Abstract
Cystine‐knot miniproteins are a class of 30–50 amino acid long peptides widespread in eukaryotic organisms. Due to their very peculiar three‐dimensional structure, they exhibit high resistance to heat and peptidase attack. The cystine‐knot peptides are well represented in several plant species including medicinal herbs and crops. The pharmacological interest in plant cystine‐knot peptides derives from their broad biological activities, mainly cytotoxic, antimicrobial and peptidase inhibitory and in the possibility to engineer them to incorporate pharmacophoric information for oral delivery or disease biomonitoring. The mechanisms of action of plant cystine‐knot peptides are still largely unknown, although the capacity to interfere with plasma membranes seems a feature common to several cystine‐knot peptides. In some cases, such as potato carboxypetidase inhibitor (PCI) and tomato cystine‐knot miniproteins (TCMPs), the cystine‐knot peptides target human growth factor receptors either by acting as growth factor antagonist or by altering their signal transduction pathway. The possibility to identify specific molecular targets of plant cystine‐knot peptides in human cells opens novel possibilities for the pharmacological use of these peptides besides their use as scaffold to develop stable disease molecular markers and therapeutic agents.
Keywords: antiangiogenic activity, cystine‐knot peptides, cytotoxicity, diagnostic agents, disease biomarkers, pharmacophoric carriers
Introduction
The analysis of the genomic sequences of several model plants has evidenced the abundance of genes coding for cysteine‐rich peptides (CRPs) 1. In some species, CRPs can represent around 2% of the expressed genes 1, 2. Plant CRPs share some common features including a small size (around 100–160 amino acids or less), the presence of an N‐terminal signal peptide, and a conserved cysteine‐rich domain in the C‐terminal region. They are grouped in several categories based on the conserved cysteine pattern and 3D structural characteristics, which are largely dependent on the arrangement of the disulfide bridges. Experimental evidence indicates that members of CRPs can play a variety of functions in plant cells, ranging from defence against biotic stress, symbiotic interactions, root growth and reproductive development, often acting as signalling molecules 3. Several plant CRPs have been classified as antimicrobial peptides (AMPs) for their cytotoxic activity against bacteria and fungi 4. Their antimicrobial activity has been related to their capacity to affect the plasma membrane functionality either by interacting with the lipid component or altering ion fluxes 4. Some plant AMPs have also been assayed for their cytotoxicity against different types of cancer cells 4, although the selectivity of these peptides towards cancer cells deserve further investigation to determine for each individual plant cysteine‐rich AMP the clinical potential.
Amongst the plant CRPs, the class of cystine‐knot peptides, that are characterized by a very peculiar three‐dimensional structure, displays interesting features for pharmaceutical applications. The cystine‐knot peptides, also referred to as knottins or inihibitor cystine‐ knot peptides or cystine‐knot miniproteins, are small proteins (less than 50 amino acids in the mature form) which contain in the C‐terminal region six conserved cysteine forming three disulfide bonds that are intertwined giving rise to a unique structural scaffold. This structure confers exceptional stability and resistance to high temperatures, proteolysis as well as chemical chaotropic agents. Cystine‐knot peptides are not restricted to plant organisms, but are present in other eukaryotes such as insects, arthropods, molluscs and arachnids 5. Plant cystine‐knot peptides exist as linear and cyclic molecules. In both types, the disulfide bridges connect the I–IV, II–V and III–VI cysteine residues forming a ring where the penetrating disulfide bridge is Cys (III–VI). The cystine‐knot motif is also present in several human growth factors, including transforming growth factor‐β (TGF‐β), nerve growth factor (NGF), glycoprotein hormones (GPHs) and vascular endothelial growth factors (VEGF) 6. The cystine‐knot motif of these growth factors has the same disulfide connectivity as the linear and cyclic molecules, but differs for the penetrating disulfide bridge 6.
The linear cystine‐knot peptides
The linear cystine‐knot peptides identified in plants have been grouped into several categories based on their sequence similarity and biological activity (Table 1) (http://knottin.cbs.cnrs.fr) 7, 8. Most of them display inhibitory activity against exo‐ and endoproteases, namely metallocarboxypeptidases and serine proteases. The potato carboxypetidase inhibitor (PCI) was the first cystine‐knot protease inhibitor characterized in plants 9. Successively, other members of this cystine‐knot class were discovered in Solanum tuberosum and Solanum lycopersicum 10, 11, 12 and in other Solanaceae species (http://knottin.cbs.cnrs.fr). Two groups of cystine‐knot serine protease inhibitors have been described; the first includes peptides common in seeds of the Cucurbitaceae family with many members in plants belonging to the Momordica genus, used as food and in Chinese traditional medicine 13, 14, and the second contains members from Spinacia oleracea and Mirabilis jalapa. Another group of linear cystine‐knot peptides is represented by inhibitors of α‐amylase; the first member of this group was identified in the medicinal herb Amaranthus hypochondriacus, and more recently other cystine‐knot α‐amylase inhibitors were isolated from the medicinal plants Allamanda cathartica and Wrightia religiosa 15, 16. The other linear cystine‐knot protein groups, ‘antimicrobial’, ‘defensins’ and ‘toxins’, have been distinguished for their antimicrobial and/or insecticidal capacity. Antimicrobial cystine‐knot proteins were identified in several plants including ginseng and poplar, whereas those belonging to ‘toxins’ have been described in some leguminous plants 7, 8. The different biological activities amongst different plant families are likely due mainly to the diverse amino acid composition of the loops (Table 1). The biological properties displayed by the linear cystine‐knot peptides can be indicative of a natural function in plant defence against microorganisms and pests 4, 17, 18.
Table 1.
The different groups of linear and cyclic cystine‐knot peptides identified in plant. The data have been obtained from the Knottin (http://knottin.cbs.cnrs.fr/) and Cybase (http://www.cybase.org.au/) databases
| Type of cystine‐knot peptides | Species | Biological activity | Organ | Ref. |
|---|---|---|---|---|
| α‐Amylase inhibitor |
Amaranthus hypochondriacus
Allamanda cathartica
Wrightia religiosa |
– α‐Amylase inhibitory activity | Seed | 15, 16 |
| – Toxic to insect larvae | ||||
| Antimicrobial |
Panax ginseng
Panax quinquefolius
Mesembryanthemum crystallinum Mirabilis jalapa Populus trichocarpa Phytolacca americana |
– Antifungal activity | Seed | 7, 8 |
| – Antimicrobial activity | ||||
| Defensin | Petunia hybrida | – Antifungal activity | Flower | 7, 8 |
| Metallo carboxypeptidase inhibitor |
Solanum lycopersicum
Solanum tuberosum
Nicotiana tabacum Hyoscyamus niger |
– Exopeptidases inhibitory activity | Fruit | 9, 10, 11, 12, 17, 19, 20 |
| – Antifungal activity | Flower | |||
| – Inhibition of cancer cell growth | Tuber | |||
| – Antiangiogenic activity | ||||
| Serine protease inhibitor 1 |
Cucumis melo
Momordica charantia
Cyclanthera pedata Lagenaria siceraria Citrullus lanatus Cucurbita maxima Luffa cylindrical Cucumis sativus |
– Serine‐type protease inhibitory activity | Seed | 13, 14 |
| Serine protease inhibitor 2 | Spinacia oleracea Mirabilis jalapa | – Serine‐type protease inhibitory activity | Seed | 7, 8 |
| Toxins | Several leguminous plants (Fabaceae family) | – Insecticidal activity | Seed | 7, 8 |
| – Cellular signal transduction (leginsulin, albumin1) | Root Nodules Leaf | |||
| Cyclotides | Several species belonging to Violaceae, Rubiaceae, Apocynaceae, Cucurbitaceae, Fabaceae, and Solanaceae families | – Pesticidal activity | Stem | 4, 21, 22, 23, 24, 25, 26, 27, 28 |
| – Antimicrobial activity | Seed | |||
| – Cytotoxic effects | Leaf | |||
| – Anti‐HIV | Root | |||
| – Inhibition of cancer cell growth | Bark |
Cyclotides
The cyclotides are a family of globular plant miniproteins characterized by a head‐to‐tail cyclized backbone and the cystine‐knot motif (http://www.cyclotide.com/knots.html/) 29, 30, 31, 32, 33. Apart from the conserved cystine‐knot motif, cyclotides are highly variable in both amino acid composition and size of their backbone loops 34, 35. The cystine‐knot occupies the core of the structure, while the majority of the other amino acids are exposed on the surface. Cyclotides are synthesized as precursor proteins; the processing of the precursor involves oxidative folding to form three disulfide bonds, excision of the mature sequence and head‐to‐tail cyclization 21. The mature proteins are typically 28–37 amino acids in length 29. To date, more than 280 cyclotides are catalogued in the Cybase database (http://www.cybase.org.au/) 22, 36 covering 55 plant species. The vast majority of cyclotides have been found in the Violaceae and Rubiaceae families, but members have also been discovered within the Fabaceae, Cucurbitaceae and Solanaceae families (Table 1) 21, 37, 38, 39. Naturally occurring cyclotides have been divided into three subfamilies: bracelet, Möbius and trypsin inhibitor (Figure 1) 30, 40. The two major families are the Möbius (e.g. Kalata B1 peptide from Viola odorata) and bracelet (e.g. cycloviolacin O1 from Viola odorata and Oldenlandia affinis), that differ in size and sequence of individual loops 40. In addition, Möbius present a Proline (P) residue in the loop 5 that is responsible for a twist in the circular backbone, that is absent in the bracelet 40. Trypsin inhibitor subfamily (e.g. trypsin inhibitor I, MCoTI‐I, from Momordica cochinchinensis) shows very little sequence similarities with the other two subfamilies, but maintains the characteristic cyclic cystine‐knot motif 40. The high number of different cyclotides within an individual plant species and the high variability of the amino acid sequences suggest that cyclotides could target a wide range of potential sites. Numerous cyclotides have been demonstrated to possess pesticidal activity against insects 23, such as Helicoverpa punctigera larvae, the major cotton pest 24, 25, parasitic helminths 41, 42 and molluscs, such as Pomacea canaliculata, a rice pest 26.
Figure 1.
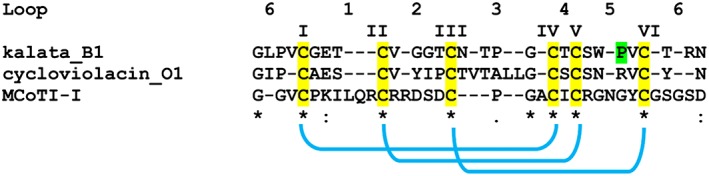
Representative sequences of cyclotides from the three subfamilies. The sequences are exported from the Cybase database (http://www.cybase.org.au/). The six cysteine residues and the three disulphide bonds are highlighted in yellow and blue, respectively. Möbius and bracelet families are distinguished by the presence in the loop 5 of a cis‐Pro (P marked in green) peptide bond that generates in the Möbius family member a twist in the tertiary structure
Biological activities and molecular targets of cystine‐knot peptides in cultured human cells
Many cystine‐knot peptides have antimicrobial properties showing inhibitory effects on microbial cell growth. Several linear and cyclic cystine‐knot peptides also displayed toxicity against a range of cell lines derived from different cancer types 4, 43. However, the cytotoxic activity against cancer cells is not specific, as some cystine‐knot peptides can also target healthy cells 43, 44. Various cyclotides from both the bracelet and Möbius subfamilies displayed anti‐HIV activity 22, 27, 28 and uterotonic activity 45. Besides this, some cyclotides also have an undesired haemolytic activity against human red blood cells 22, 46, 47.
The mechanism of action underlying the cytotoxic activities of cystine‐knot peptides remains largely unknown. Experimental evidence suggests that it is associated with the capacity of the cystine‐knot peptides to interact with plasma membranes 40. For instance, the majority of cyclotides possess a surface‐exposed cluster of hydrophobic residues as well as electrostatic patches that promote their adsorption to the lipid components of target cell membranes. After reaching a threshold concentration, cyclotides form multimeric structures, leading to pores, which could cause membrane damage and changes in ion fluxes 33, 48. In this regard, several cystine‐knot toxins produced by spiders, scorpions and sea anemone act as selective blockers of K, Na and acid‐sensing channels 49, 50, 51, 52, 53.
However, although a membrane‐based mechanism might explain many biological activities (e.g. anti‐HIV and cytotoxic activities), the current model cannot be extended for all the cystine‐knot peptides' actions, and other mechanisms could be taken into account. For instance, cyclotides belonging to the trypsin inhibitor subfamily are characterized by unrelated and distinct bioactivities. MCoTI cyclotides lack membrane binding properties and have been shown to be able to cross membranes of human macrophages, breast and ovarian cancer cell lines through various endocytic pathways 54, 55.
Several subfamilies of cystine‐knot proteins possess inhibitory capacity against different types of proteases, but there is no clear evidence how this capacity is related to their biological activities on human cells. One example is the cystine‐knot potato carboxypeptidase inhibitor (PCI). Biochemical and structural studies elucidated the inhibitory mechanism of PCI against metallocarboxypeptidase A 56, but this activity seemed unrelated to the PCI capacity to inhibit the growth of several lines of human pancreatic adenocarcinoma cells 57, 58.
In any case, the demonstration that cystine‐knot peptides can selectively target human proteases can be very interesting for pharmacological applications. In this regard, a recent study 59 reported that a cyclotide from Psychotria solitudinum acts as an inhibitor of the human prolyl oligopeptidase, which represents a promising target for the treatment of cognitive deficits associated with schizophrenia and Parkinson's disease.
In addition, cystine‐knot peptides with α‐amylase inhibitory capacity have been described in some medicinal plants. These proteins inhibit α‐amylases from the yellow mealworm (Tenebrio molitor), and did not show appreciable cytotoxic and haemolytic effects at concentrations up to100 μM. However, in the non‐toxic range of concentrations, they did not inhibit α‐amylases from mammals 15, 16. As the use of α‐amylase inhibitors could be beneficial for the control of starch intake in type 2 diabetes and obesity, these peptides could represent a scaffold for engineering metabolically stable human α‐amylase inhibitors 15.
The existence of multiple mechanisms of action for cystine‐knot peptides is corroborated by recent evidence demonstrating that some of these proteins can either target membrane receptors or affect components of growth factor‐related signalling pathways. Koehbach and collaborators 60 demonstrated that the molecular targets for the cyclotide kalata B7, found to induce contractility on human uterine smooth muscle cells, are the oxytocin and vasopressin V1a receptors, members of the G protein‐coupled receptor family. Another example came from the elucidation of the mechanism of action of PCI against tumour cells. Indeed, the capacity of PCI to restrict the growth of pancreatic adenocarcinoma cells was attributed to its action as antagonist of the human epidermal growth factor (EGF) 58. The PCI inhibits both the EGF‐induced dimerization and transphosphorylation of EGFR in pancreatic adenocarcinoma cells 58.
Recent studies carried out on two tomato metallocarboxypeptidase inhibitors 19, 20 demonstrated that these proteins exert antiangiogenic effects on human endothelial cells by targeting the vascular endothelial growth factor (VEGFA) signalling pathway.
Pharmacological applications
Until now, although displaying pharmaceutically relevant potential, none of the natural plant cystine‐knot peptides has reached the stage of clinical trial. One reason is the presence of contrasting activities in some members, such as a desired inhibitory activity against cancer cell growth often associated with undesired toxicity against normal cells. The unique example of a drug based on a cystine‐knot protein is the molecule developed from the venom of a marine core snail 5. A synthetic peptide derived from the conotoxin of Conus magus was approved by the US FDA for the treatment of chronic pain 61.
These molecules have attracted attention primarily for their possible use as scaffold for drug development due to the high flexibility of the structure, which combines an exceptional stability with the high tolerance to sequence modifications of the backbone portions 62. One of the most promising approaches to generate modified cystine‐knot peptides with new biological activities is molecular grafting 62, 63, where novel sequences – mainly small peptides – are substituted into the native loops of the natural molecule. Another widely used approach to produce variants of cystine‐knot peptides possessing novel or optimized molecular properties is the application of directed evolution‐based methods 64, 65. For instance, the use of knowledge‐based combinatorial miniprotein libraries has permitted the selection of variants of the cystine‐knot trypsin inhibitors from Momordica cochinchinensis and Spinacia oleracea with high‐affinity inhibitory activity against human matrypase‐1 66.
Peptides are potentially great drug leads, but their application as therapeutics is often ineffective because of their low oral bioavailability and instability in vivo. Grafting bioactive peptides into the backbone of cyclotides can overcome these limitations 67, 68. Most grafting studies have focused on cyclotide scaffolds from the Möbius (e.g. kalata B1) and trypsin inhibitor (e.g. MCoTI‐I or MCoTI‐II) subfamilies, but also on linear cystine‐knot peptides such as EET‐II from Ecballium elaterium 5. Furthermore, the increased interest in the cyclic trypsin inhibitors derived from the demonstration of their capability to penetrate the cells and therefore interact with intracellular targets 54, 55. Many different peptides have been inserted into cystine‐knot backbones with the aim to develop molecular probes for disease diagnosis and therapy 62, 63.
For instance, cystine‐knot proteins have been used as scaffolds to create new compounds that may be ligands for integrins and other receptors. A cystine‐knot peptide, which is a trypsin inhibitor obtained from Momordica cochinchinensis, was engineered to bind to cytotoxic T lymphocyte‐associated antigen‐4 (CTLA‐4), a target in the treatment of metastatic melanoma 69. The potential of engineered proteins as antiplatelet agents was also tested. The fibrinogen‐recognition sequence in αIIbβ3 was grafted into cystine‐knot microproteins from Ecballium elaterium by incorporating in the scaffold RGD‐ and KGD‐containing peptides. The engineered proteins inhibited in vitro the binding of fibrinogen to αIIbβ3, similarly to eptifibatide, but showed antiaggregatory activity only at high doses 70. A partially different approach was used to generate inhibitors of the fibrinogen receptors in platelets: engineered agouti‐related cystine‐knot protein containing an Arg‐Gly‐Asp integrin recognition motif sequence with high affinity and specificity for the integrins αIIbβ3 or αIIβb3 and αvβ3 expressed on platelet. The tested knottins proved to be potent inhibitors of platelet aggregation 71.
New molecular imaging probes including small molecules, peptides, proteins and nanoparticles are the object of investigation as diagnostic tools for the detection of cancer and the diagnosis of different diseases 72. Cystine‐knot miniproteins from plants, fungi, porifera as well as spiders, may act as ligands for the adhesion receptor integrins αvβ3, αvβ5, αvβ6 and α5β1, which are expressed on different cancer cells and activated endothelial cells in the tumour 73. Peptides derived from plants, in particular the Kalata B1 uterotonic peptide from Oldenlandia and trypsin inhibitors from Ecballium elaterium have been engineered to increase their affinity and specificity for integrins, usually by grafting a peptide sequence that allows effective interaction with the target molecule to become scaffolds for radioactive imaging probes to be used in positron emission tomography (PET), single photon emission computed tomography (SPECT) 73, 74 and also when combined with non‐radioactive probes, for fluorescence and ultrasound imaging.
Several studies have so far been performed in animal models, including human xenograft tumour, to evaluate the effectiveness of radio‐labelled cystine‐knot proteins as a diagnostic agent to be tested in glio‐ and medulloblastoma, melanoma, breast and pancreatic cancer and tumour neoangiogenesis. For instance, an 111In‐labelled agouti‐related protein (AgRP) with affinity for the integrin αvβ3 expressed in human glioblastoma xenograft 75 or 64Cu‐DOTA‐S02 that binds integrin αvβ6 with high affinity for pancreatic 76 and lung cancer 74. The knottin 99‐Tc‐SAAC‐S02 was tested for SPECT imaging of integrin αvβ6 positive tumours 77 and 8F‐FP‐3‐4‐A, an engineered peptide 78 that binds to integrin αvβ3, to obtain imaging of tumour angiogenesis.
Non‐radioactive derivatives of cystine‐knot proteins were developed for diagnostic purposes; an example is the protein EETI 2.5F, derived from Ecballium elaterium and conjugated to a near‐infrared imaging dye that specifically binds αvβ1 integrin receptor expressed in the brain tumour and in vivo illuminated mouse medulloblastoma tissue 79. A plant‐derived cystine‐knot peptide engineered for αvβ3 integrin binding, conjugated to the lipid shell of perfluorocarbon‐filled microbubbles was tested as a probe for contrast‐enhanced ultrasound imaging of tumour angiogenesis 80.
Different applications have been explored in the field of cardiovascular disease. A recent study evaluated a 64Cu‐labelled divalent cystine‐knot peptide as a probe for the identification of carotid atherosclerotic vulnerable plaques with PET. The knottin targeted the integrin αvβ3 which is highly expressed on activated endothelial cells and macrophages and may represent a specific biomarker of inflamed, vulnerable plaque. High and specific accumulation of the knottin was observed, suggesting a potential application as a diagnostic tool 81.
These studies demonstrated that engineered cystine‐knot proteins display enhanced knot stability and have stable pharmacokinetics with a fast renal clearance. When combined with high specificity and high affinity of the knottin for the molecular target 73, this results in low nonspecific accumulation in tissues and high tumour to normal tissue signal ratio. In the cited studies, compared with the traditional probes used in PET imaging, the cystine‐knot peptides did not accumulate in normal brain, myocardium and also in normal tissues 82.
A novel biological activity never documented before amongst plant cystine‐knot miniproteins was described for two metallocarboxypeptidase inhibitors from tomato, TCMP‐1 and TCMP‐2 19. These miniproteins are expressed in flowers and mature fruits. TCMPs have the capacity to inhibit angiogenesis at low concentrations (i.e. nanomolar range) without affecting endothelial cell proliferation and viability 19. The antiangiogenic properties of TCMPs were tested in vitro in human umbilical vascular cells (HUVEC) and in vivo in zebrafish 20. Using the Matrigel assay, a dose‐dependent inhibition of HUVEC tube formation was observed at TCMP concentrations in the range 20–100 nM, reaching a 64% reduction at the highest concentration 19. Furthermore, TCMPs were able to reduce by 50% the increase in cell migration induced by VEGFA 20. The effects of TCMPs were assayed in vivo using a transgenic line of zebrafish TG (kdr:eGFP) that allows the formation of the vasculature to be visualized. The treatment of the zebrafish embryos with 500 nM TCMPs impaired the formation of subintestinal vessels, a process controlled by VEGF and highly susceptible to the activity of compounds possessing antiangiogenic activity 20.
At the molecular level, the antiangiogenic effect of TCMPs is associated with the downregulation of integrin‐αV and β‐2‐microglobulin and the reduction in both VEGFA‐induced vascular endothelial growth factor receptor (VEGFR) phosphorylation and endothelial nitric oxide (NO) generation. This indicates that TCMPs target endothelial cell migration by acting on the VEGFA‐mediated signalling pathway. The mechanism that leads to the inhibition of NO release in TCMP‐treated endothelial cells is still unclear; experimental evidence demonstrates that it is associated with ERK1 inactivation, but is independent from Akt phosphorylation 19, 20. The structural similarity between TCMPs and VEGFA, which is a member of the cystine‐knot growth factor family 6, could lead to the hypothesis that TCMPs interfere with the binding of VEGFA to its receptor.
The cystine‐knot proteins have also been exploited as scaffold to develop antiangiogenic agents for cancer therapy 67, 68. A promising antiangiogenic agent has been obtained by grafting into kalata B1 a 6‐residue antiangiogenic sequence (an Arg‐rich sequence, RRKRRR) 67, 83. This Arg‐rich peptide is an antagonist for the interaction of VEGFA and its receptor. The grafted cyclotides showed biological activity in an in vitro VEGFA antagonism assay at low micromolar concentration 67. A similar approach was used to develop proangiogenic stable peptides 68 by grafting three different proangiogenic sequences into the plant‐derived MCoTI‐II trypsin inhibitor. The proangiogenic activity of the grafted cyclotides was tested in an in vivo chorioallantoic membrane assay using fertilized quail eggs. Promising results were obtained when the grafted sequence was a heptapeptide from osteopontin, demonstrating that the cystine‐knot scaffold improves the activity and stability of angiogenic peptide sequences 68.
Conclusions
One of the most interesting applications of cystine‐knot peptides in pharmacology is their use in replacing antibodies for medical applications, including targeted cancer therapy, regulated drug delivery and in vivo imaging 84. Their high enzymatic stability and good permeation behaviour are very promising for their use in the oral delivery of peptide agents. Several natural plant cystine‐knot proteins, some of which are present in edible parts of common crops, target human receptors or enzymes that play key roles in a variety of diseases. As it has been demonstrated that different pharmacophoric sequences can be incorporated into the exposed loops of the cystine‐knot proteins without changing their stability and resistance to proteolytic attack 63, it should be possible to widen and/or optimize their biological activities in human cells.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). This work was supported by the Joint Project research grant ‘Pharmacokinetic and pharmacodynamic characterization of tomato cystine knot miniproteins’ provided by the University of Verona. The authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Molesini, B. , Treggiari, D. , Dalbeni, A. , Minuz, P. , and Pandolfini, T. (2017) Plant cystine‐knot peptides: pharmacological perspectives. Br J Clin Pharmacol, 83: 63–70. doi: 10.1111/bcp.12932.
References
- 1. Silverstein KAT, Moskal WA Jr, Wu HC, Underwood BA, Graham MA, Town CD, VandenBosch KA. Small cysteine‐rich peptides resembling antimicrobial peptides have been under‐predicted in plants. Plant J 2007; 51: 262–80. [DOI] [PubMed] [Google Scholar]
- 2. Marshall E, Costa LM, Gutierrez‐Marcos J. Cysteine‐rich peptides (CRPs) mediate diverse aspects of cell–cell communication in plant reproduction and development. J Exp Bot 2011; 62: 1677–86. [DOI] [PubMed] [Google Scholar]
- 3. Murphy E, Smith S, De Smeta I. Small signaling peptides in arabidopsis development: how cells communicate over a short distance. Plant Cell 2012; 24: 3198–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guzmán‐Rodríguez JJ, Ochoa‐Zarzosa A, López‐Gómez R, López‐Meza JE. Plant antimicrobial peptides as potential anticancer agents. Biomed Res Int 2015; 2015: : 735087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daly NL, Craik DJ. Bioactive cystine knot proteins. Curr Opin Chem Biol 2011; 15: 362–8. [DOI] [PubMed] [Google Scholar]
- 6. Iyer S, Acharya KR. Tying the knot: the cystine signature and molecular‐recognition processes of the vascular endothelial growth factor family of angiogenic cytokines. FEBS J 2011; 278: 4304–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gracy J, Le‐Nguyen D, Gelly JC, Kaas Q, Heitz A, Chiche L. KNOTTIN: the knottin or inhibitor cystine knot scaffold in 2007. Nucleic Acids Res 2008; 36: D314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gelly JC, Gracy J, Kaas Q, Le‐Nguyen D, Heitz A, Chiche L. The KNOTTIN website and database: a new information system dedicated to the knottin scaffold. Nucleic Acids Res 2004; 32: D156–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rees DC, Lipscomb WN. Structure of potato inhibitor complex of carboxypeptidase A at 5.5‐A resolution. Proc Natl Acad Sci U S A 1980; 77: 277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hass GM, Hermodson MA. Amino acid sequence of a carboxypeptidase inhibitor from tomato fruit. Biochemistry 1981; 20: 2256–60. [DOI] [PubMed] [Google Scholar]
- 11. Pear JR, Ridge N, Rasmussen R, Rose RE, Houck CM. Isolation and characterization of a fruit‐specific cDNA and the corresponding genomic clone from tomato. Plant Mol Biol 1989; 13: 639–51. [DOI] [PubMed] [Google Scholar]
- 12. Molnár A, Lovas A, Bánfalvi Z, Lakatos L, Polgár Z, Horváth S. Tissue‐specific signal(s) activate the promoter of a metallocarboxypeptidase inhibitor gene family in potato tuber and berry. Plant Mol Biol 2001; 46: 301–11. [DOI] [PubMed] [Google Scholar]
- 13. He WJ, Chan LY, Clark RJ, Tang J, Zeng GZ, Franco OL, Cantacessi C, Craik DJ, Daly NL, Tan NH. Novel inhibitor cystine knot peptides from Momordica charantia . PLoS One 2013; 8: e75334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan LY, He W, Tan N, Zeng G, Craik DJ, Daly NL. A new family of cystine knot peptides from the seeds of Momordica cochinchinensis . Peptides 2013; 39: 29–35. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen PQ, Wang S, Kumar A, Yap LJ, Luu TT, Lescar J, Tam JP. Discovery and characterization of pseudocyclic cystine‐knot α‐amylase inhibitors with high resistance to heat and proteolytic degradation. FEBS J 2014; 281: 4351–66. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen PQ, Luu TT, Bai Y, Nguyen GK, Pervushin K, Tam JP. Allotides: proline‐rich cystine knot α‐amylase inhibitors from Allamanda cathartica . J Nat Prod 2015; 78: 695–704. [DOI] [PubMed] [Google Scholar]
- 17. Quilis J, López‐García B, Meynard D, Guiderdoni E, San Segundo B. Inducible expression of a fusion gene encoding two proteinase inhibitors leads to insect and pathogen resistance in transgenic rice. Plant Biotechnol J 2014; 12: 367–77. [DOI] [PubMed] [Google Scholar]
- 18. Tam JP, Wang S, Wong KH, Tan WL. Antimicrobial peptides from plants. Pharmaceuticals 2015; 8: 711–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cavallini C, Trettene M, Degan M, Delva P, Molesini B, Minuz P, Pandolfini T. Antiangiogenic effects of two cystine‐knot miniproteins from tomato fruit. Br J Pharmacol 2011; 162: 1261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Treggiari D, Zoccatelli G, Molesini B, Degan M, Rotino GL, Sala T, Cavallini C, MacRae CA, Minuz P, Pandolfini T. A cystine‐knot miniprotein from tomato fruit inhibits endothelial cell migration and angiogenesis by affecting vascular endothelial growth factor receptor (VEGFR) activation and nitric oxide production. Mol Nutr Food Res 2015; 59: 2255–66. [DOI] [PubMed] [Google Scholar]
- 21. Gruber CW, Elliott AG, Ireland DC, Delprete PG, Dessein S, Goransson U, Trabi M, Wang CK, Kinghorn AB, Robbrecht E, Craik DJ. Distribution and evolution of circular miniproteins in flowering plants. Plant Cell 2008; 20: 2471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang CK, Kaas Q, Chiche L, Craik DJ. CyBase: a database of cyclic protein sequences and structures, with applications in protein discovery and engineering. Nucleic Acids Res 2008; 36: D206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Craik DJ. Host‐defense activities of cyclotides. Toxins 2012; 4: 139–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jennings C, West J, Waine C, Craik D, Anderson M. Biosynthesis and insecticidal properties of plant cyclotides: the cyclic knotted proteins from Oldenlandia affinis . Proc Natl Acad Sci U S A 2001; 98: 10614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jennings CV, Rosengren KJ, Daly NL, Plan M, Stevens J, Scanlon MJ, Waine C, Norman DG, Anderson MA, Craik DJ. Isolation, solution structure, and insecticidal activity of kalata B2, a circular protein with a twist: do Mobius strips exist in nature? Biochemistry 2005; 44: 851–60. [DOI] [PubMed] [Google Scholar]
- 26. Plan MR, Saska I, Cagauan AG, Craik DJ. Backbone cyclised peptides from plants show molluscicidal activity against the rice pest Pomacea canaliculata (golden apple snail). J Agric Food Chem 2008; 56: 5237–41. [DOI] [PubMed] [Google Scholar]
- 27. Gustafson KR, Sowder RC, Henderson LE, Parsons IC, Kashman Y, Cardellina JH, McMahon JB, Buckheit RW, Pannell LK, Boyd MR. Circulins A and B. Novel human immunodeficiency virus (HIV)‐inhibitory macrocyclic peptides from the tropical tree Chassalia parvifolia . J Am Chem Soc 1994; 116: 9337–8. [Google Scholar]
- 28. Ireland DC, Wang CK, Wilson JA, Gustafson KR, Craik DJ. Cyclotides as natural anti‐HIV agents. Biopolymers 2008; 90: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Craik DJ, Daly NL, Bond T, Waine C. Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol 1999; 294: 1327–36. [DOI] [PubMed] [Google Scholar]
- 30. Craik DJ, Daly NL, Mulvenna J, Plan MR, Trabi M. Discovery, structure and biological activities of the cyclotides. Curr Protein Pept Sci 2004; 5: 297–315. [DOI] [PubMed] [Google Scholar]
- 31. Colgrave ML, Craik DJ. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry 2004; 43: 5965–75. [DOI] [PubMed] [Google Scholar]
- 32. Daly NL, Rosengren KJ, Craik DJ. Discovery, structure and biological activities of cyclotides. Adv Drug Deliv Rev 2009; 61: 918–30. [DOI] [PubMed] [Google Scholar]
- 33. Burman R, Gunasekera S, Strömstedt AA, Göransson U. Chemistry and biology of cyclotides: circular plant peptides outside the box. J Nat Prod 2014; 77: 724–36. [DOI] [PubMed] [Google Scholar]
- 34. Craik DJ, Cemazar M, Wang CK, Daly NL. The cyclotide family of circular miniproteins: nature's combinatorial peptide template. Biopolymers 2006; 84: 250–66. [DOI] [PubMed] [Google Scholar]
- 35. Gruber CW, Cemazar M, Anderson MA, Craik DJ. Insecticidal plant cyclotides and related cystine knot toxins. Toxicon 2007; 49: 561–75. [DOI] [PubMed] [Google Scholar]
- 36. Kaas Q, Craik DJ. Analysis and classification of circular proteins in CyBase. Biopolymers 2010; 94: 584–91. [DOI] [PubMed] [Google Scholar]
- 37. Park S, Strömstedt AA, Göransson U. Cyclotide structure–activity relationships: qualitative and quantitative approaches linking cytotoxic and anthelmintic activity to the clustering of physicochemical forces. PLoS One 2014; 9: : e91430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poth AG, Colgrave ML, Lyons RE, Daly NL, Craik DJ. Discovery of an unusual biosynthetic origin for circular proteins in legumes. Proc Natl Acad Sci U S A 2011; 108: 1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poth AG, Mylne JS, Grassl J, Lyons RE, Millar AH, Colgrave ML, Craik DJ. Cyclotides associate with leaf vasculature and are the products of a novel precursor in petunia (Solanaceae). J Biol Chem 2012; 287: 27033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henriques ST, Craik DJ. Cyclotides as templates in drug design. Drug Discov Today 2010; 15: 57–64. [DOI] [PubMed] [Google Scholar]
- 41. Colgrave ML, Kotze AC, Ireland DC, Wang CK, Craik DJ. The anthelmintic activity of the cyclotides: natural variants with enhanced activity. Chembiochem 2008; 9: 1939–45. [DOI] [PubMed] [Google Scholar]
- 42. Colgrave ML, Kotze AC, Kopp S, McCarthy JS, Coleman GT, Craik DJ. Anthelmintic activity of cyclotides: in vitro studies with canine and human hookworms. Acta Trop 2009; 109: 163–6. [DOI] [PubMed] [Google Scholar]
- 43. Oguis GK, Kan M‐W, Craik DJ. Natural functions and structure–activity relationships of cyclotides. Adv Bot Res 2015; 76: 187–226. [Google Scholar]
- 44. Craik DJ, Swedberg JE, Mylne JS, Cemazar M. Cyclotides as a basis for drug design. Expert Opin Drug Discov 2012; 7: 179–94. [DOI] [PubMed] [Google Scholar]
- 45. Gran L, Sletten K, Skjeldal L. Cyclic peptides from Oldenlandia affinis DC. Molecular and biological properties. Chem Biodivers 2008; 5: 2014–22. [DOI] [PubMed] [Google Scholar]
- 46. Tam JP, Lu YA, Yang JL, Chiu KW. An unusual structural motif of antimicrobial peptides containing end‐to‐end macrocycle and cystine‐knot disulfides. Proc Natl Acad Sci U S A 1999; 96: 8913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tang J, Wang CK, Pan X, Yan H, Zeng G, Xu W, He W, Daly NL, Craik DJ, Tan N. Isolation and characterization of cytotoxic cyclotides from Viola tricolor . Peptides 2010; 31: 1434–40. [DOI] [PubMed] [Google Scholar]
- 48. Strömstedt AA, Ringstad L, Schmidtchen A, Malmsten M. Interaction between amphiphilic peptides and phospholipid membranes. Curr Opin Colloid Interface Sci 2010; 15: 467–78. [Google Scholar]
- 49. Kuzmenkov AI, Vassilevski AA, Kudryashova KS, Nekrasova OV, Peigneur S, Tytgat J, Feofanov AV, Kirpichnikov MP, Grishin EV. Variability of potassium channel blockers in Mesobuthus eupeus scorpion venom with focus on Kv1.1: an integrated transcriptomic and proteomic study. J Biol Chem 2015; 290: 12195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tilley DC, Eum KS, Fletcher‐Taylor S, Austin DC, Dupré C, Patrón LA, Garcia RL, Lam K, Yarov‐Yarovoy V, Cohen BE, Sack JT. Chemoselective tarantula toxins report voltage activation of wild‐type ion channels in live cells. Proc Natl Acad Sci U S A 2014; 111: E4789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park JH, Carlin KP, Wu G, Ilyin VI, Musza LL, Blake PR, Kyle DJ. Studies examining the relationship between the chemical structure of protoxin II and its activity on voltage gated sodium channels. J Med Chem 2014; 57: 6623–31. [DOI] [PubMed] [Google Scholar]
- 52. Liu Z, Cai T, Zhu Q, Deng M, Li J, Zhou X, Zhang F, Li D, Li J, Liu Y, Hu W, Liang S. Structure and function of hainantoxin‐III, a selective antagonist of neuronal tetrodotoxin‐sensitive voltage‐gated sodium channels isolated from the Chinese bird spider Ornithoctonus hainana . J Biol Chem 2013; 288: 20392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rodríguez AA, Salceda E, Garateix AG, Zaharenko AJ, Peigneur S, López O, Pons T, Richardson M, Díaz M, Hernández Y, Ständker L, Tytgat J, Soto E. A novel sea anemone peptide that inhibits acid‐sensing ion channels. Peptides 2014; 53: 3–12. [DOI] [PubMed] [Google Scholar]
- 54. Greenwood KP, Daly NL, Brown DL, Stow JL, Craik DJ. The cyclic cystine knot miniprotein MCoTI‐II is internalized into cells by macropinocytosis. Int J Biochem Cell Biol 2007; 39: 2252–64. [DOI] [PubMed] [Google Scholar]
- 55. Contreras J, Elnagar AYO, Hamm‐Alvarez S, Camarero JA. Cellular uptake of cyclotide MCoTI‐I follows multiple endocytic pathways. J Control Release 2011; 155: 134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vendrell J, Querol E, Aviles FX. Metallocarboxy peptidases and their protein inhibitors: structure, function and biomedical processes. Biochim Biophys Acta 2000; 1477: 284–98. [DOI] [PubMed] [Google Scholar]
- 57. Blanco‐Aparicio C, Molina MA, Fernandez‐Salas E, Frazier ML, Mas JM, Querol E, Avilés FX, de Llorens R. Potato carboxypeptidase inhibitor, a T‐knot protein, is an epidermal growth factor antagonist that inhibits tumor cell growth. J Biol Chem 1998; 273: 12370–7. [DOI] [PubMed] [Google Scholar]
- 58. Sitja‐Arnaud M, Molina MA, Blanco‐Aparicio C, Ferrer‐Soler L, Lorenzo J, Aviles FX, Querol E, de Llorens R. Mechanism of action of potato carboxypeptidase inhibitor (PCI) as an EGF blocker. Cancer Lett 2005; 226: 169–84. [DOI] [PubMed] [Google Scholar]
- 59. Hellinger R, Koehbach J, Puigpinós A, Clark RJ, Tarragó T, Giralt E, Gruber CW. Inhibition of human prolyl oligopeptidase activity by the cyclotide psysol 2 isolated from Psychotria solitudinum . J Nat Prod 2015; 78: 1073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koehbach J, O'Brien M, Muttenthaler M, Miazzo M, Akcan M, Elliott AG, Daly NL, Harvey PJ, Arrowsmith S, Gunasekera S, Smith TJ, Wray S, Göransson U, Dawson PE, Craik DJ, Freissmuth M, Gruber CW. Oxytocic plant cyclotides as templates for peptide G protein‐coupled receptor ligand design. Proc Natl Acad Sci U S A 2013; 110: 21183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Williams JA, Day M, Heavner JE. Ziconotide: an update and review. Expert Opin Pharmacother 2008; 9: 1575–83. [DOI] [PubMed] [Google Scholar]
- 62. Gould A, Ji Y, Aboye TL, Camarero JA. Cyclotides, a novel ultrastable polypeptide scaffold for drug discovery. Curr Pharm Des 2011; 17: 4294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Poth AG, Chan LY, Craik DJ. Cyclotides as grafting frameworks for protein engineering and drug design applications. Biopolymers 2013; 100: 480–91. [DOI] [PubMed] [Google Scholar]
- 64. Laihti JL, Silverman AP, Cochran JR. Interrogating and predicting tolerated sequence diversity in protein folds: application to E. elaterium trypsin inhibitor‐II cystine‐knot miniprotein. PLoS Comput Biol 2009; 5: e1000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sommerhoff CP, Avrutina O, Schmoldt HU, Gabrijelcic‐Geiger D, Diederichsen U, Kolmar H. Engineered cystine knot miniproteins as potent inhibitors of human mast cell tryptase beta. J Mol Biol 2010; 395: 167–75. [DOI] [PubMed] [Google Scholar]
- 66. Glotzbach B, Reinwarth M, Weber N, Fabritz S, Tomaszowski M, Fittler H, Christmann A, Avrutina O, Kolmar H. Combinatorial optimization of cystine‐knot peptides towards high‐affinity inhibitors of human matriptase‐1. PLoS One 2013; 8: e76956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gunasekera S, Foley FM, Clark RJ, Sando L, Fabri LJ, Craik DJ, Daly NL. Engineering stabilized vascular endothelial growth factor‐A antagonists: synthesis, structural characterization, and bioactivity of grafted analogues of cyclotides. J Med Chem 2008; 51: 7697–704. [DOI] [PubMed] [Google Scholar]
- 68. Chan LY, Gunasekera S, Henriques ST, Worth NF, Le SJ, Clark RJ, Campbell JH, Craik DJ, Daly NL. Engineering pro‐angiogenic peptides using stable, disulfide‐rich cyclic scaffolds. Blood 2011; 118: 6709–17. [DOI] [PubMed] [Google Scholar]
- 69. Maaß F, Wüstehube‐Lausch J, Dickgießer S, Valldorf B, Reinwarth M, Schmoldt HU, Daneschdar M, Avrutina O, Sahin U, Kolmar H. Cystine‐knot peptides targeting cancer‐relevant human cytotoxic T lymphocyte‐associated antigen 4 (CTLA‐4). J Pept Sci 2015; 21: 651–60. [DOI] [PubMed] [Google Scholar]
- 70. Reiss S, Sieber M, Oberle V, Wentzel A, Spangenberg P, Claus R, Kolmar H, Lösche W. Inhibition of platelet aggregation by grafting RGD and KGD sequences on the structural scaffold of small disulfide‐rich proteins. Platelets 2006; 17: 153–7. [DOI] [PubMed] [Google Scholar]
- 71. Silverman AP, Kariolis MS, Cochran JR. Cystine‐knot peptides engineered with specificities for α(IIb)β(3) or α(IIb)β(3) and α(v)β(3) integrins are potent inhibitors of platelet aggregation. J Mol Recognit 2011; 24: 127–35. [DOI] [PubMed] [Google Scholar]
- 72. Moore SJ, Cochran JR. Engineering knottins as novel binding agents. Methods Enzymol 2012; 503: 223–51. [DOI] [PubMed] [Google Scholar]
- 73. Kimura RH, Levin AM, Cochran FV, Cochran JR. Engineered cystine knot peptides that bind alphavbeta3, alphavbeta5, and alpha5beta1 integrins with low‐nanomolar affinity. Proteins 2009; 77: 359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nielsen CH, Kimura RH, Withofs N, Tran PT, Miao Z, Cochran JR, Cheng Z, Felsher D, Kjær A, Willmann JK, Gambhir SS. ET imaging of tumor neovascularization in a transgenic mouse model with a novel 64Cu‐DOTA‐knottin peptide. Cancer Res 2010; 70: 9022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jiang L, Miao Z, Kimura RH, Silverman AP, Ren G, Liu H, Lu H, Cochran JR, Cheng Z. 111In‐labeled cystine‐knot peptides based on the Agouti‐related protein for targeting tumor angiogenesis. J Biomed Biotechnol 2012; 2012: 368075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kimura RH, Teed R, Hackel BJ, Pysz MA, Chuang CZ, Sathirachinda A, Willmann JK, Gambhir SS. Pharmacokinetically stabilized cystine knot peptides that bind alpha‐v‐beta‐6 integrin with single‐digit nanomolar affinities for detection of pancreatic cancer. Clin Cancer Res 2012; 18: 839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhu X, Li J, Hong Y, Kimura RH, Ma X, Liu H, Qin C, Hu X, Hayes TR, Benny P, Gambhir SS, Cheng Z. 99mTc‐labeled cystine knot peptide targeting integrin αvβ6 for tumor SPECT imaging. Mol Pharm 2014; 11: 1208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jiang L, Kimura RH, Ma X, Tu Y, Miao Z, Shen B, Chin FT, Shi H, Gambhir SS, Cheng Z. A radiofluorinated divalent cystine knot peptide for tumor PET imaging. Mol Pharm 2014; 11: 3885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Moore SJ, Hayden Gephart MG, Bergen JM, Su YS, Rayburn H, Scott MP, Cochran JR. Engineered knottin peptide enables noninvasive optical imaging of intracranial medulloblastoma. Proc Natl Acad Sci U S A 2013; 110: 14598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Willmann JK, Kimura RH, Deshpande N, Lutz AM, Cochran JR, Gambhir SS. Targeted contrast‐enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin‐binding knottin peptides. J Nucl Med 2010; 51: 433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jiang L, Tu Y, Kimura RH, Habte F, Chen H, Cheng K, Shi H, Gambhir SS, Cheng Z. 64Cu‐labeled divalent cystine knot peptide for imaging carotid atherosclerotic plaques. J Nucl Med 2015; 56: 939–44. [DOI] [PubMed] [Google Scholar]
- 82. Moore SJ, Leung CL, Cochran JR. Knottins: disulfide‐bonded therapeutic and diagnostic peptides. Drug Discov Today Tech 2012; 9: e3–e11. [DOI] [PubMed] [Google Scholar]
- 83. Bae DG, Gho YS, Yoon WH, Chae CB. Arginine‐rich anti‐vascular endothelial growth factor peptides inhibit tumor growth and metastasis by blocking angiogenesis. J Biol Chem 2000; 275: 13588–96. [DOI] [PubMed] [Google Scholar]
- 84. Banta S, Dooley K, Shur O. Replacing antibodies: engineering new binding proteins. Annu Rev Biomed Eng 2013; 15: 93–113. [DOI] [PubMed] [Google Scholar]


