Abstract
Fibrin degradation products (FDP) and D-dimer have been considered to be involved in many vascular diseases. In this study we aimed to explore the diagnostic implication of FDP and D-dimer in aortic dissection patients. 202 aortic dissection patients were collected as the case group, 150 patients with other cardiovascular diseases, including myocardial infarction (MI, n = 45), pulmonary infarction (n = 51) and abdominal aortic aneurysm (n = 54) were collected as non-dissection group, and 27 healthy people were in the blank control group. The FDP and D-dimer levels were detected with immune nephelometry. Logist regression analysis was performed to evaluate the influence of FDP and D-dimer for the aortic dissection patients. ROC curve was used to determine the diagnostic value of FDP and D-dimer. The FDP and D-dimer levels were significantly higher in aortic dissection patients than in non-dissection patients and the healthy controls. FDP and D-dimer were both the risk factors for patients with aortic dissection. From the ROC analysis, diagnostic value of FDP and D-dimer were not high to distinguish aortic dissection patients from the non-dissection patients. However FDP and D-dimer could be valuable diagnostic marker to differentiate aortic dissection patients and healthy controls with both AUC 0.863.
Aortic dissection is one of the most complex and dangerous cardiovascular diseases. The incidence and mortality are both very high, although it has well-established treatment guidelines1,2,3,4,5. It is reported that if the aortic dissection patients do not have early diagnosis or appropriate management, it is rapidly fatal6,7. But about 20% patients are without pain and with non-evocative symptoms, like syncope, cerebrovascular accidents, and congestive heart failure. Thus to evaluate the patients with suspicion of aortic dissection is very difficult6,8. Aortic dissection usually is divided into 2 types with the Stanford classification, Stanford type A and B. Type A has high mortality, but life-saving surgical repair is allowed with the rapid diagnosis9. Type B with a long-term prognosis is correlated with both higher morbidity and mortality10,11,12,13.
Thus with the high morbidity and mortality, early diagnosis is very important for improve the treatment and survival of aortic dissection patients14. D-dimer is a specific protein fiber degradation product of cross linked fibrin by the hydrolysis of fibrinolytic enzyme15,16. When thrombus degrade, D-dimer can release into the circulatory system17. In normal blood, the level of D-dimer is low, but once the thrombosis occurs, the D-dimer level is increased18. Studies have found that in the patients with cardiovascular and cerebrovascular diseases, D-dimer level is increased. And the increased D-dimer level has direct association with prognosis.
Recent years, biochemical diagnosis is widely used in the early diagnosis of many diseases, fibrin degradation products (FDP) is the degradation products of fibrous protein. Many reports have showed that FDP is involved in many vascular diseases. FDP is a mitogen of many cell types, and can promote the proliferation of endothelial cells, smooth muscle cell, and fibroblast, and cholesterol deposition19. FDP also can induce the adhesion and gather of leucocytes, which results in damage of blood vessel endothelium20.
However it is fewer reports of the both FDP and D-dimer and aortic dissection. Thus in this study, we described our preliminary experience with the diagnostic implication of FDP and D-dimer for aortic dissection.
Methods
Study Population
This was a restrospective study. The following methods were carried out in accordance with the approved guidelines. This study was approved by the Ethics Committee of the Changhai Hospital, written informed consent was obtaining from every subject.
This study recruited a total of 202 patients who were diagnosed with aortic dissection in our hospital from 2007–2016 as the case group with the age of 55.32 ± 13.59 (146 males and 56 females). And 150 patients diagnosed with other cardiovascular diseases including myocardial infarction (MI, n = 45), pulmonary infarction (n = 51) and abdominal aortic aneurysm (n = 54) were collected as non-dissection group with the age of 64.33 ± 14.31 (114 males and 36 females). There were 27 healthy volunteers as the control group with the age of 64.86 ± 10.52 (21 males and 6 females).
Aortic dissection patients were diagnosed by pathologists according to standard guidelines assisting with physical examination and contrast-enhanced computed tomography scan. The diagnosis of other cardiovascular diseases, like MI, pulmonary infraction and abdominal aortic aneurysm were performed using specific biomarkers, echocardiography, coronary angiography, CT or MRI according to respective guidelines.
Clinical studies in patients with aortic dissection
In this study, we collected the clinical data of the aortic dissection patients, and the data was all listed in Table 1. The following data obtained: age, HDL, LDL, PT, TT, FIB, INR, cholesterol and triglyceride. In the case group, there were 3 types aortic dissection patients, including patients without complications, with recurrence or endoleak, and dead patients.
Table 1. Comparison of clinical data among the three group volunteers.
| Factor | Group |
P value | ||
|---|---|---|---|---|
| Case group | Non-dissection group | Healthy group | ||
| age | 55.32 ± 13.59 | 64.33 ± 14.31 | 64.86 ± 10.52 | 0.002* |
| HDL | 1.20 ± 0.52 | 1.12 ± 0.35 | 1.24 ± 0.42 | 0.906 |
| LDL | 2.49 ± 0.77 | 2.62 ± 0.89 | 2.52 ± 0.81 | 0.665 |
| PT | 14.65 ± 5.59 | 14.35 ± 2.95 | 13.09 ± 2.65 | 0.023* |
| TT | 18.21 ± 12.12 | 16.99 ± 3.05 | 15.84 ± 1.12 | 1.000 |
| FIB | 4.18 ± 3.88 | 4.09 ± 1.49 | 4.88 ± 3.42 | 0.045* |
| INR | 1.17 ± 0.83 | 1.13 ± 0.32 | 1.06 ± 0.21 | 0.228 |
| Cholesterol (mmol/L) | 4.26 ± 1.07 | 4.43 ± 1.19 | 4.98 ± 1.21 | 0.390 |
| Triglyceride (mmol/L) | 1.32 ± 0.74 | 1.36 ± 0.85 | 1.59 ± 1.10 | 0.123 |
Measurements and analysis of FDP and D-dimer
The blood samples were collected from the patients with aortic dissection, non-dissection group and the healthy group. Immune nephelometry was performed in this detection. The FDP level and D-dimer level of all the volunteers were measured with STA R-automatic coagulometer (STAGO, Germany) using the FDP kit and D-D kit (Sekisui Medical Co. LTD).
Statistical analysis
All statistical analyses were performed using the software of SPSS 19.0 and GraphPad Prism 5. The data are summarized and presented as means ± SD. In order to compare the FDP and D-dimer level between the case group and non-dissection group and healthy group, respectively, one-way ANOVA was used. The clinicopathological characteristics among the three groups were assessed using Chi-square tests. Logist regression analysis was performed to evaluate the influence of FDP and D-dimer for the aortic dissection patients. Receiver operating characteristic (ROC) analysis was performed to determine the diagnostic value of FDP and D-dimer in distinguishing aortic dissection patients and non-dissection group and healthy group. In this study all P values < 0.05 were considered statistically significant.
Results
Clinical data of patients in the three groups
In our study, the clinical data of the three groups were listed in Table 1. And they were assessed using Chi-square tests. From the results, we could see that among the clinical data, age, PT and FIB had significantly differences among the case group, non-dissection group and healthy group. Other clinical data, including HDL, LDL, TT, INR, cholesterol and triglyceride, all had no significantly differences among the three groups.
FDP and D-dimer levels in the three groups
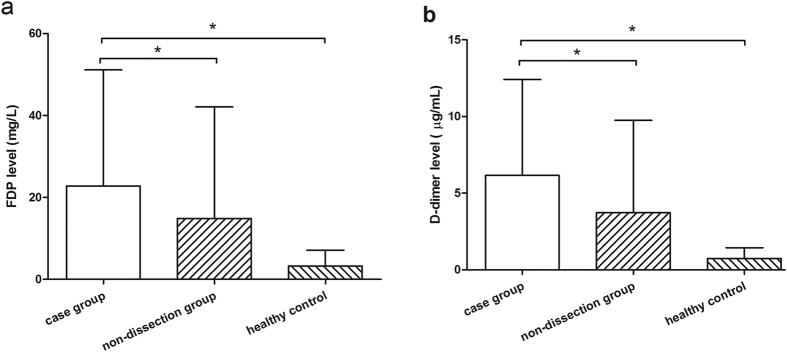
The FDP and D-dimer levels of the three groups were showed in the Fig. 1. The results of Fig. 1a revealed that the FDP level of the patients with aortic dissection (22.81 ± 28.31 mg/L) was significantly higher than that both in non-dissection group (14.81 ± 27.26 mg/L) and the healthy group (3.21 ± 3.91 mg/L) (P < 0.05), although the FDP level was also higher in non-dissection group than that of healthy group. And in Fig. 1b, the D-dimer had the same results with FDP. The D-dimer level of the patients with aortic dissection (6.16 ± 6.24 g/mL) was also significantly higher than that both in non-dissection group (3.72 ± 6.03 g/mL) and the healthy group (0.73 ± 0.70 g/mL) (P < 0.05).
Figure 1. The FDP and D-Dimer levels in the three group, the case group, non-dissection group, and the healthy group.
(a) FDP levels; (b) D-Dimer levels.
The logistic regression of FDP and D-dimer in aortic dissection patients
In the case group, the OR value of FDP (P = 0.011) and D-dimer (P = 0.006) were 1.197 and 2.040, respectively, 95%CI were 1.042–1.374 and 1.223–3.401, respectively. And in the non-dissection group, the FDP and D-dimer also were risk factors as similar with those in the case group. The results suggested that FD and D-dimer were both risk factors not only in the non-dissection group but also in the case-group according to the analysis of logist regression analysis (Table 2).
Table 2. Logist regression analysis of the FDP and D-Dimer.
| OR | 95%CI | P value | |
|---|---|---|---|
| Case group | |||
| FDP | 1.197 | 1.042–1.374 | 0.011 |
| D-Dimer | 2.040 | 1.223–3.401 | 0.006 |
| Non-dissection group | |||
| FDP | 1.186 | 1.032–1.361 | 0.016 |
| D-Dimer | 1.888 | 1.133–3.418 | 0.015 |
OR: odds ratio.
Diagnostic performance of FDP and D-dimer
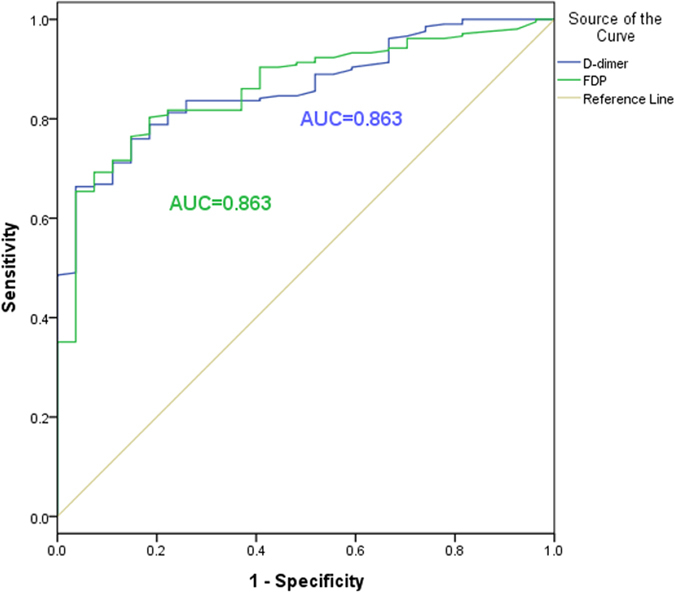
In order to estimate the diagnostic implication of FDP and D-dimer in aortic dissection patients, ROC analysis was performed (Figs 2 and 3). Figure 2 showed the ROC of FDP and D-dimer between case group and non-dissection group with the area under the curve (AUC) of 0.661 and 0.665, respectively. And in the Fig. 3, the results revealed the ROC of FDP and D-dimer between case group and healthy group with the both AUC of 0.863. For FDP, in the analysis of case group and non-dissection group, the sensitive and specificity were 53.1 and 73.6%, and in the analysis of case group and healthy group were 92.6 and 69.2%, respectively. For D-dimer, the the sensitive and specificity of case group and non-dissection group were 68.8 and 60.9%, and 68.3 and 96.4% in case group and healthy group.
Figure 2. The ROC curves of FDP and D-Dimer to distinguish aortic dissection patients and non-dissection group.
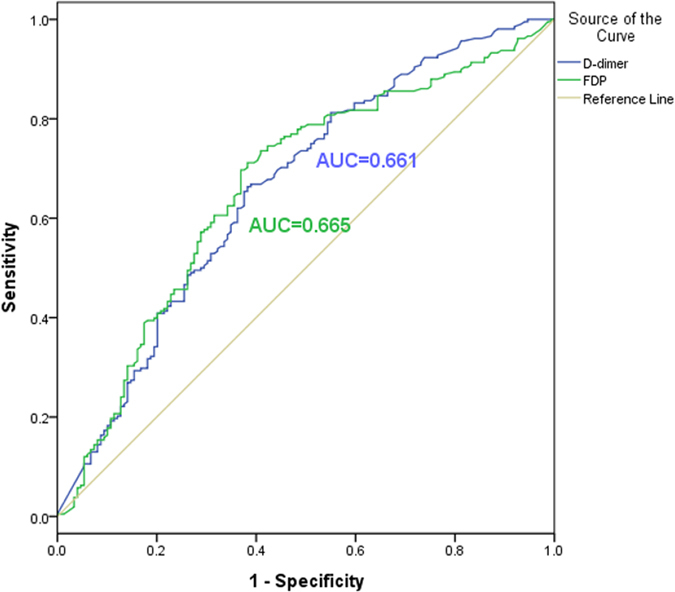
Figure 3. The ROC curves of FDP and D-Dimer to distinguish aortic dissection patients and healthy group.
Discussion
Aortic dissection is still a potentially catastrophic cardiovascular disease21,22,23. According to the foreign data, the incidence of aortic dissection can be 100–290/1000 thousand people2, and in America there are 10 thousand new case of aortic dissection every year24. Based on the statistics, the incidence of males is higher than that of females, about 2–3:1, and the patients with type A are more than those with type B25. Because of the complex clinical features, the rates of missed diagnosis and misdiagnosis are high, and the mortality of untreated acute aortic dissection is 21% per day, 37% of two days and 74% of a week26. Although diagnosis and treatment of aortic dissection have greatly improved in recent years, it is still difficult to recognize at clinical presentation for aortic dissection21,27,28,29.
FDP has been reported to be involved in vascular diseases. Fukujima et al. found that FDP participate the development and progression of atherosclerosis and thrombus30. Corban MT et al. also found that FDP was associated with larger coronary plaques and greater plaque necrotic core31. In addition, in the study of Akiyoshi Hagiwara et al., they showed that FDP was a valuable diagnosis biomarker for patent-type acute aortic dissection patients and thrombosed-type acute aortic dissection patients. They also found a strong correlation between FDP and D-dimer in acute aortic patients32. D-dimer is a kind of fibrin degradation product. In the 1990 s, it was first introduced to be a diagnostic aid for diseases such as pulmonary embolus and deep venous thrombosis33,34. Studies have found that increased D-dimer level was revealed in many diseases, including cancers, deep venous thrombosis, disseminated intravascular coagulation, aortic dissection, and so on. Weber et al. found that the increased D-dimer level had association with aortic dissection35. In the research of Eggebrecht et al., D-dimer level was elevated in all patients with aortic dissection36.
In this study, we aimed to explore the diagnostic implication of FDP and D-dimer for the aortic dissection patients. FDP and D-dimer levels were both obviously higher in aortic dissection patients than the patients with myocardial infarction, pulmonary infarction and abdominal aortic aneurysm as well as the healthy people. And among the clinical data, age, PT and FIB had significantly differences among the three groups. The results indicated FDP and D-dimer were both the risk factors for the patients with aortic dissection. From the ROC, in the analysis of case group and non-dissection group, the diagnostic value of FDP and D-dimer were not very high for distinguishing aortic dissection patients and non-dissection group. However, in the analysis of case group and healthy group, the specificity of D-dimer was 96.4%, and the sensitive of FDP was 92.6%, and the AUC of both FDP and D-dimer were 0.863. Therefore, the diagnostic value of FDP and D-dimer were outstanding to distinguishing aortic dissection patients and healthy people. Our findings were compatible with the previous studies and in order to improve the sensitive and specificity, the combined detection of FDP and D-dimer should be applied to clinic in the follow research.
Although in this study, it was revealed that FDP and D-dimer had diagnostic implication for aortic dissection patients, more and further studies for aortic dissection should be performed in the future.
Additional Information
How to cite this article: Dong, J. et al. Diagnostic implication of fibrin degradation products and D-dimer in aortic dissection. Sci. Rep. 7, 43957; doi: 10.1038/srep43957 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [81370441], and Shanghai Health System Diseases Important Joint Research Project [2014ZYJB0401].
Footnotes
The authors declare no competing financial interests.
Author Contributions J.D., X.D. and R.F. conceived and designed the experiments; Z.Z. and X.F. conceived and performed the experiments; Q.L. and Q.J. prepared figures. J.Z., J.B. and Z.J. wrote the main manuscript text. All authors reviewed the manuscript.
References
- Clouse W. D. et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 79, 176–80 (2004). [DOI] [PubMed] [Google Scholar]
- Meszaros I. et al. Epidemiology and clinicopathology of aortic dissection. Chest 117, 1271–8 (2000). [DOI] [PubMed] [Google Scholar]
- Feldman M., Shah M. & Elefteriades J. A. Medical management of acute type A aortic dissection. Ann Thorac Cardiovasc Surg 15, 286–93 (2009). [PubMed] [Google Scholar]
- Ramanath V. S., Oh J. K., Sundt T. M. 3rd & Eagle K. A. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin Proc 84, 465–81 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S. & Roselli E. E. Thoracic aortic dissection: long-term results of endovascular and open repair. Semin Vasc Surg 22, 61–8 (2009). [DOI] [PubMed] [Google Scholar]
- Erbel R. et al. Diagnosis and management of aortic dissection. Eur Heart J 22, 1642–81 (2001). [DOI] [PubMed] [Google Scholar]
- von Kodolitsch Y., Schwartz A. G. & Nienaber C. A. Clinical prediction of acute aortic dissection. Arch Intern Med 160, 2977–82 (2000). [DOI] [PubMed] [Google Scholar]
- Hagan P. G. et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 283, 897–903 (2000). [DOI] [PubMed] [Google Scholar]
- Ranasinghe A. M. & Bonser R. S. Biomarkers in acute aortic dissection and other aortic syndromes. J Am Coll Cardiol 56, 1535–41 (2010). [DOI] [PubMed] [Google Scholar]
- Tsai T. T. et al. Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 114, I350–6 (2006). [DOI] [PubMed] [Google Scholar]
- Tsai T. T. et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation 114, 2226–31 (2006). [DOI] [PubMed] [Google Scholar]
- Akutsu K. et al. Effects of the patent false lumen on the long-term outcome of type B acute aortic dissection. Eur J Cardiothorac Surg 26, 359–66 (2004). [DOI] [PubMed] [Google Scholar]
- Kodama K. et al. Tight heart rate control reduces secondary adverse events in patients with type B acute aortic dissection. Circulation 118, S167–70 (2008). [DOI] [PubMed] [Google Scholar]
- Wheat M. W. Jr., Palmer R. F., Bartley T. D. & Seelman R. C. Treatment of Dissecting Aneurysms of the Aorta without Surgery. J Thorac Cardiovasc Surg 50, 364–73 (1965). [PubMed] [Google Scholar]
- Ay C. et al. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 27, 4124–9 (2009). [DOI] [PubMed] [Google Scholar]
- Kline J. A. et al. D-dimer threshold increase with pretest probability unlikely for pulmonary embolism to decrease unnecessary computerized tomographic pulmonary angiography. J Thromb Haemost 10, 572–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 119, 2702–7 (2009). [DOI] [PubMed] [Google Scholar]
- Righini M. et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 311, 1117–24 (2014). [DOI] [PubMed] [Google Scholar]
- Naito M. Effects of fibrinogen, fibrin and their degradation products on the behaviour of vascular smooth muscle cells. Nihon Ronen Igakkai Zasshi 37, 458–63 (2000). [DOI] [PubMed] [Google Scholar]
- Yakovlev S., Zhang L., Ugarova T. & Medved L. Interaction of fibrin(ogen) with leukocyte receptor alpha M beta 2 (Mac-1): further characterization and identification of a novel binding region within the central domain of the fibrinogen gamma-module. Biochemistry 44, 617–26 (2005). [DOI] [PubMed] [Google Scholar]
- Spittell P. C. et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc 68, 642-51 (1993). [DOI] [PubMed] [Google Scholar]
- Tsai T. T., Nienaber C. A. & Eagle K. A. Acute aortic syndromes. Circulation 112, 3802–13 (2005). [DOI] [PubMed] [Google Scholar]
- Nienaber C. A. & Eagle K. A. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation 108, 628–35 (2003). [DOI] [PubMed] [Google Scholar]
- Rogers A. M. et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation 123, 2213–8 (2011). [DOI] [PubMed] [Google Scholar]
- Nienaber C. A. et al. Gender-related differences in acute aortic dissection. Circulation 109, 3014–21 (2004). [DOI] [PubMed] [Google Scholar]
- Braverman A. C. Aortic dissection: prompt diagnosis and emergency treatment are critical. Cleve Clin J Med 78, 685–96 (2011). [DOI] [PubMed] [Google Scholar]
- Nienaber C. A. et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med 328, 1–9 (1993). [DOI] [PubMed] [Google Scholar]
- Nienaber C. A. et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 340, 1539–45 (1999). [DOI] [PubMed] [Google Scholar]
- Dake M. D. et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 340, 1546–52 (1999). [DOI] [PubMed] [Google Scholar]
- Fukujima M. M., Martinez T. L., Pinto L. E., Auriemo Cdo R. & de Andrade L. A. Fibrinogen as independent risk factor for ischemic stroke. Arq Neuropsiquiatr 55, 737–40 (1997). [DOI] [PubMed] [Google Scholar]
- Corban M. T. et al. Elevated Levels of Serum Fibrin and Fibrinogen Degradation Products Are Independent Predictors of Larger Coronary Plaques and Greater Plaque Necrotic Core. Circ J 80, 931–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A. et al. Using fibrin degradation products level to facilitate diagnostic evaluation of potential acute aortic dissection. J Thromb Thrombolysis 35, 15–22 (2013). [DOI] [PubMed] [Google Scholar]
- Wells P. S. et al. A novel and rapid whole-blood assay for D-dimer in patients with clinically suspected deep vein thrombosis. Circulation 91, 2184–7 (1995). [DOI] [PubMed] [Google Scholar]
- Caprini J. A., Glase C. J., Anderson C. B. & Hathaway K. Laboratory markers in the diagnosis of venous thromboembolism. Circulation 109, I4–8 (2004). [DOI] [PubMed] [Google Scholar]
- Weber T. et al. D-dimer in acute aortic dissection. Chest 123, 1375–8 (2003). [DOI] [PubMed] [Google Scholar]
- Eggebrecht H. et al. Value of plasma fibrin D-dimers for detection of acute aortic dissection. J Am Coll Cardiol 44, 804–9 (2004). [DOI] [PubMed] [Google Scholar]