Abstract
Statement of the Problem:
Odontogenic keratocyst (OKC) is a developmental odontogenic cyst with specific histopathological features, high recurrence rate, and aggressive clinical behavior. Angiogenesis might be considered as an important factor for the growth, expansion, and distribution of this lesion.
Purpose:
The aim of the present study was to determine the mean vascular densities (MVD) of OKCs and dentigerous cysts to evaluate their relationship with the biologic behavior of these lesions.
Materials and Method:
In this cross-sectional analytical study, angiogenesis was assessed in OKC and dentigerous cyst by measuring the MVD. Immunohistochemistry was carried out using CD34 and CD105. The results were analyzed with independent samples t-test. The data were analyzed, setting p value at 0.05.
Results:
The MVDs with the use of CD34 and CD105 markers were significantly higher in OKC compared to dentigerous cyst (p< 0.05). In addition, MVDs obtained by CD105 in dentigerous cysts and OKC were significantly less than those based on CD34 (p< 0.05).
Conclusion:
Based on the results of the present study, it can be suggested that angiogenesis might be one of the possible mechanisms involved in higher aggressive biologic behavior and greater recurrence rate of OKC compared to dentigerous cysts.
Keywords: CD105 , CD34 , Dentigerous cyst , Immunohistochemistry , Odontogenic keratocyst
Introduction
Odontogenic keratocyst (OKC) is a developmental odontogenic cyst with specific histopathological features and clinical behaviors, high recurrence rate, and aggressive behavior compared to other developmental odontogenic cysts.[1] Due to its specific clinico-pathologic characteristics, its name has been changed to keratocystic odontogenic tumor in the new classification of the World Health Organization (WHO).[1-2]
Dentigerous (follicular) cyst is the most common odontogenic developmental cyst, which instigates from separation of dental follicle from crown of an unerupted tooth.[1]
The reactions between the odontogenic epithelium and its ectomesenchymal components have a role in the formation of odontogenic lesions, including odontogenic cysts.[2] Blood vessels present in the connective stroma of odontogenic cysts help the growth of their epithelium by supplying oxygen and nutrients.3 Neoplastic tissues require angiogenesis in order to continue their growth, development and progression, which can also contribute to their invasion and metastasis.[3-4] Angiogenesis is defined as the formation of new blood vessels from the existing blood vessels and has been evaluated in various lesions including breast cancer, melanoma, endometrial carcinoma, squamous cell carcinoma, lichen planus, ameloblastoma, and some odontogenic cysts.[5-11]
One of the techniques used for the quantitative analysis of angiogenesis is mean vascular density (MVD), which has been evaluated using various molecules, including vascular endothelial growth factor (VEGF), von Willebrand factor, factor VIII, CD31, CD34, and CD105 (endoglein).[12-13]
CD34 (qbend 10) is a pan-endothelial marker and trans-membranous monomeric glycoprotein of the cell surface and is expressed in the normal and neoplastic endothelial cells of blood vessels.[14-18]
CD105 (endoglein) is a hypoxia-dependent membrane hemodimer disulfide glycoprotein, associated with cell proliferation, and expressed on activated vascular endothelial cells.[19] Since the expression of this protein increases in tumoral tissues compared with normal tissues, it can be inferred that expression of CD105 is related to tumor angiogenesis.[20] Several studies have shown that CD105 antibody has higher accuracy and specificity to bind to newly formed blood vessels in tumoral tissues compared to other pan-endothelial markers such as factor VIII, von Willebrand factor, CD31 and CD34. Therefore, it is more appropriate to determine MVD. [21-22] Only a limited number of studies have investigated stromal factors such as role of angiogenesis in odontogenic lesions reported by Seifi et al[15] and de Andrade Santos et al.[23]
Hence, the aim of this study was to determine MVD by using CD34 and CD105 markers in OKCs and dentigerous cysts and evaluating their relationship with the biologic behaviors of these lesions.
Materials and Method
In this retrospective cross-sectional analytical study, the archive files of Hamadan Faculty of Dentistry were evaluated from 1997 to 2010. The study protocol was approved by our Institutional Ethics Committee. The samples diagnosed as OKC and dentigerous cysts were selected. All the paraffin blocks and slides of dentigerous cyst and OKC samples were retrieved. H&E slides were evaluated and confirmed by two oral pathologists and 15 samples from each lesion with sufficient tissues and proper fixation were selected. Samples with excessive hemorrhage, severe inflammation, and insufficient tissues were excluded. Inflammation was considered one of the factors affecting angiogenesis and therefore, as a confounding factor for the results. The files of patients with the diagnosis of odontogenic lesions, including dentigerous cysts and OKC were retrieved. Their clinical and demographic data including age, gender, and locations of the lesions were recorded in tables. Slices with 4-µ thickness were prepared from paraffin blocks for immunohistochemical staining. The slides were deparaffinized in xylene and then hydrated in graded alcohol series. For CD34endogenous peroxidase activity was blocked by incubating the slides in hydrogen peroxide (3%) in phosphate buffer and for CD105 incubating the slides with 3% H2O2 in methanol for 30 minutes. Antigen retrieval for CD105 was performed by treating sections with proteinase-K for 5 minutes. Antigen retrieval for CD34 was performed in a microwave oven for 10 minutes at 120 degree centigrade. To prevent nonspecific reactions, sections were incubated with 10% serum for 10min.Then Monoclonal Mouse anti-Human CD105 antibody (Clone: SN6h; Product code: M3527, Dako, North America Inc.) and Monoclonal Mouse anti-Human CD34 antibody (Clone: QBend 10, Product code: M7165 A/S, Glostrup, DAKO, Denmark) were applied as primary antibody. CD105 antibody was incubated at room temperature in a humidifying chamber for 90 minutes. For CD34, the sections were incubated with anti- CD34 antibody for 30 minutes at room temperature with a working dilution of 1:50, followed by incubation with secondary biotinylated antibody and streptavidin for 15 min each. Diaminobenzidine was used to produce brown staining followed by counterstaining with Mayer’s hematoxylin. After each step, the slides were placed in phosphate-buffered solution. The human tonsillar tissue was used as the positive control and the primary antibody was eliminated and replaced with phosphate buffer saline for the negative control.
The technique described by Weidner et al.[17] was used for quantitative evaluation of blood vessels. The stained slides were evaluated under a light microscope (Olympus BX41, Japan) by two oral pathologists at 100×400 magnification. In order to determine the MVD, three areas with the highest amount of vascularization, known as hot spots, were selected under low magnification (100×). Microvessels were counted in each of the three fields at 400× magnification and the mean density was reported.
Positive staining for CD105 and CD34 was defined as each brown-colored endothelial cell separately (or in aggregates) was distinctively separated from the adjacent micro-vessels. The objects which seemed to be originated from one blood vessel were counted if completely separated from it.[13] Blood vessels with muscular walls were excluded and the mean number of blood vessels in the three selected was considered as mean vascular density (MVD). Independent samples t-test was used to compare MVDs between the groups. The data were analyzed by PSS16 statistical software with setting p value at 0.05.
Results
The demographic data of the subjects are summarized in Table 1.
Table 1.
Descriptive statistics of clinical data in study groups
| Group | ||||
|---|---|---|---|---|
| Dentigerous | OKC | |||
| Frequency | 15 | 15 | ||
| Gender N(%) | Female | 5(33%) | 6(40%) | |
| Male | 10(67%) | 9(60%) | ||
| Age (year) |
|
33.3±16.4 | 32.2±17.9 | |
| Min | 14 | 13 | ||
| Max | 60 | 76 | ||
| Location | Mandibular ramus | 0(0%) | 4(26.6) | |
| Posterior mandible | 9(66.7%) | 10(66.6%) | ||
| Posterior maxilla | 0(0%) | 0(0%) | ||
| Anterior mandible | 1(3.3%) | 0(0%) | ||
| Anterior maxilla | 5(20%) | 1(3.3%) | ||
CD34 and CD105 were expressed in all samples. (Figure 1) The mean (±SD) MVDs with CD34 marker in dentigerous cysts and OKCs were 8.48±2.8 and 12.24±5.8 respectively which shows MVD in OKC was higher than in dentigerous cysts. Independent samples t-test revealed significant statistical difference between groups (p= 0.032). In addition, mean (±SD) MVDs with CD105 marker in dentigerous cysts and OKCs were 5.94±2.4 and 9.2±2.94 respectively, so MVD in OKC was higher than that in dentigerous cysts. T-test showed significant difference between groups (p= 0.002). (Table 2) The evaluation of MVDs in OKC (by CD105 and CD34 markers) showed a statistical significant differences between these markers (9.2±2.94 and 12.24±5.8 respectively). The results showed MVD with the use of CD34 marker was significantly higher than that with the use of CD105 marker. Independent samples t-test revealed significant difference between groups (p= 0.017).
Figure1.
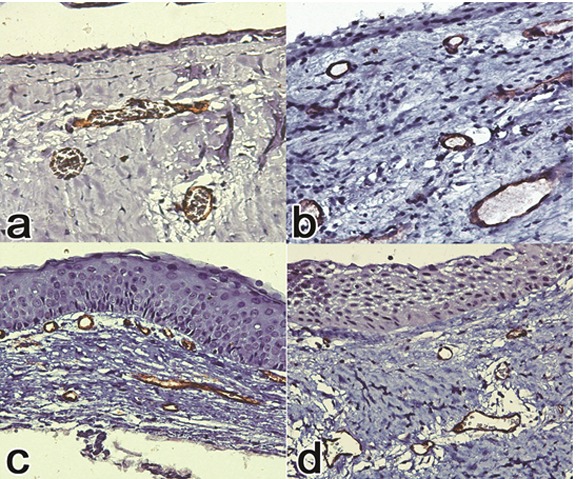
a: CD 105 positive immunoreactivity in dentigerous cyst (×400) b: CD 34 positive immunoreactivity in dentigerous cyst (×400) c: CD 34 positive immunoreactivity in odontogenic keratocyst (×400) d: CD 105 positive immunoreactivity in odontogenic keratocyst (×400)
Table 2.
CD34 and CD105 expression and MVD in studied groups
| Group | |||
|---|---|---|---|
| Dentigerous | OKC | ||
| Frequency | 15 | 15 | |
| CD34 | Mean | 8.48 | 12.24 |
| standard deviations | 2.8 | 5.8 | |
| CD105 | Mean | 5.94 | 9.2 |
| standard deviations | 2.4 | 2.94 | |
The MVDs in dentigerous cysts with the use of CD105 and CD34 markers were 5.94±2.4 and 8.48±2.8 respectively. The mean number of stained vessels in dentigerous cysts with the use of CD34 marker was significantly higher than that with the use of CD105 marker. T-test showed significant difference between groups (p= 0.007).
Discussion
OKC has aggressive behavior and a high recurrence rate compared to other developmental odontogenic cysts.[1] In recent years, various factors such as P53, MDM2, Ki67, BCL2, MMP, IL-1, and TGF-α markers which were supposed to be related to greater aggressive behavior of OKC (compared to other odontogenic cysts) have been evaluated in various studies.[24-31]
The growth mechanism of OKC is different from dentigerous cyst. Some investigators suggest the increase in some matrix metalloproteinase of connective tissue would affect the OKC growth. One of the effective factors that probably increase the angiogenesis in OKC in comparison to dentigerous cysts would be the escalation of matrix metalloproteinase.[15] Browne was one of the pioneers to propose that the connective tissue wall of OKC may have an important role in the pathogenesis of this lesion.[32] Angiogenesis is one of the well-known stromal factors contributing in lesion progression. Angiogenesis has been previously studied in OKC but the number of studies is limited.[33] Among the many angiogenic markers, CD105 has higher accuracy and specificity to bind newly formed blood vessels and CD34 is a pan endothelial marker that shows stronger staining with endothelial cells with lower error rate in comparison to CD31. [15,21-22] CD34 cannot distinguish between the newly formed blood vessels and the old host ones but it has important role in evaluation of MVD.[15] These points would justify why the current study evaluated the expression of these markers in odontogenic lesions.
In this study CD34 and CD105 were expressed in all samples but MVD in OKC was significantly higher than dentigerous cysts. Several other studies, have also confirmed the role of angiogenesis as an important factor in the aggressive behavior of OKC compared to dentigerous cysts. [15,33-35] Seifi et al.[15] showed MVD based on CD34 in OKC was higher than follicular cyst. They reported that angiogenesis has a substantial role in aggressive behavior of odontogenic lesions.[15]
Alaeddini et al.[33] determined MVD by using CD34 marker and reported that MVD was significantly higher in OKCs than in dentigerous cysts. They suggested that angiogenesis could be one of the mechanisms possibly responsible for the different biological behaviors of these lesions.[33]
Rubini et al.[34] observed a significant different expression of vascular endothelial growth factor (VEGF) in keratocysts compared to follicular cysts. They found that in all cases of OKC, there was a statistically significantly higher expression of VEGF whilst most follicular cysts were negative for VEGF expression. They concluded angiogenesis could be an active mechanism in the invasive growth of the OKC.[34] Gadbail et al.[35] found CD105 to be strongly expressed in microvessels of OKC compared with that in dentigerous cyst and normal oral mucosa. They suggested that tumor angiogenesis may be associated with locally aggressive biologic behavior of odontogenic lesions and angiogenesis can show the prognosis and clinical behavior of odontogenic lesions.[35]
Unlike the first three studies that evaluated only one marker of angiogenesis (CD34 or CD105), we studied two markers of angiogenesis and the marker used in the Rubini et al. study was different from the marker evaluated in the current study.
The results of all above mentioned studies and our study showed that angiogenesis was higher in OKC compared with dentigerous cyst. Angiogenesis is an important step in the majority of physiologic and pathologic processes and is necessary for the cells to access to oxygen and nutrients in distances more than 1‒2 mm from the blood vessels.[4]
Therefore, more angiogenesis in OKC compared to dentigerous cysts can indicate higher tissue metabolism. MVD which is considered as a quantitative analysis of angiogenesis in a lesion can predict the growth rate, metastasis, patient survival, and the aggressive behavior.[8,36]
Therefore, more angiogenesis and higher MVD in OKC compared to dentigerous cysts can indicate more aggressive biologic behavior, higher recurrence rate and more growth and proliferation. It might be possible to control the aggressive behavior of this cyst with anti-angiogenic treatments.
The result of this study was different from result of the study performed by de Andrade Santos et al.[23] de Andrade Santos et al. studied the expression of CD105 in OKCs, dentigerous cysts, and radicular cysts. They showed that micro vessel count was higher in dentigerous cyst than in OKCs and the differences in the biologic behavior of these lesions do not appear to be associated with the angiogenic index. [23] We used two markers of angiogenesis in this study. Our results based on CD105 are not in agreement with the results of the study of de Andrade Santos et al. This difference might be attributed to the differences in methodology such as counting method and number of samples.
In the present study, MVDs based on CD105 marker in OKC and dentigerous cysts were significantly less than those with CD 34. The studies of Jamshidi et al.,[37] Miyata et al.,[38] Kumagai et al.,[39 and Czekierdowski[40] which compared the expression of CD105 and CD34 antibodies in different lesions have shown a lower MVD based on CD105 marker compared to CD34 marker. The results of the current study were consistent with their results since the expression of CD105 is a prominent feature of newly formed vessels; moreover, its expression in previously formed vessels and also in the endothelium of the vessels of normal tissues is little or even negative. [41,37] Nevertheless, CD34 is expressed in the vascular endothelial cells of normal and neoplastic tissues and cannot distinguish the newly formed blood vessels from the old blood vessels of host. [8,15,17,42] Compared to CD34, these findings suggest that CD105 is a more specific and sensitive marker for angiogenesis.
The results of this study would probably contribute in better understanding of the role of angiogenesis in the biological behavior of OKC and dentigerous cyst. Further studies with larger number of cases are required to elucidate the role of angiogenesis in the aggressive behavior of OKC.
Conclusion
In the present study, MVD in OKC was higher than that in dentigerous cysts, which can indicate that angiogenesis is one of the possible mechanisms involved in the greater aggressive biologic behavior and higher recurrence rate of OKC compared to dentigerous cysts.
Acknowledgement
The authors would like to thank the Deputy Dean of Research at Hamadan University of Medical Sciences for their financial support of this study.
Conflict of Interest: None to declare.
References
- 1.Neville B, Damm DD, Allen CM, Bouquot J. Oral and Maxillofacial Pathology. 3rd ed. St Louis: Saunders Co; 2009. pp. 678–687. [Google Scholar]
- 2.Regezi JA, Sciubba JJ, Jordan RCK. Oral pathology-clinical pathologic correlations. 4th ed. St Louis: Saunders Co; 2012. p. 246, 251, 253, 255. [Google Scholar]
- 3.Kumar V, Abbas A, Fausto N, Aster JC. Robbins and cotran pathologic Basis of disease. 8th ed. Philadelphia: Saunders Co; 2010. pp. 99–102. [Google Scholar]
- 4.Rubin R, Strayer DS. Rubin's Pathology: Clinicopathologic Foundations of Medicine. 6th ed. Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins; 2012. pp. 101–192. [Google Scholar]
- 5.Nagatsuka H, Hibi K, Gunduz M, Tsujigiwa H, Tamamura R, Sugahara T, et al. Various immunostaining patterns of CD31, CD34 and endoglin and their relationship with lymph node metastasis in oral squamous cell carcinomas. J Oral Pathol Med. 2005; 34: 70–76. doi: 10.1111/j.1600-0714.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 6.Saad RS, Jasnosz KM, Tung MY, Silverman JF. Endoglin (CD105) expression in endometrial carcinoma. Int J Gynecol Pathol. 2003; 22: 248–253. doi: 10.1097/01.PGP.0000070852.25718.37. [DOI] [PubMed] [Google Scholar]
- 7.Gadbail AR, Mankar Gadbail MP, Hande A, Chaudhary MS, Gondivkar SM, Korde S, et al. Tumor angiogenesis: role in locally aggressive biological behavior of amelo-blastoma and keratocystic odontogenic tumor. Head Neck. 2013; 35: 329–334. doi: 10.1002/hed.22960. [DOI] [PubMed] [Google Scholar]
- 8.Hande AH, Gadbail AR, Sonone AM, Chaudhary MS, Wadhwan V, Nikam A. Comparative analysis of tumour angiogenesis in solid multicystic and unicystic ameloblastoma by using CD 105 (endoglin) Arch Oral Biol. 2011; 56: 1635–1640. doi: 10.1016/j.archoralbio.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Mittal N, Shankari GM, Palaskar S. Role of angiogenesis in the pathogenesis of oral lichen planus. J Oral Maxillofac Pathol. 2012; 16: 45–48. doi: 10.4103/0973-029X.92972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maschio LB, Madallozo BB, Capellasso BA, Jardim BV, Moschetta MG, Jampietro J, et al. Immunohisto-chemical investigation of the angiogenic proteins VEGF, HIF-1α and CD34 in invasive ductal carcinoma of the breast. Acta Histochem. 2014; 116: 148–157. doi: 10.1016/j.acthis.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Simonetti O, Lucarini G, Rubini C, Goteri G, Zizzi A, Staibano S, et al. Microvessel density and VEGF, HIF-1α expression in primary oral melanoma: correlation with prognosis. Oral Dis. 2013; 19: 620–627. doi: 10.1111/odi.12048. [DOI] [PubMed] [Google Scholar]
- 12.Kademani D, Lewis JT, Lamb DH, Rallis DJ, Harrington JR. Angiogenesis and CD34 expression as a predictor of recurrence in oral squamous cell carcinoma. J Oral Maxillofac Surg. 2009; 67: 1800–1805. doi: 10.1016/j.joms.2008.06.081. [DOI] [PubMed] [Google Scholar]
- 13.Fox SB, Harris AL. Histological quantitation of tumour angiogenesis. APMIS. 2004; 112: 413–430. doi: 10.1111/j.1600-0463.2004.apm11207-0803.x. [DOI] [PubMed] [Google Scholar]
- 14.Osai J. Rosai and Ackerman's Surgical Pathology. 10th ed. Printed in china: Mosby Co; 2010. pp. 51–248. [Google Scholar]
- 15.Seifi S, Shafaie S, Ghadiri S. Microvessel density in follicular cysts, keratocystic odontogenic tumours and ameloblastomas. Asian Pac J Cancer Prev. 2011;12: 351–356. [PubMed] [Google Scholar]
- 16.Kumamoto H, Ooya K. Immunohistochemical detection of platelet-derived endothelial cell growth factor/thymidine phosphorylase and angiopoietins in ameloblastic tumors. J Oral Pathol Med. 2006;35: 606–612. doi: 10.1111/j.1600-0714.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121(Pt 22): 3683–3692. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Nagai N, Siar CH, Nakano K, Nagatsuka H, Tsujigiwa H, et al. Angioarchitecture of primary oral malignant melanomas. J Histochem Cytochem. 2002;50: 1555–1562. doi: 10.1177/002215540205001116. [DOI] [PubMed] [Google Scholar]
- 19.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, et al. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14: 1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 20.Fonsatti E, Maio M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J Transl Med. 2004;2:18. doi: 10.1186/1479-5876-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagatsuka H, Hibi K, Gunduz M, Tsujigiwa H, Tamamura R, Sugahara T, et al. Various immunostaining patterns of CD31, CD34 and endoglin and their relationship with lymph node metastasis in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34: 70–76. doi: 10.1111/j.1600-0714.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 22.Nikiteas NI, Tzanakis N, Theodoropoulos G, Atsaves V, Christoni Z, Karakitsos P, et al. Vascular endothelial growth factor and endoglin (CD-105) in gastric cancer. Gastric Cancer. 2007;10: 12–17. doi: 10.1007/s10120-006-0401-8. [DOI] [PubMed] [Google Scholar]
- 23.De Andrade Santos PP, de Aquino AR, Oliveira Barreto A, de Almeida Freitas R, Galvão HC, de Souza LB. Immunohistochemical expression of nuclear factor κB, matrix metalloproteinase 9, and endoglin (CD105) in odontogenic keratocysts, dentigerous cysts, and radicular cysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112: 476–483. doi: 10.1016/j.tripleo.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Diniz MG, Gomes CC, de Castro WH, Guimarães AL, De Paula AM, Amm H, et al. miR-15a/16-1 influences BCL2 expression in keratocystic odontogenic tumors. Cell Oncol (Dordr) 2012;35: 285–291. doi: 10.1007/s13402-012-0087-3. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro AL, Nobre RM, Alves-Junior SM, Kataoka MS, Barroso RF, Jaeger RG, et al. Matrix metalloproteinases, tissue inhibitors of metalloproteinases, and growth factors regulate the aggressiveness and proliferative activity of keratocystic odontogenic tumors. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114: 487–496. doi: 10.1016/j.oooo.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Eshghyar N, Nikbin B, Amirzargar A, Dehghani Nazhvani A, Shakiba Y. Gene polymorphism of interleukin-1 alpha and beta in keratocystic odontogenic tumors. J Oral Pathol Med. 2012;41: 697–701. doi: 10.1111/j.1600-0714.2012.01162.x. [DOI] [PubMed] [Google Scholar]
- 27.Deyhimi P, Hashemzade Z. Comparative study of TGF-alpha and P53 markers' expression in odontogenic kera-tocyst and orthokeratinaized odontogenic cyst. Dent Res J (Isfahan) 2012;9(Supple 1): S39–S44. [PMC free article] [PubMed] [Google Scholar]
- 28.Piattelli A, Fioroni M, Santinelli A, Rubini C. P53 protein expression in odontogenic cysts. J Endod. 2001;27: 459–461. doi: 10.1097/00004770-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Li TJ, Browne RM, Matthews JB. Epithelial cell proliferation in odontogenic keratocysts: a comparative immunocytochemical study of Ki67 in simple, recurrent and basal cell naevus syndrome (BCNS)-associated lesions. J Oral Pathol Med. 1995;24: 221–226. doi: 10.1111/j.1600-0714.1995.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 30.Ogden GR, Kiddie RA, Lunny DP, Lane DP. Assessment of p53 protein expression in normal, benign, and malignant oral mucosa. J Pathol. 1992;166: 389–394. doi: 10.1002/path.1711660411. [DOI] [PubMed] [Google Scholar]
- 31.Sharifi-Sistani N, Zartab H, Babakoohi S, Saghravanian N, Jamshidi S, Esmaili H, et al. Immunohistochemical comparison of the expression of p53 and MDM2 proteins in ameloblastomas and keratocystic odontogenic tumors. J Craniofac Surg. 2011;22: 1652–1656. doi: 10.1097/SCS.0b013e31823188e9. [DOI] [PubMed] [Google Scholar]
- 29.Browne RM. The pathogenesis of odontogenic cysts: a review. J Oral Pathol. 1975;4: 31–46. doi: 10.1111/j.1600-0714.1975.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 30.Alaeddini M, Salah S, Dehghan F, Eshghyar N, Etemad-Moghadam S. Comparison of angiogenesis in keratocystic odontogenic tumours, dentigerous cysts and amelo-blastomas. Oral Dis. 2009;15: 422–427. doi: 10.1111/j.1601-0825.2009.01566.x. [DOI] [PubMed] [Google Scholar]
- 34.Rubini C, Artese L, Zizzi A, Fioroni M, Ascani G, Goteri G, et al. Immunohistochemical expression of vascular endothelial growth factor (VEGF) in different types of odontogenic cysts. Clin Oral Investig. 2011;15: 757–761. doi: 10.1007/s00784-010-0433-7. [DOI] [PubMed] [Google Scholar]
- 35.Gadbail AR, Hande A, Chaudhary M, Nikam A, Gawande M, Patil S, et al. Tumor angiogenesis in keratocystic odontogenic tumor assessed by using CD-105 antigen. J Oral Pathol Med. 2011;40: 263–269. doi: 10.1111/j.1600-0714.2010.00962.x. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancer: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005;46: 481–489. doi: 10.1111/j.1365-2559.2005.02142.x. [DOI] [PubMed] [Google Scholar]
- 37.Jamshidi S, Zargaran M, Baghaei F, Shojaei S, Zare Mahmoodabadi R, Dehghan A, et al. An Immunohistochemical Survey to Evaluate the Expression of CD105 and CD34 in Ameloblastoma and Odontogenic Keratocyst. J Dent (Shiraz) 2014;15: 192–198. [PMC free article] [PubMed] [Google Scholar]
- 38.Miyata Y, Mitsunari K, Asai A, Takehara K, Mochizuki Y, Sakai H. Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate. 2015;75: 84–91. doi: 10.1002/pros.22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumagai Y, Sobajima J, Higashi M, Ishiguro T, Fukuchi M, Ishibashi K, et al. Angiogenesis in superficial esophageal squamous cell carcinoma: assessment of microvessel density based on immunostaining for CD34 and CD105. Jpn J Clin Oncol. 2014;44: 526–533. doi: 10.1093/jjco/hyu039. [DOI] [PubMed] [Google Scholar]
- 40.Czekierdowski A, Czekierdowska S, Czuba B, Cnota W, Sodowski K, Kotarski J, et al. Microvessel density assessment in benign and malignant endometrial changes. J Physiol Pharmacol. 2008;59: 45–51. [PubMed] [Google Scholar]
- 41.Afshar Moghaddam N, Mahsuni P, Taheri D. Evaluation of Endoglin as an Angiogenesis Marker in Glioblastoma. Iran J Pathol. 2015;10:89–96. [PMC free article] [PubMed] [Google Scholar]
- 42.Goldiş DS, Sferdian MF, Tarţă C, Fulger LO, Totolici BD, Neamţu C. Comparative analysis of microvessel density quantified through the immunohistochemistry expression of CD34 and CD105 in rectal cancer. Rom J Morphol Embryol. 2015;56:419–424. [PubMed] [Google Scholar]


