Abstract
Peripheral blood stem cell transplantation (PBSCT) is an effective treatment for hematological malignancies. Mobilization of peripheral blood progenitor cells performs in different ways among transplantation centers. Forceful mobilization schedules are comprised of growth factor alone, chemotherapy along with growth factor and also, a newly combination of novel agent such as plerixafor with any approach. With the appearance of numerous modifications in stem cell mobilization field over the past decade and advent of novel stem cell mobilization techniques, it seems to be necessary to review recent publications about stem cell mobilization strategies to respond above cited issues. Relevant literature was identified by a PubMed search (1996–2016) of English-language literature using the terms mobilization, Allogeneic Stem Cells Transplantation, Autologous Stem Cells Transplantation and technical aspects of apheresis. Although many institutions have established their own procedures to improve stem cell mobilization success rates accompanying cost-effectiveness considerations, an optimal stem cell mobilization regimen and methods have not been well-defined, yet. Practical guidelines are required to address critical clinical issues including proper growth factor, the most Impressive chemotherapy and its dosage and appropriate time for leukapheresis initiation. Hence, based on literature, we prepared practical guidelines in this review.
Key Words: Stem cell, Mobilization, Peripheral blood, Transplantation
Introduction
Hematopoietic Stem cells transplantation (HSCT) is become a curative option for patients who suffer from hematological malignancies. 1,2 The usage of both autologous and allogeneic HSCT for adults and pediatric has exceedingly increased, over the past several decades. Small amounts of hematopoietic stem cells (HSCs) are able to circulate in Peripheral blood (PB). 3 So, HSCs mobilization from bone marrow (BM) to PB and their collection can be crucial element of HSCT programs. 4,5 Despite the vast using of peripheral stem cells transplantation (PBSCT) as therapeutic strategy, it is difficult to achieve a consensus about its parameters. These parameters are type of growth factor and its optimal dosage, effectiveness type of chemotherapy and its dosage and how to predict poor mobilize patients and which time is best to initiate leukapheresis. 6 Nowadays, most transplantation institutions have adjusted own strategies according to their priorities and resource availabilities. Therefore, there are not any standard identical approaches. Hence, this paper aims to review current literature and guide lines on mobilization strategies to underscore the importance of mentioned problems.
Methods
Mobilization guidelines for autologous and allogeneic transplantation were obtained by the way of literature search. Extracted information about mobilization schedules, laboratory monitoring protocols and technical aspects of apheresis for adults and pediatrics are main foundations of presented guide lines in our review.
Results
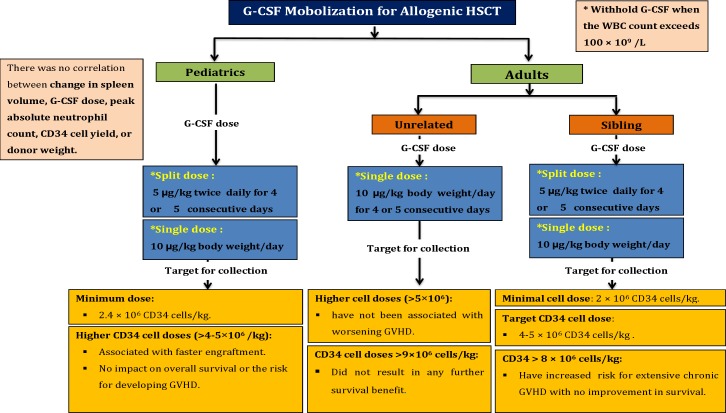
CSF dose recommendation for Allogeneic Transplantation in Adults 7-12
1- The recommended dose for sibling donors
5 µg/kg G-CSF twice per day as a split dose or 10 µg/kg/day as a single dose is advised.
Using higher split dose (12 µg/kg twice/day) results in higher collection yields with shorter collection time.
2- The recommended dose for unrelated donors
G-CSF is administered for 4 or 5 consecutive days at a dose of 10 µg/kg daily.
During the PBSCs collection, the total processed blood volume (TPBV) does not be exceeding of 24 liters and it should be collected during 1 or 2 consecutive days.
Target Stem Cells dose for Allogeneic Transplantation in Adults 14 - 19
1- Transplantation from sibling donors
The common accepted cell dose is 2×106 CD34 cells/kg at least.5,12,13 Successful engraftment has reported at dose as low as 0.75×106 CD34 cells/kg, whereas neutrophil and particularly platelet engraftments were delayed. Hence, more transfusion of blood components is required.
Based on available data, CD34 cells dose between 4 and 5×106 CD34 cells/kg seems to be most acceptable amount for allogeneic transplantation in adults. Several studies have shown that higher doses of CD34 cells infusion are associated with faster engraftment.
Any count more than 8×106 CD 34 cells/kg could enhance risk of extensive chronic GVHD without any improvement in survival of patients.
2- Transplantation from unrelated donors
Any count more than 9×106 CD 34 cells/kg did not result in any further survival benefits. Likewise, higher cell doses are not associated with worsening GVHD.
G-CSF dose recommendation for Allogeneic Transplantation in Pediatric 20-22
The most common approach makes use of G-CSF is 10 µg/kg as a single or two semi-doses per day.
Target Stem Cells dose for Allogeneic Transplantation in Pediatric 23-25
Minimum amount of collected cells are reported 2.4×106 CD34 cells/kg for allogeneic transplantation in pediatric.
Higher CD34 cell counts (>4-5×106) have been associated with faster engraftment while no impact on overall survival or the risk for developing GVHD was observed.
A summary of stem cells mobilization strategies and target cells dose for allogeneic stem cells transplantation is shown in Figure 1.
Figure 1.
A summary of stem cells mobilization strategies and target cells dose for allogeneic stem cells transplantation
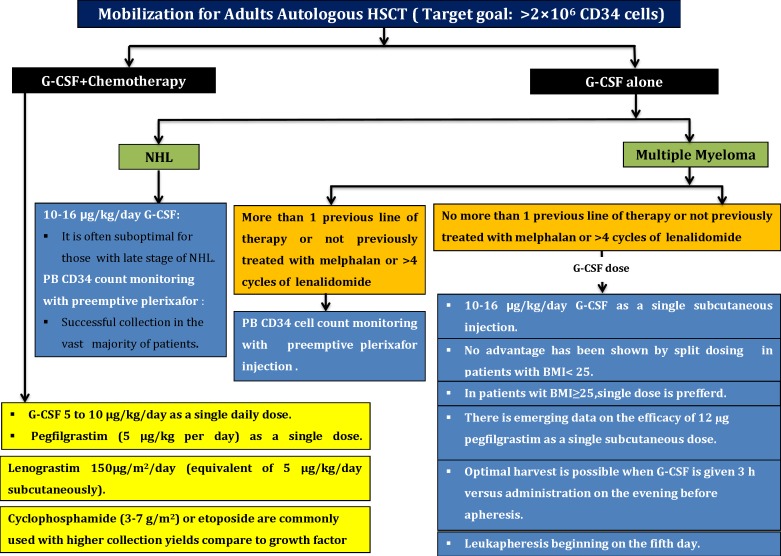
Mobilization Strategies for Autologous Transplantation in Adults
1A) G-CSF alone strategy utilization for Multiple Myeloma (MM) patients
In these subjected patients with not more than 1 previous line of therapy or negative history of previous treatment with melphalan or>4 cycles of lenalidomide, the best choice is just G-CSF with following schedule:
A daily single dose of 10-16 µg/kg G-CSF with subcutaneous injection is most common. No advantages have been observed by split dosing of G-CSF. There is evidence on efficacy of 12 µg Pegfilgrastim as a single subcutaneous dose in these patients.
Optimal harvest is possible when G-CSF is given 3 hours before apheresis versus administration was performed on the evening before apheresis. For acquire proper consequence, leukapheresis should be beginning on the fifth day.
Although growth factor alone is often adequate for patients with early-stage of MM, it is often suboptimal for them at late stage.
In patients with more than 1 previous line of therapy or history of previously treated with melphalan or >4 cycles of lenalidomide: In such patients, PB CD34 cells count monitoring with preemptive Plerixafor will lead to successful collection in the vast majority of patients.
1B) G-CSF alone and/or Plerixafor strategies utilization about None-Hodgkin Lymphoma (NHL)
Although persistent mobilization with G-CSF alone with doses of 10-16 µg/kg/day associated with higher incidence of failure rates in some patients or which thing is suboptimal but it may be an option owing to low toxicity and ease of scheduling. So that, this strategy will not suitable for patients with high risk of mobilization failure.
PB CD34 counts monitoring with preemptive plerixafor will lead to successful collection in the vast majority of patients.
2) Chemotherapy plus growth factor mobilization strategies 29-32
G-CSF and chemotherapy dose elements
In this strategy, 5 to 10 µg/kg G-CSF as a single daily dose or pegfilgrastim (5 μg/kg/day) as a single administration can be used at the beginning of leukapheresis when peripheral blood CD34 cell counts or WBC count is adequate. In addition to, Lenograstim 150μg/m2/day (equivalent of 5 μg/kg/day subcutaneously) is advised to choose.
Cyclophosphamide (3-7 gr/m2) or etoposide is commonly used for mobilization which results in higher collection yields during fewer days of apheresis than mobilization with growth factor alone. These benefits occur at the expense of increased hospitalizations for neutropenic fever, which occur in a substantial portion of patients. Current data do not declare that the concern which mobilization regimens include etoposide promote secondary malignancies.
2A) Chemotherapy plus growth factor mobilization strategy in Multiple Myeloma patients: High-dose cyclophosphamide + G-CSF are probably the most commonly used chemo mobilization strategy. Some studies also suggest that etoposide-based mobilization approaches can be considered as alternative choice.
2B) Chemotherapy plus growth factor mobilization strategy in Lymphoma patients: Chemotherapy + G-CSF as part of disease specific induction and salvage regimens have always regarded the preferred method. Such approaches can eliminate to require additional chemo-mobilizations or steady-state mobilizations before auto-HSCT in these heavily treated patients. Further, it is more effective than cyclophosphamide-based chemo-mobilization.
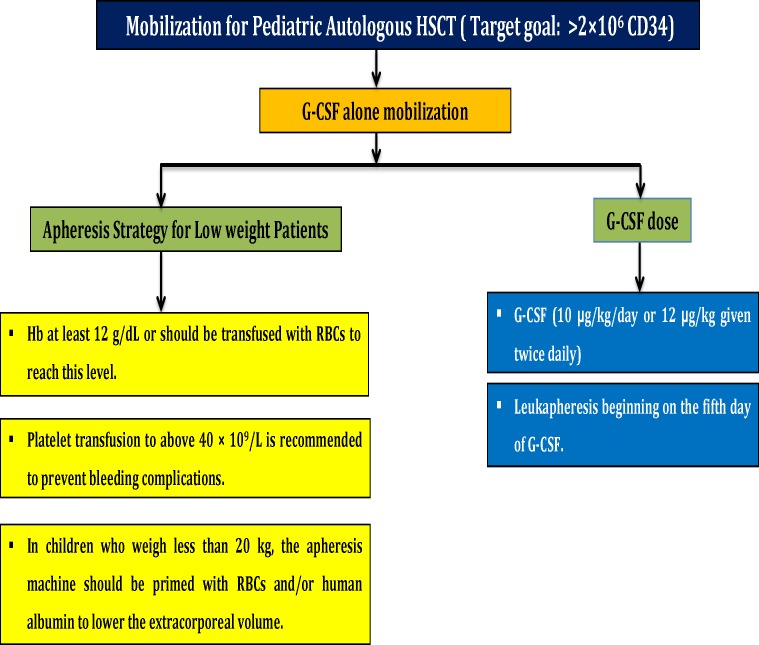
Optimal G-CSF Dose for Initial Mobilization in Pediatric Autologous Transplantation 33,34
G-CSF (10 µg/kg/day or 12 µg/kg given twice per day) with leukapheresis beginning on the fifth day of G-CSF could result in successful mobilization in one day.
Target Stem Cells Dose for Autologous Transplantation in Adults and Pediatric 35-39
2×106 CD34 cells/kg for single transplant has generally been accepted as a safe minimum count. Lower counts will concomitant the risk of delayed neutrophil and platelet engraftment.
5×106 CD34 cells/kg has been accepted as suitable optimal collected cells for successful transplantation. Higher CD34 cell doses(>6×106/kg) have been associated with faster hematopoietic recovery, more robust long-term platelet recovery, reduced blood transfusion requirements and beside improved overall survival. However, there was no significant difference at the time of platelet recovery to 20×109/L.
Special Considerations for Obese Patients 40,41
Either single daily dose (14 µg/kg/day) or split dose was suggested (2×7 µg/kg/day) for obese patients.
Patients were stratified according to body mass index (25<BMI>25). In patients with BMI>25 kg/m2, once-daily dosing resulted in a higher CD34 cells yield.
A summary of stem cells mobilization strategy and target cells dose for autologous stem cells transplantation in adults is shown in Figure 2.
Figure 2.
A summary of stem cells mobilization strategy and target cells dose for autologous stem cells transplantation in adults
Apheresis Procedure in Pediatric Patients with Low Weight 20 , 42
Patients with low weight should have characterized by hemoglobin at least level of 12 g/dL. Otherwise, it should be reached to mentioned level by RBC transfusion.
In children who weigh less than 20 kg, the apheresis machine should be primed with RBCs and/or human albumin to lower the extracorporeal volume.
In severe thrombocytopenia situation, platelet transfusion should be considered to achieve platelet count above 40×109/L in order to prevent bleeding complications.
A summary of stem cells mobilization and apheresis strategies and target cells dose for autologous stem cells transplantation in pediatrics is shown in Figure 3.
Figure 3.
A summary of stem cells mobilization and apheresis strategies and target cells dose for autologous stem cells transplantation in pediatrics
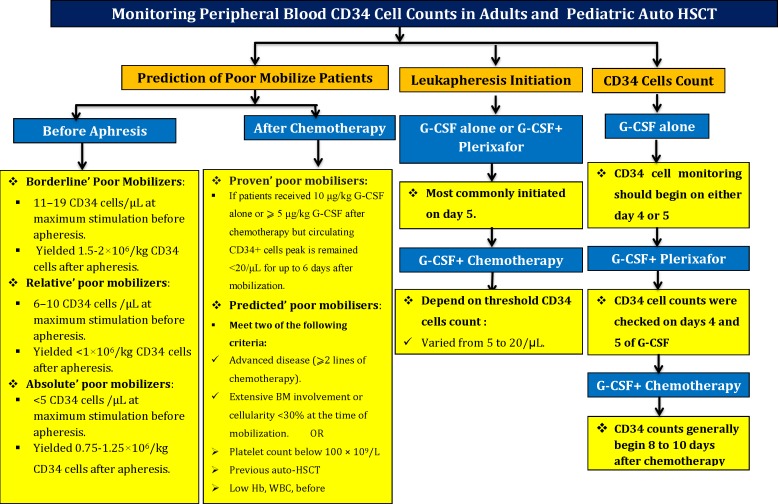
Monitoring Peripheral Blood CD34 cell Counts in Adults and Pediatrics Autologous Transplantation 45-50
1- Legitimate reasons to G-CSF alone strategy selection
CD34 cell counts peak in blood between the fourth to sixth days of therapy was observed. In this schedule, CD34 cells monitoring should begin on either day 4 or 5.29,32,43-45
2- Plerixafor plus G-CSF mobilization strategy:
CD34 cell counts were checked on days 4 and 5 of G-CSF administration.
3- Chemo mobilize strategy option
CD34 counts generally begin 8 to 10 days after chemotherapy administrations.
Golden Time to Leukapheresis Initiation 44,45,51
In chemo-mobilization strategy, the start of leukapheresis is commonly determined by threshold of CD34 cell counts. There is no consensus on optimal threshold; therefore, institutional practice or institutional local instructions have varied has varied from minimal CD34 counts of 5 to 20/µL.
Prediction of High Risk Patients for Stem Cell Mobilization Failure 51-57
Prediction of mobilization after chemotherapy
1-Proven poor mobilizes definition
If the patient received 10 μg/kg G-CSF alone or ⩾5 μg/kg G-CSF after chemotherapy but circulating CD34+ cells peak is remained <20/μL for up to 6 days after mobilization will defined as proven poor mobilisers
Furthermore, if the CD34 cells yield <2.0×106 /kg in⩽3 apheresis up to 20 days after chemo-mobilization regiment, it will define in this category.
2-Predicted poor mobilisers
Meet two main following criteria:
Advanced disease (⩾2 lines of chemotherapy).
Extensive BM involvement or cellularity <30% at the time of mobilization.
3-Other criteria are comprised
More than 60 years old
Multiple chemotherapy regimens
Prior exposure to alkylating medications
Prior radiation
Prior treatment with lenalidomide, fludarabine and other purine analogues and also melphalan
Platelet count below 100 ×109/L
Previous auto-HSCT
Low Hb level and WBC count before mobilization
Poor mobilization prediction in Multiple Myeloma patients
<12 months of therapy and a platelet count > 200×109/L, it is possible to obtain ≥4×106 CD34+ cells/kg in a single apheresis.
Patient over 70 years old with >12 months of prior therapy and platelets < 200 × 109/L; however, were considered as poor mobilizers
Prediction of Mobilization Failure According to CD34 Cells yield 44,45,58
Borderline’ poor mobilisers: CD34 cells/μL at maximum stimulation will detected in PB and it could be yielded 1.5-2×106/kg CD34 cells after apheresis.
Relative’ poor mobilisers: 6–10 CD34 cells/μL at maximum stimulation will detected in PB and it could be yielded <1×106/kg CD34 cells after apheresis.
Absolute’ poor mobilizers : ≤5 CD34 cells/μL at maximum stimulation will detected in PB and it could be yielded about 0.75×106/kg CD34 cells after apheresis.
2-After apheresis
Optimal collection: is defined as ≥5 ×106 CD34 cells/kg yield.
Low collection: ≥2 to <5 × 106 CD34 cells/kg.
Poor collection: <2 × 106 CD34 cells/kg.
Failed collection: apheresis is impossible because of low peripheral CD34 cell counts.
A summary of strategies for prediction of poor mobilize patients in autologous stem cells transplantation is shown in Figure 4.
Figure 4.
A summary of strategies for prediction of poor mobilize patients in autologous stem cells transplantation
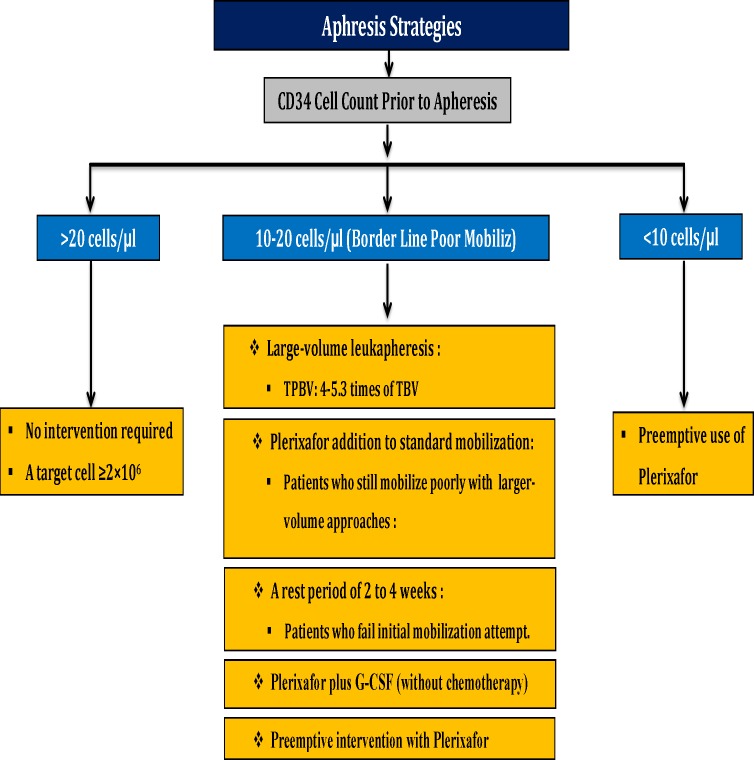
Strategies for Poor Mobilizes 59 - 65
1-Border Line Poor Mobilize
Large-volume leukopheresis: In this strategy, 4.0–5.3 folds of the patient’s total blood volume should be considered as a TPBV. No difference has observed in CD34+ cells viability compared with normal-volume apheresis (2.7–3.5 folds of the patient’s total blood volume). Relatively poor mobilisers or patients with high individual CD34+ cell collection goal (⩾3 transplants) have indication for large volume leukopheresis.
Plerixafor addition to standard mobilization strategies will be considered for patients who still mobilize poorly with larger-volume approaches.
A rest period of 2 to 4 weeks will be considered for patients who fail initial mobilization attempt.
Plerixafor plus G-CSF (without chemotherapy).
The addition of Plerixafor to G-CSF alone or G-CSF + chemotherapy: This strategy lead to mobilize more CD34+ cells, increase the proportion of more-primitive HSC subsets. Likewise, positive correlation between the number of re-infused NK cells and early absolute lymphocyte recovery after auto-HSCT has been reported.
Preemptive intervention with Plerixafor.
Relatively Poor Mobilize and Poor Mobilize
Preemptive use of plerixafor should be considered.
A summary of apheresis and mobilization strategies based on CD34 cell count prior to apheresis for poor mobilize patients in autologous stem cells transplantation is shown in Figure 5.
Figure 5.
A summary of apheresis and mobilization strategies based on CD34 cell count prior to apheresis for poor mobilize patients in autologous stem cells transplantation.
In one-third patients, WBC counts developed exceeding 50 × 109/L, while less than 1% of patients had WBC counts exceeding 75×109/L.
There was no splenic rupture or thrombosis.
During G-CSF mobilization, significant increase in spleen size was observed. The median spleen volume increased by 1.47-folds on first day of leukapheresis but declined to near pretreatment size after 7 days of leukapheresis. In only 9% of patients splenic volumes increase into more than 2-folds.
There was no correlation between change in spleen volume, G-CSF dose, absolute neutrophil count peak, CD34 cells yield, or donor weight.
Despite the lack of documentations to support the association between hematological parameters and splenic enlargement or risk of splenic rupture, current data declared that G-CSF and Plerixafor administration should withhold when the WBC count exceeds 100 × 109 and exceeds 75×109/L, respectively.
CONCLUSION
PBSC mobilization can be enhanced with a proper approach in allogeneic and autologous HSCT. In autologous HSCT, depended on the patient’s disease and treatment protocol the results of stem cell collection will be different. A low CD34+ cell count in PB before apheresis is a key predictor factor for poor PBSC collection. Hence, enumeration of CD34+ cell prior apheresis may appraisal the risk for poor PBSC collection and could permit suitable intervention to overcome of mobilization failure.
ACKNOWLEDGEMENT
This manuscript was supported by Hematology-Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran.
CONFLICT OF INTEREST
Authors deny any conflict of interest related to this study.
References
- 1.Bojanic I, Cepulic BG, Mazic S. Collection of hematopoietic progenitor cells from healthy donors. Acta Med Croatica. 2009;63(3):237–44. [PubMed] [Google Scholar]
- 2.Jalali A, Alimoghaddam K, Mahmoudi M, et al. The EBMT Risk Score in the Presence of Graft Versus Host Disease in Allogeneic Stem Cell Transplantation in Adult Acute Myelogenous Leukemia: A Multistate Model for Competing Risks. Int J Hematol Oncol Stem Cell Res. 2014;8(3):1–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Pusic I, DiPersio JF. The use of growth factors in hematopoietic stem cell transplantation. Curr Pharm Des. 2008;14(20):1950–61. doi: 10.2174/138161208785061427. [DOI] [PubMed] [Google Scholar]
- 4.Reddy RL. Mobilization and collection of peripheral blood progenitor cells for transplantation. Transfus Apher Sci. 2005;32(1):63–72. doi: 10.1016/j.transci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Jalali A, Alimoghaddam K, Mahmoudi M, et al. The Effect of GVHD on Long-term Outcomes after Peripheral Blood Allogeneic Stem Cell Transplantation from an HLA-identical Sibling in Adult Acute Lymphocytic Leukemia: A Landmark Analysis Approach in Competing Risks. Int J Hematol Oncol Stem Cell Res. 2014;8(2):1–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Rasheed W, Ghavamzadeh A, Hamladji R, et al. Hematopoietic stem cell transplantation practice variation among centers in the Eastern Mediterranean Region (EMRO): Eastern Mediterranean Bone Marrow Transplantation (EMBMT) group survey. Hematol Oncol Stem Cell Ther. 2013;6(1):14–9. doi: 10.1016/j.hemonc.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Kroger N, Renges H, Sonnenberg S, et al. Stem cell mobilisation with 16 microg/kg vs 10 microg/kg of G-CSF for allogeneic transplantation in healthy donors. Bone Marrow Transplant. 2002;29(9):727–30. doi: 10.1038/sj.bmt.1703509. [DOI] [PubMed] [Google Scholar]
- 8.Anderlini P, Donato M, Lauppe MJ, et al. A comparative study of once-daily versus twice-daily filgrastim administration for the mobilization and collection of CD34+ peripheral blood progenitor cells in normal donors. Br J Haematol. 2000;109(4):770–2. doi: 10.1046/j.1365-2141.2000.02083.x. [DOI] [PubMed] [Google Scholar]
- 9.Martinez C, Urbano-Ispizua A, Marin P, et al. Efficacy and toxicity of a high-dose G-CSF schedule for peripheral blood progenitor cell mobilization in healthy donors. Bone Marrow Transplant. 1999;24(12):1273–1278. doi: 10.1038/sj.bmt.1702073. [DOI] [PubMed] [Google Scholar]
- 10.Kroger N, Sonnenberg S, Cortes-Dericks L, et al. Kinetics of G-CSF and CD34+ cell mobilization after once or twice daily stimulation with rHu granulocyte-stimulating factor (lenograstim) in healthy volunteers: an intraindividual crossover study. Transfusion. 2004;44(1):104–10. doi: 10.1111/j.0041-1132.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 11.Lonial S, Akhtari M, Kaufman J, et al. Mobilization of hematopoietic progenitors from normal donors using the combination of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor results in fewer plasmacytoid dendritic cells in the graft and enhanced donor T cell engraftment with Th1 polarization: results from a randomized clinical trial. Biol Blood Marrow Transplant. 2013;19(3):460–67. doi: 10.1016/j.bbmt.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Bazarbachi A, Labopin M, Ghavamzadeh A, et al. Allogeneic matched-sibling hematopoietic cell transplantation for AML: comparable outcomes between Eastern Mediterranean (EMBMT) and European (EBMT) centers. Bone Marrow Transplant. 2013;48(8):1065–9. doi: 10.1038/bmt.2013.1. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Simon JA, Diez-Campelo M, Martino R, et al. Impact of CD34+ cell dose on the outcome of patients undergoing reduced-intensity-conditioning allogeneic peripheral blood stem cell transplantation. Blood. 2003;102(3):1108–13. doi: 10.1182/blood-2002-11-3503. [DOI] [PubMed] [Google Scholar]
- 14.Mohty M, Bilger K, Jourdan E, et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia. 2003;17(5):869–75. doi: 10.1038/sj.leu.2402909. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura R, Auayporn N, Smith DD, et al. Impact of graft cell dose on transplant outcomes following unrelated donor allogeneic peripheral blood stem cell transplantation: higher CD34+ cell doses are associated with decreased relapse rates. Biol Blood Marrow Transplant. 2008;14(4):449–57. doi: 10.1016/j.bbmt.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood. 2009;114(13):2606–16. doi: 10.1182/blood-2009-03-208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta J, Mehta J, Frankfurt O, et al. Optimizing the CD34 + cell dose for reduced-intensity allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2009;50(9):1434–41. doi: 10.1080/10428190903085944. [DOI] [PubMed] [Google Scholar]
- 18.Holtan SG, Hogan WJ, Elliott MA, et al. CD34+ cell dose and establishment of full donor chimerism at day +100 are important factors for survival with reduced-intensity conditioning with fludarabine and melphalan before allogeneic hematopoietic SCT for hematologic malignancies. Bone Marrow Transplant. 2010;45(12):1699–703. doi: 10.1038/bmt.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heimfeld S. HLA-identical stem cell transplantation: is there an optimal CD34 cell dose? Bone Marrow Transplant. 2003;31(10):839–45. doi: 10.1038/sj.bmt.1704019. [DOI] [PubMed] [Google Scholar]
- 20.Pulsipher MA, Levine JE, Hayashi RJ, et al. Safety and efficacy of allogeneic PBSC collection in normal pediatric donors: the pediatric blood and marrow transplant consortium experience (PBMTC) 1996-2003. Bone Marrow Transplant. 2005;35(4):361–7. doi: 10.1038/sj.bmt.1704743. [DOI] [PubMed] [Google Scholar]
- 21.Styczynski J, Balduzzi A, Gil L, et al. Risk of complications during hematopoietic stem cell collection in pediatric sibling donors: a prospective European Group for Blood and Marrow Transplantation Pediatric Diseases Working Party study. Blood. 2012;119(12):2935–2942. doi: 10.1182/blood-2011-04-349688. [DOI] [PubMed] [Google Scholar]
- 22.Sevilla J, Gonzalez-Vicent M, Lassaletta A, et al. Peripheral blood progenitor cell collection adverse events for childhood allogeneic donors: variables related to the collection and safety profile. Br J Haematol. 2009;144(6):909–16. doi: 10.1111/j.1365-2141.2008.07529.x. [DOI] [PubMed] [Google Scholar]
- 23.Kalwak K, Porwolik J, Mielcarek M, et al. Higher CD34(+) and CD3(+) cell doses in the graft promote long-term survival, and have no impact on the incidence of severe acute or chronic graft-versus-host disease after in vivo T cell-depleted unrelated donor hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant. 2010;16(10):1388–401. doi: 10.1016/j.bbmt.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Chang YJ, Xu LP, Liu DH, et al. The impact of CD34+ cell dose on platelet engraftment in pediatric patients following unmanipulated haploidentical blood and marrow transplantation. Pediatr Blood Cancer. 2009;53(6):1100–106. doi: 10.1002/pbc.22159. [DOI] [PubMed] [Google Scholar]
- 25.Liu DH, Zhao XS, Chang YJ, et al. The impact of graft composition on clinical outcomes in pediatric patients undergoing unmanipulated HLA-mismatched/ haploidentical hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2011;57(1):135–41. doi: 10.1002/pbc.23107. [DOI] [PubMed] [Google Scholar]
- 26.Smith TJ, Hillner BE, Schmitz N, et al. Economic analysis of a randomized clinical trial to compare filgrastim-mobilized peripheral-blood progenitor-cell transplantation and autologous bone marrow transplantation in patients with Hodgkin's and non-Hodgkin's lymphoma. J Clin Oncol. 1997;15(1):5–10. doi: 10.1200/JCO.1997.15.1.5. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Kim HJ, Park JS, et al. Prospective randomized comparative observation of single- vs split-dose lenograstim to mobilize peripheral blood progenitor cells following chemotherapy in patients with multiple myeloma or non-Hodgkin's lymphoma. Ann Hematol. 2005;84(11):742–7. doi: 10.1007/s00277-005-1103-8. [DOI] [PubMed] [Google Scholar]
- 28.Kim JE, Yoo C, Kim S, et al. Optimal timing of G-CSF administration for effective autologous stem cell collection. Bone Marrow Transplant. 2011;46(6):806–12. doi: 10.1038/bmt.2010.194. [DOI] [PubMed] [Google Scholar]
- 29.Simona B, Cristina R, Luca N, et al. A single dose of Pegfilgrastim versus daily Filgrastim to evaluate the mobilization and the engraftment of autologous peripheral hematopoietic progenitors in malignant lymphoma patients candidate for high-dose chemotherapy. Transfus Apher Sci. 2010;43(3):321–6. doi: 10.1016/j.transci.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Russell N, Mesters R, Schubert J, et al. A phase 2 pilot study of pegfilgrastim and filgrastim for mobilizing peripheral blood progenitor cells in patients with non-Hodgkin's lymphoma receiving chemotherapy. Haematologica. 2008;93(3):405–12. doi: 10.3324/haematol.11287. [DOI] [PubMed] [Google Scholar]
- 31.Hamadani M, Kochuparambil ST, Osman S, et al. Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony-stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transplant. 2012;18(7):1128–35. doi: 10.1016/j.bbmt.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Mahindra A, Bolwell BJ, Rybicki L, et al. Etoposide plus G-CSF priming compared with G-CSF alone in patients with lymphoma improves mobilization without an increased risk of secondary myelodysplasia and leukemia. Bone Marrow Transplant. 2012;47(2):231–5. doi: 10.1038/bmt.2011.73. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe H, Watanabe T, Suzuya H, et al. Peripheral blood stem cell mobilization by granulocyte colony-stimulating factor alone and engraftment kinetics following autologous transplantation in children and adolescents with solid tumor. Bone Marrow Transplant. 2006;37(7):661–668. doi: 10.1038/sj.bmt.1705304. [DOI] [PubMed] [Google Scholar]
- 34.Sevilla J, Gonzalez-Vicent M, Madero L, et al. Granulocyte colony-stimulating factor alone at 12 microg/kg twice a day for 4 days for peripheral blood progenitor cell priming in pediatric patients. Bone Marrow Transplant. 2002;30:417–20. doi: 10.1038/sj.bmt.1703662. [DOI] [PubMed] [Google Scholar]
- 35.Weaver CH, Hazelton B, Birch R, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86(10):3961–69. [PubMed] [Google Scholar]
- 36.Bolwell BJ, Pohlman B, Rybicki L, et al. Patients mobilizing large numbers of CD34+ cells ('super mobilizers') have improved survival in autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant. 2007;40(5):437–41. doi: 10.1038/sj.bmt.1705763. [DOI] [PubMed] [Google Scholar]
- 37.Yoon DH, Sohn BS, Jang G, et al. Higher infused CD34+ hematopoietic stem cell dose correlates with earlier lymphocyte recovery and better clinical outcome after autologous stem cell transplantation in non-Hodgkin's lymphoma. Transfusion. 2009;49(9):1890–900. doi: 10.1111/j.1537-2995.2009.02202.x. [DOI] [PubMed] [Google Scholar]
- 38.Stiff PJ, Micallef I, Nademanee AP, et al. Transplanted CD34(+) cell dose is associated with long-term platelet count recovery following autologous peripheral blood stem cell transplant in patients with non-Hodgkin lymphoma or multiple myeloma. Biol Blood Marrow Transplant. 2011;17(8):1146–53. doi: 10.1016/j.bbmt.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Mohamed SY, Fadhil I, Hamladji RM, et al. Hematopoietic stem cell transplantation in the Eastern Mediterranean Region (EMRO) 2008-2009: report on behalf of the Eastern Mediterranean Bone Marrow Transplantation (EMBMT) Group. Hematol Oncol Stem Cell Ther. 2011;4(2):81–93. doi: 10.5144/1658-3876.2011.81. [DOI] [PubMed] [Google Scholar]
- 40.Cetin T, Arpaci F, Ozet A, et al. Stem cell mobilization by G-CSF in solid and hematological malignancies: single daily dose is better than split dose in obese patients. J Clin Apher. 2003;18(3):120–4. doi: 10.1002/jca.10068. [DOI] [PubMed] [Google Scholar]
- 41.Basak GW, Wiktor-Jedrzejczak W, Apperley JF, et al. Higher BMI is not a barrier to stem cell mobilization with standard doses of plerixafor and G-CSF. Bone Marrow Transplant. 2012;47(7):1003–5. doi: 10.1038/bmt.2011.199. [DOI] [PubMed] [Google Scholar]
- 42.Orbach D, Hojjat-Assari S, Doz F, et al. Peripheral blood stem cell collection in 24 low-weight infants: experience of a single centre. Bone Marrow Transplant. 2003;31(3):171–4. doi: 10.1038/sj.bmt.1703825. [DOI] [PubMed] [Google Scholar]
- 43.Grigg AP, Roberts AW, Raunow H, et al. Optimizing dose and scheduling of filgrastim (granulocyte colony-stimulating factor) for mobilization and collection of peripheral blood progenitor cells in normal volunteers. Blood. 1995;86(12):4437–45. [PubMed] [Google Scholar]
- 44.Perez-Simon JA, Caballero MD, Corral M, et al. Minimal number of circulating CD34+ cells to ensure successful leukapheresis and engraftment in autologous peripheral blood progenitor cell transplantation. Transfusion. 1998;38(4):385–91. doi: 10.1046/j.1537-2995.1998.38498257378.x. [DOI] [PubMed] [Google Scholar]
- 45.Basquiera AL, Abichain P, Damonte JC, et al. The number of CD34(+) cells in peripheral blood as a predictor of the CD34(+) yield in patients going to autologous stem cell transplantation. J Clin Apher. 2006;21(2):92–5. doi: 10.1002/jca.20062. [DOI] [PubMed] [Google Scholar]
- 46.Mohty M, Hubel K, Kroger N, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(7):865–72. doi: 10.1038/bmt.2014.39. [DOI] [PubMed] [Google Scholar]
- 47.Gertz MA, Kumar SK, Lacy MQ, et al. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43(8):619–25. doi: 10.1038/bmt.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood WA, Whitley J, Moore D, et al. Chemomobilization with Etoposide is Highly Effective in Patients with Multiple Myeloma and Overcomes the Effects of Age and Prior Therapy. Biol Blood Marrow Transplant. 2011;17(1):141–6. doi: 10.1016/j.bbmt.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Fox CP, McMillan AK, Bishton MJ, et al. IVE (ifosfamide, epirubicin and etoposide) is a more effective stem cell mobilisation regimen than ICE (ifosphamide, carboplatin and etoposide) in the context of salvage therapy for lymphoma. Br J Haematol. 2008;141(2):244–8. doi: 10.1111/j.1365-2141.2008.07068.x. [DOI] [PubMed] [Google Scholar]
- 50.Pavone V, Gaudio F, Guarini A, et al. Mobilization of peripheral blood stem cells with high-dose cyclophosphamide or the DHAP regimen plus G-CSF in non-Hodgkin's lymphoma. Bone Marrow Transplant. 2002;29(4):285–90. doi: 10.1038/sj.bmt.1703364. [DOI] [PubMed] [Google Scholar]
- 51.Elliott C, Samson DM, Armitage S, et al. When to harvest peripheral-blood stem cells after mobilization therapy: prediction of CD34-positive cell yield by preceding day CD34-positive concentration in peripheral blood. J Clin Oncol. 1996;14(3):970–3. doi: 10.1200/JCO.1996.14.3.970. [DOI] [PubMed] [Google Scholar]
- 52.Gertz MA, Wolf RC, Micallef IN, et al. Clincal impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant. 2010;45(9):1396–403. doi: 10.1038/bmt.2009.370. [DOI] [PubMed] [Google Scholar]
- 53.Hosing C, Saliba RM, Ahlawat S, et al. Poor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphoma. Am J Hematol. 2009;84(6):335–7. doi: 10.1002/ajh.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popat U, Saliba R, Thandi R, et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15(6):718–23. doi: 10.1016/j.bbmt.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha S, Gastineau D, Micallef I, et al. Predicting PBSC harvest failure using circulating CD34 levels: developing target-based cutoff points for early intervention. Bone Marrow Transplant. 2011;46(7):943–949. doi: 10.1038/bmt.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanza F, Lemoli RM, Olivieri A, et al. Factors affecting successful mobilization with plerixafor: an Italian prospective survey in 215 patients with multiple myeloma and lymphoma. Transfusion. 2014;54(2):331–9. doi: 10.1111/trf.12265. [DOI] [PubMed] [Google Scholar]
- 57.Abhyankar S, DeJarnette S, Aljitawi O, et al. A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplant. 2012;47(4):483–7. doi: 10.1038/bmt.2011.133. [DOI] [PubMed] [Google Scholar]
- 58.Wuchter P, Ran D, Bruckner T, et al. Poor mobilization of hematopoietic stem cells-definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biol Blood Marrow Transplant. 2010;16(4):490–9. doi: 10.1016/j.bbmt.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Majado MJ, Minguela A, Gonzalez-Garcia C, et al. Large-volume-apheresis facilitates autologous transplantation of hematopoietic progenitors in poor mobilizer patients. J Clin Apher. 2009;24(1):12–17. doi: 10.1002/jca.20191. [DOI] [PubMed] [Google Scholar]
- 60.Bojanic I, Dubravcic K, Batinic D, et al. Large volume leukapheresis: Efficacy and safety of processing patient's total blood volume six times. Transfus Apher Sci. 2011;44(2):139–47. doi: 10.1016/j.transci.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Calandra G, McCarty J, McGuirk J, et al. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin's lymphoma, Hodgkin's disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant. 2008;41(4):331–8. doi: 10.1038/sj.bmt.1705908. [DOI] [PubMed] [Google Scholar]
- 62.Coluccia P, Montefusco V, Tunesi S, et al. Peripheral blood stem cell collection in multiple myeloma: a retrospective analysis of 6 years leukapheresis activity in 109 patients treated at the Istituto Nazionale dei Tumori of Milan. J Clin Apher. 2009;24(4):134–40. doi: 10.1002/jca.20203. [DOI] [PubMed] [Google Scholar]
- 63.Varmavuo V, Mantymaa P, Silvennoinen R, et al. CD34+ cell subclasses and lymphocyte subsets in blood grafts collected after various mobilization methods in myeloma patients. Transfusion. 2013;53(5):1024–32. doi: 10.1111/j.1537-2995.2012.03848.x. [DOI] [PubMed] [Google Scholar]
- 64.Varmavuo V, Mantymaa P, Kuittinen T, et al. Blood graft lymphocyte subsets after plerixafor injection in non-Hodgkin's lymphoma patients mobilizing poorly with chemotherapy plus granulocyte-colony-stimulating factor. Transfusion. 2012;52(8):1785–91. doi: 10.1111/j.1537-2995.2011.03525.x. [DOI] [PubMed] [Google Scholar]
- 65.Porrata LF, Gastineau DA, Padley D, et al. Re-infused autologous graft natural killer cells correlates with absolute lymphocyte count recovery after autologous stem cell transplantation. Leuk Lymphoma. 2003;44(6):997–1000. doi: 10.1080/1042819031000077089. [DOI] [PubMed] [Google Scholar]
- 66.Pulsipher MA, Chitphakdithai P, Miller JP, et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood. 2009;113(15):3604–11. doi: 10.1182/blood-2008-08-175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stiff PJ, Bensinger W, Abidi MH, et al. Clinical and ultrasonic evaluation of spleen size during peripheral blood progenitor cell mobilization by filgrastim: results of an open-label trial in normal donors. Biol Blood Marrow Transplant. 2009;15(7):827–34. doi: 10.1016/j.bbmt.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Platzbecker U, Prange-Krex G, Bornhauser M, et al. Spleen enlargement in healthy donors during G-CSF mobilization of PBPCs. Transfusion. 2001;41(2):184–9. doi: 10.1046/j.1537-2995.2001.41020184.x. [DOI] [PubMed] [Google Scholar]