Several recent ground-breaking papers address the deconstruction of neural circuits that regulate mammalian thermoregulation (1–3), including “A hypothalamic circuit that controls body temperature,” by Zhao et al. (3) from Wei Shen’s new laboratory at ShanghaiTech University, recently published in PNAS. In this study, Zhao et al. define neural circuit mechanisms that are novel and critically relevant for mammalian thermoregulation.
Thermoregulation is essential in all organisms, an evolutionary conditio sine qua non. In mammals and other warm-blooded animals, homeothermy became an essential physiologic feature during evolution. Homeothermy, the physiologic capability to maintain a constant core body temperature with minimal deviation from the set point, provided a critical survival advantage to mammalian and avian phyla because it brought about a thermally equilibrated internal environment for cells and organs. This in turn rendered nutrition, metabolism, and excretion more robust and efficient, and permitted more precise and powerful functioning of excitable cells in the nervous system, as well as for contractile cells in heart, muscle, and smooth muscle, and evolutionary honing of an immune-defense and wound-healing system. This change led to animals being more competitive to defend against external stress and at the same time more efficient at procreation. Thermal homeostasis coevolved with other vital homeostatic systems (4) and thermoregulation and homeothermy represent primordial–physiologic functions that have long aroused the interest of physiologists and bio-medical researchers (5). However, ground-breaking progress toward elucidation of molecular and neural-circuit mechanisms accounting for thermoregulation and homeothermy has, until recently, been elusive. As such, significant nonincremental progress toward elucidation of neural circuit mechanisms of mammalian thermoregulation, recently provided in the paper by Zhao et al., represents an important milestone (3).
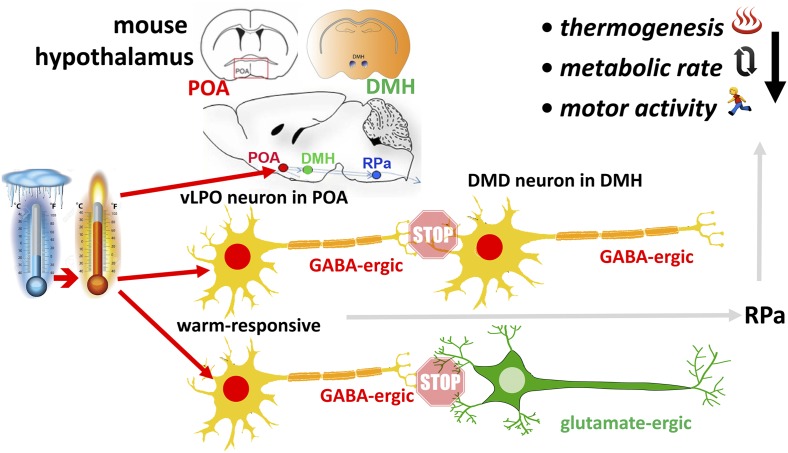
In this study (3), using cutting-edge mouse methodology to untangle neural circuitry that regulates core body temperature, Zhao et al. show that: (i) GABAergic thermally responsive neurons in an area directly rostral to the hypothalamus, the ventral lateral preoptic area (vLPO), synaptically relay neural signal to populations of both GABAergic and glutamatergic neurons in the dorsomedial hypothalamus, the so-called DMD nucleus; (ii) activation of GABAergic vLPO neurons reduces core body temperature, organismal metabolic rate, and behavioral activity, whereas inhibition causes lethal fever; and (iii) inhibition of both types of neurons in the DMD, GABAergic and glutamatergic, reduces body temperature, metabolic rate, and activity (Fig. 1). Here again, activation of these neurons has the opposite effect. This means that the thermally responsive GABAergic neurons in the vLPO subnucleus attenuate thermogenic output of the DMD neurons in the dorsomedial nucleus. In addition, using a powerful molecular method that allows physical separation of the translating ribosome (2), Zhao et al. (3) isolate warmth-activated expressed genes in the preoptic area and confirm enhanced gene expression of a neurotrophic factor, BDNF, to be warmth-activated. In addition, genes of several neuropeptides previously known to function in different homeostatic physiological systems—namely neuromedin S, galanin, and neurotensin—were also found enriched in warmth-sensitive neurons. Thus, the Zhao et al. (3) paper defines novel hypothalamic neural circuitry that controls core body temperature, organismal metabolism, and behavior in either direction.
Fig. 1.
Schematic shows a rise in temperature activating the warm-responsive, GABAergic neurons in the vLPO subnucleus of the preoptic area (POA), illustrated for schematic orientation in the mouse brain above. These GABAergic neurons relay synaptically to GABAergic and also to glutamate-ergic neurons in the DMD subnucleus of the dorsomedial hypothalamic (DMH) nucleus, schematically also shown above. GABAergic relay (i.e., inhibitory neurotransmission) to both types of DMD neurons attenuates thermogenesis, metabolic rate, and also behavioral motor activity, presumably via efferences to the rostral pallidum (RPa) in the brainstem. Note that GABAergic vLPO neurons could be intrinsically warm-sensitive, as well as receive warmth-evoked peripheral afferences.
The Zhao et al. (3) paper has to be viewed together with two other papers, also published recently: one by Tan et al. (2) from Zach Knight’s laboratory, showing that peptidergic warm-sensitive neurons in the preoptic area evoke down-regulation of core body temperature; the other by Song et al. (1) from Jan Siemens’ group, reporting that TRPM2 (transient receptor potential channel M2) ion channels function as ionotropic warm receptors in hypothalamic neurons to limit the fever response.
Taken together, these three papers significantly advance our understanding of the neural mechanisms that control core body temperature in mammals. Ground-breaking as these studies are, they also bring to mind the following “top 10” thoughts and questions, considerations that can provide some guidance as to how this very recently awakened field of inquiry can now expand, almost certainly accompanied by considerable excitement.
First on the list is the neural temperature-sensing mechanism. The sensing apparatus is largely at large. The identification of warm-activated TRPM2 functional in the fever response only constitutes the beginning of the path of discovery. Other thermally sensitive TRP ion channels could be involved, participating via yet to be discovered splice variants that do not respond to known pharmacological reagents (6). Pan-null knockout animals will very likely show compensation because thermoregulation is such a phylogenetically deeply rooted survival mechanism. In terms of sensing, brain-internal sensing will be key, but peripheral modulation will also be of importance so that peripheral input to the GABAergic vLPO neurons described by Zhao et al. (3) will have to be identified and functionally deconstructed. Thermal sensing does not have to rely on ionotropic receptors for any particular demand of the physiology, in contrast to, for example, inner-ear hair cell mechanotransduction, which relies on the speed of mechano-electrical transduction. Slower signaling systems will suffice: for example, thermally sensitive G protein-coupled receptors, and even thermally sensitive enzymatic signaling systems.
Second is robustness of the inhibitory nature of GABAergic transmission in the novel circuits. Zhao et al. (3) describe a GABAergic signaling mechanism as the preoptic area neuronal thermo-responsive element of the circuit they discover. Via GABA, these neurons signal to DMD neurons, one important target population that is also GABAergic. Membrane hyperpolarization in these neurons, in response to GABA, determines these neurons’ thermoregulatory function. Their membrane hyperpolarization will critically depend on their internal chloride ion concentration, which is maintained at low level as a function of the chloride extruding transporter molecule, KCC2 (potassium chloride transporter member 5) (7, 8). KCC2 is the only chloride-extruding transporter system in mature CNS neurons. Robust and continuously robust gene expression of KCC2 in the GABAergic DMD neurons is therefore of paramount importance for function of this circuit. How these neurons maintain their KCC2 gene expression will be interesting to learn because malfunction in this circuit is an event that would impair homeostatic stress tolerance, and therefore survival and survival advantage. Lack of KCC2 expression underlies chronic pain, epilepsy, traumatic brain injury, and other neuro-psychiatric conditions (7, 9), and for the benefit of all of these it will be interesting to learn how thermo-regulatory GABAergic neurons maintain their robust KCC2 gene expression at a constant level, whereas neurons in the aforementioned conditions can more readily become “circuit-breakers” via elevated internal chloride, which renders GABAergic transmission ineffective.
Third is fever response. We are now in a position to ask how these neural systems regulate fever, and how they are being regulated by fever, bearing in mind the evolutionary survival advantage that fever brought about as a powerful component of antiinfectious defense mechanisms.
Fourth is homeostatic response to other thermal stresses. As a related condition to fever, the same question as in the third topic applies to hyper- and hypothermia evoked by endogenous and
Significant nonincremental progress toward elucidation of neural circuit mechanisms of mammalian thermoregulation, recently provided in the paper by Zhao et al., represents an important milestone.
external conditions: for the former, thyroid disorders and immune-mediated conditions, for the latter drug-induced and climate-evoked conditions come to mind. A highly relevant external climate-related condition is hyperthermia as a result of overheating, which is invariably accompanied by dehydration. This practical issue gets us into the territory of multiple disequilibrating stresses, such as contemporaneous dehydration, lack of sodium and hyperthermia, and the respective role that the newly discovered hypothalamic thermal-sensing and thermo-regulatory machinery plays in defending against multiple stressors (10).
Fifth is translational medical significance. Hyper- and hypothermia are also dreaded medical conditions causing significant morbidity and mortality on intensive care units. Knowledge of molecular sensing and neural circuit mechanisms of hypothalamic thermo-sensing and thermo-regulatory machinery may in time guide us toward transformative medical prevention and treatment approaches that will reduce morbidity and mortality from thermal dysregulation.
Sixth is human menopause thermal disequilibrium. Considerable morbidity, less severe but affecting a higher number of people, is associated with (pre)menopausal thermal dysregulation in women (11), which we might be able to treat in an improved manner once we make more progress understanding basic neural thermoregulatory mechanisms.
Seventh is sexual dimorphism of human thermal experience. Increased insight into hypothalamic thermo-sensing and thermo-regulatory machinery will help us better understand human psychophysical responses to thermal cues as they differ between males and females (11). This engenders the prospect of an end to domestic and work-place “thermostat wars.”
Eighth are neuropeptides. The identified increased gene expression of neuropeptides by warmth can be tested for their modulatory potency on thermal sensing, thermo-regulation, energy expenditure, behavioral effects and, importantly, related physiology of equilibria and instinctive behaviors. With receptors to these neuropeptides identified, there are more transformative discoveries to be made, along the lines of a rationally guided recipe for success.
Ninth are molecular and neural circuit evolution. These studies lay a rational foundation for identifying and deconstructing what sets apart homeothermic from nonhomeothermic animals at the molecular, neuro-sensory and neural circuit level.
Tenth is hibernation. These studies lay a rational foundation to study the mechanisms and effects of hibernation on these neural systems, and how this recently identified neural organization can participate in regulation of hibernation.
A few methodological comments are provided here as a coda because this recent nonincremental leap in our understanding has been based on resourceful utilization of powerful new methodology, expertly adapted to an area of inquiry that had become rather static.
Zhao et al. (3) used Ca++ dynamics as a surrogate to measure neural activity detected with the genetically encoded Ca++ indicator protein, GCaMP6. Other powerful genetically encoded indicator proteins are available now that can be used to monitor different aspects of neural activation in intact animals. Voltage-activated fluorescent proteins allow detection of rapid changes in membrane voltage as a direct indicator of activation or inactivation of neurons (12, 13). Furthermore, activity-dependent intracellular signaling cascades can be imaged, such as CaMKII and MAP-kinase, ERK (14, 15). Whereas GABAergic and glumatergic neurons were activated or inactivated by means of opto- or chemogenetics in Zhao et al.’s paper (3), their intriguing results beg the question: what would be the consequence if these neurons were deleted, using either a rapidly acting neural toxin or a slowly acting neuro-degeneration inducing protein based on misfolding? As an intriguing method to actuate de- or hyperpolarization of the targeted neurons, and in general any neurons suspected of affecting thermal sensing or thermoregulation, magnetic actuator technology (16, 17) could be used, which will permit instantaneous change in neural activation, depending on the simple presence of a magnetic field, rendering this approach completely noninvasive.
Footnotes
The author declares no conflict of interest.
See companion article on page 2042.
References
- 1.Song K, et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. 2016;353(6306):1393–1398. doi: 10.1126/science.aaf7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan CL, et al. Warm-sensitive neurons that control body temperature. Cell. 2016;167(1):47–59.e15. doi: 10.1016/j.cell.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao ZD, et al. A hypothalamic circuit that controls body temperature. Proc Natl Acad Sci USA. 2017;114:2042–2047. doi: 10.1073/pnas.1616255114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinley MJ, et al. The median preoptic nucleus: Front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 2015;214(1):8–32. doi: 10.1111/apha.12487. [DOI] [PubMed] [Google Scholar]
- 5.Bartfai T, Conti B. Molecules affecting hypothalamic control of core body temperature in response to calorie intake. Front Genet. 2012;3:184. doi: 10.3389/fgene.2012.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaelzer C, et al. ΔN-TRPV1: A molecular co-detector of body temperature and osmotic stress. Cell Reports. 2015;13(1):23–30. doi: 10.1016/j.celrep.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 7.Medina I, et al. Current view on the functional regulation of the neuronal K(+)-Cl(-) cotransporter KCC2. Front Cell Neurosci. 2014;8:27. doi: 10.3389/fncel.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo M, Berglund K, Augustine G, Liedtke W. Novel repression of Kcc2 transcription by REST-RE-1 controls developmental switch in neuronal chloride. J Neurosci. 2009;29(46):14652–14662. doi: 10.1523/JNEUROSCI.2934-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahle KT, et al. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4(9):490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- 10.McKinley MJ, McAllen RM, Whyte D, Mathai ML. Central osmoregulatory influences on thermoregulation. Clin Exp Pharmacol Physiol. 2008;35(5-6):701–705. doi: 10.1111/j.1440-1681.2007.04833.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaciuba-Uscilko H, Grucza R. Gender differences in thermoregulation. Curr Opin Clin Nutr Metab Care. 2001;4(6):533–536. doi: 10.1097/00075197-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Gong Y, et al. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science. 2015;350(6266):1361–1366. doi: 10.1126/science.aab0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang HH, St-Pierre F. Genetically encoded voltage indicators: Opportunities and challenges. J Neurosci. 2016;36(39):9977–9989. doi: 10.1523/JNEUROSCI.1095-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458(7236):299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey CD, et al. A genetically encoded fluorescent sensor of ERK activity. Proc Natl Acad Sci USA. 2008;105(49):19264–19269. doi: 10.1073/pnas.0804598105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley SA, et al. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016;531(7596):647–650. doi: 10.1038/nature17183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler MA, et al. Genetically targeted magnetic control of the nervous system. Nat Neurosci. 2016;19(5):756–761. doi: 10.1038/nn.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]