Abstract
Aim
Glucose‐6‐phosphate dehydrogenase (G6PD) deficiency is a common genetic disorder, affecting nearly 400 million individuals worldwide. Whilst it is known that a number of drugs, foods and chemicals can trigger haemolysis in G6PD deficient individuals, the association between herbal and dietary supplements and haemolysis is less clear. The objective of this study was to evaluate the association between herbal or dietary supplements and adverse events in G6PD deficient individuals.
Methods
We searched 14 electronic databases from their inception until November 2015 for articles describing the use of herbal or dietary supplements in G6PD deficient individuals. Additional publications were identified from manually searching textbooks, conference abstracts and the grey literature. All study designs were included as long as they contained clinical information. These gathered findings were summarized narratively.
Results
Thirty‐two publications met inclusion criteria. These reported on 10 herbal and dietary supplements. Overall evidence linking haemolysis to a herbal/dietary supplement was only found for henna. No evidence of harm was observed for vitamin C, vitamin E, vitamin K, Gingko biloba and α‐lipoic acid.
Conclusions
The review showed that there was insufficient evidence to contravene the use of most herbal or dietary products at therapeutic doses in G6PD deficient subjects.
Keywords: glucose‐6‐phosphate dehydrogenase, herbal medicine, safety, systematic review
Introduction
Glucose‐6‐phosphate dehydrogenase (G6PD) deficiency is a common inherited disorder, affecting nearly 400 million individuals worldwide 1. It is characterized by an enzyme defect that plays an important role in preserving erythrocyte integrity against oxidative stress and damage 2. This predisposes G6PD deficient individuals to haemolysis when exposed to certain triggers such as fava beans and oxidant drugs. While most haemolytic attacks are generally self‐limiting, they may sometimes result in an increase of bilirubin levels that leads to kernicterus in infants or haemoglubinuria with acute renal failure in adults 3.
The effective management of G6PD deficient individuals includes educating patient and care providers about the known triggers that may increase the risk of haemolysis. Most reviews have largely focused on drugs to be avoided in G6PD deficiency 4, 5. Similarly, several reviews have suggested that certain foods are known to trigger haemolysis, including legumes, especially fava beans, as well as green tea and its extracts. However, there is scarcity of any evidence that aims to outline the potential of herbs or dietary products in worsening the condition of G6PD deficient patients. In this study, we conducted a systematic review to identify and collate evidence on herbal or dietary supplements that should be avoided by G6PD deficient individuals. This is particularly important in a contemporary environment characterized by the widespread use of such supplements.
Methods
Literature search
We searched for publications that examined the use of dietary or herbal supplements in G6PD‐deficiency. A supplement was defined as any vitamin, mineral, herb or botanical, amino acid, concentrate, metabolite or extract 6. We searched 14 electronic databases from inception until November 30 2015. We manually searched the reference lists of retrieved articles, textbooks and conference abstracts. We did not restrict the search by language and obtained full‐text translation as required. Original articles of all study designs (clinical trials, cohort studies, case–control studies, case series and case reports) were included as long as they reported clinical information regarding harm. Keywords used included glucose‐6‐phosphate deficiency, adverse events, side effects, haemolysis, herbal medicine, dietary supplements and alternative medicine. The summary of the protocol can be found on PROSPERO (CRD42016032724).
Two authors (SWHL and NML) screened the title and abstracts using a standardized data extraction form. Publications were further evaluated if they contained original data involving herbal or dietary supplement(s) and reported an outcome of interest such as haemolysis, jaundice, kernicterus or hyperbilirubinaemia in humans. Data abstracted included the exposure period, history of previous exposure to a similar substance, re‐challenge test, any laboratory findings, factors affecting the G6PD deficient subject's pharmacokinetic or pharmacodynamic response and the authors' conclusions.
Assessment of outcome, causation and quality of evidence
Outcome classifications
Outcomes were classified as major or minor. Major outcomes were death, major life‐threatening bleeding or bleeding requiring hospitalization. Minor outcomes were defined as no change in clinical status or status requiring monitoring.
Criteria for the assessment of causation
We used criteria adapted from the World Health Organization‐Uppsala Monitoring Centre causality categories 7, namely temporal association, risk of bias by other outcomes, prior exposure and outcome objectivity. Causation probability was classed as highly probable, probable, possible or highly improbable. The list of herbal or dietary supplements identified was subsequently classified into one of the three groups as proposed by Youngster and colleagues 4 as follow:
Herbs/dietary products that should be avoided in patients with G6PD deficiency. These included compounds with a well‐established association with haemolysis as evidenced by case reports and laboratory and clinical studies.
Herbs/dietary products that should be consumed with caution by G6PD deficient patients. These included all compounds that were mentioned by any author or source as causing haemolysis, but insufficient evidence existed to implicate or exclude the administration as the cause of haemolytic anaemia.
Herbs/dietary products where there was no evidence to contravene their use by G6PD deficient patients.
Results
The search identified 5511unique publications, of which 176 were evaluated as relevant. Of these, 32 publications containing original data on food or herbal use in G6PD deficient individuals met the inclusion criteria (Figure 1). These publications included five controlled studies, 18 case reports and nine case series and involved 10 dietary or herbal substances (Table 1, 2). Most of the adverse events reported were classified as major, with most patients reporting haemolysis requiring hospitalization and even death in four studies 8, 9, 10, 11. However, there was very low certainty for the evidence for harm. This may be due to reporting bias, as most of the data were based on case reports, in which only major events were likely to be reported. A discussion of individual substances is presented below.
Figure 1.
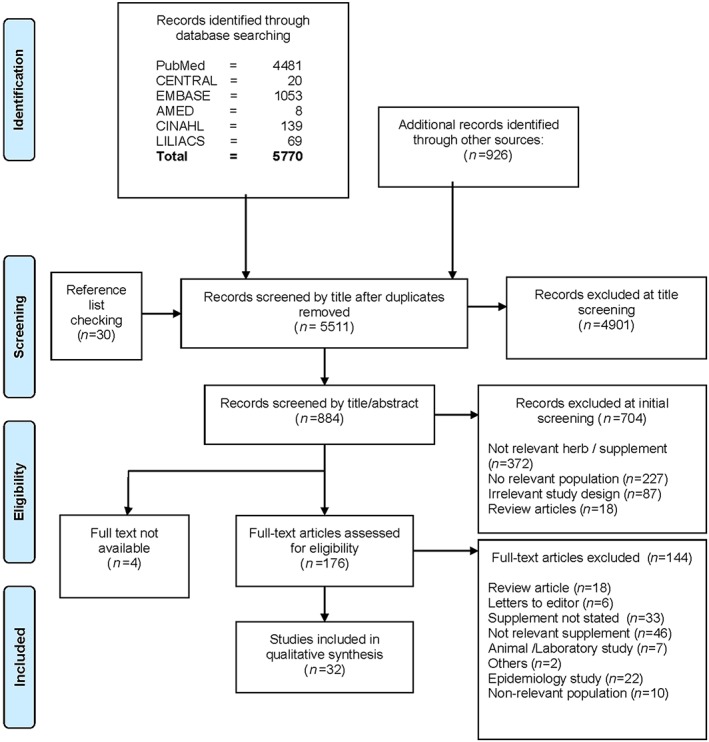
Flow diagram of study search
Table 1.
Summary of key adverse events reported
| Herbal supplement: common name | Number of studies | Adverse effect | Number of case(s) | Causality (number of studies) |
|---|---|---|---|---|
| Lawsonia inermis, Henna | 1 | Death | 1 | Possible (1) |
| 5 | Haemolysis | 20 | Probable(5) | |
| Possible(1) | ||||
| Ayurvedic medicine, Acalypha indica | 4 | Haemolysis | 12 | Possible (4) |
| Ayurvedic medicine, Mentat | 1 | Haemolysis | 1 | Highly improbable (1) |
| Ayurvedic medicine, unspecified | 1 | Haemolysis | 1 | Highly improbable (1) |
| Traditional Chinese medicine, Coptis chinensis | 1 | Death | 1 | Possible (1) |
| 1 | Hyperbilirubinaemia | 1 | Possible (1) | |
| Traditional Chinese medicine, unspecified | 1 | Haemolysis | 3 | Possible (1) |
| Ascorbic acid, vitamin C | 1 | Death | 1 | Possible (1) |
| 3 | Haemolytic anaemia | 4 | Possible (3) | |
| Vitamin K | 1 | Death | 1 | Possible (1) |
| 2 | Haemolytic anaemia | 5 | Possible (1) | |
| Highly improbable (1) | ||||
| 1 | Jaundice | 1 | Possible (1) | |
| Gingko biloba | 1 | Haemolytic anaemia | 1 | Highly improbable (1) |
Table 2.
Forms used by consumers experiencing adverse effects
| Herbal supplement | Botanical parts used, where specified | Dosage form | Comments |
|---|---|---|---|
| Henna, Lawsonia inermis | Leaves | Crude extracts | Contains 2‐hydroxy‐1, 4‐napthoquinone, which is chemically similar to 1,4 naphtoquinone, a metabolite of naphthalene |
| Ayurvedic medicine, Mentat | NA | Tablet containing 26 herbs | No mechanism of haemolysis postulated |
| Ayurvedic medicine, Acalypha indica | Leaves | Herbal tonic extract | Contains flavonoid, kaempherol, glycosides, mauritianin, clitoria and nictiflorin which has antioxidant activities |
| Ayurvedic medicine | NA | Solution containing several agents including Salix caprea | Salix caprea contains salicin, which is metabolized to salicylic acid, an inducer of haemolysis in G6PD deficient patients |
| Traditional Chinese medicine, Coptis chinensis | NA | Herbal tonic | Contains berberine, which can displace bilirubin from albumin |
| Traditional Chinese medicine, | NA | Unknown | No mechanism of haemolysis postulated |
| Ascorbic acid | NA | Fruit juice, injection | Only occurs when consumed at high concentrations, exceeding normal recommended daily allowance |
| Vitamin K | NA | Injection | Vitamin K is thought to be a strong antioxidant |
| Gingko biloba | Leaves | Injection | Use of gingko has been associated with haemorrhage in adults |
NA: Not available
Henna (Lawsonia inermis)
Henna is a dye derived from the dried leaves of the flowering plant Lawsonia inermis 12. The dye principally contains 2‐hydroxy‐1, 4‐napthoquinone, but also flavonoids and steroids. Henna continues to be widely used in Africa, Asia and the Middle East, to colour or adorn the skin, nails and hair. Its use is often associated with religio‐cultural events, including marriage.
Reports of haemolysis with the topical use of henna have been reported 8, 13, 14, 15, 16, 17. Raupp et al. presented four cases of G6PD deficient individuals who experienced haemolytic crisis following topical henna application. Acute renal failure occurred in one case and the patient died after 2 days of admission 8. In another study by Kandil and colleagues, the authors reported 15 G6PD deficient individuals who were admitted due to acute haemolysis after 24–72 h post‐henna application 13. Other authors have also reported similar features after application of henna. Taken together, existing data reviewed showed that henna could increase the risk of haemolysis in infants and children with G6PD deficiency.
Ascorbic acid (vitamin C)
Four case reports described episodes of haemolysis in G6PD‐deficient individuals following use of high doses of 3 and 40 g ascorbic acid daily 10, 18, 20. One case report described the death of a 68‐year‐old black male who was given 80 g of ascorbic acid intravenously for burns 10. In another report by Mehta and colleagues, the authors reported two boys in India who developed acute haemolysis after a ‘binge of fizzy drinks’ containing 4–6 g of ascorbic acid 18. The third report concerned a G6PD deficient individual with a history of HIV and malaria 19. He was given a course of multivitamins, essential fatty acids, glutathione supplements as well as a course of high dose intravenous antibiotics, 40 g three times weekly supplemented by 20–40 g of oral ascorbic acid daily. Acute haemolysis developed after a dose of 80 g intravenously. Our review found that at therapeutic doses, there is little evidence to preclude the use of vitamin C in G6PD deficient patients.
Vitamin E
Similar to vitamin C, vitamin E is a natural antioxidant which acts to protect cells from lysis induced by oxidative stress. Several studies have demonstrated that vitamin E deficiency can contribute to shortened red cell survival 21, 22. Seven studies examined the effects of vitamin E supplementation in G6PD deficient individuals 22, 23, 24, 25, 26, 27, 28. Five of these studies indicated that high doses of vitamin E could reduce the rate of haemolysis in G6PD deficient patients 22, 23, 24, 27, 28. Conversely, no changes in haematological status were reported in two other studies 25, 26. While the role of vitamin E on chronic haemolysis in G6PD deficient individuals remains contradictory, all seven studies reported no adverse effects with oral supplementation of vitamin E between 400 iu to 2400 iu daily. Results of these studies suggest that vitamin E is safe to be consumed at doses of up to 800 iu daily.
Vitamin K
Vitamin K is a fat soluble vitamin with a key role in the synthesis of clotting factors. As vitamin K may decrease glutathione concentrations in normal infant erythrocytes, it is suggested that it may trigger haemolysis in G6PD deficient infants 29. Three publications provided limited evidence of harm with use of vitamin K in G6PD deficient subjects 11, 30, 31. In one of the few randomized controlled studies found in this review, Capps et al.; randomized patients to receive vitamin K or no treatment. Of the 30 G6PD deficient neonates, only four treated with vitamin K and two with no treatment became jaundiced. In the other report by Dhillon et al. 11, vitamin K could not be ascertained to be the cause of all three cases reported. Taking into account the scarcity of reports and widespread use of vitamin K after birth among infants, it is likely that vitamin K can be administered safely to G6PD deficient individuals.
Gingko biloba
Gingko biloba is a commonly used phytomedicine and claims have been made for improvement in cognitive performance, prevention of Alzheimer's disease and vascular dementia. Gingko is generally well tolerated, but can increase the risk of bleeding if used combination with warfarin, antiplatelet agents or in subjects with G6PD deficiency 32. One case report discussed a 55‐year‐old woman with a history of hypertension and dementia 33, who was given a 17.5 mg injection of Gingko biloba leaf extract to improve her memory and subsequently developed jaundice. Cessation of therapy improved her condition and she was discharged 5 days later. Taking into consideration the widespread use of this supplement and paucity of reports, it is highly improbable that Gingko can lead to haemolysis in G6PD deficiency.
α‐lipoic acid
α‐lipoic acid (LA) is a naturally occurring thiol compound which has potent antioxidant activities. Studies have demonstrated that LA can act to restore intracellular gluthathoine and thus benefit G6PD deficient individuals. One study examined how LA supplementation regulated the antioxidant capacity in eight G6PD deficient adults and found no adverse events at doses of 600 mg day‐1 over a period of 28 days 34. The authors concluded that LA supplementation maybe beneficial in G6PD deficient individuals due to its antioxidant capacity and ability to modulate the blood redox status.
Ayurvedic medicine
Ayurvedic medicine is a form of Indian folk medicine which promotes universal connectedness and life forces. Practitioners often individualize treatment and include various compounds and herbs in their treatment. Two studies reported haemolysis following the administration of Ayurvedic medicine in G6PD deficient patients 35, 36. One study described the harm associated with the use of Mentat, a traditional remedy containing 26 different herbs advocated for enhancing memory. A previously healthy 21‐year‐old male had taken a Mentat tablet twice daily for his incoming examinations for the past 3 days when he complained of dark coloured urine. He subsequently developed jaundice and acute renal failure 35. The supplement was discontinued and he recovered after 28 days.
Acalypha indica
Acalypha indica is a weed found in various parts of Asia, and widely used in Ayurveda for its claimed anti‐inflammatory, antimicrobial and antitussive effects 37. Sellahewa first described Acalypha indica induced haemolysis in four patients in Sri Lanka 38. Since then, three other studies have similar reported incidences of acute haemolysis after ingestion of Acalypha indica 39, 40, 41. In all cases, the authors suggested that consumption of a broth containing Acalypha indica was the cause of haemolysis. Nevertheless, the actual dose and purity of these extracts were not reported. Indeed, toxicity studies from laboratory studies using low to very high doses of Acalypha indica extract in rats found it to be non‐toxic to major organs 42. In view of these contradictory findings, we suggest caution in the consumption of Acalypha indica .
Traditional Chinese medicines
Traditional Chinese medicine (TCM) is a type of Eastern medicine built upon the foundation of Chinese medical practice and includes herbal medicine, acupuncture, moxibustion, dietary therapy, exercise and massage. TCM is widely use worldwide for various ailments ranging from common cold to complex diseases such as arthritis and metabolic diseases. Among Asians, Chinese herbs are traditionally consumed by both the mother and child immediately after delivery, potentially endangering the newborn. We found only one study which reported haemolysis due to the use of unspecified Chinese herbal medicine in three children with G6PD deficiency 43. We found only one study which reported haemolysis due to the use of unspecified Chinese herbal medicine in three children with G6PD deficiency 43.
Coptis chinensis
Coptis chinensis is a commonly used herb in TCM for the treatment of various ailments including febrile illness as well as hepatobiliary diseases 44. It contains the alkaloid berberine, which can increase bilirubin formation and thus increase the risk of jaundice especially in infants. Two studies reported death or severe hyperbilirubinaemia in infants with G6PD deficiency following the administration of Coptis chinensis in Singapore 9, 45. However, another study by Lin and colleagues in Guangxi, China which examined 62 G6PD deficient neonates who were given Coptis chinensis found that it did not aggravate the incidence of jaundice 46. Like all reports of herbal medicines, the purity and actual doses were never reported. In view of this, we suggest caution during the consumption of Coptis chinensis.
Discussion
This review aims to provide an overview and critical evaluation on the safety of herbal products in relation to their use in G6PD deficient individuals. Most of the adverse events reported were major in nature as they required hospitalization or resulted in death. Nevertheless, we found that solid evidence associating harm with any herbal/dietary product exists only for henna. Caution should be exercised while consuming the herbal preparations such as Acalypha indica and Coptis chinensis as contradictory evidences exists. In the remaining herbal/dietary products reviewed, there was no evidence to contravene its use at therapeutic doses (Table 3).
Table 3.
Herbal/dietary supplements which should be avoided, cautioned or can be safely consumed by G6PD deficient individuals
| Herbs/dietary products that should be avoided by G6PD deficient individuals | Herbs/dietary products in which caution should be exercised during consumption | Herbs/dietary products in which there is no evidence to contravene their use |
|---|---|---|
| Henna | Acalypha indica | Vitamin C |
| Coptis chinensis | Vitamin E | |
| Vitamin K | ||
| Gingko biloba | ||
| α‐lipoic acid |
The need for rigorous safety evaluation of herbs has always been questioned, especially by those who equate ‘natural’ to be equivalent to ‘safe’. In recent years, with the increasing recognition of the role of herbal pharmacovigilance, increasing reports have appeared describing the association of herbal/dietary products with adverse events. The WHO Monitoring Centre has reported that nearly 9000 reports of adverse events over a 30 year period were associated with herbal medicines 33, 34. In light of this, supplementary studies to spontaneous reporting are needed to establish causality to the herbal/dietary product and better understand the magnitude of the risk involved especially in special populations such as G6PD deficient individuals.
Some methodological challenges were noted when conducting this study. Firstly, most of the publications found were primarily case reports and case series, from which it is generally difficult to establish causality but areimportant for assessing safety issues. In all studies especially those addressing herbal products, the actual dose of active ingredient, source of origin, purity and chemical composition to exclude the presence of contaminants are not reported. Similarly, the lack of regulation of most herbal/dietary products makes the treatment effect and even its adverse events very difficult to measure. Most plants contain a complex mixture of terpenes, alkaloids and other chemicals, which increase the risk of adverse reactions. The existence of contaminants and adulterants can also be pharmacologically active and thus responsible for toxicity. For example, some herbal plants used in Ayurvedic medicine have been known to contain heavy metals such as arsenic and mercury 47.
Strength and limitations
The primary strength of the review is the broad search strategy adopted, aiming at identifying and retrieving all available published evidence. We systematically searched 14 databases and all primary references available, to enhance the completeness of our search. We had not applied any language restriction, which allowed us to gather the most updated and relevant information from various journals since publication bias is opposite that of conventional medicine, that is negative studies ares more likely to be published in well‐known journals and positive studies in foreign language journals 48. While we hoped to be able to make robust recommendations and to provide readers with a list of potentially harmful herbal products or substances, we found instead a wide knowledge gap in their safety.
This review has several important limitations. The lack of systematic investigation and small reported sample sizes made it difficult, if not impossible, to ascertain the potential for true clinical interaction of any herbal product with G6PD deficiency. Indeed, we found that there was a paucity of evidence especially those related to TCM and Ayurvedic medicine. Similarly, we could not perform a meta‐analysis. As such it is impossible to determine if the difference in reported harm outcome is due to true pharmacological interaction or due to more independent additive effects. While the number of articles published in the literature is low, we believe that the real, clinical impact seen in these case reports and case series highlights the significance of such results.
In the course of this study, we also found several review articles that have addressed the issue concerning food which needs to be avoided by G6PD deficient individuals. In an article by Frank in 2005, the author advised against the consumption of fava beans, since they are known to be one of the precipitators of haemolysis for several decades 5. Several other articles have made similar recommendations pertaining to the consumption of fava beans, but fall short in their listing of other food sources 49, 50. While this is out of the scope of this review, future studies should look into systematically examining the possible list of foods that should be avoided by patients with G6PD deficiency. Finally, the under‐reporting of adverse events especially associated with herbal and dietary supplements is generally well documented and it is possible that such adverse events are more prevalent than the current study shows.
Conclusion
In summary, we found that evidence linking haemolytic effects in G6PD deficient patients was found only for henna. For most other herbal or dietary supplements, there is no evidence to contravene their use at therapeutic doses in G6PD deficient individuals.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
The authors wish to thank Dr Vivienne Mak and Dr Tahir Mehmood Khan for their invaluable input and feedback of the article.
Contributors
SWHL conceived the study. SWHL, NML and NC had full access to all the data and take responsibility for its integrity. SWHL and NML developed and tested the data collection forms and conducted the analysis. SWHL, LNM, NC and DWKC interpreted the data and drafted the manuscript. All authors critically reviewed the manuscript.
Supporting information
Appendix S1 Databases used in review
Supporting info item
Lee, S. W. H. , Lai, N. M. , Chaiyakunapruk, N. , and Chong, D. W. K. (2017) Adverse effects of herbal or dietary supplements in G6PD deficiency: a systematic review. Br J Clin Pharmacol, 83: 172–179. doi: 10.1111/bcp.12976.
References
- 1. Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose‐6‐phosphate dehydrogenase deficiency: a systematic review and meta‐analysis. Blood Cells Mol Dis 2009; 42: 267–78. [DOI] [PubMed] [Google Scholar]
- 2. Valaes T. Severe neonatal jaundice associated with glucose‐6‐phosphate dehydrogenase deficiency: pathogenesis and global epidemiology. Acta Paediatr Suppl 1994; 394: 58–76. [DOI] [PubMed] [Google Scholar]
- 3. Beutler E. G6PD deficiency. Blood 1994; 84: 3613–36. [PubMed] [Google Scholar]
- 4. Youngster I, Arcavi L, Schechmaster R, Akayzen Y, Popliski H, Shimonov J, et al. Medications and glucose‐6‐phosphate dehydrogenase deficiency: an evidence‐based review. Drug Saf 2010; 33: 713–26. [DOI] [PubMed] [Google Scholar]
- 5. Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Physician 2005; 72: 1277–82. [PubMed] [Google Scholar]
- 6. Dietary supplements: National Institutes of Health, Office of Dietary Supplements; 2015. https://ods.od.nih.gov/factsheets/DietarySupplements-HealthProfessional/ (last accessed on 30 November 2015).
- 7. World Health Organization (WHO) — Uppsala Monitoring Centre. The use of the WHO‐UMC system for standardized case causality assessment. Available from: http://www.who-umc.org/Graphics/24734.pdf (last accessed on 30 November 2015).
- 8. Raupp P, Hassan JA, Varughese M, Kristiansson B. Henna causes life threatening haemolysis in glucose‐6‐phosphate dehydrogenase deficiency. Arch Dis Child 2001; 85: 411–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong HB. Singapore kernicterus. Singapore Med J 1980; 21: 556–67. [PubMed] [Google Scholar]
- 10. Campbell GD, Steinberg MH, Bower JD. Ascorbic acid‐induced hemolysis in G‐6‐PD deficiency. Ann Intern Med 1975; 82: 810. [DOI] [PubMed] [Google Scholar]
- 11. Dhillon AS, Darbyshire PJ, Williams MD, Bissenden JG. Massive acute haemolysis in neonates with glucose‐6‐phosphate dehydrogenase deficiency. Arch Dis Child Fetal Neonatal Ed 2003; 88: F534–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badoni Semwal R, Semwal DK, Combrinck S, Cartwright‐Jones C, Viljoen A. Lawsonia inermis L. (henna): Ethnobotanical, phytochemical and pharmacological aspects. J Ethnopharmacol 2014; 155: 80–103. [DOI] [PubMed] [Google Scholar]
- 13. Kandil HH, Al‐Ghanem MM, Sarwat MA, Al‐Thallab FS. Henna (Lawsonia inermis Linn.) inducing haemolysis among G6PD‐deficient newborns. A new clinical observation. Ann Trop Paediatr 1996; 16: 287–91. [DOI] [PubMed] [Google Scholar]
- 14. Kok AN, Ertekin MV, Ertekin V, Avci B. Henna (Lawsonia inermis Linn.) induced haemolytic anaemia in siblings. Int J Clin Pract 2004; 58: 530–2. [DOI] [PubMed] [Google Scholar]
- 15. Zinkham WH, Oski FA. Henna: a potential cause of oxidative hemolysis and neonatal hyperbilirubinemia. Pediatrics 1996; 97: 707–9. [PubMed] [Google Scholar]
- 16. Seyedzadeh A, Hemmati M, GCheiny S. Henna induced severe hemolysis in glucose‐6‐phosphate dehydrogenase deficiency. Pak J Med Sci 2007; 23: 119–21. [Google Scholar]
- 17. Katar S, Devecioglu C, Ozbek MN, Ecer S. Henna causes life‐threatening hyperbilirubinaemia in glucose‐6‐phosphate dehydrogenase deficiency. Clin Exp Dermatol 2007; 32: 235–6. [DOI] [PubMed] [Google Scholar]
- 18. Mehta JB, Singhal SB, Mehta BC. Ascorbic acid‐induced hemolysis in G‐6‐PD deficiency. Lancet 1990; 336: 944. [DOI] [PubMed] [Google Scholar]
- 19. Rees DC, Kelsey H, Richards JD. Acute haemolysis induced by high dose ascorbic acid in glucose‐6‐phosphate dehydrogenase deficiency. BMJ 1993; 306: 841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang Y‐C, Chang T‐K, Fu Y‐C, Jan S‐L. C for colored urine: acute hemolysis induced by high‐dose ascorbic acid. Clin Toxicol 2014; 52: 984–4. [DOI] [PubMed] [Google Scholar]
- 21. Abdul‐Razzak KK, Nusier MK, Obediat AD, Salim AM. Antioxidant vitamins and hyperbilirubinemia in neonates. Ger Med Sci 2007; 5: Doc03. [PMC free article] [PubMed] [Google Scholar]
- 22. Corash L, Spielberg S, Bartsocas C, Boxer L, Steinherz R, Sheetz M, et al. Reduced chronic hemolysis during high‐dose vitamin E administration in Mediterranean‐type glucose‐6‐phosphate dehydrogenase deficiency. N Engl J Med 1980; 303: 416–20. [DOI] [PubMed] [Google Scholar]
- 23. Sultana N, Begum N, Begum S, Ferdousi S, Ali T. Effects of vitamin E supplementation on some aspects of hematological variables in patients of hemolytic anemia with glucose 6 phosphate dehydrogenase (G6PD) deficiency. Bangladesh J Physiol Pharmacol 2006; 22: 12–7. [Google Scholar]
- 24. Corash L, Sheetz M, Bieri J, Bartsocas C, Moses S, Bashan N, et al. Chronic hemolytic anemia due to glucose‐6‐phosphate dehydrogenase deficiency or glutahtione synthetase deficiency: the role of vitamin E in its treatment. Ann N Y Acad Sci 1982; 393: 348–60. [DOI] [PubMed] [Google Scholar]
- 25. Johnson GJ, Vatassery GT, Finkel B, Allen DW. High‐dose vitamin E does not decrease the rate of chronic hemolysis in glucose‐6‐phosphate dehydrogenase deficiency. N Engl J Med 1983; 308: 1014–7. [DOI] [PubMed] [Google Scholar]
- 26. Newman JG, Newman TB, Bowie LJ, Mendelsohn J. An examination of the role of vitamin E in glucose‐6‐phosphate dehydrogenase deficiency. Clin Biochem 1979; 12: 149–51. [DOI] [PubMed] [Google Scholar]
- 27. Spielberg SP, Boxer LA, Corash LM, Schulman JD. Improved erythrocyte survival with high‐dose Vitamin E in chronic Hemolyzing G6PD and glutathione synthetase deficiencies. Ann Intern Med 1979; 90: 53–4. [DOI] [PubMed] [Google Scholar]
- 28. Hafez M, Amar ES, Zedan M, Hammad H, Sorour AH, el‐Desouky ES, et al. Improved erythrocyte survival with combined vitamin E and selenium therapy in children with glucose‐6‐phosphate dehydrogenase deficiency and mild chronic hemolysis. J Pediatr 1986; 108: 558–61. [DOI] [PubMed] [Google Scholar]
- 29. Kaplan M, Waisman D, Mazor D, Hammerman C, Bader D, Abrahamov A, et al. Effect of vitamin K1 on glucose‐6‐phosphate dehydrogenase deficient neonatal erythrocytes in vitro . Arch Dis Child Fetal Neonatal Ed 1998; 79: F218–F20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Capps FP, Gilles HM, Jolly H, Worlledge SM. Glucose‐6‐phosphate dehydrogenase deficiency and neonatal jaundice in Nigeria: their relation to the use of prophylatic vitamin K. Lancet 1963; 2: 379–83. [DOI] [PubMed] [Google Scholar]
- 31. Zinkham WH, Childs B. Effect of vitamin K and naphthalene metabolites on gluthathione metabolism of erythrocytes from normal newborns and patients with napthalene hemolytic anemia. Am J Dis Child 1957; 94: 420–3. [Google Scholar]
- 32. Bent S, Goldberg H, Padula A, Avins AL. Spontaneous bleeding associated with Ginkgo biloba: a case report and systematic review of the literature. J Gen Intern Med 2005; 20: 657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lai SW, Chen JH, Kao WY. Acute hemolytic anemia in glucose‐6‐phosphate dehydrogenase deficiency complicated by Ginkgo biloba . Acta Haematol 2013; 130: 288–90. [DOI] [PubMed] [Google Scholar]
- 34. Georgakouli K, Deli CK, Zalavras A, Fatouros IG, Kouretas D, Koutedakis Y, et al. α‐lipoic acid supplementation up‐regulates antioxidant capacity in adults with G6PD deficiency. Food Chem Toxicol 2013; 61: 69–73. [DOI] [PubMed] [Google Scholar]
- 35. Sharma N, Jain S, Kumari S, Varma S. Severe haemolysis in a G6PD deficient individual following intake of ‘Mentat’. Bulletin, Postgraduate Institute of Medical Education and Research, Chandigarh 1997; 31: 95–7. [Google Scholar]
- 36. Baker S, Thomas PS. Herbal medicine precipitating massive haemolysis. Lancet 1987; 1: 1039–40. [DOI] [PubMed] [Google Scholar]
- 37. Seebaluck R, Gurib‐Fakim A, Mahomoodally F. Medicinal plants from the genus Acalypha (Euphorbiaceae)–a review of their ethnopharmacology and phytochemistry. J Ethnopharmacol 2015; 159: 137–57. [DOI] [PubMed] [Google Scholar]
- 38. Sellahewa KH. Clinical study of intravascular haemolysis at Anurahapura. Sri Lanka Medial Association Annual Session 1992: 20. [Google Scholar]
- 39. Narasimhan D, Kumar S, Murali A, Satish M, Mambatta A. Acute intravascular hemolysis triggered by herbal remedy. J Forensic Toxicol Pharmacol 2014; 3: 1. [Google Scholar]
- 40. Lamabadusuriya SP, Jayantha UK. Acalypha indica Induced haemolysis in G6PD deficiency. Ceylon Med J 1994; 39: 46–7. [PubMed] [Google Scholar]
- 41. Senanayake N, Sanmuganathan PS. Acute intravascular haemolysis in glucose‐6‐phosphate dehydrogenase deficient patients following ingestion of herbal broth containing Acalypha indica . Trop Doct 1996; 26: 32. [DOI] [PubMed] [Google Scholar]
- 42. Sathya M, Kokilavani R, Ananta Teepa KS. Acute and subacute toxicity studies of ethanolic extract of Acalypha indica Linn in male Wistar albino rats. Asian J Pharm Clin Res 2012; 5: 97–100. [Google Scholar]
- 43. Lau HK, Li CH, Lee AC. Acute massive haemolysis in children with glucose‐6‐phosphate dehydrogenase deficiency. Hong Kong Med J 2006; 12: 149–51. [PubMed] [Google Scholar]
- 44. Linn YC, Lu J, Lim LC, Sun H, Sun J, Zhou Y, et al. Berberine‐induced haemolysis revisited: safety of Rhizoma coptidis and cortex phellodendri in chronic haematological diseases. Phytother Res 2012; 26: 682–6. [DOI] [PubMed] [Google Scholar]
- 45. Yeo KL, Tan VCC. Severe hyperbilirubinaemia associated with Chinese herbs. A case report. Singapore Paediatr J 1996; 38: 180–2. [Google Scholar]
- 46. Lin N, Liu CF, Liu Y, Uang ZR, Wan R, Kong XY. Investigation of Coptis chinensis on jaundice of glucose‐6‐phosphate dehydrogenase (G6PD) deficient neonates from Guigang,. Zhongguo Zhongyao Zazhi 2007; 32: 2543–6. [PubMed] [Google Scholar]
- 47. Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, et al. Heavy metal content of ayurvedic herbal medicine products. JAMA 2004; 292: 2868–73. [DOI] [PubMed] [Google Scholar]
- 48. Pham B, Klassen TP, Lawson ML, Moher D. Language of publication restrictions in systematic reviews gave different results depending on whether the intervention was conventional or complementary. J Clin Epidemiol 2005; 58: 769–76.e2. [DOI] [PubMed] [Google Scholar]
- 49. Cappellini MD, Fiorelli G. Glucose‐6‐phosphate dehydrogenase deficiency. Lancet 2008; 371: 64–74. [DOI] [PubMed] [Google Scholar]
- 50. WHO Working Group . Glucose‐6‐phosphate dehydrogenase deficiency. Bull World Health Organ 1989; 67: 601–11. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Databases used in review
Supporting info item


