Significance
Behavior is regulated by information originating from different sensory modalities. Aggression is a universal social behavior with an important role in obtaining food, mates, territory, and social status. In this study, we demonstrate that hearing regulates aggression in Drosophila males. Further, we show that courtship and aggression songs differentially affect aggression, indicating that hearing contributes to the context-dependent regulation of aggression.
Keywords: Drosophila, hearing, aggression, behavior, sensory modalities
Abstract
Aggression is a universal social behavior important for the acquisition of food, mates, territory, and social status. Aggression in Drosophila is context-dependent and can thus be expected to involve inputs from multiple sensory modalities. Here, we use mechanical disruption and genetic approaches in Drosophila melanogaster to identify hearing as an important sensory modality in the context of intermale aggressive behavior. We demonstrate that neuronal silencing and targeted knockdown of hearing genes in the fly’s auditory organ elicit abnormal aggression. Further, we show that exposure to courtship or aggression song has opposite effects on aggression. Our data define the importance of hearing in the control of Drosophila intermale aggression and open perspectives to decipher how hearing and other sensory modalities are integrated at the neural circuit level.
Aggression is one of the most important social behaviors in nature, ensuring reproduction and survival when competing for food, territory, or mating partners (1). Aggression is a complex behavior shaped by many factors, including a complex genetic architecture, the integration of various neurotransmitter and hormone systems, and a range of environmental factors (2).
Correct integration and processing of sensory information are crucial to evoke an appropriate behavioral response. Previous studies have implicated different sensory modalities in the regulation of aggressive behavior in Drosophila melanogaster, including the olfactory, gustatory, and visual systems (3–6).
Another important sensory modality in Drosophila is hearing. Stereotypic sound patterns generated by wing vibration and their behavioral significance have been extensively studied in the context of Drosophila courtship (7–12). On the contrary, nothing is known about the impact of hearing on aggressive behavior. Furthermore, although agonistic sound pulses are known to be generated during aggressive encounters, it is unknown whether they serve as acoustic communication signals to modulate behavior (13).
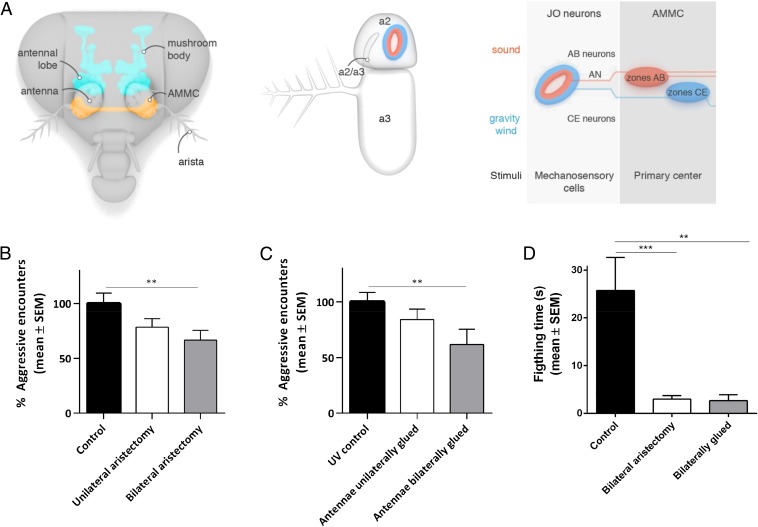
The Drosophila auditory organ, Johnston’s organ (JO), is situated in the fly’s antenna (Fig. 1A) (14–17). Antennal displacement leads to activation of ∼500 chordotonal stretch-receptor neurons in the JO, which contains AB neurons responsive to sound-evoked vibrations and CE neurons sensitive to sustained antennal deflections caused by gravity and wind (Fig. 1A) (18). The sensory neuron subclasses each innervates a particular region of the antennal mechanosensory and motor center (AMMC), the primary processing center for auditory input in the fly brain (18).
Fig. 1.
Mechanical disruption of hearing organs reduces aggressive behavior. (A) Schematic overview of the auditory system in Drosophila. (B and C) Percentage of aggressive encounters of aristectomized flies (B) and flies with glued antennal segments (preventing relative movements of the second and third segments) (100% corresponds to an average of 24.8 aggressive encounters in 2 min) (C) in groups of eight familiar males compared with intact controls. Bar graphs are presented as means ± SEM; n = 20 replicates with eight males. One-way analysis of variance (ANOVA) with post hoc Dunn’s multiple comparisons tests (100% corresponds to an average of 19.9 aggressive encounters in 2 min). (D) Time spent fighting during the first 5 min after arrival of both males on the food pad between two familiar intact males (control), bilaterally aristectomized males, or males with glued antennal segments in the presence of a decapitated virgin female. Bar graphs are presented as means ± SEM; n = 10. Kruskal–Wallis test with post hoc Dunn’s multiple comparisons tests; **P < 0.01, ***P < 0.001.
In this study, we use mechanical disruption and genetic approaches in D. melanogaster to identify hearing as an important sensory modality in the context of intermale aggressive behavior. We show that neuronal silencing and targeted knockdown of hearing genes in the fly’s auditory organ induce abnormal aggression. Further, we show that exposure to courtship or aggression song has opposite effects on aggression. Our data provide evidence on the role of hearing in the modulation of Drosophila intermale aggression and open perspectives to decipher how hearing and other sensory modalities are integrated at the neural circuit level.
Results
Mechanical Disruption of Hearing Modulates Aggression.
The Drosophila auditory organ, Johnston’s organ, is situated in the fly’s antenna (Fig. 1A) (14–17). We analyzed the effects of mechanical disruption of the antennal sound receiver on aggressive behavior in two different ways. First, we removed the arista, an essential part of the fly’s antennal ear that vibrates in response to acoustic stimulation. Bilaterally removing aristae while leaving second and third antennal segments intact resulted in a significant reduction in aggression levels in groups of eight familiar males by ∼30% compared with controls (Fig. 1B, Fig. S1A, and Movies S1 and S2). Unilateral removal also reduced aggression, albeit not at a statistically significant level (Fig. 1B). Hence, flies with only one intact hearing organ show slightly reduced aggression levels, whereas complete loss of hearing decreases aggression significantly.
Fig. S1.
Setup of aggression assays. (A) Eight-male assay in the presence of a drop of food. (B) Two-male assay in the presence of a decapitated virgin female on a food pad.
Second, in a complementary approach, we glued the distal antennal segment, which bears the arista, to the second antennal segment. This prevents the relative movement between these parts, which is required for sound-stimulated auditory receptor cell activation. The second antennal segment harbors the JO, which picks up and transduces sound-induced vibrations from the third antennal segment. Immobilizing the third antennal segment bilaterally reduced aggressive encounters by ∼40% compared with controls (Fig. 1C). Restricting antennal movement unilaterally again led to reduced aggression, albeit not significant (Fig. 1C).
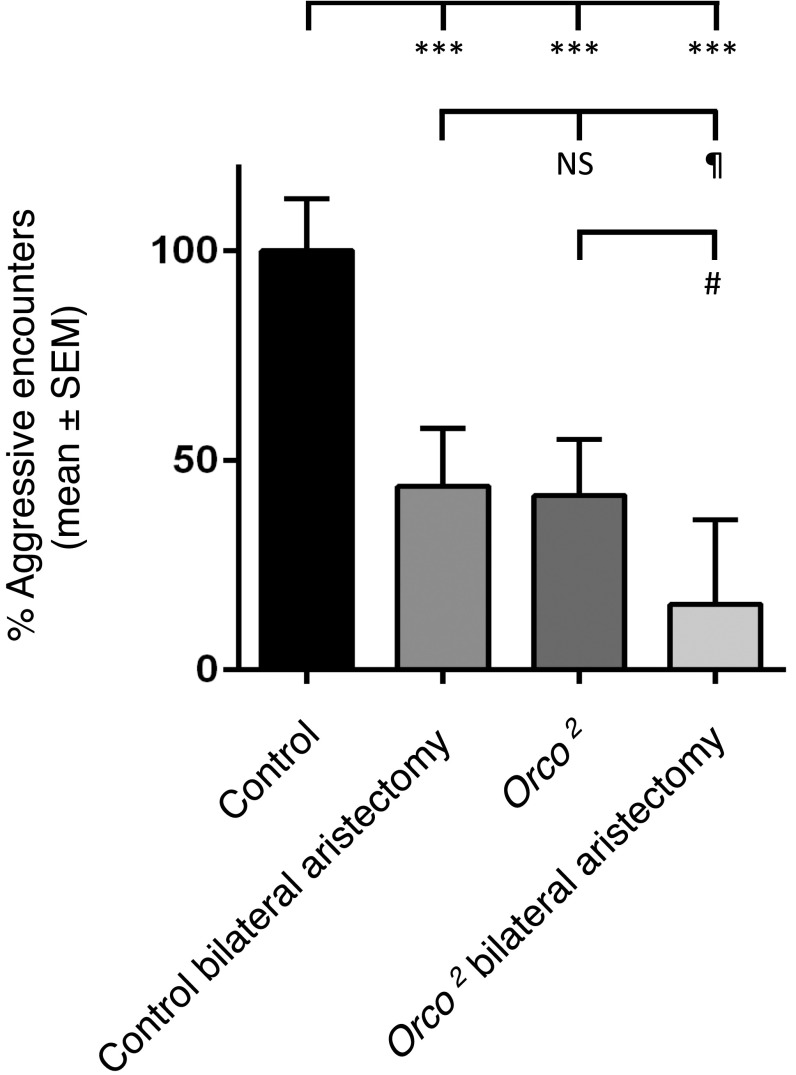
Our observation that aristectomy or antennal gluing reduces, but does not abolish, aggression seems expected given that olfactory and, to a lesser extent, gustatory and visual stimuli have been reported to modulate aggression (3–6). To determine the relative contributions of auditory and olfactory input to aggression, we quantified aggression in smell-blind Orco (odorant receptor coreceptor) mutant flies with or without bilateral aristectomy relative to controls. We observed that whereas Orco mutant flies display a 50% reduction in aggression relative to controls, bilateral aristectomy in Orco mutants further reduces this to a residual 20% of aggressive encounters (Fig. S2). We conclude that chemosensory and acoustic inputs are the major triggers for aggression in Drosophila.
Fig. S2.
Mechanical disruption of hearing organs in Orco2 smell-blind mutant flies further reduces aggressive behavior. Percentage of aggressive encounters of aristectomized wild-type and Orco2 mutant flies in groups of eight familiar males compared with intact controls. Two-way ANOVA comparison of factors genotype (wild type and Orco2) and treatment (control and aristectomy) showed a significant effect for genotype (F1,37 = 28.936; P < 0.001) and treatment (F1,37 = 26.107; P < 0.001) without significant interaction. Post hoc analysis (Tukey) revealed that all groups were significantly lower than control (***P < 0.001); Orco2 bilateral aristectomy was significantly lower than control bilateral aristectomy and Orco2 (¶P < 0.05); and Orco2 bilateral aristectomy was significantly lower than Orco2 (#P < 0.05). Bar graphs are presented as means ± SEM; n = 20 replicates with eight males. NS, not significant.
Next, we tested the effect of bilateral aristectomy or antennal gluing on aggressive behavior using an alternative aggression assay (Fig. S1B). In this assay, two socially experienced males are transferred to an arena containing both a food patch and a decapitated virgin female. For each experiment, the males were filmed for approximately 1 h, yet because the effects were strikingly robust from the beginning, we restricted our detailed analysis of behavior to the first 5 min after both males had arrived on the food pad. This analysis confirmed the role of the arista in aggressive behavior. Both bilateral aristectomy and gluing of the distal antennal segment resulted in a significant decrease of total fighting time (Fig. 1D and Movies S3–S5).
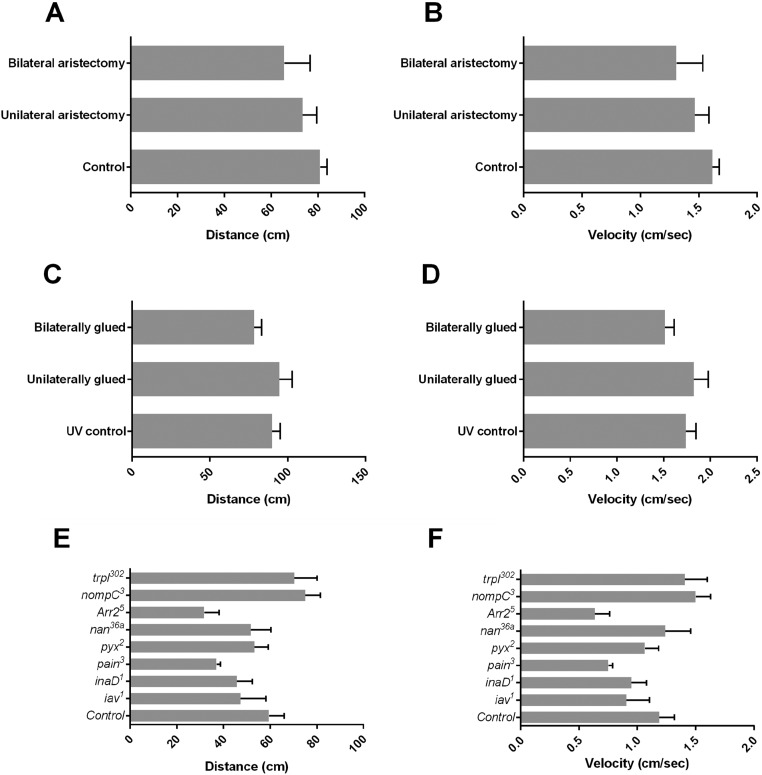
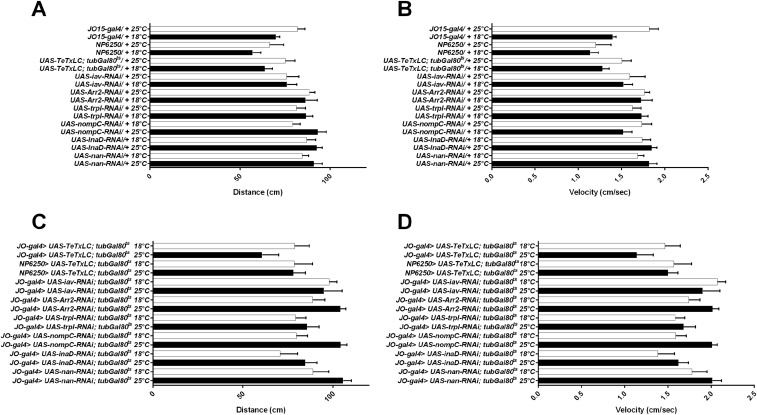
To rule out that the effects on aggression are due to a decrease in locomotor behavior, we analyzed the effects of aristectomy and antennal gluing on locomotion. We did not observe significant alterations in either walking distance or velocity (Fig. S3 A–D). We conclude that hearing is an important sense for the regulation of aggression.
Fig. S3.
Locomotor behavior upon mechanical disruption of hearing and in hearing-impaired mutants. Analyses of walking distance and walking speed of individual flies. (A and B) Walking distance (A) and walking speed (B) of aristectomized flies versus control flies. (C and D) Walking distance (C) and walking speed (D) of flies with glued antennal segments versus control flies. (E and F) Walking distance (E) and walking speed (F) of hearing-impaired mutant flies versus coisogenic control flies. Kruskal–Wallis test with post hoc Dunn’s multiple comparisons tests. Bar graphs are presented as means ± SEM; n = 10.
Functional and Genetic Disruption of the Sound-Sensitive AB Neurons Alters Aggression.
The JO contains AB neurons responsive to sound-evoked vibrations and CE neurons sensitive to sustained antennal deflections caused by gravity and wind (Fig. 1A) (18). To obtain additional support for the role of hearing in aggression and to delineate the impact of auditory input on aggression versus gravity- and wind-sensing effects, we selectively blocked neurotransmission of the AB and CE neurons in the adult brain by targeted expression of tetanus toxin light chain (TeTxLC) under the control of either JO15-Gal4 (JO-AB neurons) or NP6250-Gal4 (JO-CE neurons).
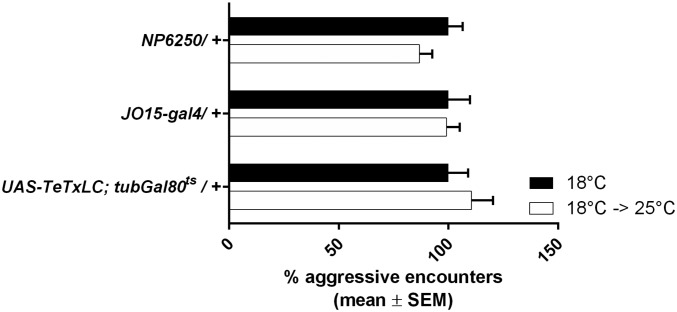
NP6250 has been shown to specifically drive expression in the CE neurons (19), and JO15 shows clear and strong expression in the JO-AB neurons. However, the latter Gal4 line has also been reported to sporadically and variably drive expression in a small number of mechanosensory neurons in the leg chordotonal organs and the mushroom bodies in the central brain (20). We confirmed strong expression in Johnston’s organ, but we did not observe any expression in the legs and only sparse expression in the brain (Fig. S4). We limited the expression of TeTxLC to the adult neurons by means of a temperature-sensitive Gal80ts allele. At its permissive temperature (18 °C), Gal80ts will repress Gal4 transcriptional activity. Switching flies to 25 °C, the nonpermissive Gal80ts temperature, will allow the expression of TeTxLC. We ruled out that the temperature shift by itself affected aggression (Fig. S5).
Fig. S4.
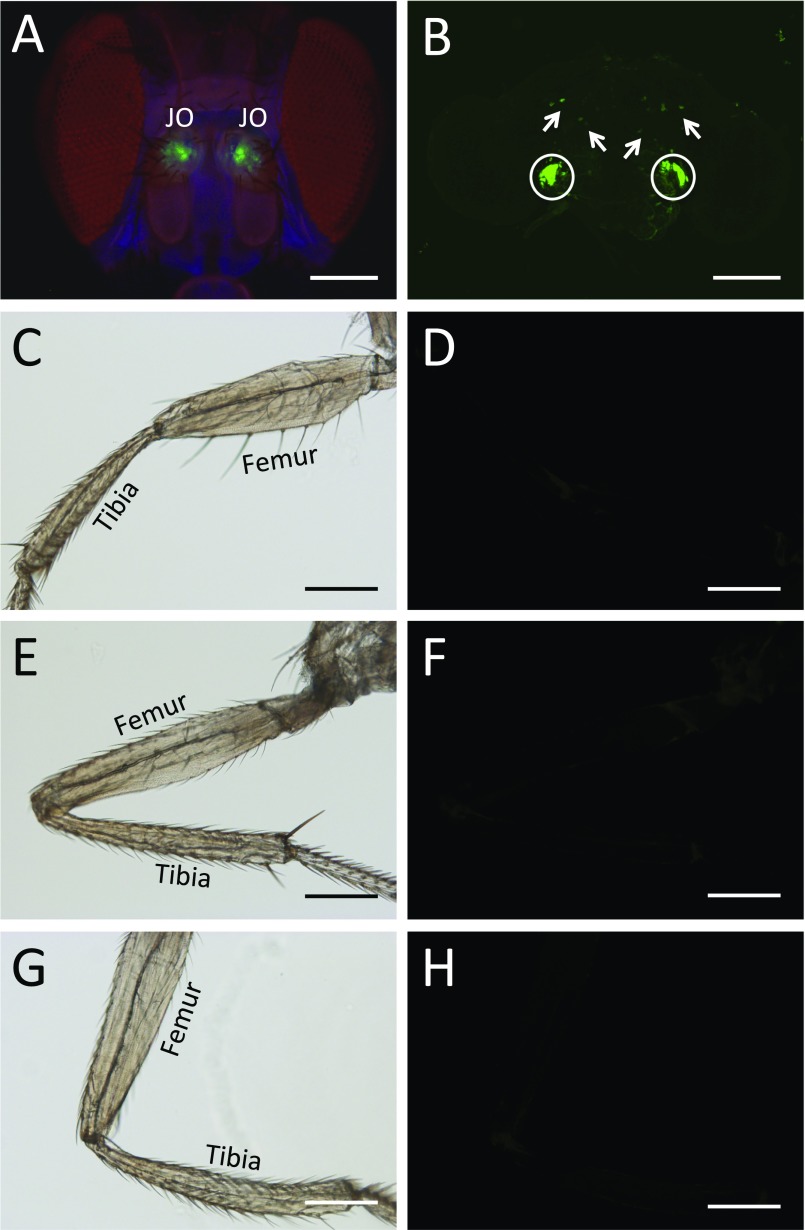
Expression pattern of JO15-Gal4. JO15-Gal4–driven expression of UAS-gfp-cd8. (A) GFP expression in Johnston’s organ in the antennae. (B) Strong GFP staining corresponding to the projections of the JO neurons to the AMMC of the brain (encircled); sparse GFP-positive cells in other regions of the central brain (arrows). (C–H) There is no GFP expression in the legs (C, E, and G, bright-field images of leg; D, F, and H, fluorescent images of leg showing no GFP expression). (C and D) Front leg. (E and F) Middle leg. (G and H) Hind leg. The femur and tibia are marked. (Scale bars, 200 μm.)
Fig. S5.
Effect of temperature switch on aggression. Percentage of aggressive encounters in groups of eight familiar males. Switching flies to 25 °C after eclosion has no effect on aggressive behavior in heterozygous NP6250, JO15-Gal4, or UAS-TeTxLC; tubGal80ts males (100% represents 12.1, 8.25, and 9.6 aggressive encounters for NP6250, JO15, and UAS-TeTxLC, respectively). Bar graphs are presented as means ± SEM; n = 20 replicates of eight males. Kruskal–Wallis with post hoc Dunn’s multiple comparisons tests.
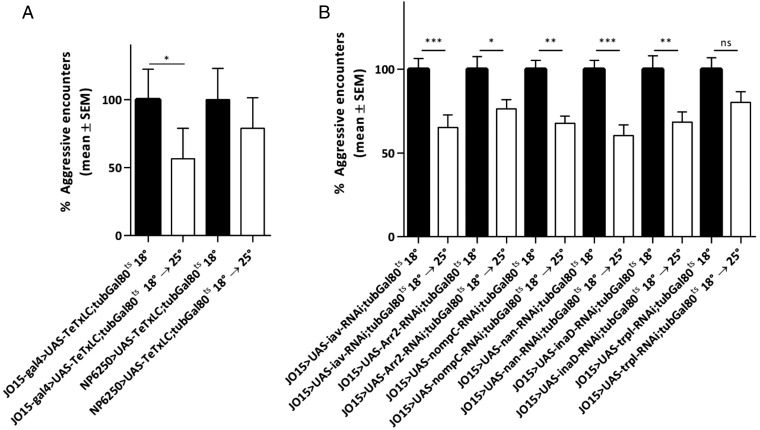
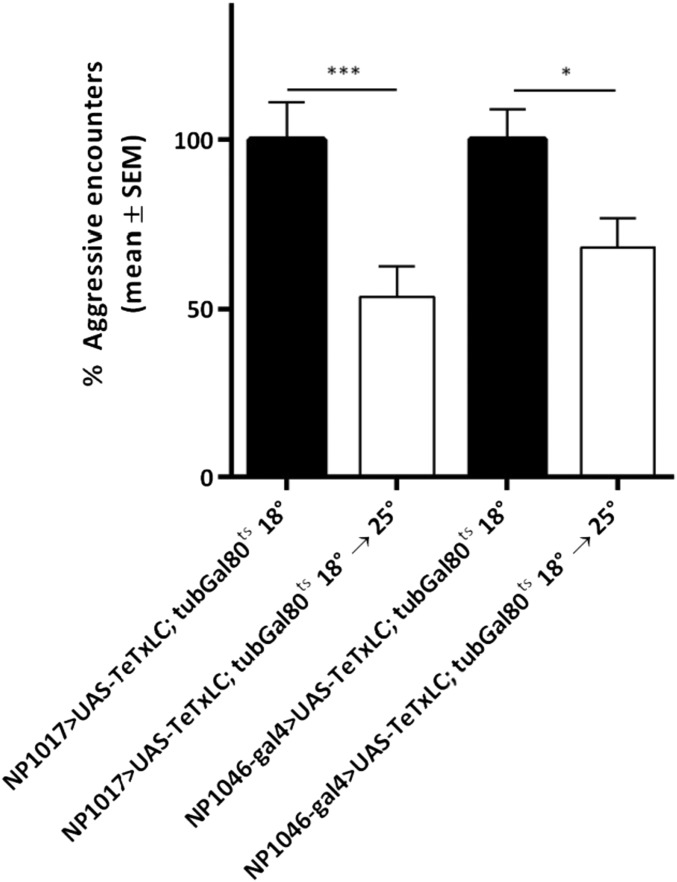
Inhibiting neurotransmitter release from the AB neurons reduced aggression levels by ∼44% (Fig. 2A). In contrast, when synaptic output from the CE neurons was blocked, no significant behavioral changes were observed (Fig. 2A). We observed a similar reduction in aggression when we blocked A (NP1017) and B (NP1046) neurons individually (Fig. S6).
Fig. 2.
Neuronal silencing and genetic disruption of Johnston’s organ results in reduced aggression. (A) Male flies expressing UAS-TeTxLC in the Johnston’s organ AB or CE neurons. Blocking neurotransmission from the hearing-specific AB neurons reduces aggression, whereas blocking the gravity-sensing CE neurons has no effect on aggression (100% represents 32.6 aggressive encounters for JO15-Gal4>UAS-TeTxLC;TubGAL80ts and 11.75 for NP6250-Gal4>>UAS-TeTxLC;TubGAL80ts). (B) RNAi-mediated knockdown in the AB neurons of the adult Johnston’s organ of the hearing genes iav, Arr2, nompC, nan, inaD, and trpl. All genotypes, except trpl, showed significantly reduced aggression. Bar graphs are presented as means ± SEM; n = 20 replicates of eight males per genotype or treatment group. Kruskal–Wallis test with post hoc Dunn’s multiple comparisons; *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant (100% represents 46.75 aggressive encounters for iav-RNAi, 38.75 for Arr2-RNAi, 47.6 for nompC-RNAi, 49.3 for nan-RNAi, 35.75 for inaD-RNAi, and 34.6 for trpl-RNAi).
Fig. S6.
A and B neurons of Johnston’s organ contribute to aggression. Male flies expressing UAS-TeTxLC in the A (NP1017) (100% represents 12.1 aggressive encounters) or B neurons (NP1046) (100% represents 10.83 aggressive encounters) of the adult Johnston’s organ show reduced aggression. Bar graphs are presented as means ± SEM; n = 20 replicates of eight males per genotype or treatment group. Kruskal–Wallis test with post hoc Dunn’s multiple comparisons; *P < 0.05, ***P < 0.001.
To rule out that the effects on aggression are due to alterations in locomotor behavior, we analyzed the effects of blocking neurotransmission in the JO neurons on locomotion. No significant effects on either walking distance or velocity were observed (Fig. S7). We conclude that blocking neurotransmission of the sound-sensitive AB neurons disrupts sound-evoked promotion of aggression in adult males.
Fig. S7.
Locomotor behavior upon functional and genetic disruption of the sound-sensitive AB neurons. Analyses of walking distance and walking speed of individual flies. (A and B) Walking distance (A) and walking speed (B) of the tested UAS and driver lines alone. Flies were reared at 18 °C versus flies switched to 25 °C after eclosion. (C and D) Walking distance (C) and walking speed (D) of flies with functional inhibition of the adult JO neurons or knockdown of a selection of hearing genes in the adult JO neurons. Compared are flies that were continuously reared at 18 °C with flies switched to 25 °C after eclosion. All flies were tested at room temperature. Kruskal–Wallis test with post hoc Dunn’s multiple comparisons tests. Bar graphs are presented as means ± SEM; n = 10.
We recently described the complex genetic architecture of Drosophila aggression and identified 1,396 genes whose transcript levels are altered in hyperaggressive Drosophila mutants (2). Surprisingly, when we compared this set of transcripts with the recently identified auditory gene set consisting of 274 genes, we found a significant overlap of 58 genes (representation factor 2.5, P < 7.661e-11; Table S1) (21). This observation further suggested that hearing might play a prominent role in Drosophila aggression.
Table S1.
Auditory genes with altered expression in hyperaggressive flies
| Gene symbol | Gene name |
| Gene transcripts down-regulated in hyperaggressive mutants | |
| a10 | antennal protein 10 |
| a5 | antennal protein 5 |
| Ank2 | Ankyrin 2 |
| boss | bride of sevenless |
| Bili | Band4.1 inhibitor LRP interactor |
| cac | cacophony |
| calx | Na/Ca-exchange protein |
| CG10362 | |
| Liprin-γ | Liprin-γ |
| CG13133 | |
| CG13924 | |
| CG14636 | |
| CG17321 | |
| CG17360 | |
| CG17378 | |
| AOX4 | Aldehyde oxidase 4 |
| CG3009 | |
| CG33203 | |
| CG3618 | |
| sigmar | salivary glands marred |
| CG4168 | |
| CG4629 | |
| CG4660 | |
| CG5687 | |
| CG6912 | |
| CG7971 | |
| CG7990 | |
| CG9279 | |
| CarT | Carcinine transporter |
| CG9150 | |
| Dhc93AB | dynein-related heavy chain at 93AB |
| dlg1 | Discslarge 1 |
| dpr19 | defective proboscis extension response 19 |
| dpr5 | defective proboscis extension response 5 |
| Eaat2 | excitatory amino acid transporter 2 |
| Dyb | Dystrobrevin |
| eya | eyes absent |
| fry | furry |
| gl | glass |
| hoe2 | hoepel2 |
| kek4 | kekkon4 |
| Klp68D | Kinesin-like protein at 68D |
| l(1)G0196 | lethal (1) G0196 |
| lectin28C//ninaE | Lectin28C /// rhodopsin |
| ninaE | neither inactivation nor afterpotential E |
| Obp59a | Odorant-binding protein 59a |
| onecut | onecut |
| Os‐C (CG3250) | Antennal-specific protein Os-C |
| Pbprp4 | Pheromone-binding protein-related protein 4 |
| Ptpmeg | Protein tyrosine phosphatase Meg |
| rdgC | retinal degeneration C |
| se | sepia |
| stops | slow termination of phototransduction |
| Sulf1 | Sulfated |
| Trl | Trithorax-like |
| Gene transcripts up-regulated in hyperaggressive mutants | |
| Arr2 | Arrestin 2 |
| B52 | B52 |
| CG8369 | |
| Pdh | Photoreceptor dehydrogenase |
| rtp | retinophilin |
From the overlapping genes, we selected a cohort of signal transduction genes with reported hearing defects for further analyses: the transient receptor potential (TRP) channel genes nan (nanchung), iav (inactive), and trpl (transient receptor potential-like) and the Ca2+ signaling-related genes Arr2 (arrestin 2) and inaD (inactivation no afterpotential D) (21). We also added the TRPN gene nompC (no mechanoreceptor potential C), which was identified in neither dataset but is crucial for Drosophila auditory receptor function (22). Flies with mutations in these genes, caused by point mutations or P-element transposons, all displayed abnormal receiver displacement and altered sound-evoked compound action potentials recorded from the antennal nerve (21).
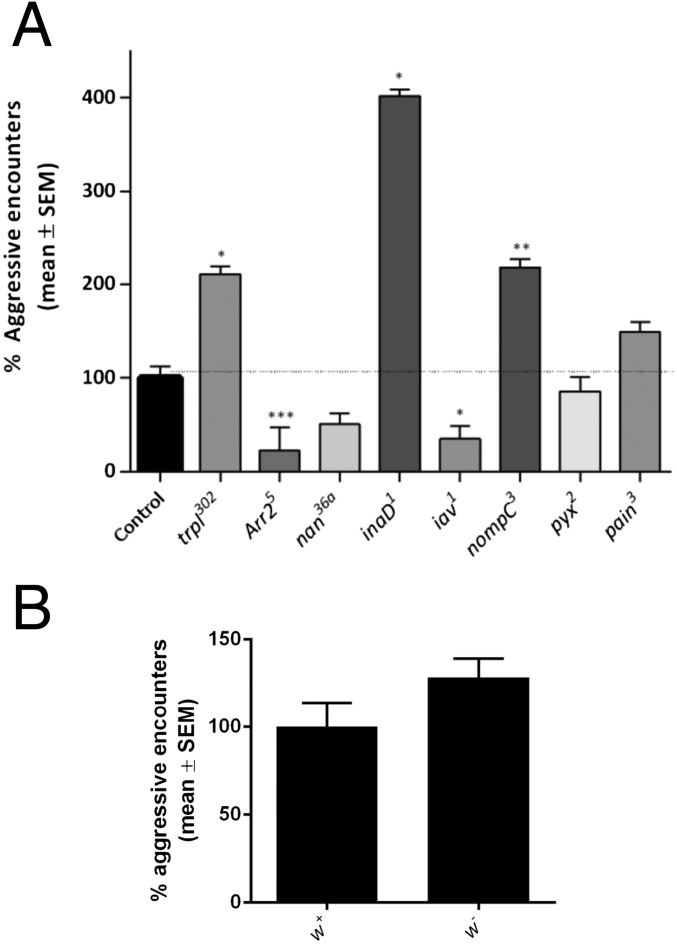
In our eight-fly behavioral paradigm, flies with these mutations showed abnormal aggressive behavior compared with the coisogenic control, with the exception of the tested nan36a mutant (Fig. S8). Although the effects of these mutations on aggressive behavior vary considerably, the results encouraged us to further investigate the specific role of hearing in the modulation of aggression in Drosophila.
Fig. S8.
Hearing-impaired mutants show aberrant behavior. (A) Percentage of aggressive encounters of lines with gene mutations that cause particular alterations of Johnston’s organ function (abnormal receiver displacement and altered sound-evoked compound action potentials). Mutant lines were compared with the coisogenic control. Kruskal–Wallis test with post hoc Dunn’s multiple comparisons; *P < 0.05, **P < 0.01, ***P < 0.001 (100% represents an average of 15.48 aggressive encounters in 2 min). (B) The tested alleles and their isogenic control contained a w1118 allele. Different reports show variable and contradictory effects of this mutation on aggression (3, 45–47). Although this mutation was present in both the control and mutant lines and should thus not be able to explain the observed variations in aggression, we ruled out possible effects of the w1118 mutation by comparing the aggression levels of coisogenic w1118 and w+ males. We could not observe significant differences in aggression between the two genotypes in our assay. Student’s t test (100% represents an average of 10.9 aggressive encounters in 2 min). Bar graphs are presented as means ± SEM; n = 20 replicates with eight males.
We also included mutant alleles for the TRPA genes pyrexia and painless, which were shown to display disrupted gravity sensing but normal auditory responses (23). We observed no behavioral changes for the pyx2 and pain3 mutant lines compared with the isogenic control, confirming that gravity sensing by the JO does not specifically contribute to aggressive behavior (Fig. S8).
To rule out that the effects on aggression are due to alterations in locomotor behavior, we analyzed the effects of these mutant alleles on locomotion and found no significant effects on either walking distance or velocity (Fig. S3 E and F).
Many of the analyzed genes have been shown to mediate pleiotropic functions that might confound the effects on aggression (24–29). Therefore, to specifically analyze the roles of these genes in the adult Johnston’s organ, we made use of RNAi-mediated knockdown (Fig. 2B). When expressing RNAi in the AB neurons of the adult Johnston’s organ targeted against the signal transduction genes iav, nompC, Arr2, inaD, nan, and trpl, we observed reduced aggression levels for all genes except trpl.
To rule out that the effects on aggression are due to alterations in locomotor behavior, we analyzed the effects of knockdown of these genes in Johnston’s organ on locomotion. We observed no significant effects on either walking distance or velocity (Fig. S7).
We conclude that genetically impairing auditory transduction in these cells disrupts the sound-evoked promotion of aggression in adult males. Combined with the mechanical disruption and the blocked neurotransmission data, our results demonstrate that hearing is an important regulatory modality in Drosophila intermale aggressive behavior.
Agonistic Sound Promotes Aggressive Behavior.
Drosophila males generate sound pulses during agonistic behavior by flicking both wings in a stereotyped manner (13). However, it is unknown whether aggression songs serve as acoustic communication signals between Drosophila males to modulate their subsequent behavior.
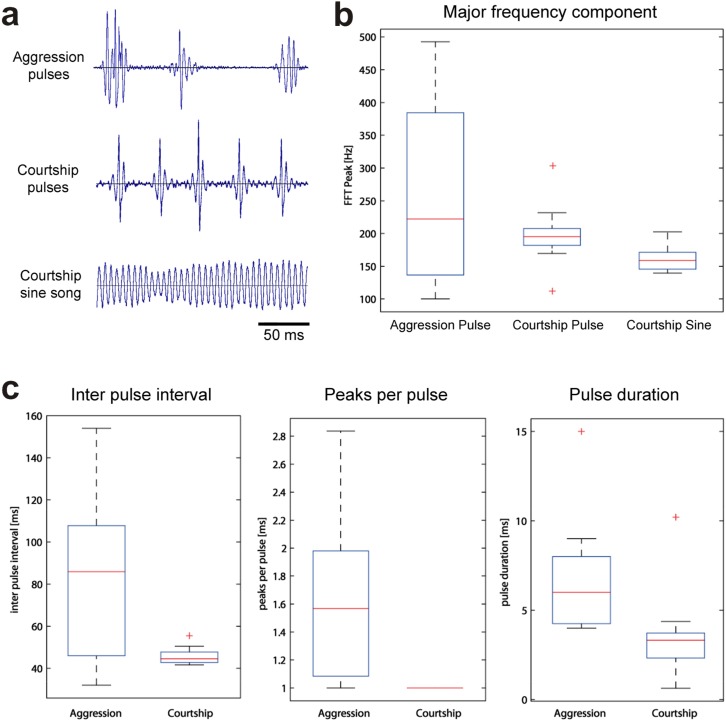
We first analyzed in detail the pulse shapes in aggression songs, compared these to the pulse shapes in courtship songs, and then made quantitative comparisons of song traits (Fig. S9). Courtship sound consists of sine song and pulse song with very regular interpulse intervals. Aggression sound differs from courtship sound in that it does not contain sine song but instead only consists of pulse song, which is also distinct with much larger interpulse intervals, a higher number of peaks per pulse, and longer pulse duration. The distinct characteristics of both types of sound allow Drosophila males to differentiate both signals.
Fig. S9.
Comparison of aggression and courtship songs of male Canton-S D. melanogaster. (A) Oscillograms of aggression pulses, courtship pulses, and part of a sine-song sequence. (B) Major frequency components of aggression song, courtship pulse song, and courtship sine song. FFT Peak, Fast Fourier Transform Peak. (C) Comparison of interpulse intervals, numbers of peaks per individual pulse, and pulse duration between aggression songs and courtship pulse songs. Data from aggression songs derive from 383 pulses from 11 different males. Data from courtship songs derive from 3,713 pulses and 107 sine sequences from 13 different males. The boxes represent the interquartile range from the 25th to the 75th percentile, the lines in the boxes show the median, the whiskers indicate maximum and minimum values, and the crosses are extreme values.
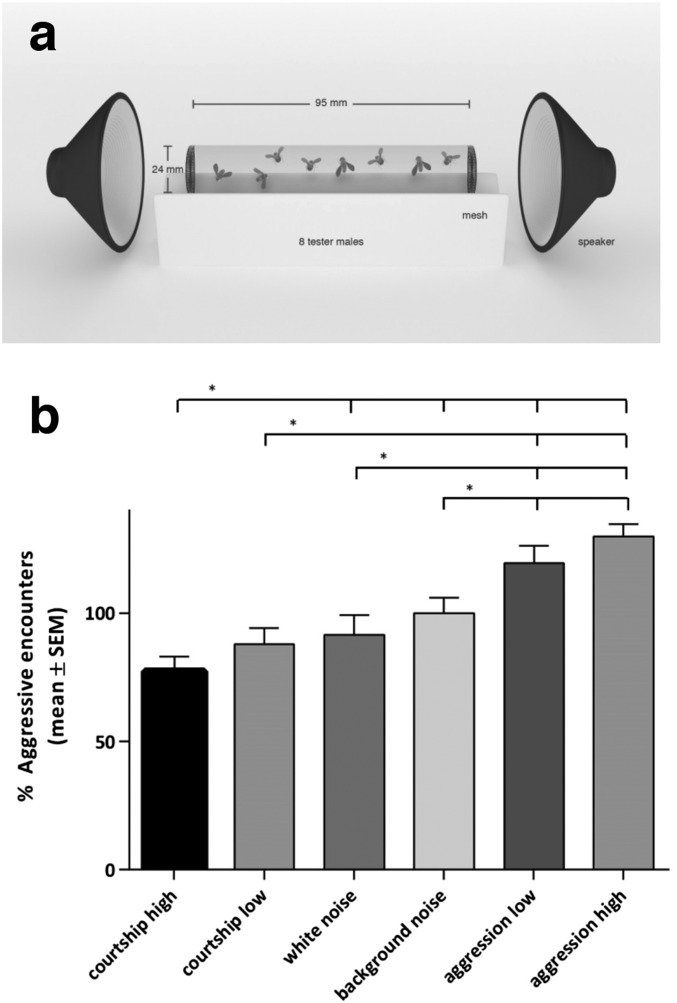
To investigate whether aggression songs modulate behavior, we next presented groups of eight male Drosophila with both types of sound stimuli (referred to as aggression and courtship), each with two different repetition rates (referred to as high and low), for 2 min (Fig. 3A). The results of our behavioral tests demonstrate a positive relation between type and repetition rate of acoustic stimuli and the number of aggressive encounters (Fig. 3B).
Fig. 3.
Agonistic sound promotes aggression in flies. (A) Assay design. (B) Percentage of aggressive encounters of male flies exposed to agonistic sound versus courtship songs compared with background noise (2 min). Agonistic sound with the highest acoustic repetition rate triggers the most prominent response. Male flies stimulated with intermediary agonistic sound are more aggressive than background noise- or white noise-stimulated flies but less aggressive than flies stimulated with a higher repetition rate. Acoustic stimulation of flies with courtship songs reduces aggressive behavior. Male flies presented with a courtship stimulus with a lower repetition rate are less aggressive than background noise-stimulated flies but more aggressive than flies exposed to a courtship stimulus with a higher repetition rate. Bar graphs are presented as means ± SEM; n = 20 replicates of eight males per treatment group. Fisher’s exact permutation test; *P < 0.05 (100% represents 43.86 aggressive encounters).
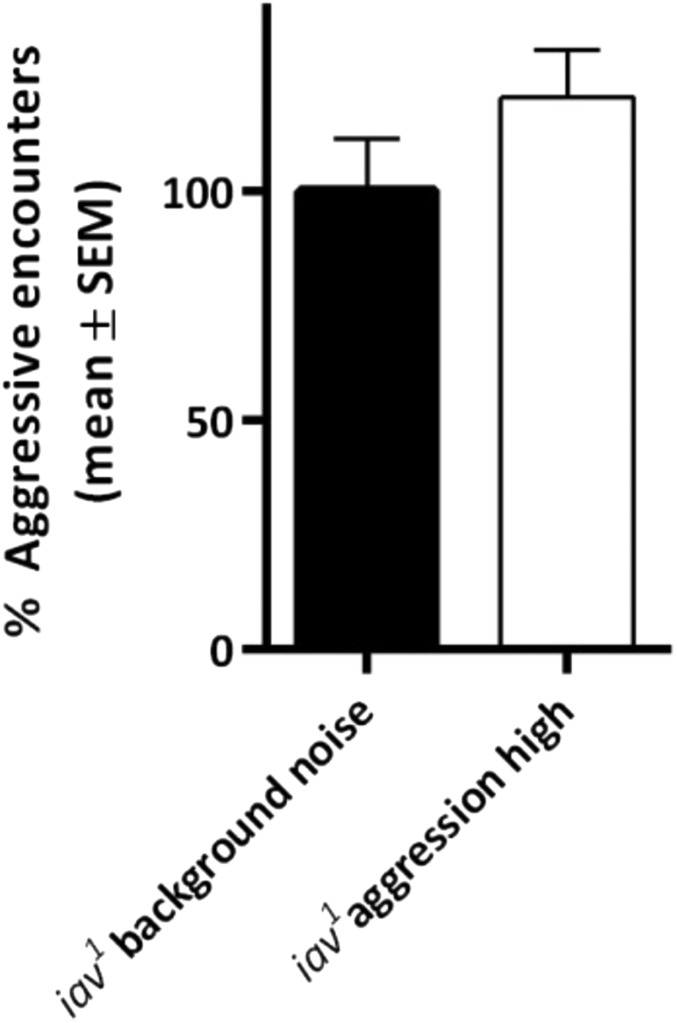
When flies were exposed to aggression songs, they became more aggressive compared with flies presented with background noise or white noise. This increase was already significant when agonistic songs were presented with the lower repetition rate and even more pronounced when they were presented with the higher repetition rate. We observed an opposite impact on aggressive behaviors when male flies were presented with courtship songs, although only courtship songs with high repetition rate induced significant reduction of aggression in comparison with the two control treatment groups (Fig. 3B). In contrast, deaf iav1 mutants did not respond to agonistic stimuli (Fig. S10).
Fig. S10.
Agonistic sound does not promote aggression in iav1 flies. Percentage of aggressive encounters of iav1 mutants that were stimulated with background noise or agonistic sound with the highest repetition rate. Flies displayed no significant difference in aggression levels when stimulated with either acoustic stimulus. Student’s t test (100% represents an average of 5.47 aggressive encounters in 2 min). The bar graph is presented as means ± SEM; n = 20 replicates with eight males.
From these results, we conclude that agonistic sound promotes aggressive behavior and that this effect varies with the poignancy of the acoustic signal presented. In contrast, courtship songs reduce agonistic behavioral responses.
Conclusions
In this study, we have identified an important role for hearing in the regulation of Drosophila intermale aggressive behavior. We show that mechanical, functional, and genetic disruption of Johnston’s organ, the Drosophila auditory organ, alters intermale aggression. Furthermore, we provide evidence that agonistic sounds promote aggression, thus demonstrating that the previously described agonistic sounds (13) serve as acoustic communication signals to modulate behavior.
We demonstrate that hearing impacts male fruit fly aggressive behavior in a context-dependent manner. When males compete for mates, courtship and aggression have been shown to be mutually exclusive. The choice between courtship and aggressive behavior is biased by situation-dependent acoustic signals that enhance motivation for one behavior while reducing motivation for the other. In light of recent work describing responsiveness of specific classes of projection neurons and local interneurons in the AMMC to courtship songs in both males and females, our results suggest that other neurons in the AMMC could be responsive to aggression songs in Drosophila males and thus that distinct neural circuits may exist that jointly control responsiveness to sound (30).
Courtship and aggression are important social behaviors that determine male reproductive success. Previous studies demonstrated the importance of species-specific sound patterns generated by wing vibration for Drosophila male courtship success (11, 12). Courtship songs are generated by vibrations of one extended wing and include two different patterns, the sine and pulse song (7–10). Although nothing was known about their role in communication, agonistic sounds have been previously observed in Drosophila (13, 31, 32). We confirm that these agonistic sounds consist of recurrent, stereotypical components. In contrast to courtship sounds, these do not contain sine-like components, are produced by movement of both wings, and vary in pulse duration and interval length between pulses. Agonistic sounds have been reported in multiple insects but have been most intensely studied in crickets (33–35). Interestingly, crickets also produce very strictly regulated courtship songs (intended to select mating partners of the same species) and more variable aggression sounds (34) (that may also be directed against other species competing for similar resources), suggesting that this could be a more general phenomenon in insects.
Previous reports described courtship song-induced chaining behavior between males (36–38). In our assay, we did not observe this behavior. We attribute these differences to the fact that the earlier studies used flies with their wings removed, which reduced or eliminated male-generated visual and auditory stimuli, thereby allowing normally suppressed courtship behavior to be executed. In our locomotion control experiments for the different mutant alleles, we observed no significant differences in free locomotion even though several of them have previously been reported to affect locomotion (39–41). These differences may be due to the different experimental paradigms or behavioral analyses that were used. In addition, a further explanation may lie in the fact that in our experiments the animals were starved for 90 min (as in the aggression assays). This condition of mild starvation is likely to provoke higher motivational levels and thus increased locomotion compared with the sated state, with as a consequence there being no longer significant differences with the wild-type controls.
In summary, our results show that male-derived acoustic signals are perceived and interpreted by male D. melanogaster to promote context-appropriate behavior. We conclude that hearing is an important sensory modality in intermale aggressive behavior and that auditory discrimination of agonistic and courtship songs (but not noise) biases behavioral choice and performance toward either courtship or aggression.
Experimental Procedures
Fly Stocks.
Flies were maintained on standard media. Crosses were cultured on a 12:12-h light/dark cycle at either 18 °C for all experiments including tubgal80ts or 25 °C for P-element insertion lines. Experiments only included male flies. UAS-TeTxLC and tubgal80ts were obtained from the Bloomington Drosophila Stock Center. F-gal4 (40), JO15-Gal4 (20), NP1017, NP1046, and NP6250 originated from the Kei Ito stock collection. RNAi lines for nompC [Transgenic RNAi Project (TRiP) JF01067] and trpl (TRiP JF02264) are part of the TRiP collection and were obtained from the Bloomington Drosophila Stock Center. Arr2 [Vienna Drosophila RNAi Center (VDRC) GD40999], inaD (VDRC GD26211), iav (VDRC GD7126), and nan (VDRC GD5261) RNAi lines originated from the VDRC stock center. All driver and RNAi lines were backcrossed into the Canton-S (B) genetic background for 10 generations. P-element lines were compared with their appropriate isogenic background.
Temperature-Shift Experiments.
For inactivation of synaptic output from JO neuronal subgroups and RNAi experiments, crosses were reared until adulthood at 18 °C. Immediately after eclosion, male offspring were divided into a control group, shifted for 3 d to the permissive temperature of 18 °C, and the test group, exposed to 25 °C, the nonpermissive temperature.
Aggression Assays.
Behavioral assays between eight males were performed according to standard protocols by an experimenter who was blinded to the type of tested fly strain (2, 42). Flies were not anesthetized for at least 24 h before the assay. All tests were performed between 10 and 11:30 AM, except for the analysis of courtship versus aggression songs, where flies were tested between 10 AM and 1 PM. Flies were aged in mixed groups until 3 d before testing, when males were separated into groups of eight 3- to 7-d-old males. For testing, males were placed in a vial without food for 90 min, after which they were transferred (without anesthesia) to a test arena containing a droplet of food and allowed to acclimate for 2 min. After the acclimation period, the flies were observed for 2 min. The aggression score for each replicate was the total number of aggressive interactions observed among all eight flies in the 2-min observation period. Kicking, chasing, wing threats, boxing, and head butts were scored as aggressive encounters. Results are shown as percentages of aggressive encounters, normalized to control, of 100%. The assay was performed with 20 replicate measurements for each line or treatment group.
Aggression assays between two males were performed as previously described (43). The time spent fighting was analyzed for the first 5 min after the two males first met on the food pad. The assay was performed with 10 replicate measurements for each line or treatment group.
Locomotion Assays.
Free locomotion was analyzed in single 3- to 7-d-old socially experienced males, which were starved 90 min before testing. Arenas consisted of the lid of a 5.5-cm-diameter petri dish placed in the bottom of a 9-cm-diameter petri dish. Flies were transferred to the arena using an aspirator and allowed to acclimatize for 1 min. Next, the flies were filmed from above for 1 min. All experiments were done between 10 and 11:30 AM and at room temperature. Walking distance and speed were analyzed using FlyTracker, Matlab-based software written by Ben Vermaercke, Laboratory for Biological Psychology, KU Leuven, Leuven, Belgium.
Mechanical Disruption of Hearing.
Aristectomy and restricting movement of the third antennal segments were performed under CO2 anesthesia. Nontoxic UV-cured glue (Heliobond) was used to fix antennae at the a2/a3 joint. Flies were tested for aggression after a 24-h recovery period.
Sound Recordings and Stimulation.
Acoustic signals of courtship and aggressive encounters were recorded as described (13). Oscillograms were generated and modified using Audacity software (audacity.sourceforge.net). The aggression arena was adapted to allow sound stimulus entry by sealing the vials with transparent mesh on both ends. Acoustic stimuli were presented for 2 min (in the absence of food) with speakers located on both sides of the arena and aggressive encounters were measured. Courtship sequences used for acoustic stimulation consisted of a pulse (2.5 s) and sine song (1.7 s) and were presented with a repetition rate of 10 per min (high-intensity courtship song) or 4 per min (low-intensity courtship song). Agonistic sound stimuli consisted of a continuous recording of 25 bouts in 2 min of agonistic pulses (high-intensity aggression song) or only 15 of these bouts per 2 min total stimulation time (low-intensity aggression song). As unstimulated control, flies were exposed to a background recorded under the same conditions as courtship and aggression stimuli. As control for unspecific activation of the auditory system not related to communication sounds, white noise was presented during behavioral assays.
Dissections and Immunofluorescence.
JO-gal4; UAS-gfp-cd8 males were dissected in PBS and tissues were subsequently fixed using 37% (vol/vol) formaldehyde (1:10). GFP fluorescence was analyzed using an Olympus FV1000 microscope.
Statistical and Bioinformatic Analyses.
Significant overlap between identified genes was analyzed using a Fisher’s exact hypergeometric test (nemates.org/MA/progs/overlap_stats.html). Statistical analysis of behavior was performed with Prism 4 software (GraphPad), implementing parametric one-way ANOVA or nonparametric Kruskal–Wallis models depending on the distribution of the data with corresponding post hoc tests. To assess the effects of courtship and aggression songs on aggression levels, we used Fisher’s exact permutation test (44).
Supplementary Material
Acknowledgments
We thank Veerle Vulsteke and Margret Winkler for technical assistance, and Ben Vermaercke for the FlyTracker program. This work was supported by grants from the VIB, Fonds Wetenschappelijk Onderzoek-Vlaanderen (FWO G.0789.14N to P.C.), and German Science Foundation (DFG; GO 1092/3 and SFB 889, A1 to M.C.G.). M.V. was the recipient of a predoctoral fellowship from the Instituut voor Innovatie door Wetenschap en Technologie-Vlaanderen (IWT). L.V.B. was supported by the FWO. We thank the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic RNAi fly stocks and plasmid vectors used in this study.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605946114/-/DCSupplemental.
References
- 1.Kravitz EA, Huber R. Aggression in invertebrates. Curr Opin Neurobiol. 2003;13(6):736–743. doi: 10.1016/j.conb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Zwarts L, et al. Complex genetic architecture of Drosophila aggressive behavior. Proc Natl Acad Sci USA. 2011;108(41):17070–17075. doi: 10.1073/pnas.1113877108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoyer SC, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18(3):159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, et al. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat Neurosci. 2011;14(7):896–902. doi: 10.1038/nn.2836. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463(7278):227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan Q, Song Y, Yang CH, Jan LY, Jan YN. Female contact modulates male aggression via a sexually dimorphic GABAergic circuit in Drosophila. Nat Neurosci. 2014;17(1):81–88. doi: 10.1038/nn.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shorey HH. Nature of the sound produced by Drosophila melanogaster during courtship. Science. 1962;137(3531):677–678. doi: 10.1126/science.137.3531.677. [DOI] [PubMed] [Google Scholar]
- 8.Hall JC. The mating of a fly. Science. 1994;264(5166):1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 9.Bennet-Clark HC, Dow M, Ewing AW, Manning A, von Schilcher F. Letter: Courtship stimuli in Drosophila melanogaster. Behav Genet. 1976;6(1):93–95. doi: 10.1007/BF01065681. [DOI] [PubMed] [Google Scholar]
- 10.von Schilcher F. The function of pulse song and sine song in the courtship of Drosophila melanogaster. Anim Behav. 1976;24(3):622–625. [Google Scholar]
- 11.Ewing AW. Functional aspects of Drosophila courtship. Biol Rev Camb Philos Soc. 1983;58(2):275–292. [Google Scholar]
- 12.Crossley SA, Bennet-Clark HC, Evert HT. Courtship song components affect male and female Drosophila differently. Anim Behav. 1995;50(3):827–839. [Google Scholar]
- 13.Jonsson T, Kravitz EA, Heinrich R. Sound production during agonistic behavior of male Drosophila melanogaster. Fly (Austin) 2011;5(1):29–38. doi: 10.4161/fly.5.1.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20(16):5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Göpfert MC, Robert D. Biomechanics. Turning the key on Drosophila audition. Nature. 2001;411(6840):908. doi: 10.1038/35082144. [DOI] [PubMed] [Google Scholar]
- 16.Göpfert MC, Robert D. The mechanical basis of Drosophila audition. J Exp Biol. 2002;205(Pt 9):1199–1208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- 17.Göpfert MC, Robert D. Motion generation by Drosophila mechanosensory neurons. Proc Natl Acad Sci USA. 2003;100(9):5514–5519. doi: 10.1073/pnas.0737564100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yorozu S, et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458(7235):201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamikouchi A, Shimada T, Ito K. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J Comp Neurol. 2006;499(3):317–356. doi: 10.1002/cne.21075. [DOI] [PubMed] [Google Scholar]
- 20.Sharma Y, Cheung U, Larsen EW, Eberl DF. PPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in Drosophila. Genesis. 2002;34(1–2):115–118. doi: 10.1002/gene.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senthilan PR, et al. Drosophila auditory organ genes and genetic hearing defects. Cell. 2012;150(5):1042–1054. doi: 10.1016/j.cell.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 22.Effertz T, Wiek R, Göpfert MC. NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol. 2011;21(7):592–597. doi: 10.1016/j.cub.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, et al. TRPA channels distinguish gravity sensing from hearing in Johnston’s organ. Proc Natl Acad Sci USA. 2009;106(32):13606–13611. doi: 10.1073/pnas.0906377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randall AS, et al. Speed and sensitivity of phototransduction in Drosophila depend on degree of saturation of membrane phospholipids. J Neurosci. 2015;35(6):2731–2746. doi: 10.1523/JNEUROSCI.1150-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SJ, Xu H, Kang LW, Amzel LM, Montell C. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 2003;39(1):121–132. doi: 10.1016/s0896-6273(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450(7167):294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 27.Shieh BH, Zhu MY. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron. 1996;16(5):991–998. doi: 10.1016/s0896-6273(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 28.O’Dell KM. The effect of the inactive mutation on longevity, sex, rhythm and resistance to p-cresol in Drosophila melanogaster. Heredity (Edinb) 1993;70(Pt 4):393–399. doi: 10.1038/hdy.1993.55. [DOI] [PubMed] [Google Scholar]
- 29.Yan Z, et al. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493(7431):221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaughan AG, Zhou C, Manoli DS, Baker BS. Neural pathways for the detection and discrimination of conspecific song in D. melanogaster. Curr Biol. 2014;24(10):1039–1049. doi: 10.1016/j.cub.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 31.Paillette M, Ikeda H, Jallon J-M. A new acoustic signal of the fruit-flies Drosophila simulans and D. melanogaster. Bioacoustics. 1991;3(4):247–254. [Google Scholar]
- 32.Tauber E, Eberl D. The effect of male competition on the courtship song of Drosophila melanogaster. J Insect Behav. 2002;15(1):109–120. [Google Scholar]
- 33.Rudinsky JA, Michael RR. Sound production in scolytidae: ‘Rivalry’ behaviour of male Dendroctonus beetles. J Insect Physiol. 1974;20(7):1219–1230. doi: 10.1016/0022-1910(74)90228-5. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel B, Hedwig B. Neurochemical control of cricket stridulation revealed by pharmacological microinjections into the brain. J Exp Biol. 1999;202(Pt 16):2203–2216. doi: 10.1242/jeb.202.16.2203. [DOI] [PubMed] [Google Scholar]
- 35.Jones MD. The acoustic behaviour of the bush cricket Pholidoptera griseoaptera. I. Alternation, synchronism and rivalry between males. J Exp Biol. 1966;45(1):15–30. doi: 10.1242/jeb.45.1.15. [DOI] [PubMed] [Google Scholar]
- 36.Yoon J, et al. Selectivity and plasticity in a sound-evoked male-male interaction in Drosophila. PLoS One. 2013;8(9):e74289. doi: 10.1371/journal.pone.0074289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Schilcher F. The role of auditory stimuli in the courtship of Drosophila melanogaster. Anim Behav. 1976;24(1):18–26. [Google Scholar]
- 38.Eberl DF, Duyk GM, Perrimon N. A genetic screen for mutations that disrupt an auditory response in Drosophila melanogaster. Proc Natl Acad Sci USA. 1997;94(26):14837–14842. doi: 10.1073/pnas.94.26.14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Dell K, Burnet B. The effects on locomotor activity and reactivity of the hypoactive and inactive mutations of Drosophila melanogaster. Heredity. 1988;61:199–207. [Google Scholar]
- 40.Kim J, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424(6944):81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 41.Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67(3):373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards AC, Rollmann SM, Morgan TJ, Mackay TF. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2006;2(9):e154. doi: 10.1371/journal.pgen.0020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mundiyanapurath S, Certel S, Kravitz EA. Studying aggression in Drosophila (fruit flies) J Vis Exp. 2007;25(2):155. doi: 10.3791/155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jowett GH. Book reviews: Statistical methods for research workers, by Ronald A. Fisher. J R Stat Soc Ser C Appl Stat. 1956;5(1):68–70. [Google Scholar]
- 45.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One. 2010;5(5):e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11(9):1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 47.Zwarts L, Versteven M, Callaerts P. Genetics and neurobiology of aggression in Drosophila. Fly (Austin) 2012;6(1):35–48. doi: 10.4161/fly.19249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.