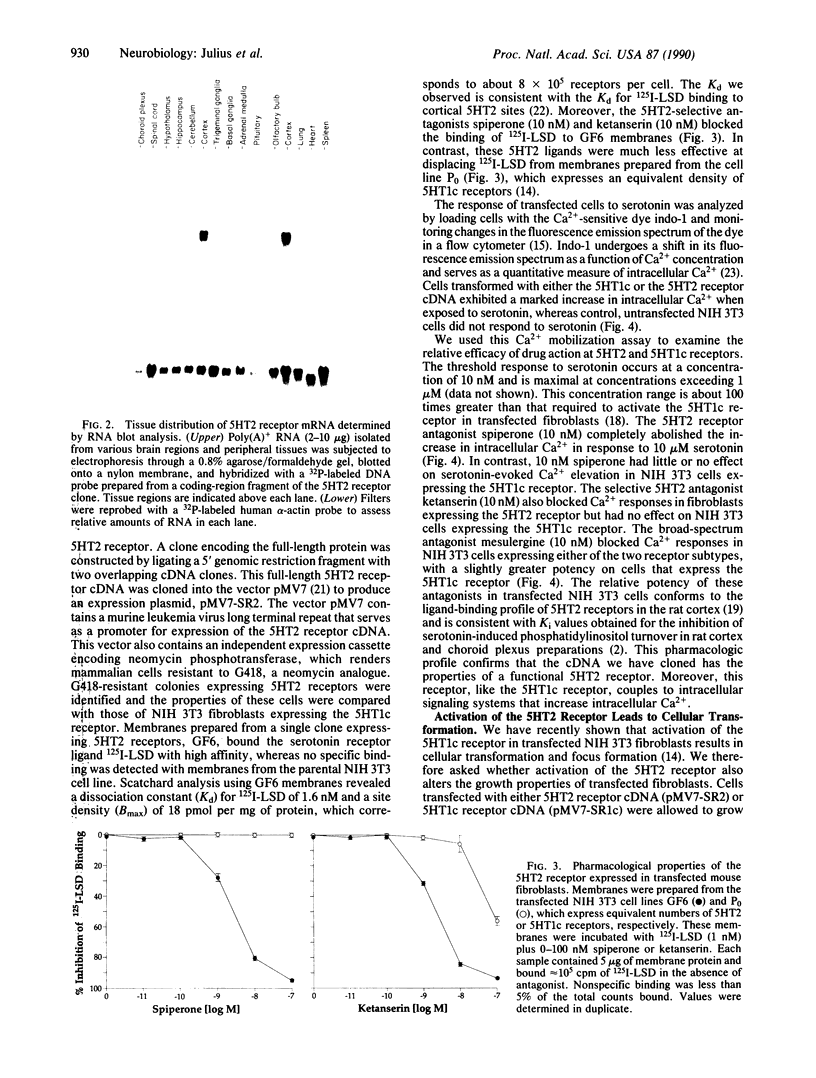
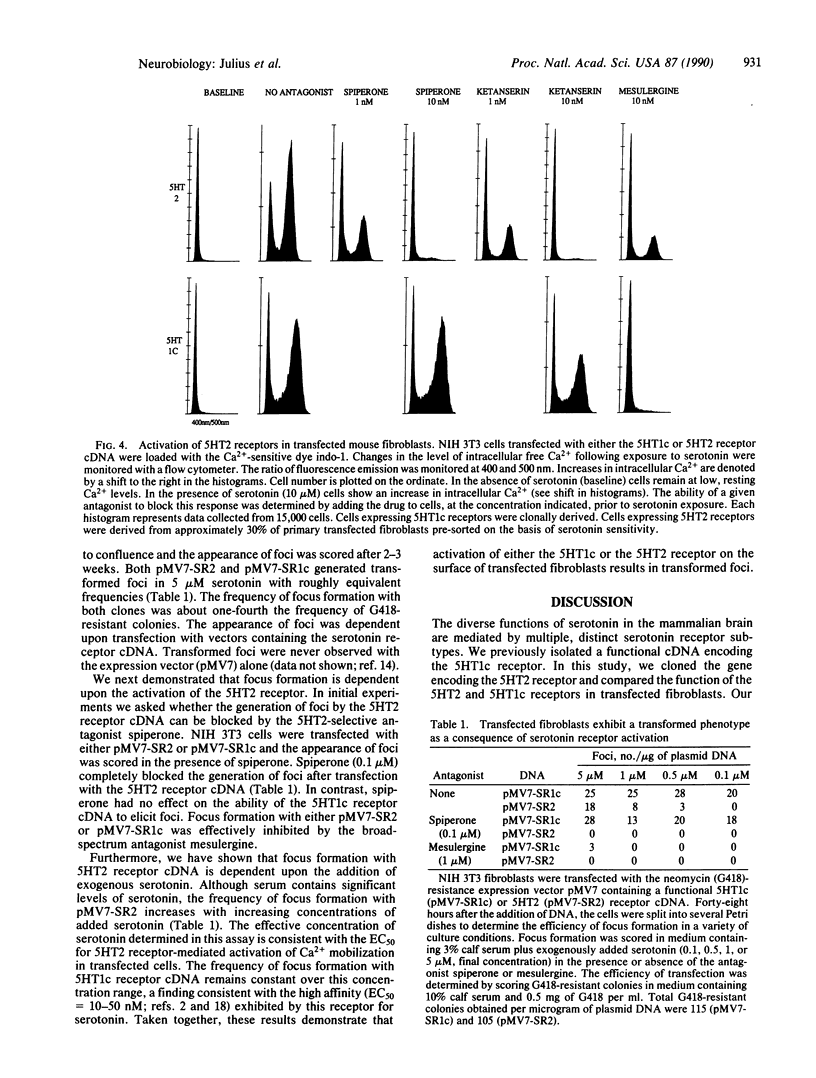
Abstract
Serotonin exerts its diverse physiological effects by interacting with multiple distinct receptor subtypes. We have isolated a rat brain 5HT2 serotonin receptor cDNA by virtue of its homology with the 5HT1c receptor. The 5HT2 receptor is a member of the family of receptors that are linked to guanine nucleotide-binding proteins and are predicted to span the lipid bilayer seven times. Overall sequence identity between the 5HT2 and 5HT1c receptors is 49%, but identity within the transmembrane domains is 80%. Expression of both the 5HT2 and 5HT1c receptors in transfected mouse fibroblasts activates phospholipase C signaling pathways and promotes cellular transformation. However, RNA blotting shows that these two receptor subtypes are differentially expressed in the central nervous system. In this manner, structurally and functionally homologous receptor subtypes may elicit distinct physiologic actions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arquint M., Roder J., Chia L. S., Down J., Wilkinson D., Bayley H., Braun P., Dunn R. Molecular cloning and primary structure of myelin-associated glycoprotein. Proc Natl Acad Sci U S A. 1987 Jan;84(2):600–604. doi: 10.1073/pnas.84.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functional role of muscarinic acetylcholine receptor subtype diversity. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):263–272. doi: 10.1101/sqb.1988.053.01.033. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Conn P. J., Sanders-Bush E., Hoffman B. J., Hartig P. R. A unique serotonin receptor in choroid plexus is linked to phosphatidylinositol turnover. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4086–4088. doi: 10.1073/pnas.83.11.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vivo M., Maayani S. Characterization of the 5-hydroxytryptamine1a receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in guinea pig and rat hippocampal membranes. J Pharmacol Exp Ther. 1986 Jul;238(1):248–253. [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987 May 19;26(10):2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- Engel G., Müller-Schweinitzer E., Palacios J. M. 2-[125Iodo]LSD, a new ligand for the characterisation and localisation of 5-HT2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1984 Apr;325(4):328–336. doi: 10.1007/BF00504377. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Lohse M. J., Kobilka B. K., Caron M. G., Lefkowitz R. J. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988 Sep 22;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hoyer D., Schoeffter P. 5-HT1D receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in calf substantia nigra. Eur J Pharmacol. 1988 Feb 16;147(1):145–147. doi: 10.1016/0014-2999(88)90645-0. [DOI] [PubMed] [Google Scholar]
- Julius D., Livelli T. J., Jessell T. M., Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989 Jun 2;244(4908):1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Jessel T. M., Huang K., Molineaux S., Schieren I., Axel R. Functional expression of the 5-HT1c receptor in neuronal and nonneuronal cells. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):385–393. doi: 10.1101/sqb.1988.053.01.046. [DOI] [PubMed] [Google Scholar]
- Kirschmeier P. T., Housey G. M., Johnson M. D., Perkins A. S., Weinstein I. B. Construction and characterization of a retroviral vector demonstrating efficient expression of cloned cDNA sequences. DNA. 1988 Apr;7(3):219–225. doi: 10.1089/dna.1988.7.219. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Frielle T., Collins S., Yang-Feng T., Kobilka T. S., Francke U., Lefkowitz R. J., Caron M. G. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature. 1987 Sep 3;329(6134):75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- Molineaux S. M., Jessell T. M., Axel R., Julius D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Pazos A., Palacios J. M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985 Nov 4;346(2):205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett D. B., Bach A. W., Wozny M., Taleb O., Dal Toso R., Shih J. C., Seeburg P. H. Structure and functional expression of cloned rat serotonin 5HT-2 receptor. EMBO J. 1988 Dec 20;7(13):4135–4140. doi: 10.1002/j.1460-2075.1988.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieren I., MacDermott A. Flow cytometric identification and purification of cells by ligand-induced changes in intracellular calcium. J Neurosci Methods. 1988 Nov;26(1):35–44. doi: 10.1016/0165-0270(88)90127-6. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Weiss S., Sebben M., Kemp D. E., Bockaert J. Serotonin 5-HT1 receptors mediate inhibition of cyclic AMP production in neurons. Eur J Pharmacol. 1986 Jan 21;120(2):227–230. doi: 10.1016/0014-2999(86)90544-3. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D., Leysen J. E., De Clerck F., Van Belle H., Janssen P. A. Evidence that phospholipid turnover is the signal transducing system coupled to serotonin-S2 receptor sites. J Biol Chem. 1985 Jun 25;260(12):7603–7608. [PubMed] [Google Scholar]





