Abstract
Bioactive peptides derived from milk proteins are food components that, in addition to their nutritional value, retain many biological properties and have therapeutic effects in several health disorders, including cardiovascular disease. Amongst these, atherosclerosis is the underlying cause of heart attack and strokes. It is a progressive dyslipidaemic and inflammatory disease where accumulation of oxidized lipids and inflammatory cells leads to the formation of an atherosclerotic plaque in the vessel wall. Milk‐derived bioactive peptides can be released during gastrointestinal digestion, food processing or by enzymatic and bacterial fermentation and are considered to promote diverse beneficial effects such as lipid lowering, antihypertensive, immnomodulating, anti‐inflammatory and antithrombotic effects. In this review, an overview of the diverse biological effects of these compounds is given, particularly focusing on their beneficial properties on cardiovascular disease and proposing novel mechanisms of action responsible for their bioactivity. Attempts to prevent cardiovascular diseases target modifications of several risk factors such as high blood pressure, obesity, high blood concentrations of lipids or insulin resistance. Milk‐derived bioactive peptides are a source of health‐enhancing components and the potential health benefit of these compounds has a growing commercial potential. Consequently, they have been incorporated as ingredients in functional foods, as dietary supplements and as pharmaceuticals to promote health and reduce risk of chronic diseases.
Keywords: atherosclerosis, bioactive peptides, cardiovascular disease, milk proteins, immunomodulation, inflammation
Introduction
In the last two decades many studies have demonstrated that bovine milk is a source of bioactive compounds. These bioactive molecules are naturally contained in milk. This is the case of lysozyme, lactoferrin, immunoglobulins, growth factors and hormones 1, and they may also be generated by hydrolysis of native proteins. Milk‐derived bioactive peptides are usually encrypted and kept inactive within the primary structure of milk protein and they are generated by proteolysis of casein (α‐, β‐, γ‐ and κ‐casein) and whey proteins (β‐lactoglobulin, α‐lactalbumin, serum albumin, immunoglobulins, lactoferrin and protease‐peptone fractions) 2. The increasing number of and health promoting effects attributed to milk‐derived bioactive peptides make them potential ingredients of functional food 3.
Generation of peptides may occur by enzymatic hydrolysis or microbial fermentation, either in vivo during digestion by digestive enzymes like trypsin and by gut microbial enzymes, or during food processing or ripening or by in vitro hydrolysis using isolated enzymes. During digestion, the bioactive peptides can be absorbed from the intestine to the blood stream and exert either local effects in the gastrointestinal system or systemic effects. In addition, microbial enzymes found in the gut or found in the food target different cleavage sites in comparison with isolated enzymes and so the peptides generated by these enzymes may be different from those generated during digestion. This is, for example, the case of KVLPVP peptide from casein. An additional pancreatic digestion results in a much higher angiotensin‐converting enzyme (ACE) inhibitory activity than that generated by only L. helveticus proteolysis (KVLPVPQ) 4.
Numerous health promoting effects have been attributed to milk‐derived bioactive peptides released from dairy proteins by enzymatic proteolysis, including antithrombotic, antihypertensive, anti‐inflammatory, anti‐oxidative, antimicrobial and anti‐obesity properties 5, 6, 7. Several characterized bioactive peptides are multifunctional and show two or more different biological activities 8, 9. Thus they may have health promoting effects on many body systems such as the cardiovascular, digestive, endocrine, immune and nervous systems (Figure 1) 5, 7, 10. In particular, the cardiovascular system is a main target of milk‐derived bioactive peptides and many in vitro and in vivo studies have demonstrated beneficial cardiovascular effects of these peptides, for example, reducing arterial stiffness and improving endothelial activity 11, 12, 13.
Figure 1.
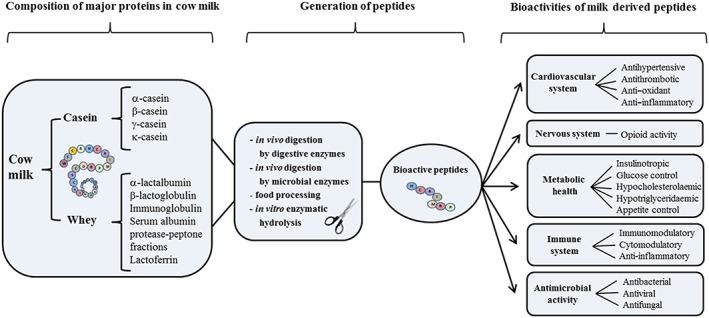
Schematic illustrating generation of milk‐derived bioactive peptides and their physiological functionalities. Milk‐derived bioactive peptides can be encrypted in both casein (α‐, β‐, γ‐ and κ‐casein) and whey proteins (β‐lactoglobulin, α‐lactalbumin, serum albumin, immunoglobulins, lactoferrin, protease‐peptone fractions). Generation of peptides may be induced in several ways, by enzymatic hydrolysis or microbial fermentation, in vivo during digestion by digestive enzymes, like trypsin, and by gut microbial enzymes, during food processing, by ripening or by in vitro hydrolysis using isolated enzymes. Numerous health promoting effects have been attributed to milk derived bioactive peptides released from dairy proteins by enzymatic proteolysis, including antithrombotic, antihypertensive, anti‐inflammatory, anti‐oxidative, antimicrobial and anti‐obesity
Cardiovascular diseases are multifactorial diseases linked to genetics and life‐style, such as diet, smoking and exercise. Some risk factors are controllable and pharmacological drugs are widely used in that context, for example to manage elevated cholesterol, triglycerides and blood pressure. This review provides an overview of the potential impact of milk‐derived peptides on cardiovascular disease and the mechanisms of action of such bioactive peptides. Studies for inclusion in this article were identified by searching PubMed and Scopus data bases for relevant articles examining the effects of milk derived peptides in the cardiovascular system from 1986 up to the present.
Bioactivities of milk‐derived peptides
Milk bioactive peptides may influence several risk factors for cardiovascular disease, including blood pressure, thrombosis (16.17), inflammation and lipid metabolism, providing an alternative to synthetic pharmaceuticals. Numerous bioactivities have been reported for peptides generated from in vivo and in vitro hydrolysis and enzymatic digestion of milk. Milk peptides have been shown to have antihypertensive effects, to influence insulin secretion and glucose control, and to have anti‐oxidant and antithrombotic properties. They also influence lipid concentrations, immune response, inflammation and markers of oxidative stress (Table 1), Figure 2.
Table 1.
Bioactivity of milk‐derived peptides on cardiovascular system
| Bioactivity | Precursor product | Preparation | Peptide fragment | Effect | Reference |
|---|---|---|---|---|---|
| Antihypertensive | αs1‐, β‐casein | L. helveticus and Saccharomyces cerevisiae | VPP | ACE inhibitor | 106, 107 |
| IPP | |||||
| α‐lactalbumin | Proteolytic enzymes | VAGTWY HIRL | ACE inhibitor | 108, 109 | |
| β‐lactoglobulin | |||||
| β‐lactoglobulin | Trypsin digestion | ALPMHIR | Inhibition of endothelin‐1, ACE inhibitor | 104, 108, 109 | |
| LAMA | |||||
| VKF | |||||
| β‐casein | Trypsin, pepsin, intestinal digestion | YPFPGPI | ACE inhibitor, opioid | 17 | |
| YPFPGPIPNSL | |||||
| α‐lactalbumin | Trypsin, pepsin, chimotrypsin | YG | ACE inhibitor, opioid | 110, 111 | |
| YLLF | |||||
| YGLF | |||||
| VGINYWLAHK | |||||
| αs1‐casein | Crescenza cheese | FFVAPFPEVFGK | ACE inhibitor | 112 | |
| FFVAP | |||||
| Yak milk casein | Qula cheese + alcalase hydrolysis | PPEIN | ACE inhibitor | 113 | |
| PLPLL | |||||
| β‐casein | Enzyme‐modified cheese Lactobacillus casei | LTLTDVE YPQRDMPIQ | ACE inhibitor | 114 | |
| PGPIP | |||||
| Antithrombotic | κ‐casein | Chymosin and trypsin digestion | MAIPPKKNQDK | Inhibition of platelet aggregation and fibrinogen binding | 29, 115 |
| NQDK | |||||
| MAIPPK | |||||
| Sheep κ‐casein | Enzymatic hydrolysis | TAQVTSTEV | Inhibition of thrombin‐induced platelet aggregation | 31 | |
| KDQDK | |||||
| QVTSTEV | |||||
| Sheep lactoferrin | Pepsin hydrolysates | RGDX | 116 | ||
| Human lactoferrin | KRDS | ||||
| α‐lactalbumin | Instestinal digestion | GLF | Inhibition of collagen‐induced platelet aggregation | 117, 118 | |
| RGDGLF | |||||
| Antioxidant | Casein | Pepsin digestion | YFYPEL | Superoxide anion scavenging activity | 42 |
| FYPEL | |||||
| YPEL | |||||
| PEL | |||||
| EL | |||||
| α‐lactalbumin | Corolase PP | WYSLAMAAS—DI | Free radical scavenging activity | 43 | |
| Casein | Pepsin digestion | RYLGY | Free radical scavenging activity and ACE inhibitor | 119 | |
| AYFYPEL | |||||
| YQKFQY | |||||
| Antilipaemic | β‐lactoglobulin | Trypsin digestion | IIAEK | Cholesterol lowering | 5 |
| ALPMH | |||||
| GLNIQK | |||||
| Whey protein | Whey protein | Cholesterol lowering | 120, 121 | ||
| Anti‐inflammatory | Casein | Enterococcus faecalis hydrolysis | Casein hydrolysate | Leukocyte recruitment and PPAR‐γ dependent NF‐κB inhibition | 80 |
| Bacterial fermentation | VPP | Leukocyte recruitment and JNK inhibition | 68 | ||
| Pepsin and corolase | Casein hydrolysate | TGF‐β1, COX‐2, and NFκB inhibition | 69 | ||
| Aspergillus oryzae hydrolysis | Casein hydrolysate | Suppressive effect on adjuvant arthritis | 79 | ||
| Whey protein | Pressurisation of whey | Pressurized whey | Reduced expression of cytokines | 70, 71 | |
| Enzymatic hydrolysis | Whey protein hydrolysate | Reduced atopic dermatitis skin lesions | 78 | ||
| Lactoferrin | Proteolytic cleavage | Lactoferricin | Anti‐catabolic and Anti‐arthritis | 74, 75 | |
| Lactoferrin | Inhibition of cytokine production | 72 |
Figure 2.
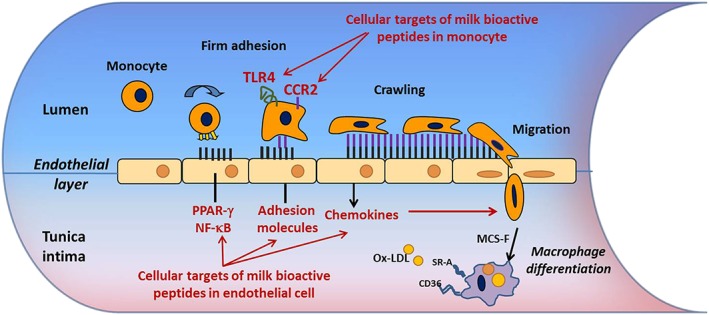
The role of the endothelial cell and monocyte in early stages of atherosclerosis: potential mechanisms of milk derived bioactive peptides. Activated ECs express selectins (P‐selectin and E‐selectin) and cell adhesion molecules (VCAM‐1 and ICAM‐1), which mediate the rolling and firm adhesion of the monocyte along the vessel wall. Firmly adhered monocytes undergo transendothelial monocyte migration from the lumen to the intima in response to chemokines such as MCP‐1. Upon entering the underlying intima the monocyte is exposed to the growth factor MCS‐F, which differentiates the monocyte into a macrophage. Macrophage differentiation results in the up‐regulation of the scavenger receptors, CD36 and SRA1, which are necessary for the subsequent formation of foam cells following the uptake of ox‐LDL and other modified lipids. Bioactive peptides have anti‐inflammatory effects on ECs by inhibiting activation of the NF‐κB pathway in a PPARγ dependent manner, and by modifying the expression of CCR2 and TLR4 receptors in monocytes
Antihypertensive effects
Elevated blood pressure represents a major and controllable risk factor for the development of cardiovascular disease (CVD) 14. ACE is a multifunctional enzyme which plays a central role in the regulation of endogenous pathways regulating blood pressure such as the renin‐angiotensin and bradykinin systems. Cleavage of angiotensinogen by renin produces angiotensin I which is subsequently hydrolyzed by ACE to angiotensin II (a potent vasoconstrictor). ACE also inactivates the vasodilator bradykinin, contributing to an increased blood pressure. Synthetic ACE inhibitors have been developed as antihypertensive agents, but their use is also associated with side effects such as hypotension, cough, skin rash and reduced renal function. Recently, some food derived bioactive peptides from milk, fish and plants have been found to behave as ACE inhibitors 4. Milk proteins contain a number of ACE inhibitory peptides and potent ACE inhibitors from milk casein (casokinins) and whey proteins (lactokinins) have been described 15, 16, 17. In particular, two potent inhibitory tri‐peptides Val‐Pro‐Pro and Ile‐Pro‐Pro from bovine casein were isolated from sour milk fermented with L. helveticus and Saccharomyces cerevisiae 18. In vivo studies in spontaneously hypertensive rats and in hypertensive humans have demonstrated that several ACE inhibitor peptides reduced blood pressure in a dose‐dependent manner after intravenous or oral administration 18, 19, 20. Interestingly, there was little effect on the blood pressure of normotensive subjects, so that hypotension is an unlikely side effect. Therefore, ACE inhibitor peptides may be used to treat mild hypertension or as supplemental therapy. In addition, in vivo studies showed that ACE activity was lower in aortas of hypertensive rats after oral administration of fermented milk than in a control group, demonstrating that these peptides were absorbed without further cleavage by digestive enzymes, reached the abdominal aorta and exerted antihypertensive activity 21, 22. Antihypertensive peptides may also influence blood pressure through mechanisms that are independent of ACE inhibition. These include the vascular release of endogenous vasodilators, including prostaglandin I2 23, nitric oxide (NO) 24 and carbon monoxide (CO) 25, and activation by α‐lactorphin of opioid receptors 26. More details about antihypertensive peptides are given in Table 1. While many antihypertensive peptides have been generated by in vitro digestion with specific proteases, further in vivo work is needed to address if these undergo gastrointestinal hydrolysis and inactivation when orally administered.
Antithrombotic properties
Thrombosis arises from increased platelet activity and aggregations or defective fibrinolysis in arteries or veins. Arterial thrombosis is the main cause of myocardial infarction and stroke, and arises when the blood vessel is damaged by atherosclerosis. Thus, antithrombotic drugs are used to inhibit platelet functions and enhance fibrinolysis. Many peptides derived from lactoferrin and κ‐casein have been shown to inhibit platelet aggregation and to have antithrombotic activity 27, 28. Whole κ‐casein has inhibitory effects on thrombin‐induced platelet aggregation and secretion, and caseinoglycopeptide has been shown to inhibit platelet aggregation, while para‐κ‐casein is inactive 28, 29, 30, 31. In particular three peptides obtained from enzymatic hydrolysis of caseinoglycopeptide from sheep milk, KDQDK, TAQVTSTEV and QVTSTEV, completely inhibited platelet aggregation induced by thrombin in in vitro experiments 31. Interestingly, KDQDK peptide also known as casoplatelin possesses a similar sequence to the corresponding bovine peptide KNQDK which also showed antiplatelet activity 32. The main antithrombotic peptide MAIPPKKNQDK of bovine κ‐casein inhibits ADP‐induced platelet fibrinogen by binding to its platelet receptor αIIbβ3 protein and so prevents platelet aggregation 29. In addition, a peptide derived from human lactoferrin contains the amino acid sequence KRDS. This is similar to a sequence of fibrinogen α‐chain, RGDS, which mediates its binding to αIIbβ3. KRDS and RGDS inhibit platelet aggregation in vitro by preventing fibrinogen binding to αIIbβ3, although KRDS is less effective 33, 34. KRDS also inhibits serotonin release from platelet dense granules, but the corresponding fibrinogen analogue RGDS has no effect on the granule release, suggesting that the two peptides differ in their impact on platelet cellular pathways 35. In vivo antithrombotic activity has been demonstrated for peptides from k‐casein and for lactoferrin‐derived peptides, with no detectable toxic effects 35. Current therapeutic drugs used to treat atherothrombosis are also associated with severe side effects, such as bleeding 36. Hence, milk bioactive peptides could provide novel ingredients in functional foods as safer alternatives to the current therapies used to prevent thrombosis.
Anti‐oxidant peptides
Oxidative stress is another factor contributing to the development and progression of cardiovascular disease. It is characterized by the generation of reactive oxygen species (ROS), including free radicals superoxide, hydroxyl radicals and non‐radical hydrogen peroxide ‐ due to their increased production and to decreased antioxidant defence mechanisms. ROS primary targets are cellular macromolecules, including DNA, RNA, proteins and lipids and have been implicated in ageing and a number of human diseases, including atherosclerosis 37. On the contrary, low ROS concentrations have physiological effects through regulation of cell signalling, through the redox regulation of protein phosphorylation, ion channels and transcription factors 38. Consumption of dietary antioxidants, containing for example vitamin C, E polyphenols and carotenoids, has been shown to reduce oxidative stress by boosting natural antioxidant defences 39, 40, 41. Interestingly, recent in vitro studies showed that milk peptides from casein and whey proteins are a source of antioxidant peptides such as, for example, YFYPEL peptide, a hexapeptide generated by pepsin hydrolysis of bovine casein which scavenges superoxide radicals 42. Enzymatic peptides identified in the hydrolysate of whey proteins by Corolase or other commercial proteases also showed strong capacity to scavenge free radicals. A total of 42 peptide fragments were identified containing the sequence WYSLAMAASDI evincing the highest activity 43. The potency of antioxidant activity of bioactive peptides has been attributed to the content of specific amino acids, in particular to high amounts of histidine, which has peroxyradical trapping and chelating abilities and to hydrophobic amino acids which increase the accessibility of peptides to hydrophobic targets 44, 45.
Antilipaemic peptides
Elevated blood lipids, such as hypercholesterolaemia and hyperglyceridaemia, represent an important risk factor in the pathogenesis of CVD, in particular atherosclerosis 46, 47. The pharmacological treatment of hypercholesterolaemia and hyperglyceridaemia is a mainstay in the management of CVD 48. Many dietary proteins can improve the blood lipid profile, especially soy proteins 49. Numerous studies showed that milk bioactive peptides derived from whey may reduce serum cholesterol concentrations similarly to soy proteins. In particular the peptide IIAEK (lactostatin) derived from bovine milk β‐lactoglobulin showed strong cholesterol‐lowering effects in in vivo animal studies, exhibiting a greater activity in comparison with that of the drug β‐sitosterol 50. To clarify the mechanism of the hypocholesterolaemic action of lactostatin, Morikawa et al. performed in vitro studies screening for the target gene and signal transduction pathway targeted by lactostatin in human liver cells, and found that lactostatin regulated the phosphorylation of extracellular signal‐regulated kinase (ERK) and intracellular Ca2+ concentration, demonstrating the involvement of the calcium‐channel‐related MAPK signalling pathway in the lactostatin‐mediated cholesterol degradation 51. In addition, it has been shown that water‐soluble lactostatin activates the transcription of the cholesterol 7α‐hydroxylase (CYP7A1) gene, inducing hypocholesterolaemic effects by increasing cholesterol metabolism 51. On the contrary, casein proteins induce elevation of cholesterol concentrations, possibly due to their higher ratios of methionine‐glycine and lysine‐arginine, but the mechanism responsible for this effect has not been elucidated.
Insulin secretion and glucose control
Type 2 diabetes mellitus (T2DM) is associated with an increased risk of cardiovascular disease together with other risk factors such as hypertension and dyslipidaemia 52. Impaired insulin sensitivity is common in T2DM, although other mechanisms are involved. Several studies have shown beneficial effects of milk‐derived components including the regulation of insulin secretion and on the control of blood glucose. Ingestion of both whey and casein proteins induces increased insulin secretion 53, but ingestion of whey protein leads to more rapid secretion of insulin than micellar casein 54. The insulinotropic effect of intact whey protein is similar to whey protein hydrolysate at doses of 20–50 g of proteins in healthy individuals 55. In T2DM individuals, 18 g of whey protein ingested with a meal induced greater insulinotropic and gut peptide glucose‐dependent insulinotropic polypeptide response compared with ingestion of isoenergetic non‐dairy protein 56. As little as 55 g ingested before a carbohydrate meal can stimulate insulin and incretin hormone secretion and slow gastric emptying, leading to marked reduction in post‐prandial glycaemia in type 2 diabetes 57. In vivo studies in rats also showed that insulin sensitivity increased after 6 weeks administration of whey proteins 58. The insulinotropic effect of whey proteins may be mediated by the high content of amino acids generated from its hydrolysis, which can induce an amino acid‐mediated insulin secretion from pancreatic β‐cells 59. The insulinotropic effect can also be mediated by the activation of the incretin system by inducing a glucose‐dependent insulinotropic polypeptide response 56, 60. The effects of casein are not as consistent as whey proteins. Co‐ingestion of a casein hydrolysate/leucine mixture following each meal substantially reduces the prevalence of hyperglycaemia in type 2 diabetic patients, accompanied by a significant reduction in average 24 h blood glucose concentration 61. Another study showed that co‐ingestion of a casein hydrolysate with each main meal does not improve glucose homeostasis over a 24 h period in long‐standing type 2 diabetes patients, possibly as a consequence of β‐cells ‘exhaustion’ in chronic disease 62. Also, casein showed no effects on the incretin responses while it suppressed the triglyceride response and increased glucagon 63.
Anti‐inflammatory peptides
Inflammation plays a physiological role in wound healing and microbial resistance, but uncontrolled and chronic inflammation underpins chronic disease such as rheumatoid arthritis and atherosclerosis (Figure 2) and has been linked to cancer. Non‐steroidal anti‐inflammatory drugs, with the exception of aspirin, are not widely used to treat cardiovascular disease 64, 65. They are ineffective in this setting and in fact are associated with severe side effects such as gastric ulceration, bleeding 66, 67 and in some cases, thrombosis. Indeed there is no pharmacological treatment for the inflammation underlying atherosclerosis.
Recently Val‐Pro‐Pro peptide, previously examined for its antihypertensive effect through inhibition of the ACE enzyme, has also shown direct anti‐inflammatory effects in reducing leukocyte‐endothelial cell interaction in vitro, an effect attributable to the inhibition of the MAP kinase signalling pathway 68. In addition, peptides derived from Corolase casein digestion showed anti‐inflammatory effects on macrophages 69 while hydrolysates generated from whey proteins showed similar effects on respiratory and intestinal epithelial cells 70, 71. Lactoferrin derived from whey likewise showed a number of anti‐inflammatory effects. Lactoferrin down‐regulates the LPS‐induced cytokine production in monocytic cells, thought to involve NF‐κB activation 72. Another study showed inhibition of intracellular adhesion molecules 1 and cytokines, by lactoferrin, as well as inhibition of the proliferation and migration of bovine aortic endothelial cells. Lactoferrin has also been shown to exert a potent anti‐inflammatory activity by driving monocyte differentiation towards dendritic cells with impaired capacity to undergo activation and to promote Th1 responses 73. Lactoferrin hydrolysate also showed anti‐inflammatory effects on human cartilage and synovial cells suggesting a potential role in the treatment of arthritis 74, 75. On the basis of several in vitro studies, the anti‐inflammatory properties of milk‐derived peptides have been tested in in vivo models. In particular, Val‐Pro‐Pro and Ile‐Pro‐Prp peptides showed anti‐inflammatory bioactivity in a model of intestinal enterocolitis 76, and also protected against atherosclerosis in the ApoE knockout mice where it showed suppression of the mRNA for inflammatory cytokines, oxidized low density lipoprotein receptor and transcription regulators 77. In addition, whey and casein hydrolysates attenuated dermatitis in NC/Nga mice 78 and showed modulation of inflammatory responces in models of adjuvant arthritis in rats 79. Our group recently explored the effects of milk‐derived bioactive peptides on the expression of the inflammatory phenotype of human endothelial cells and their effects on monocyte adherence to endothelial cells. We found that milk‐derived bioactive peptides worked as anti‐inflammatory agents by inhibiting the NF‐κB pathway through a PPAR‐γ dependent mechanism 80.
Immunomodulating activities
Several immunomodulating peptides have been found in bovine milk with effects on specific immune system cell types. Lymphocyte proliferation has been shown to be modulated by a number of milk components such α‐β‐κ‐casein, whey protein and lactoferrin 81, 82, 83. In addition, β‐casein fermented with lactic acid bacteria exhibited immonomodulating activity on mononuclear cells and T‐helper cells, especially Th1 cells 84, while synthetic peptides corresponding to fragments of bovine κ‐ casein, beta‐casomorphin‐7 and beta‐casomorphin‐10 enhanced proliferation of human lymphocytes in a dose dependent manner 85. In particular, Tyr‐Gly and Tyr‐Gly‐Gly significantly enhanced the proliferation of lymphocytes, while with beta‐casomorphin‐7 and beta‐casomorphin‐10, lymphocyte proliferation was suppressed at lower concentrations, but stimulated at higher concentrations 85. In addition to the bioactivities previously described, lactoferrin and lactoferrin‐derived peptides also showed immunomodulating activity, by inhibiting granulopoiesis and antibody production, and by regulating natural killer cell activity 86. Interestingly, ACE inhibitor peptides have been implicated in the stimulation of the immune system as inhibition of ACE increases bradykinin concentrations which, in turn, stimulate macrophages, and enhance lymphocyte migration and lymphokine secretion 87, 88. The mechanism by which milk‐derived peptides exert their Immunomodulating activities is not clear yet, but it has been suggested that the presence of arginine in the N‐ or C‐terminal region of the bioactive peptides may be important for this activity 87.
Characterization of anti‐atherogenic molecular mechanisms of bioactive peptides
Several milk‐derived bioactive peptides reviewed above show bioactivities that could be beneficial in the management of atherosclerosis but little is known about their mechanism of action or effectiveness. Further characterization of their molecular mechanisms may help to optimize their application. Atherosclerosis is a chronic disease characterized by the formation of an atherosclerotic plaque in the vessel wall and it is the leading cause of heart attack and strokes 89. Development of atherosclerosis is characterized by an inflammatory process (Figure 2), aggravated by well‐known risk factors such as hypertension, obesity, hyperlipidaemia and diabetes 89, 90, 91, which in turn promotes endothelial dysfunction 92. Early stages of atherosclerosis are characterized by recruitment of monocytes to vascular endothelial cells (EC) following minor injury 93, 94 and the subsequent migration of adherent monocytes through the intima of the damaged vessel 95. These mechanisms are regulated by an increased expression of adhesion molecules (VCAM‐1, ICAM‐1 and E‐selectin) and chemokines (IL‐8 and MCP‐1) in EC 96. Many signalling pathways have been identified in the development of atherosclerosis, including peroxisome proliferator‐activator receptor‐gamma (PPAR‐γ), c‐Jun N‐terminal kinases (JNK) and Ras‐Raf‐MEK‐ERK, and STAT3, amongst others. In particular, PPAR‐γ is a nuclear receptor which plays a regulatory role in the early stages of atherosclerosis development 97, in that activation of PPAR‐γ by natural or synthetic agonists prevents the signal transduction and activation of pro‐inflammatory transcription factors such as NF‐κB 98. Our group is studying the molecular mechanisms of milk‐derived bioactive peptides by which they exert anti‐inflammatory and anti‐atherogenic effects. In one study we examined the inhibitory effects of the hydrolysate obtained by Enterococcus faecalis fermentation of sodium caseinate on the expression of inflammatory phenotypes of endothelial cells and on monocyte adhesion and migration, and we found that the hydrosylate's effects were mediated by an inhibition of the NF‐κB pathway via activation of PPAR‐γ 80. We also found that the hydrolysate suppresses the NF‐κB pathway and inhibited the expression of the endothelial cells inflammatory phenotype induced by TNF‐α, by down‐regulating the expression of endothelial cell adhesion molecules (VCAM‐1, ICAM1 and E‐sel) and chemokines (IL‐8 and MCP‐1). These effects were completely reversed by the specific PPAR‐γ inhibitor, GW9662, suggesting that the casein hydrolysate component(s) may be ligands for PPAR‐γ and through this mechanism inhibit NF‐κB activation 80. However, we cannot exclude that the anti‐inflammatory effects described in our study may also be due to PPAR‐γ‐ and NF‐κB‐independent mechanisms as the hydrolysate generated by Enterococcus faecalis fermentation of sodium caseinate is a complex mixture of peptides, which can act at different molecular levels and target other cellular pathways such as ERK 99 and AP‐1 100.
The analysis of the hydrolysate fractions further revealed that the samples containing peptides of 0.5–5 KDa were the most bioactive in reducing the gene expression of adhesion molecules in activated endothelial cells, thus suggesting that the anti‐inflammatory activity of the hydrolysate may be limited to peptides in those fractions 80. Similarly, a recent in vitro study showing an anti‐inflammatory effect of casein hydrolysates 101. Showed that whole β‐casein hydrolysate and the fractions containing peptides between 1 and 5 kDa had the strongest inhibitory effect in a cell‐based assay, supporting our studies on fractions obtained by bacterial fermentation of sodium caseinate 80.
Our group is now focusing on the effects of milk derived bioactive peptides on the monocyte toll‐like receptor 4 (TLR4), a monocyte surface receptor for LPS, which primes immune cells to secrete pro‐inflammatory cytokines and chemokines 102. TLR4 can signal through both JNK and NF‐κB and up‐regulate chemokines and adhesion molecules 103. It is also responsible for CCR2 receptor expression, a membrane receptor involved in the recruitment of monocytes to activated endothelial cells. We therefore explored whether the milk‐derived peptides could utilize this surface receptor to influence gene regulation in monocytes. New, unpublished data show that the milk peptides are signalling through TLR4 in THP‐1 monocytes, and that casein hydrolysate inhibited LPS‐induced cytokine synthesis. Interestingly, the casein hydrolysate significantly inhibited the binding of LPS to THP‐1 monocytes while flow cytometric analysis of its counter receptor (TLR4) indicated that milk hydrolysates do not alter TLR4 surface expression, and therefore do not result in internalization of TLR4. Taken together, these data suggest that the milk hydrolysates are more likely to be inhibiting the binding of LPS to TLR4.
These findings point to the potential of milk derived peptides as nutraceuticals in the prevention and management of atherosclerosis. Identification of bioactive peptides using this bioassay of monocyte migration and adhesion to endothelial cells could help identify more specific peptides, which in turn could be incorporated into functional food ingredients.
Conclusions
Work on the diverse and specific bioactivities of milk derived peptides shows that they may have important functional food effects that in turn may impact on cardiovascular disease. Atherosclerosis complicated by arterial thrombosis is a major cause of human morbidity and mortality, with the disease contributing to more than 50% of all deaths. What triggers atherothrombosis is unknown, but there is evidence for an interaction between hereditary and environmental factors, specifically high fat diets, obesity and smoking. Pharmaceuticals, such as antiplatelet and lipid lowering agents, play an important role in the management of atherothrombosis, but their effects are limited and associated with complications. The combined effects of milk derived bioactive peptides on inflammation, hypertension, cholesterol concentration, suppression of free radical formation and platelet activation may represent a novel and feasible strategy for the management of these diverse risk factors in large populations. Specifically, identification and characterization of novel compounds from natural sources, including milk, which exert beneficial effects and possibly with fewer side effects, may represent an alternative to drugs. Numerous products containing bioactive peptides are already on the market and casein derived peptides have found applications as dietary supplements and in pharmaceutical preparations 104. However, while it is known that bioactive compounds are generated during digestion in vivo it is not known if these peptides have any physiological effects. Moreover, while many studies have described promising health promoting effects of milk derived peptides in CVD, further studies, and in particular more in vivo research with a focus on toxicity, will be required before their application in the management of disease is considered. Currently, little is still known about the bioavailability and pharmacokinetics of bioactive peptides 105 and it is difficult to determine dosage and frequency of administration. In addition, while intuitively milk derived peptides may be considered safe, and no toxic effects have been reported at the doses used for in vitro and in vivo experiments, we cannot exclude the potential for toxicity when given in high dose and over a long period of time. More studies are needed to understand fully the biological activity of the milk‐derived bioactive peptides, and the elucidation of the specific molecular mechanisms involved. This is critical in refining the application of bioactive peptides and optimizing their use for health and wellbeing.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by Enterprise Ireland as part of the Food for Health Ireland Project [Grant Number TC20130001]. We also would like to acknowledge funding from the Programme for Research in Third‐Level Institutions (PRTLI), administered by the Higher Education Authority of Ireland (DJF).
Marcone, S. , Belton, O. , and Fitzgerald, D. J. (2017) Milk‐derived bioactive peptides and their health promoting effects: a potential role in atherosclerosis. Br J Clin Pharmacol, 83: 152–162. doi: 10.1111/bcp.13002.
References
- 1. Schanbacher FL, Talhouk RS, Murray FA. Biology and origin of bioactive peptides in milk. Livest Prod Sci 1997; 50: 105–23. [Google Scholar]
- 2. Wong DW, Camirand WM, Pavlath AE. Structures and functionalities of milk proteins. Crit Rev Food Sci Nutr 1996; 36: 807–44. [DOI] [PubMed] [Google Scholar]
- 3. Korhonen H, Pihlanto A. Technological options for the production of health‐promoting proteins and peptides derived from milk and colostrum. Curr Pharm Des 2007; 13: 829–43. [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto N. Antihypertensive peptides derived from food proteins. Biopolymers 1997; 43: 129–34. [DOI] [PubMed] [Google Scholar]
- 5. Lahov E, Regelson W. Antibacterial and immunostimulating casein‐derived substances from milk: casecidin, isracidin peptides. Food Chem Toxicol 1996; 34: 131–45. [DOI] [PubMed] [Google Scholar]
- 6. Meisel H. Biochemical properties of regulatory peptides derived from milk proteins. Biopolymers 1997; 43: 119–28. [DOI] [PubMed] [Google Scholar]
- 7. Dziuba J, Minkiewicz P, Nalecz D, Iwaniak A. Database of biologically active peptide sequences. Nahrung 1999; 43: 190–5. [DOI] [PubMed] [Google Scholar]
- 8. Gobbetti M, Stepaniak L, De Angelis M, Corsetti A, Di Cagno R. Latent bioactive peptides in milk proteins: proteolytic activation and significance in dairy processing. Crit Rev Food Sci Nutr 2002; 42: 223–39. [DOI] [PubMed] [Google Scholar]
- 9. Meisel H. Multifunctional peptides encrypted in milk proteins. Biofactors 2004; 21: 55–61. [DOI] [PubMed] [Google Scholar]
- 10. Malkoski M, Dashper SG, O'Brien‐Simpson NM, Talbo GH, Macris M, Cross KJ, et al. Kappacin, a novel antibacterial peptide from bovine milk. Antimicrob Agents Chemother 2001; 45: 2309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jakala P, Pere E, Lehtinen R, Turpeinen A, Korpela R, Vapaatalo H. Cardiovascular activity of milk casein‐derived tripeptides and plant sterols in spontaneously hypertensive rats. J Physiol Pharmacol 2009; 60: 11–20. [PubMed] [Google Scholar]
- 12. Sipola M, Finckenberg P, Santisteban J, Korpela R, Vapaatalo H, Nurminen ML. Long‐term intake of milk peptides attenuates development of hypertension in spontaneously hypertensive rats. J Physiol Pharmacol 2001; 52: 745–54. [PubMed] [Google Scholar]
- 13. Hirota T, Ohki K, Kawagishi R, Kajimoto Y, Mizuno S, Nakamura Y, et al. Casein hydrolysate containing the antihypertensive tripeptides Val‐Pro‐Pro and Ile‐Pro‐Pro improves vascular endothelial function independent of blood pressure‐lowering effects: contribution of the inhibitory action of angiotensin‐converting enzyme. Hypertension Res 2007; 30: 489–96. [DOI] [PubMed] [Google Scholar]
- 14. Pearson T. Cardiovascular update: risk, guidelines, and recommendations. Workplace Health Saf 2015; 63: 376–80. [DOI] [PubMed] [Google Scholar]
- 15. FitzGerald RJ, Murray BA, Walsh DJ. Hypotensive peptides from milk proteins. J Nutr 2004; 134: 980S–8S. [DOI] [PubMed] [Google Scholar]
- 16. Vercruysse L, Van Camp J, Smagghe G. ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: a review. J Agric Food Chem 2005; 53: 8106–15. [DOI] [PubMed] [Google Scholar]
- 17. FitzGerald RJ, Meisel H. Milk protein‐derived peptide inhibitors of angiotensin‐I‐converting enzyme. Br J Nutr 2000; 84 (Suppl 1): S33–7. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura Y, Yamamoto N, Sakai K, Takano T. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I‐converting enzyme. J Dairy Sci 1995; 78: 1253–7. [DOI] [PubMed] [Google Scholar]
- 19. Seppo L, Jauhiainen T, Poussa T, Korpela R. A fermented milk high in bioactive peptides has a blood pressure‐lowering effect in hypertensive subjects. Am J Clin Nutr 2003; 77: 326–30. [DOI] [PubMed] [Google Scholar]
- 20. Murakami M, Tonouchi H, Takahashi R, Kitazawa H, Kawai Y, Negishi H, et al. Structural analysis of a new anti‐hypertensive peptide (beta‐lactosin B) isolated from a commercial whey product. J Dairy Sci 2004; 87: 1967–74. [DOI] [PubMed] [Google Scholar]
- 21. Boelsma E, Kloek J. Lactotripeptides and antihypertensive effects: a critical review. Br J Nutr 2009; 101: 776–86. [DOI] [PubMed] [Google Scholar]
- 22. Boelsma E, Kloek J. IPP‐rich milk protein hydrolysate lowers blood pressure in subjects with stage 1 hypertension, a randomized controlled trial. Nutr J 2010; 9: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujita H, Usui H, Kurahashi K, Yoshikawa M. Isolation and characterization of ovokinin, a bradykinin B1 agonist peptide derived from ovalbumin. Peptides 1995; 16: 785–90. [DOI] [PubMed] [Google Scholar]
- 24. Matoba N, Usui H, Fujita H, Yoshikawa M. A novel anti‐hypertensive peptide derived from ovalbumin induces nitric oxide‐mediated vasorelaxation in an isolated SHR mesenteric artery. FEBS Lett 1999; 452: 181–4. [DOI] [PubMed] [Google Scholar]
- 25. Erdmann K, Grosser N, Schipporeit K, Schroder H. The ACE inhibitory dipeptide Met‐Tyr diminishes free radical formation in human endothelial cells via induction of heme oxygenase‐1 and ferritin. J Nutr 2006; 136: 2148–52. [DOI] [PubMed] [Google Scholar]
- 26. Nurminen ML, Sipola M, Kaarto H, Pihlanto‐Leppala A, Piilola K, Korpela R, et al. Alpha‐lactorphin lowers blood pressure measured by radiotelemetry in normotensive and spontaneously hypertensive rats. Life Sci 2000; 66: 1535–43. [DOI] [PubMed] [Google Scholar]
- 27. Fiat AM, Levy‐Toledano S, Caen JP, Jolles P. Biologically active peptides of casein and lactotransferrin implicated in platelet function. J Dairy Res 1989; 56: 351–5. [DOI] [PubMed] [Google Scholar]
- 28. Leonil J, Molle D. Liberation of tryptic fragments from caseinomacropeptide of bovine kappa‐casein involved in platelet function. Kinetic study. Biochem J 1990; 271: 247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jolles P, Levy‐Toledano S, Fiat AM, Soria C, Gillessen D, Thomaidis A, et al. Analogy between fibrinogen and casein. Effect of an undecapeptide isolated from kappa‐casein on platelet function. Eur J Biochem 1986; 158: 379–82. [DOI] [PubMed] [Google Scholar]
- 30. Manso MA, Escudero C, Alijo M, Lopez‐Fandino R. Platelet aggregation inhibitory activity of bovine, ovine, and caprine kappa‐casein macropeptides and their tryptic hydrolysates. J Food Prot 2002; 65: 1992–6. [DOI] [PubMed] [Google Scholar]
- 31. Qian ZY, Jolles P, Migliore‐Samour D, Schoentgen F, Fiat AM. Sheep kappa‐casein peptides inhibit platelet aggregation. Biochim Biophys Acta 1995; 1244: 411–7. [DOI] [PubMed] [Google Scholar]
- 32. Bal dit Sollier C, Drouet L, Pignaud G, Chevallier C, Caen J, Fiat AM, et al. Effect of kappa‐casein split peptides on platelet aggregation and on thrombus formation in the guinea‐pig. Thromb Res 1996; 81: 427–37. [DOI] [PubMed] [Google Scholar]
- 33. Raha S, Dosquet C, Abgrall JF, Jolles P, Fiat AM, Caen JP. KRDS–a tetrapeptide derived from lactotransferrin–inhibits binding of monoclonal antibody against glycoprotein IIb‐IIIa on ADP‐stimulated platelets and megakaryocytes. Blood 1988; 72: 172–8. [PubMed] [Google Scholar]
- 34. Mazoyer E, Levy‐Toledano S, Rendu F, Hermant L, Lu H, Fiat AM, et al. KRDS, a new peptide derived from human lactotransferrin, inhibits platelet aggregation and release reaction. Eur J Biochem 1990; 194: 43–9. [DOI] [PubMed] [Google Scholar]
- 35. Drouet L, Bal dit Sollier C, Cisse M, Pignaud G, Mazoyer E, Fiat AM, et al. The antithrombotic effect of KRDS, a lactotransferrin peptide, compared with RGDS. Nouv Rev Fr Hematol 1990; 32: 59–62. [PubMed] [Google Scholar]
- 36. Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs 2012; 72: 2087–116. [DOI] [PubMed] [Google Scholar]
- 37. Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India 2004; 52: 794–804. [PubMed] [Google Scholar]
- 38. Brieger K, Schiavone S, Miller FJ Jr, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly 2012; 142: w13659. [DOI] [PubMed] [Google Scholar]
- 39. Machlin LJ, Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J 1987; 1: 441–5. [PubMed] [Google Scholar]
- 40. de Lorgeril M, Salen P, Monjaud I, Delaye J. The 'diet heart' hypothesis in secondary prevention of coronary heart disease. Eur Heart J 1997; 18: 13–8. [DOI] [PubMed] [Google Scholar]
- 41. Simopoulos AP. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. J Nutr 2001; 131: 3065S–73S. [DOI] [PubMed] [Google Scholar]
- 42. Suetsuna K, Ukeda H, Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J Nutr Biochem 2000; 11: 128–31. [DOI] [PubMed] [Google Scholar]
- 43. Hernandez‐Ledesma B, Davalos A, Bartolome B, Amigo L. Preparation of antioxidant enzymatic hydrolysates from alpha‐lactalbumin and beta‐lactoglobulin. Identification of active peptides by HPLC‐MS/MS. J Agric Food Chem 2005; 53: 588–93. [DOI] [PubMed] [Google Scholar]
- 44. Chen HM, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. Antioxidative properties of histidine‐containing peptides designed from peptide fragments found in the digests of a soybean protein. J Agric Food Chem 1998; 46: 49–53. [DOI] [PubMed] [Google Scholar]
- 45. Jimenez‐Ruiz EI, Calderon de la Barca AM, Sotelo‐Mundo RR, Arteaga‐Mackinney GE, Valenzuela‐Melendez M, Pena‐Ramos EA. Partial characterization of ultrafiltrated soy protein hydrolysates with antioxidant and free radical scavenging activities. J Food Sci 2013; 78: C1152–8. [DOI] [PubMed] [Google Scholar]
- 46. Grundy SM, Balady GJ, Criqui MH, Fletcher G, Greenland P, Hiratzka LF, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA Task Force on Risk Reduction, American Heart Association. Circulation 1998; 97: 1876–87. [DOI] [PubMed] [Google Scholar]
- 47. Martin MJ, Hulley SB, Browner WS, Kuller LH, Wentworth D. Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet 1986; 2: 933–6. [DOI] [PubMed] [Google Scholar]
- 48. Jabir NR, Siddiqui AN, Firoz CK, Md Ashraf G, Zaidi SK, Khan MS, et al. Current updates on therapeutic advances in the management of cardiovascular diseases. Curr P harm Des 2016; 22: 566–71. [DOI] [PubMed] [Google Scholar]
- 49. Hori G, Wang MF, Chan YC, Komatsu T, Wong Y, Chen TH, et al. Soy protein hydrolyzate with bound phospholipids reduces serum cholesterol levels in hypercholesterolemic adult male volunteers. Biosci Biotechnol Biochem 2001; 65: 72–8. [DOI] [PubMed] [Google Scholar]
- 50. Nagaoka S, Futamura Y, Miwa K, Awano T, Yamauchi K, Kanamaru Y, et al. Identification of novel hypocholesterolemic peptides derived from bovine milk beta‐lactoglobulin. Biochem Biophys Res Commun 2001; 281: 11–7. [DOI] [PubMed] [Google Scholar]
- 51. Morikawa K, Kondo I, Kanamaru Y, Nagaoka S. A novel regulatory pathway for cholesterol degradation via lactostatin. Biochem Biophys Res Commun 2007; 352: 697–702. [DOI] [PubMed] [Google Scholar]
- 52. Woodward M, Zhang X, Barzi F, Pan W, Ueshima H, Rodgers A, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia‐Pacific region. Diabetes Care 2003; 26: 360–6. [DOI] [PubMed] [Google Scholar]
- 53. Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose‐equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 2004; 80: 1246–53. [DOI] [PubMed] [Google Scholar]
- 54. Calbet JA, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr 2004; 43: 127–39. [DOI] [PubMed] [Google Scholar]
- 55. Claessens M, Saris WH, van Baak MA. Glucagon and insulin responses after ingestion of different amounts of intact and hydrolysed proteins. Br J Nutr 2008; 100: 61–9. [DOI] [PubMed] [Google Scholar]
- 56. Frid AH, Nilsson M, Holst JJ, Bjorck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 2005; 82: 69–75. [DOI] [PubMed] [Google Scholar]
- 57. Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet‐controlled type 2 diabetes. Diabetes Care 2009; 32: 1600–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Belobrajdic DP, McIntosh GH, Owens JA. A high‐whey‐protein diet reduces body weight gain and alters insulin sensitivity relative to red meat in wistar rats. J Nutr 2004; 134: 1454–8. [DOI] [PubMed] [Google Scholar]
- 59. Liu Z, Jeppesen PB, Gregersen S, Chen X, Hermansen K. Dose‐ and glucose‐dependent effects of amino acids on insulin secretion from isolated mouse islets and clonal INS‐1E beta‐cells. Rev Diabet Stud 2008; 5: 232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Phillips LK, Prins JB. Update on incretin hormones. Ann N Y Acad Sci 2011; 1243: E55–74. [DOI] [PubMed] [Google Scholar]
- 61. Manders RJ, Praet SF, Meex RC, Koopman R, de Roos AL, Wagenmakers AJ, et al. Protein hydrolysate/leucine co‐ingestion reduces the prevalence of hyperglycemia in type 2 diabetic patients. Diabetes Care 2006; 29: 2721–2. [DOI] [PubMed] [Google Scholar]
- 62. Manders RJ, Praet SF, Vikstrom MH, Saris WH, van Loon LJ. Protein hydrolysate co‐ingestion does not modulate 24 h glycemic control in long‐standing type 2 diabetes patients. Eur J Clin Nutr 2009; 63: 121–6. [DOI] [PubMed] [Google Scholar]
- 63. Brader L, Holm L, Mortensen L, Thomsen C, Astrup A, Holst JJ, et al. Acute effects of casein on postprandial lipemia and incretin responses in type 2 diabetic subjects. Nutr Metab Cardiovasc Dis 2010; 20: 101–9. [DOI] [PubMed] [Google Scholar]
- 64. Cohen AT, Imfeld S, Markham J, Granziera S. The use of aspirin for primary and secondary prevention in venous thromboembolism and other cardiovascular disorders. Thromb Res 2015; 135: 217–25. [DOI] [PubMed] [Google Scholar]
- 65. Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep 2014; 16: 435. [DOI] [PubMed] [Google Scholar]
- 66. Pellicano R. Gastrointestinal damage by non‐steroidal anti‐inflammatory drugs: updated clinical considerations. Minerva Gastroenterol Dietol 2014; 60: 255–61. [PubMed] [Google Scholar]
- 67. Wehling M. Non‐steroidal anti‐inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur J Clin Pharmacol 2014; 70: 1159–72. [DOI] [PubMed] [Google Scholar]
- 68. Aihara K, Ishii H, Yoshida M. Casein‐derived tripeptide, Val‐Pro‐Pro (VPP), modulates monocyte adhesion to vascular endothelium. J Atheroscler Thromb 2009; 16: 594–603. [DOI] [PubMed] [Google Scholar]
- 69. Nielsen DS, Theil PK, Larsen LB, Purup S. Effect of milk hydrolysates on inflammation markers and drug‐induced transcriptional alterations in cell‐based models. J Anim Sci 2012; 90 (Suppl 4): 403–5. [DOI] [PubMed] [Google Scholar]
- 70. Iskandar MM, Dauletbaev N, Kubow S, Mawji N, Lands LC. Whey protein hydrolysates decrease IL‐8 secretion in lipopolysaccharide (LPS)‐stimulated respiratory epithelial cells by affecting LPS binding to Toll‐like receptor 4. Br J Nutr 2013; 110: 58–68. [DOI] [PubMed] [Google Scholar]
- 71. Piccolomini AF, Iskandar MM, Lands LC, Kubow S. High hydrostatic pressure pre‐treatment of whey proteins enhances whey protein hydrolysate inhibition of oxidative stress and IL‐8 secretion in intestinal epithelial cells. Food Nutr Res 2012; 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haversen L, Ohlsson BG, Hahn‐Zoric M, Hanson LA, Mattsby‐Baltzer I. Lactoferrin down‐regulates the LPS‐induced cytokine production in monocytic cells via NF‐kappa B. Cell Immunol 2002; 220: 83–95. [DOI] [PubMed] [Google Scholar]
- 73. Puddu P, Latorre D, Carollo M, Catizone A, Ricci G, Valenti P, et al. Bovine lactoferrin counteracts Toll‐like receptor mediated activation signals in antigen presenting cells. PLoS One 2011; 6: e22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yan D, Kc R, Chen D, Xiao G, Im HJ. Bovine lactoferricin‐induced anti‐inflammation is, in part, via up‐regulation of interleukin‐11 by secondary activation of STAT3 in human articular cartilage. J Biol Chem 2013; 288: 31655–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yan D, Chen D, Shen J, Xiao G, van Wijnen AJ, Im HJ. Bovine lactoferricin is anti‐inflammatory and anti‐catabolic in human articular cartilage and synovium. J Cell Physiol 2013; 228: 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti‐inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol 2013; 45: 1730–47. [DOI] [PubMed] [Google Scholar]
- 77. Nakamura T, Hirota T, Mizushima K, Ohki K, Naito Y, Yamamoto N, et al. Milk‐derived peptides, Val‐Pro‐Pro and Ile‐Pro‐Pro, attenuate atherosclerosis development in apolipoprotein e‐deficient mice: a preliminary study. J Med Food 2013; 16: 396–403. [DOI] [PubMed] [Google Scholar]
- 78. Shimizu N, Dairiki K, Ogawa S, Kaneko T. Dietary whey protein hydrolysate suppresses development of atopic dermatitis‐like skin lesions induced by mite antigen in NC/Nga mice. Allergol Int 2006; 55: 185–9. [DOI] [PubMed] [Google Scholar]
- 79. Hatori M, Ohki K, Hirano S, Yang XP, Kuboki H, Abe C. Effects of a casein hydrolysate prepared from Aspergillus oryzae protease on adjuvant arthritis in rats. Biosci Biotechnol Biochem 2008; 72: 1983–91. [DOI] [PubMed] [Google Scholar]
- 80. Marcone S, Haughton K, Simpson PJ, Belton O, Fitzgerald DJ. Milk‐derived bioactive peptides inhibit human endothelial‐monocyte interactions via PPAR‐gamma dependent regulation of NF‐kappaB. J Inflamm 2015; 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wong CW, Seow HF, Liu AH, Husband AJ, Smithers GW, Watson DL. Modulation of immune responses by bovine beta‐casein. Immunol Cell Biol 1996; 74: 323–9. [DOI] [PubMed] [Google Scholar]
- 82. Barta O, Barta VD, Crisman MV, Akers RM. Inhibition of lymphocyte blastogenesis by whey. Am J Vet Res 1991; 52: 247–53. [PubMed] [Google Scholar]
- 83. Otani H, Monnai M, Kawasaki Y, Kawakami H, Tanimoto M. Inhibition of mitogen‐induced proliferative responses of lymphocytes by bovine kappa‐caseinoglycopeptides having different carbohydrate chains. J Dairy Res 1995; 62: 349–57. [DOI] [PubMed] [Google Scholar]
- 84. Laffineur E, Genetet N, Leonil J. Immunomodulatory activity of beta‐casein permeate medium fermented by lactic acid bacteria. J Dairy Sci 1996; 79: 2112–20. [DOI] [PubMed] [Google Scholar]
- 85. Kayser H, Meisel H. Stimulation of human peripheral blood lymphocytes by bioactive peptides derived from bovine milk proteins. FEBS Lett 1996; 383: 18–20. [DOI] [PubMed] [Google Scholar]
- 86. Crouch SP, Slater KJ, Fletcher J. Regulation of cytokine release from mononuclear cells by the iron‐binding protein lactoferrin. Blood 1992; 80: 235–40. [PubMed] [Google Scholar]
- 87. Paegelow I, Werner H. Immunomodulation by some oligopeptides. Methods Find Exp Clin Pharmacol 1986; 8: 91–5. [PubMed] [Google Scholar]
- 88. McFadden RG, Vickers KE. Bradykinin augments the in vitro migration of nonsensitized lymphocytes. Clin Invest Med 1989; 12: 247–53. [PubMed] [Google Scholar]
- 89. Anogeianaki A, Angelucci D, Cianchetti E, D'Alessandro M, Maccauro G, Saggini A, et al. Atherosclerosis: a classic inflammatory disease. Int J Immunopathol Pharmacol 2011; 24: 817–25. [DOI] [PubMed] [Google Scholar]
- 90. Tousoulis D, Kampoli AM, Papageorgiou N, Androulakis E, Antoniades C, Toutouzas K, et al. Pathophysiology of atherosclerosis: the role of inflammation. Curr Pharm Des 2011; 17: 4089–110. [DOI] [PubMed] [Google Scholar]
- 91. Mizuno Y, Jacob RF, Mason RP. Inflammation and the development of atherosclerosis. J Atheroscler Thromb 2011; 18: 351–8. [DOI] [PubMed] [Google Scholar]
- 92. Ait‐Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol 2011; 31: 969–79. [DOI] [PubMed] [Google Scholar]
- 93. Kruth HS. Lipoprotein cholesterol and atherosclerosis. Curr Mol Med 2001; 1: 633–53. [DOI] [PubMed] [Google Scholar]
- 94. Le Brocq M, Leslie SJ, Milliken P, Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal 2008; 10: 1631–74. [DOI] [PubMed] [Google Scholar]
- 95. Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 1999; 38: 12959–68. [DOI] [PubMed] [Google Scholar]
- 96. Rao RM, Yang L, Garcia‐Cardena G, Luscinskas FW. Endothelial‐dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res 2007; 101: 234–47. [DOI] [PubMed] [Google Scholar]
- 97. Sasaki M, Jordan P, Welbourne T, Minagar A, Joh T, Itoh M, et al. Troglitazone, a PPAR‐gamma activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF‐alpha. BMC Physiol 2005; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator‐activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res 2000; 49: 497–505. [DOI] [PubMed] [Google Scholar]
- 99. Wilmer WA, Dixon C, Lu L, Hilbelink T, Rovin BH. A cyclopentenone prostaglandin activates mesangial MAP kinase independently of PPARgamma. Biochem Biophys Res Commun 2001; 281: 57–62. [DOI] [PubMed] [Google Scholar]
- 100. Perez‐Sala D, Cernuda‐Morollon E, Canada FJ. Molecular basis for the direct inhibition of AP‐1 DNA binding by 15‐deoxy‐delta 12,14‐prostaglandin J2. J Biol Chem 2003; 278: 51251–60. [DOI] [PubMed] [Google Scholar]
- 101. Malinowski J, Klempt M, Clawin‐Radecker I, Lorenzen PC, Meisel H. Identification of a NFkappaB inhibitory peptide from tryptic beta‐casein hydrolysate. Food Chem 2014; 165: 129–33. [DOI] [PubMed] [Google Scholar]
- 102. Yi L, Chandrasekaran P, Venkatesan S. TLR signaling paralyzes monocyte chemotaxis through synergized effects of p38 MAPK and global Rap‐1 activation. PLoS One 2012; 7: e30404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll‐like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 2003; 37: 1043–55. [DOI] [PubMed] [Google Scholar]
- 104. Pihlanto A, Korhonen H. Bioactive peptides and proteins. Adv Food Nutr Res 2003; 47: 175–276. [DOI] [PubMed] [Google Scholar]
- 105. Sanchez‐Rivera L, Ares I, Miralles B, Gomez‐Ruiz JA, Recio I, Martinez‐Larranaga MR, et al. Bioavailability and kinetics of the antihypertensive casein‐derived peptide HLPLP in rats. J Agric Food Chem 2014; 62: 11869–75. [DOI] [PubMed] [Google Scholar]
- 106. Maeno M, Yamamoto N, Takano T. Identification of an antihypertensive peptide from casein hydrolysate produced by a proteinase from Lactobacillus helveticus CP790. J Dairy Sci 1996; 79: 1316–21. [DOI] [PubMed] [Google Scholar]
- 107. Yamamoto N, Akino A, Takano T. Antihypertensive effect of the peptides derived from casein by an extracellular proteinase from Lactobacillus helveticus CP790. J Dairy Sci 1994; 77: 917–22. [DOI] [PubMed] [Google Scholar]
- 108. Pihlanto‐Leppala A, Koskinen P, Piilola K, Tupasela T, Korhonen H. Angiotensin I‐converting enzyme inhibitory properties of whey protein digests: concentration and characterization of active peptides. J Dairy Res 2000; 67: 53–64. [DOI] [PubMed] [Google Scholar]
- 109. Mullally MM, Meisel H, FitzGerald RJ. Identification of a novel angiotensin‐I‐converting enzyme inhibitory peptide corresponding to a tryptic fragment of bovine beta‐lactoglobulin. FEBS Lett 1997; 402: 99–101. [DOI] [PubMed] [Google Scholar]
- 110. FitzGerald RJ, Meisel H. Lactokinins: whey protein‐derived ACE inhibitory peptides. Nahrung 1999; 43: 165–7. [DOI] [PubMed] [Google Scholar]
- 111. Mullally MM, Meisel H, FitzGerald RJ. Synthetic peptides corresponding to alpha‐lactalbumin and beta‐lactoglobulin sequences with angiotensin‐I‐converting enzyme inhibitory activity. Biol Chem Hoppe Seyler 1996; 377: 259–60. [DOI] [PubMed] [Google Scholar]
- 112. Smacchi E, Gobbetti M. Bioactive peptides in dairy products: synthesis and interaction with proteolytic enzymes. Food Microbiol 2000; 17: 129–41. [Google Scholar]
- 113. Mao X‐Y, Ni J‐R, Sun W‐L, Hao P‐P, Fan L. Value‐added utilization of yak milk casein for the production of angiotensin‐I‐converting enzyme inhibitory peptides. Food Chem 2007; 103: 1282–7. [Google Scholar]
- 114. Haileselassie SS, Lee BH, Gibbs BF. Purification and identification of potentially bioactive peptides from enzyme‐modified cheese. J Dairy Sci 1999; 82: 1612–7. [DOI] [PubMed] [Google Scholar]
- 115. Chabance B, Jolles P, Izquierdo C, Mazoyer E, Francoual C, Drouet L, et al. Characterization of an antithrombotic peptide from kappa‐casein in newborn plasma after milk ingestion. Br J Nutr 1995; 73: 583–90. [DOI] [PubMed] [Google Scholar]
- 116. Qian ZY, Jolles P, Migliore‐Samour D, Fiat AM. Isolation and characterization of sheep lactoferrin, an inhibitor of platelet aggregation and comparison with human lactoferrin. Biochim Biophys Acta 1995; 1243: 25–32. [DOI] [PubMed] [Google Scholar]
- 117. Chabance B, Marteau P, Rambaud JC, Migliore‐Samour D, Boynard M, Perrotin P, et al. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 1998; 80: 155–65. [DOI] [PubMed] [Google Scholar]
- 118. Fiat AM, Migliore‐Samour D, Jolles P, Drouet L, Bal dit Sollier C, Caen J. Biologically active peptides from milk proteins with emphasis on two examples concerning antithrombotic and immunomodulating activities. J Dairy Sci 1993; 76: 301–10. [DOI] [PubMed] [Google Scholar]
- 119. Contreras MM, Carrón R, Montero MJ, Ramos M, Recio I. Novel casein‐derived peptides with antihypertensive activity. Int Dairy J 2009; 19: 566–73. [Google Scholar]
- 120. Nagaoka S, Kanamaru Y, Kuzuya Y, Kojima T, Kuwata T. Comparative studies on the serum cholesterol lowering action of whey protein and soybean protein in rats. Biosci Biotechnol Biochem 1992; 56: 1484–5. [Google Scholar]
- 121. Zhang X, Beynen AC. Lowering effect of dietary milk‐whey protein v. casein on plasma and liver cholesterol concentrations in rats. Br J Nutr 1993; 70: 139–46. [DOI] [PubMed] [Google Scholar]


