 (228.0) | 290.3 (202.1)* | |||
Data are given as means (standard deviation). Different letters in rows shows significant difference between groups by the Bonferroni post‐hoc test (P< 0.05). Values with asterisks are statistically different from baseline by the Bonferroni post‐hoc test
P < 0.05;
P < 0.01.
Data analysed by repeated‐measures two‐factor analysis of variance (ANOVA) (P < 0.05).
Data analysed by analysis of covariance (P < 0.05) and adjusted for energy intake and the baseline value of each variable. GAE, gallic acid equivalent; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
Cardiovascular risk factors and inflammatory biomarkers
Table 4 shows the changes from baseline in urine TPs, anthropometric measurements and cardiovascular risk factors. By definition of the groups, urinary TPE, expressed as mg GAE per gram creatinine, increased significantly in T2 and T3, and decreased in T1. In all groups, participants’ systolic and diastolic BP decreased significantly. No significant changes were observed in blood glucose levels, total cholesterol or triglycerides. Low‐density lipoprotein (LDL) cholesterol was reduced significantly and HDL‐cholesterol increased significantly in all groups. Inflammatory biomarkers were reduced significantly in the groups with larger changes in urinary TPE (T2 and T3), with larger decreases in the T3 group. Figure 1 shows the change from baseline values in VCAM‐1, ICAM‐1, IL‐6, TNF‐α and MCP‐1 concentrations in the three groups.
Table 4.
Baseline level and 1‐year changes in total polyphenol excreted, lipid profiles, blood pressure and inflammatory biomarkers, according to tertiles of changes in total urinary polyphenols excreted†
| Δ Urine mg GAE per gram creatinine tertiles | Repeated measures ANOVA‡ | P value for differences§ | ||||
|---|---|---|---|---|---|---|
| T1 (<–14.03) | T2 (–14.04 to 31.56) | T3 (>31.57) | Time x Group | T2 vs. T1 | T3 vs. T1 | |
| No. of subjects | 379 | 380 | 380 | |||
| Urine total polyphenol (mg GAE per gram creatinine) | ||||||
| Baseline | 166.1 (84.7) | 102.4 (40.6) | 107.5 (47.3) | <0.001 | <0.001 | <0.001 |
| 1 year | 102.9 (51.6)**,a | 110.2 (41.7)**,a | 209.0 (95.9)**,b | |||
| Systolic BP (mmHg), mean (SD) | ||||||
| Baseline | 150.2 (17.3) | 151.6 (17.4) | 153.2 (18.5) | 0.594 | 0.034 | 0.005 |
| 1 year | 149.8 (18.1) | 147.8 (16.2)** | 148.6 (17.6)** | |||
| Diastolic BP (mmHg), mean (SD) | ||||||
| Baseline | 83.3 (9.3) | 84.9 (10.2) | 85.2 (9.8) | 0.409 | 0.044 | 0.004 |
| 1 year | 83.4 (9.4) | 83.0 (10.1)** | 83.4 (9.4)** | |||
| Fasting glucose, mg dl –1 | ||||||
| Baseline | 122.5 (36.8) | 117.6 (37.2) | 114.1 (33.4) | 0.024 | 0.118 | 0.455 |
| 1 year | 121.5 (39.0) | 116.4 (34.4) | 114.7 (35.9) | |||
| Total cholesterol, mg dl–1 | ||||||
| Baseline | 204.3 (37.7) | 206.4 (35.2) | 207.9 (35.4) | 0.250 | 0.734 | 0.648 |
| 1 year | 206.1 (36.6) | 208.0 (34.2) | 211.4 (34.5) | |||
| LDL‐cholesterol, mg dl–1 | ||||||
| Baseline | 132.3 (33.6) | 134.9 (31.4) | 136.8 (32.7) | 0.313 | 0.539 | 0.163 |
| 1 year | 124.1 (32.3)** | 126.8 (29.4)** | 126.8 (30.2)** | |||
| HDL‐cholesterol, mg dl–1 | ||||||
| Baseline | 53.4 (11.0) | 53.3 (11.2) | 53.9 (10.9) | 0.086 | 0.712 | 0.004 |
| 1 year | 56.6 (13.4)**,a | 55.9 (13.5)**,a | 59.5 (14.3)**,b | |||
| Triglyceride, mg dl–1 | ||||||
| Baseline | 126.5 (58.9) | 127.5 (69.6) | 132.9 (66.3) | 0.122 | 0.382 | 0.553 |
| 1 year | 128.8 (58.8) | 124.8 (59.6)a | 138.4 (80.5)b | |||
| VCAM‐1, ng ml–1 | ||||||
| Baseline | 176.1 (44.1) | 177.5 (49.8) | 179.8 (46.2) | 0.877 | 0.045 | 0.019 |
| 1 year | 180.3 (47.2) | 174.1 (46.8) | 173.2 (46.3)* | |||
| ICAM‐1, ng ml–1 | ||||||
| Baseline | 183.9 (64.4) | 170.8 (78.3) | 180.9 (68.8) | 0.039 | 0.034 | 0.002 |
| 1 year | 187.5 (73.3)a | 166.6 (64.7)b | 173.5 (64.2)* | |||
| IL‐6, pg ml–1 | ||||||
| Baseline | 6.0 (6.7) | 6.0 (5.6) | 5.3 (6.4) | 0.031 | 0.019 | 0.004 |
| 1 year | 6.1 (8.7)a | 5.1 (3.9)** | 4.4 (4.6)**,b | |||
| TNF‐α, pg ml–1 | ||||||
| Baseline | 15.5 (21.5) | 15.7 (21.8) | 12.9 (22.2) | 0.040 | <0.001 | <0.001 |
| 1 year | 16.7 (27.2)a | 12.4 (16.8)** | 9.2 (13.7)**,b | |||
| MCP‐1, pg ml–1 | ||||||
| Baseline | 37.0 (17.5) | 38.9 (26.2) | 41.7 (25.2) | 0.306 | 0.012 | 0.017 |
| 1 year | 40.2 (18.4)* | 37.7 (18.5) | 39.2 (17.0)* | |||
Data are given as means [standard deviation (SD)]. Different letters in rows shows significant difference between groups by the Bonferroni post‐hoc test (P < 0.05). Values with asterisks are statistically different from baseline by the Bonferroni post‐hoc test
P < 0.05;
P < 0.01.
Data analysed by repeated‐measures two‐factor analysis of variance (ANOVA) (P < 0.05).
Data analysed by analysis of covariance (P < 0.05) and adjusted by the baseline value of each variable, intervention group, gender, age, body mass index, smoking status, physical activity, medication use (antihypertensive agents, statins or other hypolipidaemic drugs, insulin, oral hypoglycaemic drugs, and aspirin or other antiplatelet drugs), supplements taken in the previous month, and sodium, potassium, total energy, monounsaturated fatty acid, polyunsaturated fatty acid and saturated fatty acid intake. BP, blood pressure; GAE: gallic acid equivalent; ICAM‐1: soluble intercellular adhesion molecule‐1; IL‐6, plasma interleukin 6; MCP‐1, monocyte chemotactic protein 1; Med diet, Mediterranean diet; LDL, low‐density lipoprotein; TNF‐α, tumour necrosis factor alpha; VCAM‐1, vascular cell adhesion molecule 1.
Figure 1.
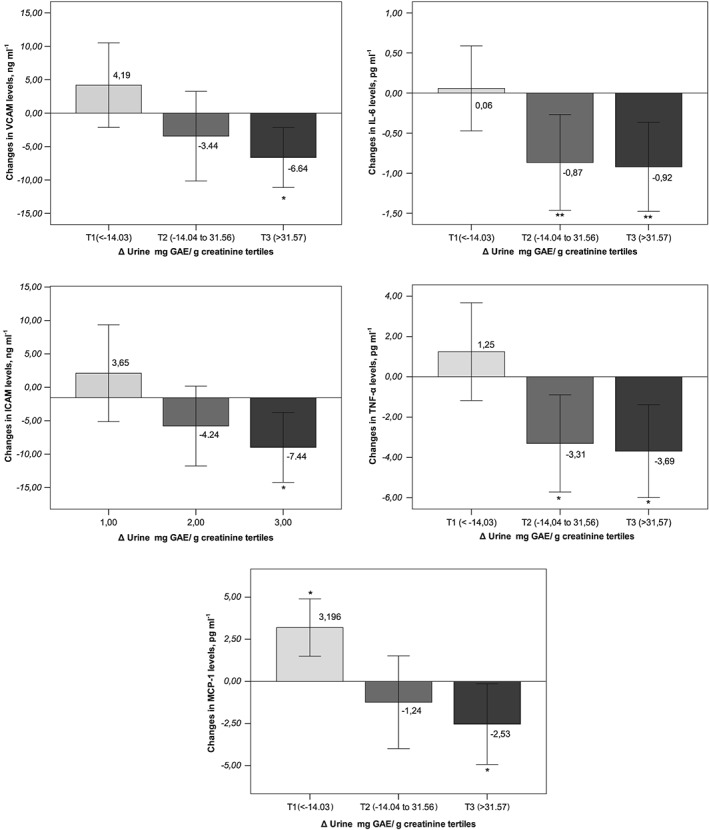
Changes from baseline in plasma concentrations of the inflammatory biomarkers (A) vascular cell adhesion molecule (VCAM), (B) soluble intercellular adhesion molecule (ICAM), (C) plasma interleukin 6 (IL‐6), (D) tumour necrosis factor alpha (TNF‐α) and (E) monocyte chemotactic protein 1 (MCP‐1) after 1 year, according to tertiles of changes in total urinary polyphenols excreted. *P < 0.05; **P < 0.01. indicates statistical significance between the baseline and 1 year of intervention period, with a confidence interval of 95%
After the intervention with the Med diet + EVOO and the Med diet + nuts, participants showed significant increases in urinary TPE, of 22.82 mg GAE per gram creatinine (95% CI 16.63, 29.01; P < 0.001) and 21.83 mg GAE per gram creatinine (95% CI 12.68, 30.98; P < 0.001), respectively. Figure 2 shows the changes from baseline values in VCAM‐1, ICAM‐1, IL‐6, TNF‐α and MCP‐1 concentrations in the three intervention groups. In the covariate analysis with VCAM‐1, ICAM‐1, IL‐6, TNF‐α and MCP‐1 concentrations at 1 year as dependent variables, circulating VCAM‐1, ICAM‐1, IL‐6, TNF‐α and MCP‐1 concentrations decreased in both Med diets and increased in the control diet group.
Figure 2.
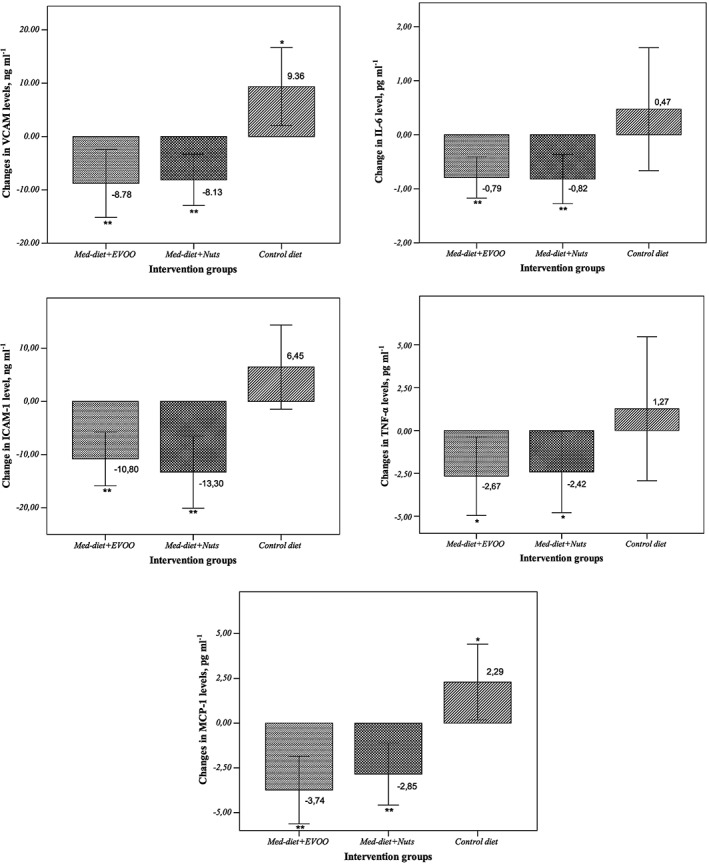
Changes from baseline in plasma concentrations of the inflammatory biomarkers (A) vascular cell adhesion molecule (VCAM), (B) soluble intercellular adhesion molecule (ICAM), (C) plasma interleukin 6 (IL‐6), (D) tumour necrosis factor alpha (TNF‐α) and (E) monocyte chemotactic protein 1 (MCP‐1) after 1 year, according to intervention groups. EVOO, extra‐virgin olive oil; Med‐diet, Mediterranean diet. **P < 0.01, *P < 0.05 indicates statistical significance between the baseline and 1 year of intervention period, with a confidence interval of 95%
Table 5 shows the 1‐year changes in VCAM‐1, ICAM‐1, IL‐6, TNF‐α and MCP‐1 concentrations, associated with tertiles of changes in urinary TPE (T1 compared with T2 and T3). After the covariate analysis of the changes in inflammatory biomarkers, we analysed 1‐year measures with respect to baseline, with inflammatory biomarkers at year 1 as the dependent variables, tertiles of changes in TPE as the fixed factor, and other measurements as additional covariates. We observed the effects of the larger changes in urinary TPE (T2 and T3) with respect to T1. The nonstandardized coefficient (B) represents the differences in T2 and T3 with respect to T1. In model 2, adjusted by all possible confounders, participants with similar VCAM‐1 levels at baseline experienced a statistically significant reduction of –8.45 (95% CI –16.73, –0.18) ng ml–1 and –9.47 (95% CI –17.38, –1.56) ng ml–1 for those in T2 and T3, respectively, compared with T1. In this model, participants in T2 and T3 experienced a reduction in ICAM–1 of –10.18 (95% CI –19.60, –0.77) ng ml–1 and –14.71 (95% CI –23.88, –5.53) ng ml–1, respectively. IL–6 was reduced significantly, by –0.99 (95% CI –2.03, –0.40) pg ml–1 and –1.21 (95% CI –1.85, –0.21) pg ml–1, in T3 and T2 compared with T1, respectively. Statistically significant reductions in TNF–α of –5.88 (95% CI –9.17, –2.58) pg ml–1 and –7.05 (95% CI –10.35, –3.75) pg/ml–1 were observed in T2 and T3, respectively, compared with T1. Participants in T2 and T3 experienced a reduction in MCP–1 of –3.55 (95% CI –4.68, –0.82) ng ml–1 and –3.36 (95% CI: –4.55, –0.92) ng ml–1, respectively. Finally, a significantly inverse correlation was observed between changes in urinary TPE and changes in the five inflammatory biomarkers analysed (Table 6).
Table 5.
Changes in inflammatory biomarkers after 1 year, associated with tertiles of changes in total polyphenol excreted, T2 (–14.04 to 31.56) and T3 (>31.57) compared with T1 (<–14.03)
| Model | B | P | 95% CI | |
|---|---|---|---|---|
| VCAM‐1, ng ml –1 | Model 1 | |||
| T2 vs. T1 | –7.18 | 0.066 | –14.84 to 0.48 | |
| T3 vs. T1 | –9.64 | 0.016 | –17.46 to –1.82 | |
| Model 2 | ||||
| T2 vs. T1 | –8.45 | 0.045 | –16.73 to –0.18 | |
| T3 vs. T1 | –9.47 | 0.019 | –17.38 to –1.56 | |
| ICAM‐1, ng ml –1 | Model 1 | |||
| T2 vs. T1 | –10.91 | 0.009 | –19.10 to –2.72 | |
| T3 vs. T1 | –11.78 | 0.006 | –20.20 to –3.36 | |
| Model 2 | ||||
| T2 vs. T1 | –10.18 | 0.034 | –19.60 to –0.77 | |
| T3 vs. T1 | –14.71 | 0.002 | –23.88 to –5.53 | |
| IL‐6, pg ml –1 | Model 1 | |||
| T2 vs. T1 | –0.95 | 0.009 | –1.67 to –0.24 | |
| T3 vs. T1 | –1.23 | 0.001 | –1.96 to –0.51 | |
| Model 2 | ||||
| T2 vs. T1 | –0.99 | 0.019 | –2.03 to –0.40 | |
| T3 vs. T1 | –1.21 | 0.004 | –1.85 to –0.21 | |
| TNF‐α, pg ml –1 | Model 1 | |||
| T2 vs. T1 | –4.46 | 0.003 | –7.39 to –1.53 | |
| T3 vs. T1 | –5.93 | <0.001 | –8.92 to –2.94 | |
| Model 2 | ||||
| T2 vs. T1 | –5.88 | <0.001 | –9.17 to –2.58 | |
| T3 vs. T1 | –7.05 | <0.001 | –10.35 to –3.75 | |
| MCP‐1, pg ml –1 | Model 1 | |||
| T2 vs. T1 | –3.32 | 0.009 | –4.20 to 0.75 | |
| T3 vs. T1 | –2.98 | 0.021 | –3.84 to 1.20 | |
| Model 2 | ||||
| T2 vs. T1 | –3.55 | 0.012 | –4.68 to 0.82 | |
| T3 vs. T1 | –3.36 | 0.017 | –4.55 to 0.92 |
B, nonstandardized coefficient; CI, confidence interval; Model 1, unadjusted; Model 2: adjusted by baseline inflammatory biomarkers, intervention group, gender, age, body mass index, smoking status, physical activity, medication use (antihypertensive agents, statins or other hypolipidaemic drugs, insulin, oral hypoglycaemic drugs, and aspirin or other antiplatelet drugs) supplements taken in the previous month, and sodium, potassium, total energy, monounsaturated fatty acid, polyunsaturated fatty acid and saturated fatty acid intake; P, two‐sided test of significance.
Table 6.
Correlation between changes in total polyphenol excreted (mg gallic acid per gram creatinine) and changes in inflammatory biomarkers, after 1 year
| Inflammatory biomarkers | r | P |
|---|---|---|
| VCAM‐1, ng ml–1 | –0.301 | <0.001 |
| ICAM‐1, ng ml–1 | –0.159 | <0.001 |
| IL‐6, pg ml–1 | –0.092 | 0.006 |
| TNF‐α, pg ml–1 | –0.138 | 0.001 |
| MCP‐1, pg ml–1 | –0.077 | 0.019 |
ICAM‐1, soluble intercellular adhesion molecule‐1; IL‐6, plasma interleukin 6; MCP‐1, monocyte chemotactic protein 1; TNF‐α, tumour necrosis factor alpha; VCAM‐1, vascular cell adhesion molecule 1
Discussion
In the present cohort of older participants at high cardiovascular risk, we observed a significant inverse correlation between changes in polyphenol intake, as measured by the objective biomarker urinary TPE, and circulating inflammatory molecules related to atherosclerosis. Thus, participants with larger increases in urinary TPE showed significant reductions in the plasma concentrations of VCAM‐1, ICAM‐1, IL‐6, TNF‐α and MCP‐1 compared with participants with smaller changes in urinary TPE. In addition, other cardiovascular risk factors, such as systolic and diastolic BP, were decreased significantly, and HDL‐cholesterol increased when polyphenol intake increased (T2 and T3).
Polyphenols are the most abundant antioxidants in human diets. They are secondary metabolites of plants. Fruit and vegetables, particularly seeds, and derived foods and beverages make up the main sources of polyphenols 26. The most common polyphenols in the human diet are not necessarily the most active in vivo 26 as most dietary polyphenols (75–99%) are not found in the urine, and the quantities detected intact vary from one phenolic compound to another 27. This fact may be due to their reduced absorption through the gut barrier, their excretion to the bile or their metabolism by the colonic microflora or in the tissues.
EVOO, one of the supplemental foods given in the present study, contains considerable amount of polyphenols that have a large effect on the stability and nutritional characteristics of the oil. Some of the most representative simple phenols are hydroxytyrosol and tyrosol. However, most phenolic compounds are removed when the oil is refined 26. The anti‐inflammatory properties of EVOO have been attributed to its content in polyphenols 28, 29, and some phenolic compounds, such as oleocanthal, exhibit strong anti‐inflammatory properties 30. Epidemiological studies have related olive oil consumption to various health indices. A recent comprehensive meta‐analysis of 32 cohort studies relating the exposure to MUFA (of both plant and animal origin), olive oil, oleic acid and the MUFA : SFA ratio to various health outcomes indicated that, when comparing the upper to the lower tertile of consumption, olive oil, but not MUFA, was associated with a reduced risk of all‐cause mortality, cardiovascular disease events and stroke 31. When focusing on virgin olive oil consumption, an additional inverse association with CHD risk was observed. A recent report from the prospective cohort of the Nurses' Health Study, a US population with low average olive oil consumption, suggested a modest inverse relationship between exposure to olive oil and the risk of type‐2 diabetes 32, and recent evidence from the PREDIMED study indicated that the Med diet enriched with EVOO protects from incident diabetes 33.
Cardiovascular protection from olive oil is attributable, in part, to effects on cardiovascular risk factors. In fact, there is evidence that polyphenol‐rich olive oils decrease BP and improve the lipid profile 34, having a significant HDL‐cholesterol‐raising effect 12. The beneficial effect of virgin olive oil on HDL‐cholesterol is due partly to MUFA and partly to polyphenols, but the latter appear to have additional favourable effects on HDL functionality by promoting reverse cholesterol transport 35 and are also implicated in improving endothelial function 36. Clinical trials have also demonstrated beneficial effects of olive oil on markers of endothelial function and inflammation, such as C‐reactive protein and IL‐6 37. All of these effects might be attributed to the antioxidant and anti‐inflammatory effects of EVOO components, particularly polyphenols 34.
Nuts, the other supplemented food in the present study, also contain highly bioactive molecules, including unsaturated fatty acids, high‐quality protein, fibre, tocopherols, nonsodium minerals, phytosterols and phenolic compounds 38. In all nuts, most of the antioxidants are located in the pellicle or outer soft shell, and more than 50% of them are lost when the skin is removed 39. Nut consumption also plays a role in the prevention of chronic age‐related diseases 40. There is a growing body of evidence suggesting a beneficial role of nut consumption on CHD and mortality 41, 42, 43. A pooled analysis of epidemiological studies showed that subjects in the highest quantiles of nut consumption had an approximately 35% reduced risk of CHD incidence, with a reduction in fatal CHD that was due primarily to a decrease in sudden cardiac death 40.
Several feeding trials have also analysed the mechanisms of the health effects of nuts. Thus, diets enriched with nuts improved the lipid profile 44, 45, reduced endothelial dysfunction 46, 47 and ameliorated glycaemic control in diabetes 48. While cholesterol lowering by nut diets can be ascribed both to unsaturated fatty acids and phytosterols 45, the improvement in vascular reactivity is likely to be due to their richness in polyphenols, in addition to the fact that they contain L‐arginine, the precursor of the endogenous vasodilator nitric oxide 46. In addition, nuts ameliorate inflammatory status at the vascular level, reducing levels of ICAM‐1, VCAM‐1 and E‐selectin, which are released from the activated endothelium and circulating monocytes 47.
Berry et al. 49 showed that there was less oxidation of plasma and LDL lipids in healthy volunteers after an almond diet compared with a low‐fat diet. Jenkins et al. 50, in a dose–response study, compared two doses of almonds with a low‐fat diet in hyperlipidaemic subjects. The full‐dose almonds produced the greatest reduction in blood lipid levels. Significant reductions from baseline in LDL‐cholesterol and in the ratio LDL‐ : HDL‐cholesterol were seen in participants on both half‐ and full‐dose almonds, and in lipoprotein and oxidized LDL concentrations in those on full‐dose almonds alone, with no significant reductions in those on the control diet. Other previous studies have also reported that some components of the Med diet, such as EVOO or nuts, may downregulate inflammatory markers related to atherosclerosis, such as VCAM‐1, ICAM‐1, E‐ and P‐selectin, C‐reactive protein and IL‐6 51, 52, 53.
Finally, the role of dietary patterns on the prevention of chronic diseases should be emphasized. The Med diet and the DASH diet have been considered as healthy dietary patterns, useful in the prevention of cardiovascular disease 4, 5. Both diets include a high intake of fruit, vegetables, whole‐grain cereals, legumes and nuts, together with a moderate consumption of fish and low‐fat dairy products, and low consumption of meat, meat products, sweets and commercial pastries. The Med diet also includes a high intake of olive oil and a moderate consumption of wine, mainly with meals, as specific and distinguishing features. Given that the Med diet is plant based and enriched in olive oil and wine, it comprises, by definition, a polyphenol‐rich dietary pattern, and this is likely to play a role in its anti‐inflammatory effect and downregulation of cellular and circulating inflammatory biomarkers related to atherosclerosis 54, 55.
The present study had limitations. First, as the study subjects included were older people at high risk of cardiovascular disease, the results may not be generalizable to other populations. A second limitation was the size of the study population, which was relatively small in comparison with other studies. The present study also had strengths, including the design as a randomized controlled clinical trial, which is considered to be the most rigorous method of determining whether a cause–effect relationship exists between an intervention and an outcome. In randomized studies, the conclusions reached achieve the highest level of scientific evidence. Another strength was the use of TPE as a biomarker of TP intake, as this is more precise than self‐reported information based on recalled dietary assessments, thus providing a more objective measurement of specific nutrient intake than the subjective information obtained by an FFQ.
In conclusion, our results suggest that a Med diet intervention is associated with increased TPE in spot urine samples. After adjustment for potential confounders, larger compared with smaller changes in TPE in urine samples are associated with decreased levels of inflammatory biomarkers and an improvement in cardiovascular risk factors such as LDL‐cholesterol, HDL‐cholesterol and systolic and diastolic BP. Thus, a polyphenol‐rich diet may help to reduce cardiovascular risk.
Competing Interests
Dr Ros reports grants, nonfinancial support and other from the California Walnut Commission; grants, personal fees, nonfinancial support and other from Merck, Sharp & Dohme; grants, personal fees, nonfinancial support and other from Alexion; personal fees, nonfinancial support and other from Aegerion; grants and personal fees from Sanofi Aventis; grants, personal fees, nonfinancial support and other from Ferrer International; grants from Amgen; and grants from Pfizer, outside the submitted work. Dr Estruch reports grants from the Spanish Institute of Health ‘Carlos III’, nonfinancial support from Patrimonio Comunal Olivarero, Spain; nonfinancial support from the California Walnut Commission, Spain; nonfinancial support from Borges SA, Spain; grants and nonfinancial support from FIS, Government of Spain; nonfinancial support from Fundacion Bosch i Gimpera, Spain, during the conduct of the study; personal fees from Fundación Dieta Mediterránea, Spain; personal fees from FIVIN, Spain; personal fees from Cerveceros de España, Spain; personal fees from Brewers of Europe, Belgium; grants from Bicentury, SA, Spain; grants from Grand Fountaine, Spain; grants from Novartis Farmaceutica, SA; grants from Amgen SA; personal fees from Lilly Laboratories, Spain; personal fees from Instituto Cervantes, Albuquerque, NM, USA; personal fees from Instituto Cervantes, Milan, Italy; personal fees from the Wine and Culinary International Forum; nonfinancial support from the Harvard School of Public Health, Boston, MA, USA, and from University of Columbia, New York, NY, USA, outside the submitted work.
We would like to thank all the volunteers involved in the PREDIMED study for their valuable cooperation. This study was supported in part by CICYT (AGL2010‐22319‐C03) from the Spanish Ministry of Science and Innovation (MICINN); and the Instituto de Salud Carlos III, ISCIII (CIBERobn‐CB06/03, PI1002658, and PI1001407). The CIBEROBN is an initiative of the ISCIII, Spain. AT‐R received support from ISCIII (FI10/00265). A. M.‐R. thanks the ‘Juan de la Cierva’ postdoctoral program (JCI‐2012‐13463) from MEC (Ministerio de Economía y Competitividad). The MICINN, MEC and ISCIII had no role in the design, analysis or writing of this article.
Medina‐Remón, A. , Casas, R. , Tressserra‐Rimbau, A. , Ros, E. , Martínez‐González, M. A. , Fitó, M. , Corella, D. , Salas‐Salvadó, J. , Lamuela‐Raventos, R. M. , Estruch, R. , and on behalf of the PREDIMED Study Investigators (2017) Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. Br J Clin Pharmacol, 83: 114–128. doi: 10.1111/bcp.12986.
References
- 1. Lawes CM, Vander HS, Rodgers A. Global burden of blood‐pressure‐related disease, 2001. Lancet 2008; 371: 1513–8. [DOI] [PubMed] [Google Scholar]
- 2. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685–95. [DOI] [PubMed] [Google Scholar]
- 3. Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem 2008; 54: 24–38. [DOI] [PubMed] [Google Scholar]
- 4. Estruch R, Martínez‐González MA, Corella D, Salas‐Salvadó J, Ruiz‐Gutiérrez V, Covas MI, et al. Effects of a Mediterranean‐style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 2006; 145: 1–11. [DOI] [PubMed] [Google Scholar]
- 5. Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006; 47: 296–308. [DOI] [PubMed] [Google Scholar]
- 6. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–87. [DOI] [PubMed] [Google Scholar]
- 7. Alonso A, de la Fuente C, Martín‐Arnau AM, de Irala J, Martínez JA, Martínez‐González MA. Fruit and vegetable consumption is inversely associated with blood pressure in a Mediterranean population with a high vegetable‐fat intake: the Seguimiento Universidad de Navarra (SUN) Study. Br J Nutr 2004; 92: 311–9. [DOI] [PubMed] [Google Scholar]
- 8. Harnden KE, Frayn KN, Hodson L. Dietary Approaches to Stop Hypertension (DASH) diet: applicability and acceptability to a UK population. J Hum Nutr Diet 2010; 23: 3–10. [DOI] [PubMed] [Google Scholar]
- 9. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003; 348: 2599–608. [DOI] [PubMed] [Google Scholar]
- 10. Agudo A, Cabrera L, Amiano P, Ardanaz E, Barricarte A, Berenguer T, et al. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC‐Spain). Am J Clin Nutr 2007; 85: 1634–42. [DOI] [PubMed] [Google Scholar]
- 11. Covas MI, Fitó M, Marrugat J, Miró E, Farré M, de la Torre R, et al. Coronary disease protective factors: antioxidant effect of olive oil. Therapie 2001; 56: 607–11. [PubMed] [Google Scholar]
- 12. Covas MI, Nyyssönen K, Poulsen HE, Kaikkonen J, Zunft HJ, Kiesewetter H, et al. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann Intern Med 2006; 145: 333–41. [DOI] [PubMed] [Google Scholar]
- 13. Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium‐dependent vasodilation in hypertensives. Hypertension 2005; 46: 398–405. [DOI] [PubMed] [Google Scholar]
- 14. Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol 2005; 16: 77–84. [DOI] [PubMed] [Google Scholar]
- 15. Tresserra‐Rimbau A, Rimm EB, Medina‐Remón A, Martínez‐González MA, López‐Sabater MC, Covas MI, et al. Polyphenol intake and mortality risk: a re‐analysis of the PREDIMED trial. BMC Med 2014; 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tresserra‐Rimbau A, Rimm EB, Medina‐Remón A, Martínez‐González MA, de la Torre R, Corella D, et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis 2014; 24: 639–47. [DOI] [PubMed] [Google Scholar]
- 17. Mena MP, Sacanella E, Vazquez‐Agell M, Morales M, Fitó M, Escoda R, et al. Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr 2009; 89: 248–56. [DOI] [PubMed] [Google Scholar]
- 18. Vendrame S, Klimis‐Zacas D. Anti‐inflammatory effect of anthocyanins via modulation of nuclear factor‐κB and mitogen‐activated protein kinase signaling cascades. Nutr Rev 2015; 73: 348–58. [DOI] [PubMed] [Google Scholar]
- 19. Joven J, Micol V, Segura‐Carretero A, Alonso‐Villaverde C, Menéndez JA, Bioactive Food Components Platform . Polyphenols and the modulation of gene expression pathways: can we eat our way out of the danger of chronic disease? Crit Rev Food Sci Nutr 2014; 54: 985–1001. [DOI] [PubMed] [Google Scholar]
- 20. Estruch R, Ros E, Salas‐Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–90. [DOI] [PubMed] [Google Scholar]
- 21. Martínez‐González MA, Corella D, Salas‐Salvadó J, Ros E, Covas MI, Fiol M, et al. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol 2012; 41: 377–85. [DOI] [PubMed] [Google Scholar]
- 22. Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. AHA dietary guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000; 102: 2284–99. [DOI] [PubMed] [Google Scholar]
- 23. Iglesias‐Bonilla P, Mayoral‐Sánchez E, Lapetra‐Peralta J, Iborra‐Oquendo M, Villalba‐Alcalá F, Cayuela‐Domínguez A. Validación de dos sistemas de automedida de presión arterial, modelos OMRON HEM 705 CP y OMRON MI (HEM 422C2‐E). Aten Primaria 2002; 30: 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medina‐Remón A, Barrionuevo‐González A, Zamora‐Ros R, Andres‐Lacueva C, Estruch R, Martínez‐González MA, et al. Rapid Folin‐Ciocalteu method using microtiter 96‐well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal Chim Acta 2009; 634: 54–60. [DOI] [PubMed] [Google Scholar]
- 25. Mataix J. Tabla de composición de alimentos (Food composition tables). Granada, Spain: Universidad de Granada, 2003. [Google Scholar]
- 26. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004; 79: 727–47. [DOI] [PubMed] [Google Scholar]
- 27. Kris‐Etherton PM, Hu FB, Ros E, Sabaté J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr 2008; 138: 1746S–51S. [DOI] [PubMed] [Google Scholar]
- 28. Tovar MJ, Motilva MJ, Romero MP. Changes in the phenolic composition of virgin olive oil from young trees (Olea europaea L. cv. Arbequina) grown under linear irrigation strategies. J Agric Food Chem 2001; 49: 5502–8. [DOI] [PubMed] [Google Scholar]
- 29. Abe R, Beckett J, Abe R, Nixon A, Rochier A, Yamashita N, et al. Olive oil polyphenol oleuropein inhibits smooth muscle cell proliferation. Eur J Vasc Endovasc Surg 2011; 41: 814–20. [DOI] [PubMed] [Google Scholar]
- 30. Covas MI, Ruiz‐Gutiérrez V, de la Torre R, Kafatos A, Lamuela‐Raventós RM, Osada J, et al. Minor components of olive oil: evidence to date of health benefits in humans. Nutr Rev 2006; 64: S20–30. [Google Scholar]
- 31. Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta‐analysis of cohort studies. Lipids Health Dis 2014; 13: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guasch‐Ferré M, Hu FB, Martínez‐González MA, Fitó M, Bulló M, Estruch R, et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED study. BMC Med 2014; 12: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salas‐Salvadó J, Bulló M, Estruch R, Ros E, Covas MI, Ibarrola‐Jurado N, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med 2014; 160: 1–10. [DOI] [PubMed] [Google Scholar]
- 34. López‐Miranda J, Pérez‐Jiménez F, Ros E, De Caterina R, Badimón L, Covas MI, et al. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr Metab Cardiovasc Dis 2010; 20: 284–94. [DOI] [PubMed] [Google Scholar]
- 35. Hernáez Á, Fernández‐Castillejo S, Farràs M, Catalán Ú, Subirana I, Montes R, et al. Olive oil polyphenols enhance high‐density lipoprotein function in humans: a randomized controlled trial. Arterioscler Thromb Vasc Biol 2014; 34: 2115–9. [DOI] [PubMed] [Google Scholar]
- 36. Ruano J, Lopez‐Miranda J, Fuentes F, Moreno JA, Bellido C, Perez‐Martinez P, et al. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J Am Coll Cardiol 2005; 46: 1864–8. [DOI] [PubMed] [Google Scholar]
- 37. Schwingshackl L, Christoph M, Hoffmann G. Effects of olive oil on markers of inflammation and endothelial function − a systematic review and meta‐analysis. Nutrients 2015; 7: 7651–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lucas L, Russell A, Keast R. Molecular mechanisms of inflammation. Anti‐inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr Pharm Des 2011; 17: 754–68. [DOI] [PubMed] [Google Scholar]
- 39. Blomhoff R, Carlsen MH, Andersen LF, Jacobs DR Jr. Health benefits of nuts: potential role of antioxidants. Br J Nutr 2006; 96 (Suppl. 2): S52–60. [DOI] [PubMed] [Google Scholar]
- 40. Grosso G, Estruch R. Nut consumption and age‐related disease. Maturitas 2016; 84: 11–6. [DOI] [PubMed] [Google Scholar]
- 41. Hshieh TT, Petrone AB, Gaziano JM, Djousse L. Nut consumption and risk of mortality in the Physicians' Health Study. Am J Clin Nutr 2015; 101: 407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van den Brandt PA, Schouten LJ. Relationship of tree nut, peanut and peanut butter intake with total and cause‐specific mortality: a cohort study and meta‐analysis. Int J Epidemiol 2015; 44: 1038–49. [DOI] [PubMed] [Google Scholar]
- 43. Bonaccio M, Di Castelnuovo A, De Curtis A, Costanzo S, Bracone F, Persichillo M, et al. Nut consumption is inversely associated with both cancer and total mortality in a Mediterranean population: prospective results from the Moli‐sani study. Br J Nutr 2015; 114: 804–11. [DOI] [PubMed] [Google Scholar]
- 44. Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med 2010; 170: 821–7. [DOI] [PubMed] [Google Scholar]
- 45. Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D. Are phytosterols responsible for the low‐density lipoprotein‐lowering effects of tree nuts? A systematic review and meta‐analysis. J Am Coll Cardiol 2015; 65: 2765–7. [DOI] [PubMed] [Google Scholar]
- 46. Ros E, Núñez I, Pérez‐Heras A, Serra M, Gilabert R, Casals E, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation 2004; 109: 1609–14. [DOI] [PubMed] [Google Scholar]
- 47. Casas‐Agustench P, Lopez‐Uriarte P, Ros E, Bullo M, Salas‐Salvado J. Nuts, hypertension and endothelial function. Nutr Metab Cardiovasc Dis 2011; 21: S21–33. [DOI] [PubMed] [Google Scholar]
- 48. Viguiliouk E, Kendall CW, Blanco Mejia S, Cozma AI, Ha V, Mirrahimi A, et al. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta‐analysis of randomized controlled dietary trials. PLoS One 2014; 9: .e103376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Berry EM, Eisenberg S, Friedlander Y, Harats D, Kaufmann NA, Norman Y, et al. Effects of diets rich in monounsaturated fatty acids on plasma lipoproteins – the Jerusalem Nutrition Study. II. Monounsaturated fatty acids vs carbohydrates. Am J Clin Nutr 1992; 56: 394–403. [DOI] [PubMed] [Google Scholar]
- 50. Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low‐density lipoproteins, lipoprotein (a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation 2002; 106: 1327–32. [DOI] [PubMed] [Google Scholar]
- 51. Casas R, Sacanella E, Urpí‐Sardà M, Chiva‐Blanch G, Ros E, Martínez‐González MA, et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS One 2014; 9: .e100084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr 2009; 89: 1649S–56S. [DOI] [PubMed] [Google Scholar]
- 53. Urpi‐Sarda M, Casas R, Chiva‐Blanch G, Romero‐Mamani ES, Valderas‐Martínez P, Arranz S, et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomarkers related to atherosclerosis. Pharmacol Res 2012; 65: 577–83. [DOI] [PubMed] [Google Scholar]
- 54. Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low‐grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets 2014; 14: 245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ros E, Martínez‐González MA, Estruch R, Salas‐Salvadó J, Fitó M, Martínez JA, et al. Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Adv Nutr 2014; 5: 330S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]


