Abstract
South Africa aims to eliminate malaria within its borders by 2018. Despite well-coordinated provincial vector control programmes that are based on indoor residual insecticide spraying, low-level residual malaria transmission continues in the low-altitude border regions of the north-eastern sector of the country. In order to identify the underlying causes of residual transmission, an enhanced vector surveillance system has been implemented at selected sites in the Mpumalanga and KwaZulu-Natal (KZN) provinces. The collection periods for the data presented are March 2015 to April 2016 for Mpumalanga and January 2014 to December 2015 for KZN. The mosquito collection methods used included indoor and outdoor traps based on the use of traditional ceramic pots, modified plastic buckets and exit window traps (KZN only). All Anopheles funestus species group mosquitoes collected were identified to species and all females were screened for the presence of Plasmodium falciparum sporozoites. Two An. vaneedeni females, one from each surveillance site, tested positive for P. falciparum sporozoites. These are the first records of natural populations of An. vaneedeni being infective with P. falciparum. As both specimens were collected from outdoor-placed ceramic pots, these data show that An. vaneedeni likely contributes to residual malaria transmission in South Africa.
Malaria is a parasitic disease caused by Plasmodium protozoa and transmitted by female Anopheles mosquitoes (Diptera: Culicidae)1. The populations most at risk live in sub-Saharan Africa which accounts for 80% of cases and 90% of total deaths2.
Malaria transmission in South Africa is limited to the low-altitude northern and north-eastern border regions of the country which span the Limpopo, Mpumalanga and KwaZulu-Natal (KZN) provinces3. Historically, only the major malaria vector Anopheles funestus was directly implicated in malaria transmission in South Africa4. In addition, the malaria vectors An. arabiensis and An. merus were provisionally implicated based on their occurrence in South Africa’s malaria affected regions and because they have been directly implicated in transmission in neighbouring southern Mozambique5,6,7,8. Recently, several An. arabiensis and one An. merus specimen collected outdoors during 2014–2016 were also found to be infected with P. falciparum sporozoites in KwaZulu-Natal (Dandalo et al., submitted), thus expanding the number of species directly implicated in malaria transmission within South Africa.
Malaria vector control in South Africa’s malaria affected provinces is primarily based on indoor spraying of long-lasting residual insecticides8. The indoor residual spraying (IRS) method has been the mainstay of malaria vector control in South Africa since the 1940s and has remained effective owing to carefully co-ordinated provincial IRS programmes9. Despite this, low-level residual malaria transmission continues and is likely caused by outdoor feeding and resting Anopheles vector mosquitoes that are unaffected by indoor applications of insecticide10. Maintaining effective control whilst scaling up control methods to address ongoing residual malaria transmission within South Africa are high priority activities because the country has adopted a malaria elimination agenda and aims to eliminate malaria within its borders by 201811.
Continuing residual malaria transmission and the burgeoning incidence of insecticide resistance in malaria vector populations within South Africa’s borders4,12 have necessitated an intensification of vector surveillance activities in the affected provinces. The principle objectives of these enhanced surveillance activities are to compare and establish optimal methods of collecting adult Anopheles mosquitoes, to establish which Anopheles species are responsible for ongoing residual malaria transmission, to assess the extent of residual malaria transmission within South Africa and to assess the range and geographical extent of insecticide resistance in incriminated vector populations. Within these broad objectives, the aim of this project was to assess whether An. vaneedeni, a member of the An. funestus species group13, contributes to residual malaria transmission in South Africa.
Results
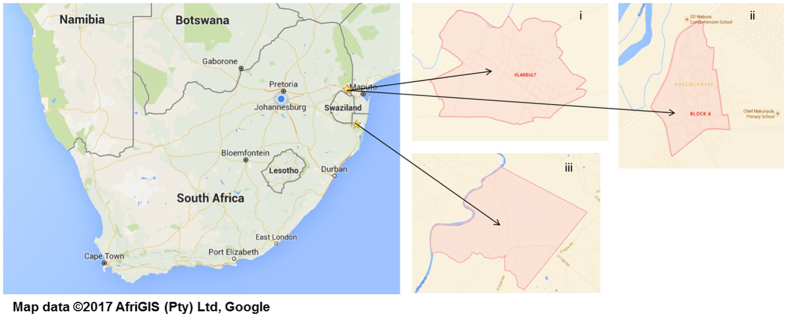
Anopheles vector surveillance for indoor and outdoor-resting mosquitoes was conducted in two villages in Mpumalanga Province (Tonga - Block A and Vlakbult) (S25°42′03″; E31°48′31″ and S25°38′42″; E31°42′01″) for one year (March 2015–April 2016) and in Mamfene, KwaZulu-Natal (KZN) Province (S27°23′50.5″; E032°12′20.1″) for two years (January 2014–December 2015) (Fig. 1).
Figure 1.
Anopheles mosquito surveillance sites at Vlakbult (i) and Block A (ii) (Ehlanzeni District of Mpumalanga) and Mamfene (iii) (KwaZulu-Natal) South Africa. Map source data were obtained from Map data (c) 2016 AfriGIS (Pty) Ltd, Google (https://www.google.co.za/maps/place/South+Africa/).
A total of 255 and 77 An. funestus group specimens was collected from the KZN and Mpumalanga sites respectively (Table 1). Of these, 45 of the adult females collected from Block A and Vlakbult (Mpumalanga) and 51 of the adult females collected from Mamfene (KZN) were positively identified as An. vaneedeni, first by morphology10 to An. funestus group followed by PCR14 to species (Table 1). Two of these An. vaneedeni specimens, one from each province, tested positive for the presence of Plasmodium falciparum sporozoites through two ELISA assays. These data give P. falciparum infectivity rates for An. vaneedeni of 2.44% and 1.96% for the Mpumalanga and KZN sites respectively.
Table 1. Distribution of Anopheles funestus group collected by species and gender from the Mpumalanga (Vlakbult and Block A: March 2015–April 2016) and KwaZulu-Natal (Mamfene: January 2014–December 2015) Anopheles mosquito surveillance sites, South Africa.
| Province | Site | Species | Total |
|
|---|---|---|---|---|
| Males | Females | |||
| Mpumalanga | Vlakbult | An. vaneedeni | 11 | 41 |
| An. rivulorum | 1 | 3 | ||
| An. leesoni | 0 | 2 | ||
| Block A | An. vaneedeni | 0 | 3 | |
| An. rivulorum | 0 | 16 | ||
| An. leesoni | 0 | 0 | ||
| KwaZulu-Natal | Mamfene | An. vaneedeni | 25 | 51 |
| An. rivulorum | 5 | 13 | ||
| An. leesoni | 13 | 53 | ||
| An. parensis | 34 | 61 | ||
The Anopheles species identification of these two specimens as An. vaneedeni was further confirmed by sequencing their internal transcribed spacer 2 (ITS2) region14. There was a 99% sequence identity between the ITS2 region of the KZN and Mpumalanga specimens and the published An. vaneedeni sequence originating from South Africa (GenBank accession number JN994152.115). The presence of P. falciparum sporozoite DNA was confirmed by nested PCR for the KZN specimen16. Sequence analysis of the nested PCR product revealed that there was a 99% identity to the published sequence for P. falciparum (GenBank accession number KT991235.117).
None of the An. leesoni, An. rivulorum and An. parensis female specimens from either field site (Table 1) showed positive for P. falciparum sporozoites based on ELISA analysis.
Discussion
The perennial occurrence of several An. funestus species group members–An. vaneedeni, An. rivulorum, An. leesoni and An. parensis–at the Mpumalanga and KwaZulu-Natal field sites warrants investigation into their possible contribution to malaria transmission in these regions, especially given that An. rivulorum has been implicated in malaria transmission in Tanzania18 and Kenya19, and a previous survey has shown that An. parensis will readily rest indoors in the Mamfene region20. The evident absence of An. funestus sensu stricto at these sites can be attributed to ongoing annual IRS based control activities, particularly the use of DDT which has effectively eradicated this species from South Africa. This is because An. funestus in South Africa is highly susceptible to DDT4,8 and has a strong tendency to rest indoors, making this species especially susceptible to IRS programmes that utilize DDT7,9.
The data summarised here represent the first record of wild-caught P. falciparum sporozoite positive An. vaneedeni females, directly implicating this species in malaria transmission in South Africa. Although this species is considered to be to be primarily zoophilic10, it will readily feed on humans outdoors and has previously been experimentally infected with P. falciparum under laboratory conditions21. The outdoor-resting and feeding traits of this species are reinforced by the fact that most of the An. vaneedeni specimens collected in these surveys, including the two that tested sporozoite positive, were found in outdoor-placed ceramic pots (Fig. 2) deployed at randomly selected households at the two sites.
Figure 2.
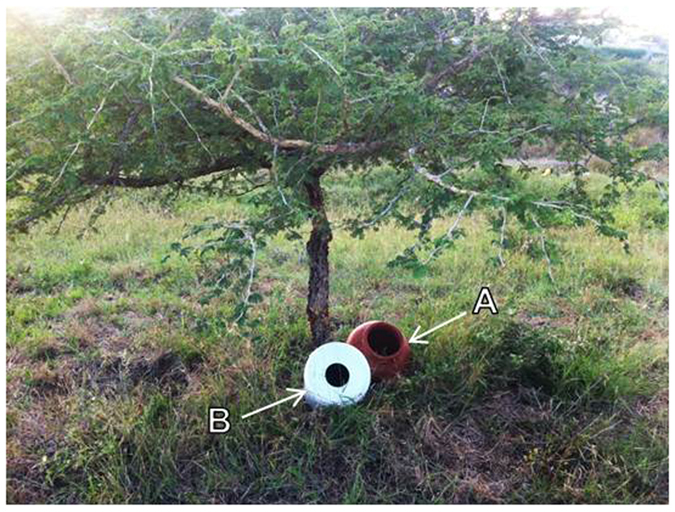
Ceramic pot (A) and modified plastic bucket (B) used for adult Anopheles mosquito surveillance, Mpumalanga and KwaZulu-Natal Provinces, South Africa.
The geographical range of An. vaneedeni primarily includes the north-eastern low-altitude regions of South Africa, and likely extends into southern and eastern Zimbabwe and southern Mozambique22. However, there are collection records of An. vaneedeni in the western highlands of Kenya23, suggesting that its range may be substantially more extensive.
The collection of sporozoite-positive, outdoor-resting An. vaneedeni supports the hypothesis of ongoing outdoor residual malaria transmission in South Africa, as first proposed by De Meillon et al. in ref. 21, and tentatively suggests that this species may also be contributing to malaria transmission in other malaria-endemic countries in which it occurs. This information highlights the need to intensify malaria vector control in South Africa by including methods designed to target outdoor feeding vector populations without compromising the efficacy of the IRS programme.
Materials and Methods
Ethical statement
Informed consent was obtained from all household owners involved in this study. Ethical clearance for the collection of mosquito specimens was obtained from the University of the Witwatersrand (M141023 & W-CJ-150520-2) and the KwaZulu-Natal Department of Health (HRKM337/14).
Mosquito collections
Adult Anopheles mosquitoes were collected using traditional ceramic pots and modified plastic buckets (Fig. 2). These were placed both inside and outside selected households in Vlakbult and Block A in Mpumalanga (March 2015–April 2016), and only outside at households in Mamfene, KZN (January 2014–December 2015). Traps were not deployed indoors at Mamfene because homeowners consent did not include this provision and because exit window traps were instead used to collect indoor resting mosquitoes at this site (Fig. 3). The traps were cleared at sunrise weekly by the malaria vector surveillance teams based near the collection sites in each province.
Figure 3. Window exit trap used for adult Anopheles mosquito surveillance, KwaZulu-Natal Province, South Africa.

Anopheles species identification and vector incrimination
All Anopheles specimens collected were preserved on silica and initially identified by external morphology using dichotomous keys10. Those identified as belonging to the An. funestus group were subsequently identified to species level by PCR14. All females were screened for the presence of Plasmodium falciparum sporozoites by ELISA24. Plasmodium falciparum infectivity, where indicated by ELISA, was confirmed with a nested Plasmodium PCR16.
Sequence analysis of mosquito ITS2 and Plasmodium falciparum ssRNA
In order to confirm Anopheles species identity, the internal transcribed spacer region 2 (ITS2) region of the rDNA of each An. vaneedeni sporozoite positive sample was amplified using the following primers: ITS2A: 5′-TGTGAACTGCAGGACA-CAT-3′; and ITS2B: 5′-TATGCTTAAATTCAGGGGGT-3′. PCR conditions were the same as those used in the An. funestus species-specific PCR14. In order to confirm Plasmodium species identity, Plasmodium ssRNA from the KwaZulu-Natal An. vaneedeni sporozoite positive sample was amplified using the following primers: rPLU5: 5′-CCTGTTGTTGCCTTAAACTTC-3′; rPLU6: 5′-TTAAAATTGTTGCAGTTAAAACG-3 for the first amplification step, and rFAL1: 5′-TTAAACTGGTTTGGGAAAACCAAATATATT-3′; and rFAL2: 5′-ACACAATGAACTCAATCATGACTACCCGTC-3′ for the second amplification step and sequencing. PCR conditions were the same as those previously described16. All amplicons were electrophoresed on 2.5% agarose gels stained with ethidium bromide and product sizes were confirmed using a molecular weight marker (Thermo Scientific O’GeneRuler 100 bp DNA Ladder; 0.1 μg/μl concentration, supplied with 1Ml 6x Orange DNA Loading Dye). ITS2 PCR products were purified and sequenced by Macrogen. The sequences were manually edited by BioEdit version 7.2.525. Subsequently, the sequences were aligned with sequences stored in GenBank using nucleotide BLAST (BLASTn) (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Additional Information
How to cite this article: Burke, A. et al. A new malaria vector mosquito in South Africa. Sci. Rep. 7, 43779; doi: 10.1038/srep43779 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
Entomology team members of the provincial Malaria Control Programmes of KwaZulu-Natal and Mpumalanga are thanked for their assistance with the collection of specimens. Mr Jabulani Zhikali, Dr. Maria Kaiser, Mrs Leanne Lobb and Mr Oliver Wood are especially thanked for their assistance with the collection of specimens in KwaZulu-Natal. Dr Riann Christian is thanked for assisting with the validation of mosquito identifications by PCR. Mr Nelius Venter is thanked for his assistance with the deployment of mosquito traps in Mpumalanga. Mr Keith Hargreaves is thanked for his assistance with the identification of participating households in Mamfene. These activities were sponsored by the National Institute for Communicable Diseases, a CDC/GDD (Global Diseases Detection programme) grant (U19GH000622-01 MAL01), the MRC South Africa, the International Atomic Energy Agency (Research Contract No. 17904; 19099 and SAF 5013/5014), the Industrial Development Corporation and the South African Nuclear Energy Corporation (NECSA) through its Nuclear Technologies in Medicine Biosciences Initiative (NTeMBI)–a national platform funded by the Department of Science and Technology.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.C., L.K., B.B., F.M., S.N. and G.M. designed the mosquito surveillance system and associated experiments. A.B., L.D., G.M., L.K., B.B. and F.M. conducted the field work. A.B. and L.D. conducted the laboratory work and initial data analysis. Y.D. co-ordinated the sequence analyses. B.B., L.K. and G.M. finalised the data analysis. A.B., L.D., Y.D. and B.B. wrote the initial drafts of the manuscript. B.B. produced the final version of the manuscript. All authors read and approved the final manuscript.
References
- Harrison G. Mosquitoes, malaria and man: a history of the hostilities since 1880. EP Dutton, New York (1978). [Google Scholar]
- World Health Organization. World Malaria Report 2015. World Health Organisation, Geneva (2015). [Google Scholar]
- Morris N. et al. Re-defining the extent of malaria transmission in South Africa: implications for chemoprophylaxis. S Afr Med J 103, 861–864 (2013). [DOI] [PubMed] [Google Scholar]
- Hargreaves K. et al. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol 14(2), 181–189 (2000). [DOI] [PubMed] [Google Scholar]
- Mendis C. et al. Anopheles arabiensis and An. funestus are equally important vectors of malaria in Matola coastal suburb of Maputo, southern Mozambique. Med Vet Entomol 14(2), 171–80 (2000). [DOI] [PubMed] [Google Scholar]
- Cuamba N. & Mendis C. The role of Anopheles merus in malaria transmission in an area of southern Mozambique. J Vector Borne Dis 46(2), 157–159 (2009). [PubMed] [Google Scholar]
- Maharaj R. et al. Epidemiology of malaria in South Africa: From control to elimination. S Afr Med J 103 (10 Suppl 2), 779–783 (2013). [DOI] [PubMed] [Google Scholar]
- Brooke B. et al. Malaria vector control in South Africa. S Afr Med J 103 (10 Suppl 2), 784–788 (2013). [DOI] [PubMed] [Google Scholar]
- Coetzee M. et al. Malaria in South Africa: 110 years of learning to control the disease. S Afr Med J 103 (10 Suppl 2), 770–778 (2013). [DOI] [PubMed] [Google Scholar]
- Gillies M. T. & Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara (Afro tropical region). South African Institute for Medical Research 55 (1987). [Google Scholar]
- South Africa National Department of Health, Malaria elimination strategy for South Africa 2012–2018, Pretoria, NDoH (2012).
- Brooke B. D. et al. Insecticide resistance in the malaria vector Anopheles arabiensis in Mamfene, KwaZulu-Natal. S Afr J Sci 111(11/12) (2015). [Google Scholar]
- Coetzee M. & Koekemoer L. L. Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu Rev Entomol 58, 393–412 (2013). [DOI] [PubMed] [Google Scholar]
- Koekemoer L. L., Kamau L., Hunt R. H. & Coetzee M. A cocktail polymerase chain reaction (PCR) assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg 66, 804–811 (2002). [DOI] [PubMed] [Google Scholar]
- Norris L. C. & Norris D. E. Phylogeny of anopheline (Diptera: Culicidae) species in southern Africa, based on nuclear and mitochondrial genes. J Vector Ecol 40, 16–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snounou G. et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Molecular and Biochemical Parasitol 61, 315–320 (1993). [DOI] [PubMed] [Google Scholar]
- Zhang C. et al. Species identification and sequence analysis of Plasmodium spp. in border areas of Yunnan Province by 18S rRNA-based nested PCR. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 34, 220–226 (2011). [PubMed] [Google Scholar]
- Wilkes T. J., Matola Y. G. & Charlwood J. D. Anopheles rivulorum, a vector of human malaria in Africa. Med Vet Entomol 10, 108–110 (1996). [DOI] [PubMed] [Google Scholar]
- Kawada H. et al. Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in western Kenya: some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasit Vectors 5, 230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouatcho J. C. et al. Indoor collections of the Anopheles funestus group (Diptera: Culicidae) in sprayed houses in northern KwaZulu-Natal, South Africa. Malar J 6, 30 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meillon B. et al. Observations on a species of the Anopheles funestus subgroup, a suspected exophilic vector of malaria parasites in Northeastern Transvaal, South Africa. Mosquito News 37(4), 657–661 (1977). [Google Scholar]
- Choi K. S., Koekemoer L. L. & Coetzee M. Population genetic structure of the major malaria vector Anopheles funestus s.s. and allied species in southern Africa. Parasit Vectors. 5, 283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweka E. J. et al. A first report of Anopheles funestus sibling species in western Kenya highlands. Acta Trop. 128(1), 158–61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz R. A. et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bulletin of the World Health Organisation 65, 39–45 (1987). [PMC free article] [PubMed] [Google Scholar]
- Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41, 95–98 (1999). [Google Scholar]