Abstract
There are widespread inconsistencies and contradictions in the many published definitions of ‘nutraceuticals’ and ‘functional foods’, demonstrating wholesale uncertainty about what they actually are. Furthermore, in a 2014 lecture, the inventor of the term ‘nutraceutical’, confessing that nutraceuticals do not work, said that ‘the quest to demonstrate whether … long‐term supplementation [with nutraceuticals] can prevent serious diseases … has come to an end’. Definitions of ‘nutraceuticals’ and related terms, still widely used, should therefore be explored systematically. There are no internationally agreed definitions of ‘nutraceuticals’ and ‘functional foods’, or of similar terms, such as ‘health foods’, or of terms related to herbal products, which are sometimes referred to as ‘nutraceuticals’, compounding the confusion. ‘Nutraceuticals’ and ‘functional foods’ are vague, nondiscriminatory, unhelpful terms; the evidence suggests that they should be abandoned in favour of more precise terms. The term ‘dietary supplement’ is widely used to designate formulations that are also called ‘nutraceuticals’ but it would be better restricted to individual compounds used to treat or prevent deficiencies. ‘Fortified foods’, sometimes called ‘designer foods’, are foods to which compounds of proven therapeutic or preventive efficacy (e.g. folic acid) have been added. Other terms, such as ‘food’, ‘foodstuffs’, ‘eat’, ‘drink’, and ‘nutrition’, are well defined, as are ‘medicinal products’ and ‘pharmaceutical formulations’. Dietary regimens, such as Mediterranean or nitrate‐rich diets or vegetarianism, can affect health. A dietary regimen of this kind can be defined as a programme of food, of a defined kind and/or quantity, prescribed or adopted for the restoration or preservation of health.
Keywords: designer foods, dietary regimens, dietary supplements, fortified foods, functional foods, nutraceuticals
Introduction
‘So where do we go from here? Well, I think, again I'm not sure, that the quest to demonstrate whether chronic administration, long‐term diet, long‐term supplementation, can prevent serious diseases like cancer, heart disease, dementia, arthritis has come to an end’.
Stephen L. DeFelice, 2014
The above quotation comes from a 2014 lecture, quoted more fully below 1, by the man who invented the word ‘nutraceutical’. The evidence that nutraceuticals are efficacious is vague and often conflicting, and their reputation is inflated by poor trial design and exaggerated for commercial purposes 2, 3.
Here, I explore definitions of ‘nutraceuticals’ and related words.
Methods
I use a fourfold linguistic approach to definition 4, exploring etymology, usage, previous attempts at definition, and operational aspects of the definiendum. Synthesis of these generally yields satisfactory definitions.
For etymologies, I generally begin with the earliest identifiable linguistic root. In English, that is usually a theoretical construct in the system known as Indo‐European, which hypothesizes that a large range of languages, including Albanian, Armenian, Balto‐Slavic, Celtic, Germanic, Hellenic, Indo‐Iranian, Italic, and some extinct languages, have descended from a single parent language, known as Proto‐Indo‐European. For usage (i.e. ‘senses relating to habit, custom or practice’), I consult the Oxford English Dictionary (OED) 5, which gives the earliest known occurrences of specific meanings; I chart these on time lines. For previous definitions, I undertake systematic searches of the usual databases (such as Pubmed, Embase, and the Cochrane Library). I synthesize these different pieces of information, combining them, when appropriate, with the Ramsey–Lewis method, which recognizes that the meaning of a term is implied by the relevant scientific theory, including all the assertions that it makes about the term 6 and, in the case of clinical terms, an understanding of the practical aspects of the relevant theory.
Using these approaches, I have explored the definitions of six groups of interrelated words:
Food and foodstuffs, eat and drink, diet and dietary regimen, nutrition.
Medicinal products and pharmaceutical formulations.
Herbal products.
Nutraceuticals and functional foods.
Fortified foods.
Dietary supplements.
Examples
Examples of compounds that have been derived from plants, animals, and micro‐organisms are listed in Table 1. Other compounds are also included in the table, to demonstrate the confusion that can arise when substances from similar sources are classified differently. For example, digoxin from foxglove leaves is formulated as a medicinal product (as are several other plant‐derived drugs 8), while extracts from green tea leaves are regarded as nutraceuticals 9.
Table 1.
Examples of compounds derived from plants, animals, and micro‐organisms
| Natural source | Compounds of proven efficacy (indication) | Compounds of unproven or doubtful efficacy, or an unfavourable benefit/harm balance, or not used because of toxicity |
|---|---|---|
| Plants | ||
| Herbs | Hypericum perforatum (mild depression) | Echinacea; Piper methysticum; Aristolochia |
| Nonherbal plants | ||
| Trees/vines | ||
| Leaves | Artemisinin (malaria); digoxin (atrial fibrillation) | Green tea (catechins, polyphenols*) |
| Bark | Quinine (malaria); salicylates (fevers); taxol (cancers) | |
| Seeds/pods | Ispaghula (laxative); senna (laxative) | Guaraná |
| Roots | Emetine (amoebiasis) | |
| Fruits | Raspberry ketone; resveratrol (a polyphenol) | |
| Nuts | Omega‐3 fatty acids; arecoline | |
| Cereals | Prebiotics and dietary fibre; Zea mays | |
| Vegetables | Capsaicin (postherpetic neuralgia) | Prebiotics and dietary fibre |
| Mammals | ||
| Dairy products | Milk hydrolysates; ghee | |
| Tissues | Insulin (diabetes mellitus); growth hormone (growth impairment); melatonin (jet lag) | Melatonin (aid to sleep); bear bile; gangliosides; glycosaminoglycans |
| Fish, shellfish | Calcitonin | Omega‐3 fatty acids; tetrodotoxin; carp bile; green tipped mussel; imedeen; oyster extract; shark cartilage; squalene |
| Reptiles | Dendrotoxin; rattlesnake meat; toad venom | |
| Worms | Hirudin (anticoagulant) | |
| Insects | Apamin; cantharides; charybdotoxin; propolis; royal jelly | |
| Micro‐organisms | ||
| Fungi | Penicillin (infections) | Psilocybin; mycotoxins |
| Algae | Laminaria | |
| Bacteria | Antibiotics from Actinomycetes, e.g. streptomycin, tetracyclines, macrolides (infections) | Probiotics (e.g. bifidobacteria, Escherichia coli Nissle 1917, lactobacilli); Kombucha ‘mushroom’ |
Natural polyphenols form a diverse group of hundreds of different substances, including flavonoids and nonflavonoids, found widespread in plants, insects, crustaceans, and other animals 7.
Definitions
Foodstuffs are at the heart of the definitions of the first group of words.
Food and foodstuffs
The etymology and time line of usage of ‘food’ are shown in Figure 1. Food is typically something that is consumed by animals or humans, or applied to plants for purposes of nutrition. A legal definition of food is laid down in Article 2 of EC regulation No 178/2002, restricted to human uses 10. This definition specifies that food is ‘any item that is to be processed, partially processed or unprocessed for consumption, and that is intended to be, or reasonably expected to be, ingested by humans’. The definition specifically excludes the following categories:
Animal feed;
Live animals (unless being prepared for sale in a market);
Plants before harvesting;
Medicinal products;
Cosmetics;
Tobacco and tobacco products;
Narcotic or psychotropic substances;
Residues and contaminants.
Figure 1.
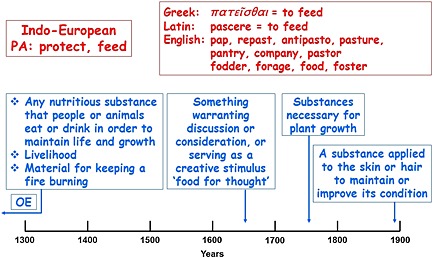
The etymology (in red) and time line of usage (in blue) of ‘food’; OE, Old English; the dates are the earliest citations listed in the Oxford English Dictionary
The definition encompasses liquid foods, but not water, which is not conventionally regarded as a foodstuff.
The exclusion of medicinal products in this definition is noteworthy. However, the definition does not exclude the therapeutic use of foods in other types of product.
Foodstuffs are defined as ‘particular substances suitable for consumption as food’. Foods consist of primary foodstuffs (particularly meat, fish, eggs, dairy products, fruits, vegetables, and cereals, containing proteins, carbohydrates, fats, vitamins, and minerals) and additives (herbs and spices, sweeteners, colourings, preservatives, antioxidants, emulsifiers, thickeners, stabilizers, and carrier solvents).
Eat and drink
The etymology and time line of usage of ‘eat’ are shown in Figure 2. Its main sense relates to the consumption of food. Other meanings attested to by its usage over the centuries are figurative. In passing, it is worth noting that the Greek word, ὀδύνη, which is related to ἔδειν, to eat, means pain, as anything that eats you will cause you pain; this gives us painful words such as glossodynia, mastodynia, oneirodynia, and pleurodynia; anodyne means free of pain. Dr Collis Browne's Chlorodyne was a patent medicine, advertised as having anodyne and hypnotic properties, which it did, by virtue of the laudanum, Indian hemp, and chloroform it contained, which also explained its adverse effects 11. All this, incidentally, reminds us that interventions that have beneficial effects, and some that do not, can also have harmful ones.
Figure 2.
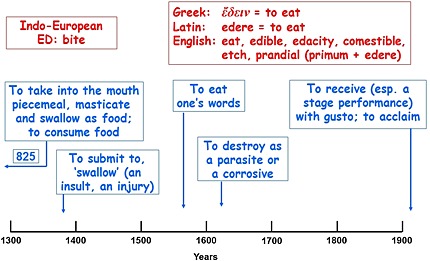
The etymology (in red) and time line of usage (in blue) of ‘eat’; the dates are the earliest citations listed in the Oxford English Dictionary
To drink is defined in the OED as ‘to take (liquid) into the stomach; … to swallow down or imbibe water or other liquid, for nourishment or quenching of thirst’. Its Indo‐European root DHREG, to drag or slide, also gives words such as drag, draught, draw, drench, and drown.
Diet and dietary regimen
The etymology and time line of usage of ‘diet’ are shown in Figure 3. It usually refers to the use of a range of foods, although it can have figurative meanings. Even when it means a way of life or a regimen, it specifically refers to a therapeutic, or at least a beneficial, one. Dietary regimens, such as the Mediterranean diet 12, vegetarianism 13, 14, or diets that are high in nitrates and nitrites 15, which affect health in different ways, should be distinguished from individual compounds with supposed but unproven efficacy. Taking together the relevant definitions of ‘diet’ and ‘regimen’ from the OED, and recognizing that in treating malnutrition the amount of food in a regimen may need to be increased rather than decreased, a dietary regimen specifically implemented for therapeutic purposes in this way can be defined as ‘a programme of food, of a defined kind or quantity, prescribed or adopted for the restoration or preservation of health’.
Figure 3.
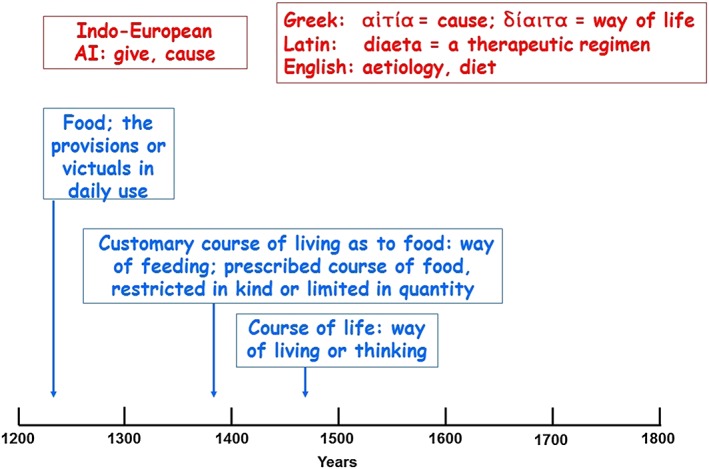
The etymology (in red) and time line of usage (in blue) of ‘diet’; the dates are the earliest citations listed in the Oxford English Dictionary
Nutrition and nutrient
The etymology and time line of usage of the nouns ‘nutrition’ and ‘nutrient’ are shown in Figure 4. Apart from a technical pharmaceutical meaning, the preparation of an ointment by the mixing (emulsification) of an oil and vinegar or other liquid, the action of providing nutrition generally refers to the use of foodstuffs to nourish, which is ‘to sustain the life or health of (a person, animal, or part of the body) with food or proper nutriment’. ‘Nutrient’ is defined as ‘a nutritious substance’, reflecting the role of nutrients as functionally active components of the diet; the first citation in the OED, from 1828, cites Noah Webster's American Dictionary of the English Language, in which nutrient is defined as ‘any substance which nourishes by promoting the growth or repairing the waste of animal bodies’.
Figure 4.
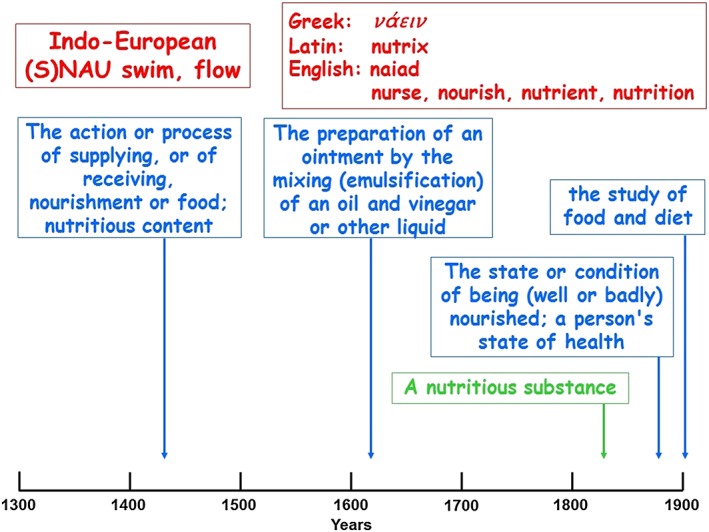
The etymology (in red) and time line of usages of ‘nutrition’ (in blue) and ‘nutrient’ (in green); the dates are the earliest citations listed in the Oxford English Dictionary
Medicinal products and pharmaceutical formulations
The definitions of these terms have previously been discussed in detail 16.
A medicinal product is one that contains a compound with proven pharmacological and beneficial therapeutic effects, a drug, prodrug, or a cellular element, plus excipients, or excipients only; it may also contain adulterants or contaminants.
A codicil to this definition states that a medicinal product is one that is intended to be taken by or administered to a person or animal for one or more of the following reasons:
as a placebo;
to prevent a disease;
to make a diagnosis;
to test for the possibility of an adverse effect or reaction;
to modify a physiological, biochemical or anatomical function or abnormality;
to replace a missing factor;
to ameliorate a symptom;
to treat a disease;
to induce anaesthesia.
The terms ‘medicine’ and ‘medicament’ are acceptable synonyms for ‘medicinal product’. The term ‘drug’ is often used colloquially to mean a medicinal product (as in ‘adverse drug reaction’) but it is important to remember the distinction between the drug itself (the active component) and the whole product. For definitive regulatory or legislative purposes, the more precise term ‘medicinal product’ is preferable.There are five broad categories of the drugs contained in medicinal products:
Naturally occurring compounds derived in pure form from plants, animals or micro‐organisms (e.g. digoxin, melatonin, antibiotics, vaccines).
Elemental salts (e.g. lithium chloride, zinc sulfate).
Semisynthetic compounds derived from or related to compounds in plants (e.g. docetaxel).
Small synthetic molecules (i.e. most licensed medicines).
Biologics (e.g. monoclonal antibodies).
A pharmaceutical formulation, also called a ‘dosage form’, is the form in which a medicinal product is presented – e.g. as a tablet, capsule, elixir, solution for injection, transdermal formulation, cream, or ointment. The commonly used term ‘preparation’ is ambiguous, as it can refer to the pure substance itself (e.g. as prepared from a plant) or to the formulation. However, the term ‘preparation’ is justifiably used in referring to herbal products, which are often crude extracts and not pharmaceutical formulations.
Herbal products
The definition of a herb in the OED is ‘a plant of which the stem does not become woody and persistent (as in a shrub or a tree) but remains more or less soft and succulent, and dies down to the ground (or entirely) after flowering; spec. applied to plants of which the leaves, or stem and leaves, are used for food or medicine, or in some way for their scent or flavour’.
This definition makes it clear that many herbal products are prepared from plants that are not strictly speaking herbs.
There is no internationally recognized definition of a herbal medicine 17. The European Union (EU) has official definitions for terms related to herbal products – namely, ‘herbal substances (or herbal drugs)’, ‘herbal preparations’, and ‘herbal medicinal products’ 18.
A herbal substance consists of mainly whole, fragmented, or cut plants, plant parts, algae, fungi, or lichen in an unprocessed, usually dried form but sometimes fresh.
A herbal preparation is one that is obtained by subjecting herbal substances to treatments such as extraction, distillation, expression, fractionation, purification, concentration, or fermentation.
A herbal medicinal product is any medicinal product exclusively containing as active ingredients one or more herbal substances or one or more herbal preparations, or one or more such herbal substances in combination with one or more such herbal preparations.
To the last definition is added a note that ‘the product may contain vitamins or minerals for which there is well‐documented evidence for safety, provided that the action of the vitamins or minerals is ancillary to that of the herbal active ingredients regarding the specific claimed indications’.
The constituents of herbal medicines have been described by the World Health Organization as including ‘herbs, herbal materials, herbal preparations and finished herbal products’ and ‘in some countries … by tradition, natural organic or inorganic active ingredients that are not of plant origin (e.g. animal and mineral materials)’ 19.
Nutraceuticals and functional foods
Nutraceuticals
The term ‘nutraceutical’ was invented in 1989 by Stephen L. Defelice, who established The Foundation for Innovation in Medicine in 1976 20. Use of the word, judged from frequencies in papers indexed in Pubmed, has increased steeply since about 2000 (Figure 5).
Figure 5.
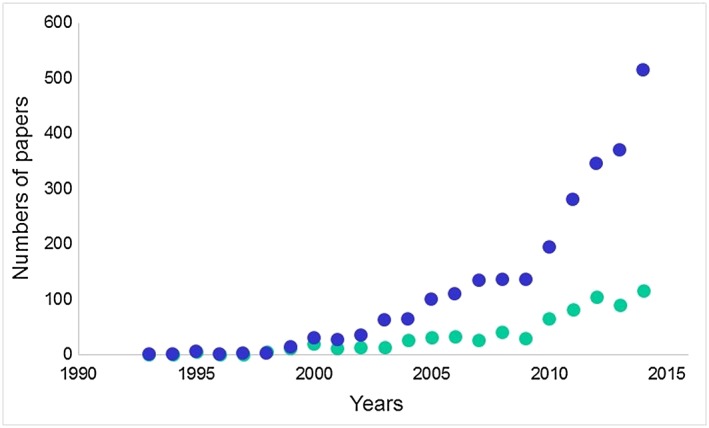
The numbers of papers in Pubmed retrieved by searching for ‘nutraceutical[s]’ in papers (blue symbols) or titles only (green symbols)
There is no internationally recognized definition of a nutraceutical, and various confusing and contradictory definitions have appeared. All of the proposed meanings are covered more precisely by other terms.
In an interview with Sheldon Baker on 28 October 2011, DeFelice defined a nutraceutical as ‘a food or part of a food, such as a dietary supplement, that has a medical or health benefit, including the prevention and treatment of disease’ 21. It is defined in the OED as ‘a foodstuff, food additive, or dietary supplement that has beneficial physiological effects but is not essential to the diet. Also called functional food. And in the proposed US Nutraceutical Research and Education Act, which was presented to the House of Representatives in the first session of the 106th Congress of 1999–2000 but not promulgated, it was defined as ‘a dietary supplement, food or medical food … that (1) has a benefit which prevents or reduces the risk of a disease or health condition, including the management of a disease or health condition or the improvement of health; and (2) is safe for human consumption in the quantity, and with the frequency required to realize such properties’ 22.
A nutraceutical has elsewhere been defined as ‘a functional food that aids in the prevention and treatment of disease(s) and or disorders (except anemia)’ 23. The author of this definition explained that he had singled out anaemia for omission ‘since most of the functional foods act in some way or the other as antianemic, [so that] the exception to anemia [makes] a clear distinction between the two terms, functional food and nutraceutical’, adding the confusing rider that ‘a functional food for one consumer can act as a nutraceutical for another consumer’. This imprecise statement seems to suggest that anaemia has been singled out for omission because it is often a deficiency disorder, treatable by supplementation with one or other deficient substance. However, there are many other deficiencies that could have been similarly excluded for this reason (see below and Table 2). The same author defined a functional food as ‘food that is cooked or prepared with ‘scientific intelligence’ with or without knowledge of why it is being used’. The precise meaning of this is unclear, but the author followed it with a statement that ‘functional food provides the body with the required amount of vitamins, fats, proteins, carbohydrates, etc, needed for its healthy survival’, which sounds no different from ‘food’ or ‘dietary regimen’.
Table 2.
Examples of normal constituents of the diet or enzymes used as dietary supplements to treat or prevent illnesses or diseases associated with deficiencies
| Compound | Deficiency disease |
|---|---|
| Normal dietary constitutents | |
| Carnitine | Primary deficiency 24; valproate toxicity 25 |
| Cereal starch | Glycogen storage disease 26 |
| Cobalamins | Pernicious anaemia 27 |
| Ferrous salts | Iron deficiency anaemia 28 |
| Folic acid * | Folate deficiency 29 |
| Vitamin C | Scurvy 30 |
| Vitamin D analogues | Osteomalacia and rickets 31 |
| Zinc | Acrodermatitis enteropathica 32 |
| Enzymes 33 | |
| Agalsidase | Fabry's disease |
| Alglucosidase alfa | Pompe's disease |
| Galsulfase | Mucopolysaccharidosis type VI |
| Idursulfase | Mucopolysaccharidosis type II |
| Imiglucerase | Gaucher's disease types I and III |
| Lactase | Lactose intolerance 34 |
| Laronidase | Mucopolysaccharidosis type I |
| Pancreatic enzymes | Exocrine pancreatic insufficiency 35 |
| Velaglucerase alfa | Gaucher's disease type I |
Also used in pregnancy to prevent neural tube defects, a use that is not relevant to this table.
The European Nutraceutical Association defines nutraceuticals as ‘nutritional products which have effects that are relevant to health … which are not synthetic substances or chemical compounds formulated for specific indications … contain[ing] nutrients (partly in concentrated form)’ 36. This definition contrasts with definitions of functional foods, which some have equated with nutraceuticals. It ignores the fact that all food is ‘relevant to health’, offers a vague definition of dosage (‘in concentrated form’) and implies that nutraceuticals should not have specific indications. The Association also states that ‘dietary supplements are a typical example for nutraceuticals, but also dietetic and functional foods may be counted among these products’, which compounds the confusion. Their definition would also exclude such dietary non‐nutrient compounds as polyphenols (e.g. isoflavones and resveratrol) and some carotenoids (lycopene and lutein), which are commonly marketed as nutraceuticals.
Functional foods
The term ‘functional food’ (or ‘physiologically functional food’) was introduced in the Japanese literature in 1984, to distinguish a so‐called tertiary function of foods, different, so it was said, from the primary function of nutrition and the secondary function of preference. It was supposed to be directly involved in modifying physiological systems, such as the immune, endocrine, nervous, circulatory, and digestive systems 37.
A functional food is defined in the OED as ‘a foodstuff containing chemical or biological additives intended to have beneficial physiological effects on the consumer; a nutraceutical’. This suggests that the terms ‘functional food’ and ‘nutraceutical’ are indistinguishable and that the key feature of a nutraceutical is that it is a foodstuff with nonfoodstuff additives. Such additives have been described as ‘chemicals to aid digestion, added dietary fibre, new artificial sweeteners and a wide variety of largely untested anti‐cholesterol agents’ 38.
Health Canada has defined a functional food as one that ‘is similar in appearance to, or may be, a conventional food that is consumed as part of a usual diet, and is demonstrated to have physiological benefits and/or reduce the risk of chronic disease beyond basic nutritional functions’ 39. An accompanying definition says that a nutraceutical is ‘a product isolated or purified from foods that is generally sold in medicinal forms not usually associated with food, demonstrated to have a physiological benefit or provide protection against chronic disease’. This seems to differentiate the two terms ‘functional food’ and ‘nutraceutical’.
By contrast, the Special Committee of the Food and Nutrition Board of the Institute of Medicine, National Academy of Sciences defines a functional food as ‘food in which concentrations of one or more food constituents have been manipulated to enhance their contributions to a healthful diet’ 40. Elsewhere, the same Institute has defined functional foods differently, as ‘those that encompass potentially healthful products [including] any modified food or food ingredient that may provide a health benefit beyond the traditional nutrients it contains’ 41.
In China and Japan, the equivalent term ‘health foods’ has been used but they are classified in Japan as nondrugs without health claims 42.
All this illustrates a lack of consistency and major confusion in attempts to define ‘nutraceuticals’ and ‘functional foods’. It is clear that all those who attempt definitions of these terms assume that the terms are worthy of definition, when they really go no further than the definitions of food or foodstuffs and dietary regimens.
Fortified foods
Fortified foods, also called ‘designer foods’, have been defined as ‘normal foods fortified with health promoting ingredients’ 43. This definition would encompass bread with added folic acid, used to prevent neural tube defects 44, salt with added iodide to prevent hypothyroidism 45, and butter substitutes containing plant sterols as lipid‐modifying agents 46, as well as beverages and fruit juices fortified with milk, soy, or other proteins for nutritional purposes. These are examples with good evidence of efficacy; the evidence base for other such foods is not always as good. The addition of fluoride to toothpaste to prevent dental caries 47 is also an example of preventive fortification, although not with a food.
Dietary supplements
The etymology and time line of usage of ‘supplement’ are shown in Figure 6. The hallmark of the definitions is the inclusion of the word ‘deficiency’. The term ‘dietary supplement’ should therefore be restricted to describing individual compounds used in the therapy or prevention of deficiencies, or when a constant supply of a nutrient is required (e.g. glucose in glycogen storage disease to prevent hypoglycaemia), or when a missing enzyme is replaced (e.g. lactase in lactose intolerance, pancreatic enzymes). Examples are given in Table 2.
Figure 6.
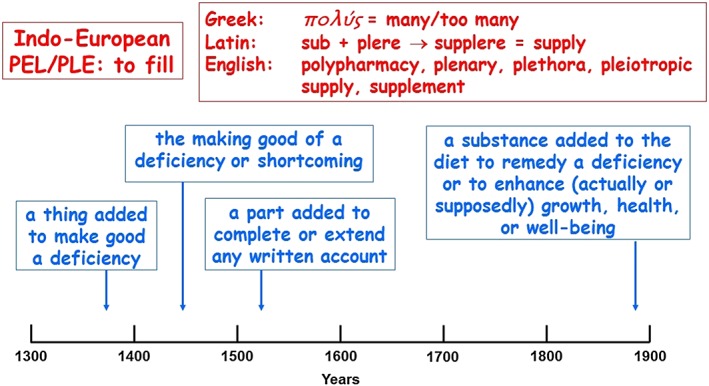
The etymology (in red) and time line of usage (in blue) of ‘supplement’; the dates are the earliest citations listed in the Oxford English Dictionary
However, the US Dietary Supplement Health and Education Act of 1994 48 was promulgated under the assumptions that ‘the importance of nutrition and the benefits of dietary supplements to health promotion and disease prevention have been documented increasingly in scientific studies’, that ‘there is a link between the ingestion of certain nutrients or dietary supplements and the prevention of chronic diseases such as cancer, heart disease and osteoporosis’, that ‘clinical research has shown that several chronic diseases can be prevented simply with a healthful diet, such as a diet that is low in fat, saturated fat, cholesterol and sodium, with a high proportion of plant‐based foods’, and other related assumptions. This Act extends the definition of ‘dietary supplement’ from the definition proposed above to ‘products (other than tobacco) intended to supplement the diet that bear or contain one or more of the following dietary ingredients:
a vitamin;
a mineral;
a herb or other botanical;
an amino acid;
a dietary substance for use by man to supplement the diet by increasing the total dietary intake;
a concentrate, metabolite, constituent, extract, or combination of any of the aforementioned ingredients;
a product that … is labeled as a dietary supplement;
[and]
[several other categories].’
This definition is based on unwarranted assumptions of efficacy. It blurs the distinction between dietary regimens and individual compounds marketed as dietary supplements, and also between compounds that are used to remedy deficiencies and the same compounds when used to treat or prevent diseases, such as the appropriate use of vitamin B1 (thiamine) to treat beri‐beri and its inclusion in multivitamin formulations with no proven efficacy in the absence of deficiency. Furthermore, the final catch‐all clause quoted above opens the door to inappropriate description of any other substance as a dietary supplement: if you label it as a dietary supplement, it is one! For example, melatonin is often marketed as a nutritional or dietary supplement 49, 50 but it is in fact formulated as medicinal products that are claimed to aid sleep 51 and may have efficacy in preventing or ameliorating jet lag 52. The dangers of using high doses of so‐called dietary supplements in the absence of deficiency are illustrated by evidence that high doses of vitamin D analogues not only did not prevent functional decline in elderly subjects who had previously fallen, but also increased the risk of falls 53.
Another category of so‐called ‘nutritional supplements’ includes formulations that contain essential and non‐essential amino acids from which specific amino acids have been omitted for use in certain metabolic disorders (Table 3). These are not supplements as defined above and would be better termed nutritional medicinal products. Gluten‐free formulations and low‐protein foods also come under this heading.
Table 3.
Formulations of essential and non‐essential amino acids from which specific amino acids have been omitted for treatment of metabolic disorders
| Disorder | Amino acids omitted |
|---|---|
| Glutaric aciduria type I | Lysine (and tryptophan reduced) |
| Homocysteinuria and hypermethioninaemia | Methionine |
| Hyperlysinaemia | Lysine |
| Isovaleric acidaemia | Leucine |
| Maple syrup urine disease | Isoleucine, leucine, and valine |
| Methylmalonic acidaemia and propionic acidaemia | Methionine, threonine, and valine (and isoleucine reduced) |
| Phenylketonuria | Phenylalanine |
| Tyrosinaemia | Phenylalanine and tyrosine |
Synthesis
The relationships that link these terms are shown diagrammatically as a Venn diagram in Figure 7. The EU definition of food specifically excludes medicinal products, so those two categories do not overlap, except in the case of fortified foods, when an active medicinal substance is added to a foodstuff. The overlap between herbal substances and medicinal products is less clear, but as some herbal substances are formulated as herbal medicinal products they can be included in the overlap; other forms of herbal substances could include a range of nonpharmaceutical preparations (e.g. herbal teas). Herbal substances are not specifically excluded in the EU definition of food, but I do not regard the two categories as overlapping. ‘Nutraceuticals’ and ‘functional foods’ find no places in this schema that are not otherwise occupied by other categories. The two terms are often used interchangeably, which is potentially confusing, and the term ‘fortified foods’ can be used to describe functional foods that consist of foods with added nutrients or other substances that are not conventionally regarded as nutrients (e.g. plant stanols in margarines).
Figure 7.
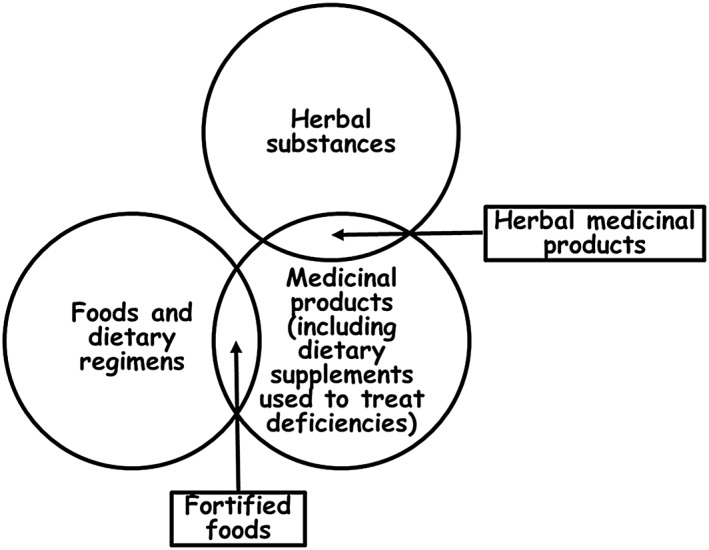
A Venn diagram showing the relationships linking foods and dietary regimens, herbal substances and medicinal products, as discussed in the text; the sizes of the several discrete areas in the diagram are not proportional to the number of elements in each area
An independent expert view
In a 2014 lecture 1, Stephen L. DeFelice, the inventor of the term ‘nutraceutical’, confessed that nutraceuticals do not work: ‘Within the past decade, the past ten years, many studies now have been published on dietary supplements and diets … and most of them have proven that these things do not work. Not proven. The results of clinical studies have shown that they do not work. They may work. But the studies may not have been designed properly. … Now, is it due to poorly designed clinical trials? Perhaps. Is it due to the fact that they don't work? I have problems with that. But I will say “perhaps”. I have to be intellectually honest. You know, I can't be an advocate of something I believe in when the proof's not there.’
Later Defelice wondered ‘why these things don't work, these dietary supplements don't work, these diets don't work. And then I said to myself, “Well, the cell doesn't need them”. If the cell's not deficient in them, it doesn't need them. That's why multivitamins don't work – perhaps – because the cells don't need them. That's why diets don't work. Because the cells don't need them. The cells just do what they want to do. … There's a lack of efficacy. Why is there a lack of toxicity? And then I came up with my theory. It's called the cell–nutraceutical acceptance–rejection theory’. DeFelice did not explain his theory; he merely said, ‘It's self‐explanatory’. But a more probable explanation is that any medication that does not cause harms is unlikely to produce benefits.
Summing up, DeFelice said, ‘So where do we go from here? Well I think, and again I'm not sure, that the quest to demonstrate whether chronic administration, long‐term diet, long‐term supplementation [with nutraceuticals] can prevent serious diseases … has come to an end’.
Although showing occasional reluctance, DeFelice was admirably open‐minded, given that he was admitting that his idea had not been supported by evidence from clinical trials. He ended by apologizing to his audience for having probably confused them. After all, they would surely have been expecting him to talk about how successful his idea had been.
Conclusions
There are widespread inconsistencies and contradictions in the many published definitions of ‘nutraceuticals’ and ‘functional foods’, demonstrating wholesale uncertainty about what they actually are.
There are no internationally agreed definitions of ‘nutraceuticals’ and ‘functional foods’, or of similar terms, such as ‘health foods’, or terms related to herbal products, which are sometimes referred to as ‘nutraceuticals’, compounding the confusion. ‘Nutraceuticals’ and ‘functional foods’ are vague, nondiscriminatory, unhelpful terms; the evidence suggests that they should be abandoned in favour of more precise terms. Indeed, there are no meanings that have been attached to these terms that are not encompassed more clearly by other terms in the field.
The complete set of definitions that emerges from this analysis is listed in Table 4.
Table 4.
Definitions
| Definiendum | Definition(s) |
|---|---|
| Diet | Customary course of living as to food: way of feeding; a prescribed programme of food of a defined kind and/or quantity |
| Dietary regimen | A programme of food, of a defined kind and/or quantity, prescribed or adopted for the restoration or preservation of health or the prevention of illness and disease |
| Dietary supplement | 1. A substance added to the diet, often taken as a pharmaceutical formulation, to treat or prevent a deficiency |
| 2. [Also used to mean a substance added to the diet to enhance (actually or supposedly) growth, health, or well‐being] | |
| Eat | To take into the mouth piecemeal, masticate, and swallow as food; to consume food |
| Food | 1. Any nutritious substance that people or animals eat or drink in order to maintain life and growth |
| 2. Any item that is to be processed, partially processed, or unprocessed for consumption, and that is intended to be, or reasonably expected to be, ingested by humans | |
| Foodstuffs | Particular substances suitable for consumption as food |
| Fortified foods (also called ‘designer foods’) | Foodstuffs to which compounds of proven therapeutic or preventive efficacy have been added |
| Functional food | [No satisfactory definition] |
| Herb | A plant of which the stem does not become woody and persistent (as in a shrub or a tree) but remains more or less soft and succulent, and dies down to the ground (or entirely) after flowering; spec. applied to plants of which the leaves, or stem and leaves, are used for food or medicine, or in some way for their scent or flavour |
| Herbal medicinal product | Any medicinal product, exclusively containing as active ingredients one or more herbal substances or one or more herbal preparations, or one or more such herbal substances in combination with one or more such herbal preparations |
| Herbal preparation | A preparation that is obtained by subjecting herbal substances to treatments such as extraction, distillation, expression, fractionation, purification, concentration, or fermentation |
| Herbal substance | A substance that consists of mainly whole, fragmented, or cut plants, plant parts, algae, fungi, or lichen in an unprocessed, usually dried form but sometimes fresh |
| Medicinal product (synonyms medicine and medicament) | A product that contains a compound with proven pharmacological and beneficial therapeutic effects, a drug, prodrug, or a cellular element, plus excipients, or excipients only; it may also contain adulterants or contaminants |
| Nutraceutical | [No satisfactory definition] |
| Nutrition | 1. The action or process of supplying, or of receiving, nourishment or food; nutritious content |
| 2. The state or condition of being (well or badly) nourished; a person's state of health | |
| Nutritional medicinal products | Foods or formulations containing nutritional ingredients from which specific nutrients have been omitted |
| Pharmaceutical formulation (also called a ‘dosage form’) | The form in which a medicinal product is presented, e.g. a tablet, capsule, elixir, solution for injection, transdermal formulation, cream, or ointment |
Competing Interests
The author has completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declares no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Aronson, J. K. (2017) Defining ‘nutraceuticals’: neither nutritious nor pharmaceutical. Br J Clin Pharmacol, 83: 8–19. doi: 10.1111/bcp.12935.
References
- 1. DeFelice SL. Nutrition stymied: the nutraceutical solution. Plenary Lecture September 9, 2014. XXV National Congress of the Italian Chemical Society‐SCI‐The University of Calabria. Available at: http://www.fimdefelice.org (last accessed 13 February 2016).
- 2. Schmitt J, Ferro A. Nutraceuticals: is there good science behind the hype? Br J Clin Pharmacol 2013; 75: 585–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ioannidis JP. Implausible results in human nutrition research. BMJ 2013; 347: f6698. [DOI] [PubMed] [Google Scholar]
- 4. Aronson JK. Medication errors: definitions and classification. Br J Clin Pharmacol 2009; 67: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oxford English Dictionary . The definitive record of the English language. Available at: http://ezproxy‐prd.bodleian.ox.ac.uk:2355 (last accessed 13 February 2016).
- 6. Lewis D. How to define theoretical terms. J Philos 1970; 67: 427–46. [Google Scholar]
- 7. Zamora‐Ros R, Knaze V, Rothwell JA, Hémon B, Moskal A, Overvad K, Tjønneland A, Kyrø C, Fagherazzi G, Boutron‐Ruault MC, Touillaud M, Katzke V, Kühn T, Boeing H, Förster J, Trichopoulou A, Valanou E, Peppa E, Palli D, Agnoli C, Ricceri F, Tumino R, de Magistris MS, Peeters PH, Bueno‐de‐Mesquita HB, Engeset D, Skeie G, Hjartåker A, Menéndez V, Agudo A, Molina‐Montes E, Huerta JM, Barricarte A, Amiano P, Sonestedt E, Nilsson LM, Landberg R, Key TJ, Khaw KT, Wareham NJ, Lu Y, Slimani N, Romieu I, Riboli E, Scalbert A. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr 2015. doi:10.1007/s00394‐015‐0950‐x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aronson JK. Plant poisons and traditional medicines In: Manson's Tropical Diseases, 23rd edn, eds Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo DG, White NJ. London: Harcourt International, 2014. [Google Scholar]
- 9. Sato T, Miyata G. The nutraceutical benefit, part I: green tea. Nutrition 2000; 16: 315–7. [DOI] [PubMed] [Google Scholar]
- 10. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002. Laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Official J Eur Communities 2002: L 31/7. Available at: http://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:031:0001:0024:en:PDF (last accessed 13 February 2016).
- 11. Aronson JK. Patent medicines and secret remedies. BMJ 2009; 339: b5415. [DOI] [PubMed] [Google Scholar]
- 12. Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean diet; a literature review. Nutrients 2015; 7: 9139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnard ND, Levin SM, Yokoyama Y. A systematic review and meta‐analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet 2015; 115: 954–69. [DOI] [PubMed] [Google Scholar]
- 14. Sabaté J, Wien M. A perspective on vegetarian dietary patterns and risk of metabolic syndrome. Br J Nutr 2015; 113 (Suppl. 2): S136–43. [DOI] [PubMed] [Google Scholar]
- 15. Omar SA, Webb AJ, Lundberg JO, Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J Intern Med 2016; 279: 315–36. [DOI] [PubMed] [Google Scholar]
- 16. Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf 2005; 28: 851–70. [DOI] [PubMed] [Google Scholar]
- 17. Barnes J. Adverse drug reactions and pharmacovigilance of herbal medicines In: Stephens' Detection and Evaluation of Adverse Drug Reactions: Principles and Practice, 6th edn, eds Talbot J, Aronson JK. Oxford: Wiley‐Blackwell, 2011. [Google Scholar]
- 18. The European Parliament and the Council of the European Union . Directive 2004/24/EC of the European Parliament and of the Council of 31 March 2004, amending, as regards traditional herbal medicinal products, Directive 2001/83/EC on the Community code relating to medicinal products for human use. Off J Eur Union 2004; L136: 85–90. [Google Scholar]
- 19. World Health Organization . WHO Traditional Medicine Strategy 2002–2005. Geneva: World Health Organization, 2002. [Google Scholar]
- 20. The Foundation for Innovation in Medicine. Available at: http://www.fimdefelice.org (last accessed 13 February 2016).
- 21. An Interview with Dr. Stephen DeFelice by Sheldon Baker. Available at: http://www.nutraceuticalsworld.com/contents/view_health‐e‐insights/2011‐10‐28/an‐interview‐with‐dr‐stephen‐defelice (last accessed 13 February 2016).
- 22. H.R.3001 – Nutraceutical Research and Education Act. Available at: https://www.congress.gov/bill/106th‐congress/house‐bill/3001/all‐actions?overview=closed (last accessed 13 February 2016).
- 23. Kalra EK. Nutraceutical – definition and introduction. AAPS Pharm Sci 2003; 5: E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magoulas PL, El‐Hattab AW. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis 2012; 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid‐induced toxicity. Clin Toxicol (Phila) 2009; 47: 101–11. [DOI] [PubMed] [Google Scholar]
- 26. Shah KK, O'Dell SD. Effect of dietary interventions in the maintenance of normoglycaemia in glycogen storage disease type 1a: a systematic review and meta‐analysis. J Hum Nutr Diet 2013; 26: 329–39. [DOI] [PubMed] [Google Scholar]
- 27. Shipton MJ, Thachil J. Vitamin B12 deficiency – a 21st century perspective. Clin Med (Lond) 2015; 15: 145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs 2015; 75: 101–27. [DOI] [PubMed] [Google Scholar]
- 29. Yakoob MY, Bhutta ZA. Effect of routine iron supplementation with or without folic acid on anemia during pregnancy. BMC Public Health 2011; 11 (Suppl. 3): S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agarwal A, Shaharyar A, Kumar A, Bhat MS, Mishra M. Scurvy in pediatric age group – a disease often forgotten? J Clin Orthop Trauma 2015; 6: 101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reid IR. What diseases are causally linked to vitamin D deficiency? Arch Dis Child 2016; 101: 185–9. [DOI] [PubMed] [Google Scholar]
- 32. Kasana S, Din J, Maret W. Genetic causes and gene–nutrient interactions in mammalian zinc deficiencies: acrodermatitis enteropathica and transient neonatal zinc deficiency as examples. J Trace Elem Med Biol 2015; 29: 47–62. [DOI] [PubMed] [Google Scholar]
- 33. Baldo BA. Enzymes approved for human therapy: indications, mechanisms and adverse effects. BioDrugs 2015; 29: 31–55. [DOI] [PubMed] [Google Scholar]
- 34. Anonymous . Efficacy of exogenous lactase for lactose intolerance. Nutr Rev 1988; 46: 150–2. [DOI] [PubMed] [Google Scholar]
- 35. Hammer HF. Pancreatic exocrine insufficiency: diagnostic evaluation and replacement therapy with pancreatic enzymes. Dig Dis 2010; 28: 339–43. [DOI] [PubMed] [Google Scholar]
- 36. European Nutraceutical Association . Health, wellness and fitness. Available at: https://www.linkedin.com/company/european‐nutraceutical‐association (last accessed 13 February 2016).
- 37. Arai S. Studies on functional foods in Japan. Biosci Biotechnol Biochem 1996; 60: 9–15. [DOI] [PubMed] [Google Scholar]
- 38. Daily Telegraph . 14 April 1990: 3/7.
- 39. Health Canada . Policy Paper – Nutraceuticals/functional foods and health claims on foods. Available at: http://www.hc‐sc.gc.ca/fn‐an/label‐etiquet/claims‐reclam/nutra‐funct_foods‐nutra‐fonct_aliment‐eng.php.
- 40. Special Committee of the Food and Nutrition Board of the Institute of Medicine, National Academy of Sciences . Opportunities in the nutrition and food sciences: research challenges and the next generation of investigators. J Nutr 1994; 124: 763–9 (last accessed 13 February 2016). [DOI] [PubMed] [Google Scholar]
- 41. Thomas PR, Earl R, eds. Opportunities in the Nutrition and Food Sciences. Washington, DC: Institute of Medicine/National Academy of Sciences, National Academy Press, 1994; 109. [Google Scholar]
- 42. Ohama H, Ikeda H, Moriyama H. Health foods and foods with health claims in Japan. Toxicology 2006; 221: 95–111. [DOI] [PubMed] [Google Scholar]
- 43. Rajasekaran A, Kalaivani M. Designer foods and their benefits: A review. J Food Sci Technol 2013; 50: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Obeid R, Pietrzik K, Oakley GP Jr, Kancherla V, Holzgreve W, Wieser S. Preventable spina bifida and anencephaly in Europe. Birth Defects Res A Clin Mol Teratol 2015; 103: 763–71. [DOI] [PubMed] [Google Scholar]
- 45. Angermayr L, Clar C. Iodine supplementation for preventing iodine deficiency disorders in children. Cochrane Database Syst Rev 2004; CD003819. [DOI] [PubMed] [Google Scholar]
- 46. Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R, Stresa Workshop Participants . Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 2003; 78: 965–78. [DOI] [PubMed] [Google Scholar]
- 47. Bowen WH. Dental caries – not just holes in teeth! A perspective. Mol Oral Microbiol 2015; [Epub ahead of print]. doi:10.1111/omi.12132 [DOI] [PubMed] [Google Scholar]
- 48. National Institutes of Health . US Dietary Supplement Health and Education Act of 1994. Public Law 103‐417. 103rd Congress. Available at: https://ods.od.nih.gov/About/DSHEA_Wording.aspx (last accessed 13 February 2016).
- 49. Hahm H, Kujawa J, Augsburger L. Comparison of melatonin products against USP's nutritional supplements standards and other criteria. J Am Pharm Assoc (Wash) 1999; 39: 27–31. [DOI] [PubMed] [Google Scholar]
- 50. Tresguerres IF, Tamimi F, Eimar H, Barralet JE, Prieto S, Torres J, Calvo‐Guirado JL, Tresguerres JA. Melatonin dietary supplement as an anti‐aging therapy for age‐related bone loss. Rejuvenation Res 2014; 17: 341–6. [DOI] [PubMed] [Google Scholar]
- 51. Wright A, Diebold J, Otal J, Stoneman C, Wong J, Wallace C, Duffett M. The effect of melatonin on benzodiazepine discontinuation and sleep quality in adults attempting to discontinue benzodiazepines: a systematic review and meta‐analysis. Drugs Aging 2015; 32: 1009–18. [DOI] [PubMed] [Google Scholar]
- 52. Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev 2002; 2: CD001520. [DOI] [PubMed] [Google Scholar]
- 53. Bischoff‐Ferrari HA, Dawson‐Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A. Monthly high‐dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med 2016; 176: 175–83. [DOI] [PubMed] [Google Scholar]


