Abstract
The bacterium Bacillus thuringiensis (Bt) produces a wide range of toxins that are effective against a number of insect pests. Identifying the mechanisms responsible for resistance to Bt toxin will improve both our ability to control important insect pests and our understanding of bacterial toxicology. In this study, we investigated the role of MAPK pathways in resistance against Cry1Ca toxin in Chilo suppressalis, an important lepidopteran pest of rice crops. We first cloned the full-length of C. suppressalis mitogen-activated protein kinase (MAPK) p38, ERK1, and ERK2, and a partial sequence of JNK (hereafter Csp38, CsERK1, CsERK2 and CsJNK). We could then measure the up-regulation of these MAPK genes in larvae at different times after ingestion of Cry1Ca toxin. Using RNA interference to knockdown Csp38, CsJNK, CsERK1 and CsERK2 showed that only knockdown of Csp38 significantly increased the mortality of larvae to Cry1Ca toxin ingested in either an artificial diet, or after feeding on transgenic rice expressed Cry1Ca. These results suggest that MAPK p38 is responsible for the resistance of C. suppressalis larvae to Bt Cry1Ca toxin.
Pore-forming toxins (PFT) play an important role in bacterial pathogenesis and the development of pest resistant strains of crops1,2,3. Several previous studies have shown that PFTs such as streptolysin O (Streptococcus pyogenes), α-hemolysin (Escherichia coli), α-toxin (Staphylococcus aureus) and Crystal (Cry) toxin (Bacillus thuringiensis) (Bt) have high toxicity to insect pests4,5,6,7. Among these, Bt Cry toxins are the most widely used bacterial pesticides8.
The use of transcriptomic and proteomic approaches has been a recent advance in investigating the mechanisms underlying host responses to Bt toxins. For example, the non-olfactory, odorant binding protein C12 has been reported to play a role in the resistance of Tribolium castaneum larvae to Cry3Ba and Cry23Aa/Cry37Aa toxins9. Knockdown of the ATP synthase subunits beta and actin in Aedes aegypti larvae increased their susceptibility to Cry11Aa but silencing the heat shock protein caused larvae to become resistant to this toxin10. Moreover, transcriptional profiling has demonstrated that cells trigger different survival mechanisms to counteract the effects of non-lethal doses of Bt toxins11,12,13,14,15.
Besides high throughput approaches, a few studies have focused on analyzing the roles of specific pathways and genes in resistance to Bt toxins. For example, exposure to Cry1Ab and Cry11Aa activated p38 phosphorylation in both Manduca sexta and A. aegypti, thereby increasing resistance to these toxins16. The unfolded protein (UPR) pathway, induced after activation of Mitogen-activated protein kinase (MAPK) p38, has since been implicated in the resistance of A. aegypti to Cry11Aa17. In T. castaneum, the hemolymph protein Apolipophorin-III acts as an immune response protein following challenge by Cry3Ba18,19. Insect midgut alkaline phosphatase and cadherin, which are generally thought to be Cry toxin receptors, have also been found to be involved in the immune response to Cry toxins20,21.
The striped rice borer, Chilo suppressalis, is a lepidopteran pest that causes major damage to rice crops in Asia22,23,24,25. Previous studies have demonstrated that the Cry1Ca toxin is effective against this pest26, and that transgenic Bt crops producing Cry1Ca (T1C-19) are resistant to C. suppressalis27. In this paper we present the results of experiments designed to determine the function of specific C. suppressalis MAPK pathway genes in resistance to Cry1Ca. The results suggest that the p38 pathway plays a major role in resistance to Cry1Ca toxin in C. suppressalis.
Results
Cloning of Cs-p38, -JNK, -ERK1/2 cDNA sequences and sequence analysis
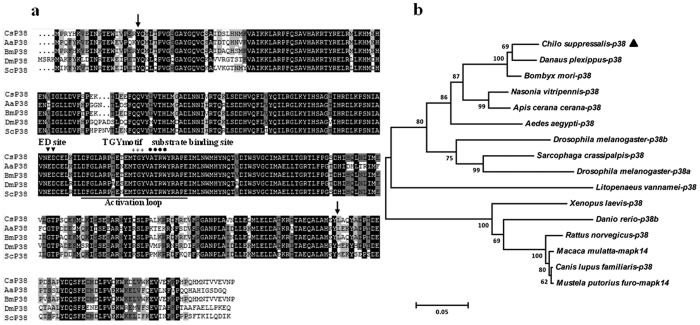
The full-length of C. suppressalis p38 cDNA (GenBank accession No.: KU358549) consists of an 82 bp 5′ untranslated region (UTR), a 981 bp 3′ UTR, a TGA terminator sequence, and a 1,080 bp open reading frame (ORF) encoding 360 amino acid residues with a molecular mass of 41.58 kDa and a pI value of 5.84. Pair-wise and multiple sequence alignments revealed that Csp38 contains an activation loop structure, a conserved Thr-Gly-Tyr (TGY) phosphorylation motif, the substrate binding site Ala-Thr-Arg-Trp (ATRW), and the ERK docking (ED) site (Fig. 1a). Csp38 protein was most similar to that in Bombyx mori (95.8%), followed by A. aegypti (83.5%), Sarcophaga crassipalpis (79.3%) and Drosophila melanogaster (76.5%). The predicted serine/threonine protein kinase (S_TKc) domain was found at position 20–304 (Fig. 1a). To assess the evolutionary relationship between Csp38 and its homologs, a phylogenetic tree was constructed using the neighbor-joining method based on the amino acid sequences of p38 from selected species. This showed that Csp38 was most homologous to that of Danaus plexippus and B. mori, which collectively comprised a relatively distinct clade (Fig. 1b).
Figure 1. Comparison of C. suppressalis p38 (Csp38) with that of other species.
(a) Multiple-sequence alignment of the deduced amino acid sequence of Csp38 MAPK with other known p38 MAPKs. Amino acids with 100%, 75%, and 50% conservation are shaded in black, dark grey and light grey. The predicted serine/threonine protein kinase (S_TKc) domain was found at position 20–304 (arrow). The characteristic p38 structure, activation loop (underline), substrate binding site (dark spot), ED site (inverted triangle), and TGY motif, are indicated by asterisks. (b) Phylogenetic tree of relationships between Csp38 and MAPKs from other species (Supplementary Table S2). The bar indicates a phylogenetic distance of 0.05, the position of Csp38 is indicated by a black triangle.
In this study, we used RT-PCR and rapid amplification of cDNA ends (RACE) technology to clone C. suppressalis c-Jun N-terminal kinase (JNK) from midgut of 3rd instar larvae. We did not, however, obtain the full-length of this gene. The 1,392 bp partial CsJNK cDNA (GenBank accession number: KU358550) we obtained contains a 400 bp 5′ UTR and a partial ORF encoding a predicted protein of 331 amino acids. Multiple sequence alignments showed that CsJNK includs an activation loop structure and a conserved Thr-Pro-Tyr (TPY) phosphorylation motif (Fig. 2a). Its identity with its homologs in B. mori, Helicoverpa armigera, Culex quinquefasciatus and D. melanogaster is 97.7%, 97.4%, 93.4% and 88.8%, respectively. The predicted S_TKc domain was found at position 20–304 (Fig. 2a). The associated phylogenetic tree shows that C. suppressalis JNK clusters with other lepidopteran JNK, and is most closely related to that of B. mori and H. armigera (Fig. 2b).
Figure 2. Comparison of C. suppressalis JNK (CsJNK) with that of other species.
(a) Amino acids with 100%, 75%, and 50% conservation are shaded in black, dark grey and light grey. The characteristic JNK structure, activation loop (underline), substrate binding site (dark spot), and TPY motif (asterisks) are found at position 35–331 in the S_TKc domain (arrow). (b) Phylogenetic tree of relationships between CsJNK and MAPKs from other species (Supplementary Table S2). The bar indicates a phylogenetic distance of 0.05, the position of CsJNK is indicated by a black triangle.
The full length of C. suppressalis extracellular signal-regulated kinase 1 (ERK1) (GenBank accession number: KU358551) cDNA is 1,892 bp and contains a 164 bp 5′ UTR and a 516 bp 3′ UTR. The ORF is 1,209 bp encoding 403 amino acid proteins with a calculated molecular weight of about 44.51 kDa and a PI value of 6.34. The full-length of CsERK2 (GenBank accession No.: KU358552) is 2,015 bp. The ORF encodes 364 amino acids, the calculated molecular weight is about 41.96 kDa and the estimated PI was 6.09. The predicted S_TKc domain is shown in Fig. 3a. Alignment of CsERK1 and CsERK2 with ERK proteins of other species shows that CsERK2 includes an activation loop structure and a conserved Thr-Glu-Tyr (TEY) phosphorylation motif, and a substrate binding site (Fig. 3a). However, the predicted protein of CsERK1 has no typical conserved motif. Phylogenetic analysis indicates that CsERK2 has relatively highest identity with that of B. mori (95.1%) and lowest with that of D. plexippus (21.8%), whereas CsERK1 has highest identity with that of D. plexippus (98.0%) and lowest with that of B. mori (21.2%). Moreover, CsERK1 and CsERK2 has very low identity with each other (21.5%) (Fig. 3a). The associated phylogenetic tree places CsERK1 in a distinct clade with that of D. plexippus and that CsERK2 is most homologous to that of B. mori (Fig. 3b).
Figure 3. Comparison of C. suppressalis ERK1 and ERK2 MAPK genes (CsERK1 and CsERK2) with known ERK MAPKs from other species.
(a) Amino acids with 100%, 75%, and 50% conservation are shaded in black, dark grey and light grey. The predicted S_TKc domain was found at position 90–369 (arrow) of CsERK1 and 28–316 (arrow) of ERK2. The characteristic ERK structure, activation loop (underline), substrate binding site (dark spot), and TEY motif, are indicated by asterisks. (b) Phylogenetic tree of relationships between CsERKs and MAPKs from other species (Supplementary Table S2). The bar indicates a phylogenetic distance of 0.05, the position of CsERK is indicated by a black triangle.
Induction of Csp38, CsJNK and CsERK1/2 by Cry1Ca toxin
In order to determine whether Csp38, CsJNK and CsERK1/2 were activated by Cry1Ca toxin, we quantified expression of these genes at different periods of time after C. suppressalis larvae had ingested this toxin. According to a preliminary dosage screening, we chose the dosages of 20 and 60 μg of final Cry1Ca digested product (FDP) to each gram of artificial food to induce MAPK gene expressions. Ingestion of a diet containing of 20 μg of FDP to each gram of artificial food was followed by a 2-fold increase in Csp38 expression within 30 min compared to a control group fed on a diet comprised of the usual artificial food plus water (Fig. 4a). A 3-fold Up-regulation of CsERK1 also occurred within 30 min of ingesting a diet containing 20 μg of FDP to each gram of artificial food (Fig. 4c). CsJNK transcription increased after 1 h and 48 h of consuming artificial diet containing 60 μg of FDP to each gram of artificial food (Fig. 4b). Slight alteration of CsERK2 expression was observed within 1 h of feeding on artificial diet containing 60 μg of FDP to each gram of artificial food (Fig. 4d). In contrast, down-regulation of the CsJNK and CsERK2 transcription were observed within 30 min of feeding on a diet containing 20 μg of FDP to each gram of artificial food (Fig. 4b,d), and down-regulation of the CsERK2 gene expression was induced within 30 min of feeding on a diet containing 60 μg of FDP to each gram of artificial food (Fig. 4d).
Figure 4. Effects of Cry1Ca toxin on expression of C. suppressalis MAPK genes (CsMAPK).
(a) Estimates of relative Csp38 transcription levels determined by qRT-PCR of C. suppressalis larvae sampled at 30 min, 1 h and 48 h after ingesting Cry1Ca toxin. (b) Estimates of relative CsJNK transcription levels determined by qRT-PCR of C. suppressalis larvae sampled at 30 min, 1 h and 48 h after ingestion of Cry1Ca toxin. (c) Estimates of relative CsERK1 transcription levels determined by qRT-PCR of C. suppressalis larvae sampled at 30 min, 1 h and 48 h after ingestion of Cry1Ca toxin. (d) Estimates of relative CsERK2 transcription levels determined by qRT-PCR of C. suppressalis larvae sampled at 30 min, 1 h and 48 h after ingestion of Cry1Ca toxin. Relative levels of gene transcription were normalized to that of EF1 (the internal reference). Asterisks indicate P values < 0.05 (ANOVA).
RNA interference (RNAi) knockdown of Csp38 caused increased susceptibility to Cry1Ca
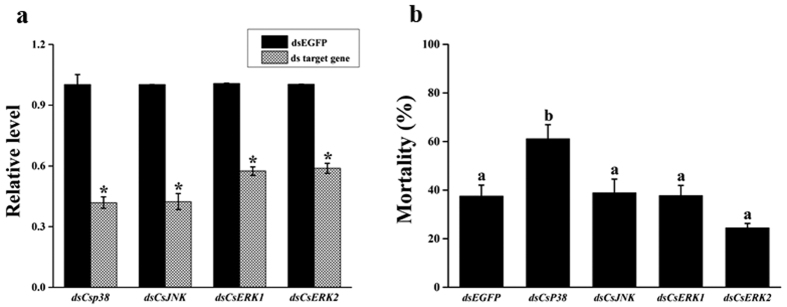
We used RNAi to test the roles of Csp38, CsJNK, CsERK1 and CsERK2 in resistance to Cry1Ca toxicity in C. suppressalis larvae. Expression of Csp38, CsJNK, CsERK1 and CsERK2 significantly decreased in larvae in which these target genes had been knocked down 48 h compared to a control diet containing EGFP dsRNA (Fig. 5a). dsRNA knockdown of Csp38 led to a significant increase in mortality (61.1%) compared to that in the EGFP dsRNA control (37.5%). In contrast, knockdown of CsJNK, CsERK1 and CsERK2 did not result in a significant increase in mortality relative to the control (Fig. 5b).
Figure 5. Effect of RNA interference knockdown of p38, JNK, ERK1 and ERK2 transcripts on the resistance of C. suppressalis larvae to Cry1Ca toxin.
(a) Estimates of target gene transcription as determined by qRT-PCR and normalized to the expression of a reference gene (EF1). qRT-PCR was performed 48 h after C. suppressalis neonate larvae had fed on an artificial diet mixed with either EGFP dsRNA (the control), or target gene dsRNA. Asterisks indicate P values < 0.05 (Student’s t-test). (b) Larval mortality. Larvae were first fed their usual artificial food with the addition of either EGFP dsRNA (the control), or target gene dsRNA, then their usual artificial food mixed with FDP (30 μg FDP to each gram of food). Corrected mortality was calculated from 5 replicates. Bars with different letters are significantly different (P < 0.05; ANOVA).
dsRNA knockdown of Csp38, CsJNK, CsERK1 and CsERK2 also caused a significant reduction in the transcription of these genes compared to the EGFP dsRNA control (Fig. 6a). Mortalities in the Csp38, CsJNK, CsERK1 and CsERK2 knockdown treatment groups after feeding on transgenic rice was 55.1%, 26.7%, 20.3% and 20.6%, respectively. However, only the Csp38 knockdown treatment group had significantly higher mortality (55.1%) than the control (15.8%) (Fig. 6b).
Figure 6. Effect of RNA interference knockdown of p38, JNK, ERK1 and ERK2 transcripts on the susceptibility of C. suppressalis larvae to Cry1Ca toxin expressed in transgenic rice.
(a) Estimates of target gene transcription as determined by qRT-PCR and normalized to the expression of a reference gene (EF1). qRT-PCR was performed 48 h after C. suppressalis neonate larvae had fed on non-transgenic rice (Minghui 63) treated with either EGFP dsRNA (the control), or target gene dsRNA. Asterisks indicate P values < 0.05 (Student’s t-test). (b) Larval mortality. Larvae were first fed on non-transgenic rice (Minghui 63) treated with either EGFP dsRNA (the control), or target gene dsRNA, then transgenic rice expressing the Cry1Ca toxin. Corrected mortality was estimated from 5 replicates. Bars with different letters are significantly different (P < 0.05; ANOVA).
Discussion
MAPK signaling pathways are comprised of serine-threonine protein kinases that regulate a variety of cellular processes28,29,30. Multicellular organisms have three subfamilies of MAPKs, including ERK, JNK and p38 MAPKs31. Among these, the p38 pathway is the most important in regulating resistance to PFTs.
Since the activation of the p38 pathway by Cry5B toxin in Caenorhabditis elegans was first described2, several studies have demonstrated that low doses of other PFTs (e.g., aerolysin, pneumolysin, streptolysin and a-hemolysin) can induce the activation of this pathway in cultured-epithelial cell lines2,32. Similar responses have also been observed in insects, for example, both M. sexta and A. aegypti were found to activate p38 phosphorylation to protect themselves against Cry toxins16. As expected, our results also demonstrate that p38 plays a key role in resistance to Cry1Ca in C. suppressalis. This may be related to K+ transmission; it has been shown that K+ efflux throughout the toxin pore is likely to activate the p38 defensive response to α-toxin, cytolysin or hemolysin33. Besides, the different extend of the induced Csp38 up-regulation between diet mixed with toxin and transgenic rice might be led by different amount of Cry1Ca in artificial diet and transgenic rice.
We also investigated the role of the JNK and ERK pathways in resistance to Cry1Ca toxin in C. suppressalis. Although low doses of Cry1Ca induced csJNK and csERK expression (Fig. 4), no significant difference in mortality were observed between control larvae and those in which CsJNK and CsERK had been knocked down with RNAi (Figs 5 and 6). We conclude therefore that the JNK and ERK pathways are not involved in resistance to Cry1Ca in C. suppressalis. Besides, we did not obtain the full length of CsJNK, the sequencing data was always noised in the 3 end part, which may be caused by the secondary structure at the 3′ terminal region of CsJNK RNA.
Cellular responses to Cry toxins are complex and require further intensive research. The results of this study show that knockdown of p38, but not other MAPK genes, significantly increased the mortality of C. suppressalis larvae following ingestion of Cry1Ca. This suggests that p38 is responsible for resistance to Cry1Ca in C. suppressalis, and potentially other insect pests. Future work should focus on searching for genes downstream of the p38 pathway that play a role in PFT resistance.
Materials and Methods
Insect rearing and Cry1Ca toxin
The founder population of C. suppressalis larvae were collected in Dawu County, Hubei Province, China in 2012 and propagated in a laboratory for 4 years. Larvae were kept at 28 ± 1 °C under a 16-h photoperiod, > 80% relative humidity and fed on an artificial diet34. The Cry1Ca toxin used in this study is a gift from Dr. Jie Zhang (Institute of Plant Protection, China Academy of Agricultural Science). The toxin is a protein crude extract from a genetic modified Bacillus thuringiensis strain, in which Cry1Ca is the only toxin it expressed. The crude extraction product was then digested with trypsin without purification. The FDP was used directly in all bioassays.
Cloning of three MAPK genes
Total RNA was isolated from actively feeding 4th instar C. suppressalis larvae using RNAiso reagent (TaKaRa, Dalian, China). Contaminating genomic DNA was eliminated with RNase-free DNase, and the RNA preparation was then subjected to reverse transcription using a PrimeScriptTM RT reagent Kit (TaKaRa, China) according to the manufacturer’s instructions. Partial p38, JNK, ERK1 and ERK2 cDNA sequences had been obtained from the C. Suppressalis transcriptome determined in previous studies35. A 5′ and 3′ RACE kit (TaKaRa, Dalian, China) was used to amplify full-length MAPK genes from C. Suppressalis larvae, and pairs of gene-specific and degenerate primers were designed based on the partial sequences using Primer 5.0 software (Supplementary Table S1). PCR products were subcloned into a PMD (18)-T vector (Takara, Dalian, China) and sequenced by the Nanjing Genscript Company, China.
Sequence analysis
Sequence analysis was conducted on full-length cDNA using an ORF finder tool (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Sequence alignment was performed with DNAMAN software and phylogenetic analysis conducted with MEGA4.036,37. The molecular weight and pI were calculated using the Compute pI/Mw (http://us.expasy.org/tools/pi_tool.html). Phosphorylation sites were determined using the NetPhos 2.0 Server (http://www.cbs.dtu.dk/services/NetPhos/) and protein domains or motifs identified using SMART software (http://smart.embl-heidelberg.de).
RNAi knockdown of Csp38, CsJNK, and CsERK1/2
The partial Csp38, CsJNK, and CsERK1/2 sequences were cloned into pET-2P vectors with flanking T7 promoter and T7 terminator sites to produce Csp38, CsJNK, and CsERK1/2 dsRNA38. The plasmid pET-2P/EGFP was used to produce EGFP dsRNA for the control group38. Briefly, dsRNA fragments were amplified from the cDNA of C. suppressalis larvae with PrimeSTAR HS DNA polymerase (TaKaRa Bio Inc., Dalian, China). PCR products were digested with restriction enzymes (see Supplementary Table S1) and ligated into the previously digested pET-2P. The correct inserts of the recombinant plasmid were confirmed by sequencing by the Genscript Biology Company, Nanjing, China. Plasmids were transformed into Escherichia coli HT115 (DE3) competent cells and individual colonies were cultured at 37 °C in 500 ml of LB medium and induced to generate dsRNA with 0.4 mM isopropyl-D-thiogalactoside (IPTG). dsRNA was extracted from aliquots of bacteria with a recently described protocol39,40.
All subsequent experiments were conducted at 27 °C. To silence RNA, newly hatched larvae were fed a diet comprised of 30 μg of Cs-target genes dsRNA, or EGFP dsRNA as a control, to each gram of their usual artificial food. Larvae were allowed to feed on this diet for 48 h, then transferred to wells in a 6-well plate where they were fed a diet containing 30 μg FDP toxin to each gram of their usual artificial food (the approximate LC25 determined in a pilot study) for 7 days. The control was the usual artificial food mixed with water. Five replicates were carried out using a total of 120 larvae per treatment. The toxicity to larvae of transgenic rice expressing Cry1Ca toxin was also determined41. Newly hatched larvae were fed non-transgenic rice (Minghui 63) smeared with purified dsRNA on the surface of rice plants for 48 h, then transferred to feed on transgenic Cry1Ca rice plants for 7 days. Each treatment was replicated 5 times as described above.
Real-time quantitative PCR
To examine the effect of Cry toxin on MAPK activation, ten larvae midguts from each treatment were sampled after 30 min, 1 h, and 48 h of FDP (20 μg/g, 60 μg/g) challenge. RNAi knockdown of Cs-p38, -JNK, -ERK1 and Cs-ERK2 was conducted using qRT-PCR with three replicates for each treatment. Total RNA was extracted from larvae and cDNA synthesized using a PrimeScript RT reagent kit with gDNA eraser (perfect real time) (Takara, Dalian, China) according to the manufacturer’s instructions. Gene-specific primers (Supplementary Table S1) were designed by NCBI profile Server (http://www.ncbi.nlm.nih.gov/tools/primer-blast) for qRT-PCR. The C. suppressalis elongation factor-1 (EF-1) gene was used as the internal reference42,43. The standard qPCR protocol was as follows: denaturing at 95 °C for 30 sec, followed by 40 cycles of 95 °C for 10 sec and 59 °C for 30 sec. Real-time quantitative PCR was performed in triplicate for each sample using the SYBR® Premix Ex Taq™ (TaKaRa, Dalian, China) and a Bio-rad Detection iQ2 System. Melting curve analysis was performed from 55 °C to 95 °C to determine the specificity of qPCR primers. To determine the efficiency of the qRT-PCR primers, a 5-fold dilution series of 3rd instar larvae cDNA corresponding to one microgram total RNA was used to produce a standard curve (cDNA concentration vs. Ct) with efficiencies calculated from the slope using linear regression. The corresponding qRT-PCR efficiencies were calculated according to the equation: E = (10[−1/slope] − 1)*10044,45 (Supplementary Table S3).
Data analysis
Gene expression data were analyzed with the 2−ΔΔCt method44. Means and variances of treatments were analyzed with a one-way ANOVA implemented in the SPSS program for windows (SPSS, Chicago, IL, USA).
Additional Information
How to cite this article: Qiu, L. et al. Knockdown of the MAPK p38 pathway increases the susceptibility of Chilo suppressalis larvae to Bacillus thuringiensis Cry1Ca toxin. Sci. Rep. 7, 43964; doi: 10.1038/srep43964 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was funded by grants from the Special Fund for Agro-scientific Research in the Public Interest of China (201303017) and the National Natural Science Foundation of China (grant no. 31201510).
Footnotes
The authors declare no competing financial interests.
Author Contributions L.Q., J.F., L.L. and B.Z. performed the experiments. X.W., C.L., Y.L. and W.M. conceived and designed the experiments. L.Q. and W.M. analyzed the data and wrote the manuscript.
References
- Alouf J. E. Molecular features of the cytolytic pore-forming bacterial protein toxins. Folia Microbiol (Praha) 48, 5–16 (2003). [DOI] [PubMed] [Google Scholar]
- Huffman D. L., Bischof L. J., Griffitts J. S. & Aroian R. V. Pore worms: Using Caenorhabditis elegans to study how bacterial toxins interact with their target host. Int J Med Microbiol 293, 599–607 (2004). [DOI] [PubMed] [Google Scholar]
- Gonzalez M. R., Bischofberger M., Pernot L., van der Goot F. G. & Freche B. Bacterial pore-forming toxins: The (w)hole story? Cell Mol Life Sci 65, 493–507 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Derbyshire D. J., Promdonkoy B. & Ellar D. J. Structural implications for the transformation of the Bacillus thuringiensis delta-endotoxins from water-soluble to membrane-inserted forms. Biochem Soc Trans 29, 571–577 (2001). [DOI] [PubMed] [Google Scholar]
- Wellmer A. et al. Decreased virulence of a pneumolysin-deficient strain of Streptococcus pneumoniae in murine meningitis. Infect Immun 70, 6504–6508 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeva A. et al. Binding of Escherichia coli hemolysin and activation of the target cells is not receptor-dependent. J Biol Chem 280, 36657–36663 (2005). [DOI] [PubMed] [Google Scholar]
- Valeva A. et al. Evidence that clustered phosphocholine head groups serve as sites for binding and assembly of an oligomeric protein pore. J Biol Chem 281, 26014–26021 (2006). [DOI] [PubMed] [Google Scholar]
- Bravo A., Likitvivatanavong S., Gill S. S. & Soberon M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem Mol Biol 41, 423–431 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras E., Rausell C. & Real M. D. Proteome response of Tribolium castaneum larvae to Bacillus thuringiensis toxin producing strains. PloS one 8, e55330 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino-Rodezno A. et al. Comparative proteomic analysis of Aedes aegypti larval midgut after intoxication with Cry11Aa toxin from Bacillus thuringiensis. PloS one 7, e37034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppert B. et al. Transcriptome profiling of the intoxication response of Tenebrio molitor larvae to Bacillus thuringiensis Cry3Aa protoxin. PloS one 7, e34624 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel Y., Jakubowska A. K., Costa J., Herrero S. & Escriche B. Comprehensive analysis of gene expression profiles of the beet armyworm Spodoptera exigua larvae challenged with Bacillus thuringiensis Vip3Aa toxin. PloS one 8, e81927 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks M. E., Blackburn M. B., Kuhar D. & Gundersen-Rindal D. E. Transcriptome of the Lymantria dispar (gypsy moth) larval midgut in response to infection by Bacillus thuringiensis. PloS one 8, e61190 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton P. E., Cancino-Rodezno A., Gill S. S., Soberon M. & Bravo A. Transcriptional cellular responses in midgut tissue of Aedes aegypti larvae following intoxication with Cry11Aa toxin from Bacillus thuringiensis. BMC genomics 16, 1042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F. et al. Transcriptional profiling analysis of Spodoptera litura larvae challenged with Vip3Aa toxin and possible involvement of trypsin in the toxin activation. Sci Rep 6, 23861 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino-Rodezno A. et al. The mitogen-activated protein kinase p38 is involved in insect defense against Cry toxins from Bacillus thuringiensis. Insect Biochem Mol Biol 40, 58–63 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya-Perez L. P., Cancino-Rodezno A., Flores-Escobar B., Soberon M. & Bravo A. Role of UPR pathway in defense response of Aedes aegypti against Cry11Aa toxin from Bacillus thuringiensis. Int J Mol Sci 14, 8467–8478 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras E., Rausell C. & Real M. D. Tribolium castaneum Apolipophorin-III acts as an immune response protein against Bacillus thuringiensis Cry3Ba toxic activity. J Invertebr Pathol 113, 209–213 (2013). [DOI] [PubMed] [Google Scholar]
- Contreras E., Benito-Jardon M., Lopez-Galiano M. J., Real M. D. & Rausell C. Tribolium castaneum immune defense genes are differentially expressed in response to Bacillus thuringiensis toxins sharing common receptor molecules and exhibiting disparate toxicity. Dev Comp Immunol 50, 139–145 (2015). [DOI] [PubMed] [Google Scholar]
- Yao J., Buschman L. L., Lu N., Khajuria C. & Zhu K. Y. Changes in gene expression in the larval gut of Ostrinia nubilalis in response to Bacillus thuringiensis Cry1Ab protoxin ingestion. Toxins 6, 1274–1294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalinski R., Laporte F., Despres L. & Tetreau G. Alkaline phosphatases are involved in the response of Aedes aegypti larvae to intoxication with Bacillus thuringiensis subsp. israelensis Cry toxins. Environ Microbiol 18, 1022–1036 (2016). [DOI] [PubMed] [Google Scholar]
- Goto M., Li Y.-P. & Honma T. Changes of diapause and cold hardiness in the Shonai ecotype larvae of the rice stem borer, Chilo suppressalis Walker (Lepidoptera: Pyralidae) during overwintering. Appl Entomol Zool 36, 323–328 (2001). [Google Scholar]
- Zhu Z. R. et al. Integrated management of rice stem borers in the Yangtze Delta, China. (Springer, Po Box 17, 3300 Aa Dordrecht, Netherlands, 2007). [Google Scholar]
- Hou M. L., Han Y. Q. & Lin W. Influence of soil moisture on supercooling capacity and associated physiological parameters of overwintering larvae of rice stem borer. Entomol Sci 12, 155–161 (2009). [Google Scholar]
- Xu S. et al. Relationships between body weight of overwintering larvae and supercooling capacity; diapause intensity and post-diapause reproductive potential in Chilo suppressalis Walker. J Insect Physiol 57, 653–659 (2011). [DOI] [PubMed] [Google Scholar]
- Jiao Y., Yang Y., Meissle M., Peng Y. & Li Y. Comparison of susceptibility of Chilo suppressalis and Bombyx mori to five Bacillus thuringiensis proteins. J Invertebr Pathol 136, 95–99 (2016). [DOI] [PubMed] [Google Scholar]
- Wang Y. N. et al. Comparison of three transgenic Bt rice lines for insecticidal protein expression and resistance against a target pest, Chilo suppressalis (Lepidoptera: Crambidae). Insect Sci(2014). [DOI] [PubMed] [Google Scholar]
- Zhang W. & Liu H. T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12, 9–18 (2002). [DOI] [PubMed] [Google Scholar]
- Roux P. P. & Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68, 320–344 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G. H., Shi L. Z. & Chi H. B. Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine 48, 161–169 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. L. & Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912 (2002). [DOI] [PubMed] [Google Scholar]
- Ratner A. J. et al. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J Biol Chem 281, 12994–12998 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloft N. et al. Pore-forming toxins activate MAPK p38 by causing loss of cellular potassium. Biochem Biophys Res Commun 385, 503–506 (2009). [DOI] [PubMed] [Google Scholar]
- Han L., Li S., Liu P., Peng Y. & Hou M. New artificial diet for continuous rearing of Chilo suppressalis (Lepidoptera: Crambidae). Ann Entomol Soc Am 105, 253–258 (2012). [Google Scholar]
- Ma W. et al. Exploring the midgut transcriptome and brush border membrane vesicle proteome of the rice stem borer, Chilo suppressalis (Walker). PloS one 7, e38151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N. & Nei M. The neighbor-joining method: a new method for recons tructing phylogenetic trees. Mol Biol Evol 4, 406–425 (1987). [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M. & Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24, 1596–1599 (2007). [DOI] [PubMed] [Google Scholar]
- Qiu L. et al. Cadherin is involved in the action of Bacillus thuringiensis toxins Cry1Ac and Cry2Aa in the beet armyworm, Spodoptera exigua. J Invertebr Pathol 127, 47–53 (2015). [DOI] [PubMed] [Google Scholar]
- Timmons L., Court D. L. & Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103–112 (2001). [DOI] [PubMed] [Google Scholar]
- Dong X., Li Q. & Zhang H. The noa gene is functionally linked to the activation of the Toll/Imd signaling pathways in Bactrocera dorsalis (Hendel). Dev Comp Immunol 55, 233–240 (2016). [DOI] [PubMed] [Google Scholar]
- Tang W. et al. Development of insect-resistant transgenic indica rice with a synthetic cry1C* gene. Mol Breeding 18, 1–10 (2006). [Google Scholar]
- Hui X. M. et al. RNA interference of ace1 and ace2 in Chilo suppressalis reveals their different contributions to motor ability and larval growth. Insect Mol Biol 20, 507–518 (2011). [DOI] [PubMed] [Google Scholar]
- Zhu J., Dong Y. C., Li P. & Niu C. Y. The effect of silencing 20E biosynthesis relative genes by feeding bacterially expressed dsRNA on the larval development of Chilo suppressalis. Sci rep 6, 28697 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29, e45 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonić A. et al. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 313, 856–862 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.