Significance
Simian immunodeficiency virus (SIV)-specific follicular CD8 T cells represent a unique subset of antiviral CD8 T cells that rapidly expand during pathogenic SIV infection, localize within B-cell follicles, and contribute to control of chronic SIV replication. The potential for these cells to infiltrate sites of ongoing viral replication and viral persistence and the ability to induce these cells by vaccination provide a tremendous opportunity to develop and optimize therapeutic strategies to target and reduce the HIV reservoirs in lymphoid tissues.
Keywords: SIV, follicular CD8 T cells, CXCR5+CD8+ T cells, lymphoid follicles, HIV
Abstract
A significant challenge to HIV eradication is the elimination of viral reservoirs in germinal center (GC) T follicular helper (Tfh) cells. However, GCs are considered to be immune privileged for antiviral CD8 T cells. Here, we show a population of simian immunodeficiency virus (SIV)-specific CD8 T cells express CXCR5 (C-X-C chemokine receptor type 5, a chemokine receptor required for homing to GCs) and expand in lymph nodes (LNs) following pathogenic SIV infection in a cohort of vaccinated macaques. This expansion was greater in animals that exhibited superior control of SIV. The CXCR5+ SIV-specific CD8 T cells demonstrated enhanced polyfunctionality, restricted expansion of antigen-pulsed Tfh cells in vitro, and possessed a unique gene expression pattern related to Tfh and Th2 cells. The increase in CXCR5+ CD8 T cells was associated with the presence of higher frequencies of SIV-specific CD8 T cells in the GC. Following TCR-driven stimulation in vitro, CXCR5+ but not CXCR5– CD8 T cells generated both CXCR5+ as well as CXCR5– cells. However, the addition of TGF-β to CXCR5– CD8 T cells induced a population of CXCR5+ CD8 T cells, suggesting that this cytokine may be important in modulating these CXCR5+ CD8 T cells in vivo. Thus, CXCR5+ CD8 T cells represent a unique subset of antiviral CD8 T cells that expand in LNs during chronic SIV infection and may play a significant role in the control of pathogenic SIV infection.
Numerous studies conducted to date have demonstrated the critical nature of antiviral CD8 T cells in the control of human and simian immunodeficiency virus (HIV/SIV) replication (1–3). Studies also showed a direct relationship between higher frequency and function of HIV-specific CD8 T cells and enhanced viral control (4–6). In particular, early induction of HIV-specific CD8 T cells resulted in a concomitant decline in plasma viremia (7, 8), suggesting that antiviral CD8 T-cell responses elicited early after HIV/SIV infection can significantly modulate viral control outcome. Consistent with this, contemporary vaccine strategies designed to elicit high frequencies of antiviral CD8 T cells have contained pathogenic SHIV (9, 10) and SIV challenges (11, 12) in macaques. Despite a pronounced antiviral CD8 T-cell response elicited early after HIV infection and the subsequent decline in set-point viremia, the majority of HIV-infected individuals do not control HIV replication in the absence of ART and inevitably progress to disease.
It is now well appreciated that lymphoid sites, in particular B-cell follicles and T follicular helper (Tfh) cells, serve as important sites of productive HIV/SIV infection (13–15). The density of infection that is localized to secondary lymphoid sites and germinal centers (GCs), even under continuous ART, underscores the need to better understand T-cell dynamics at lymphoid sites and specific immune factors that may limit effective clearance of virally infected CD4 T cells. Studies in unvaccinated SIV-infected rhesus macaques (RMs) and HIV-infected humans indicated that antiviral CD8 T cells have a limited capacity to migrate to B-cell follicles and GCs of the lymphoid tissue during chronic infection (16–18), and the exclusion of CD8 T cells from GC sites has been posited as an important mechanism of immune evasion by HIV/SIV. However, recent studies have reported the emergence of CD8 T cells expressing the C-X-C chemokine receptor type 5 (CXCR5) that is required for homing to B-cell follicles (19, 20) during chronic LCMV and HIV infections (21–23). A remaining critical question to be addressed is whether CD8 T cells can gain access to GCs of B-cell follicles during chronic HIV/SIV infection and, if so, whether these cells can impact levels of viral replication in vivo.
Recently, others and we reported an aberrant accumulation of virus-infected Tfh cells in the lymph nodes (LNs) and rectal mucosa of SIV-infected RMs with high viral load (VL) (14, 15, 24–27), which was not evident in vaccinated SIV-infected RMs with low VL during a pathogenic SIVmac251 infection (15). In the current study, we sought to understand the role of antiviral CD8 T cells in limiting the virus-infected Tfh cells. In particular, we studied the nature of CXCR5 expression on SIV-specific CD8 T cells in blood and LNs. The chemokine receptor CXCR5 is required for homing to B-cell follicles/GCs (19, 20), and a prior human study showed the presence of CXCR5+ SIV-specific CD8 T cells in tonsils (28). We also sought to understand phenotypic and functional differences in the CD8 T cells based on CXCR5 expression. We observed a strong induction of CXCR5 on SIV-specific CD8 T cells in the blood and LNs of animals that exhibited superior viral control. These CXCR5+ CD8 T cells showed a unique gene expression profile, were able to limit the expansion of antigen-pulsed Tfh cells in vitro, and were associated with a lower viral burden within the Tfh subset. These findings demonstrate that CXCR5+ CD8 T cells represent a unique subset of vaccine-induced antiviral CD8 T cells with the potential to home to B-cell follicles and limit HIV replication in vivo.
Results
Study Overview.
Despite comprehensive analyses on the role of CXCR5 on CD4 T cells during chronic SIV/HIV infection (14, 15, 24–26), much less is known about the role of CXCR5 on CD8 T cells. Moreover, previous studies have not characterized antigen-specific CXCR5+ CD8 T cells and their role in HIV/SIV pathogenesis and viral control. We therefore studied the CXCR5 expression on SIV-specific CD8 T cells in the LNs during chronic SIVmac251 infection in a group of 20 DNA/MVA (Modified Vaccinia Ankara)-vaccinated SIV-infected RMs with a spectrum of viral control (Fig. 1 and Fig. S1). We considered animals with set point (week 24) VL at or below 104 copies per mL of plasma as low-VL animals, as described previously. There were 12 animals with low VL and 8 animals with high VL. This was a retrospective study, and thus, we used samples from LNs and blood where available.
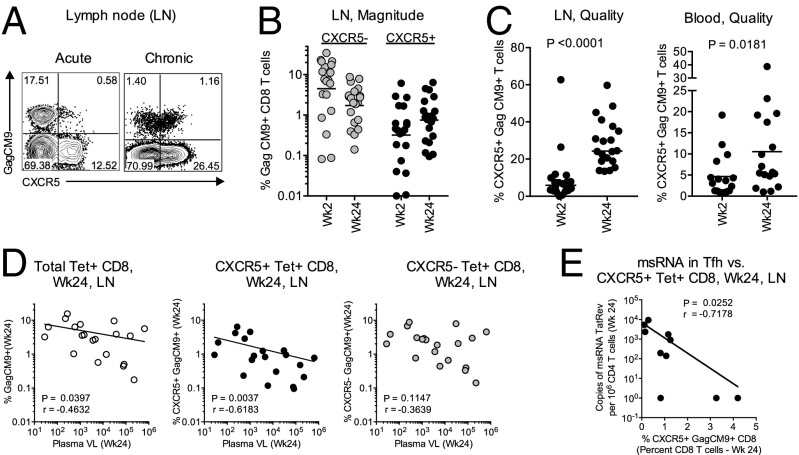
Fig. 1.
Rapid expansion of CXCR5+ SIV-specific CD8 T cells is associated with enhanced control of chronic SIV infection. (A) Representative FACS plots showing CXCR5 expression on Gag CM9 tetramer+ CD8 T cells (Tet+) following SIVmac251 infection in LNs. (B) Magnitude of CXCR5– and CXCR5+ Tet+ CD8 T cells in the LNs during acute (Wk2) and chronic (Wk24) phases of SIV infection (n = 20). (C) The proportion of Gag CM9 tetramer+ cells expressing CXCR5 in LNs (n = 20) and blood (n = 17). (D) Correlation between the magnitude of total, CXCR5+, or CXCR5– tetramer+ CD8 T cells and set-point plasma VLs in the LNs (n = 20). (E) Correlation between the frequency of CXCR5+ Tet+ CD8 T cells and the msRNA of TatRev in GC–Tfh cells in the LNs at week 24 postinfection (n = 10). Horizontal lines show the median.
Fig. S1.
(A) Kinetics of plasma VLs in vaccinated animals. (B) Correlation of CXCR5+ Gag CM9 tetramer+ cells between LNs and blood during the chronic phase (n = 17). (C) Correlation between Tet+, CXCR5+, and CXCR5– CD8 T-cell subsets in the LNs and plasma VLs at week 24 post-SIV infection. (D) Correlation between the frequency of CXCR5+ Tet+ CD8 T cells and cell-associated DNA within the GC–Tfh subsets at week 24 postinfection (n = 10). (E) Comparison of CXCR5 expression on CD8 T cells on unstimulated and Gag CM9 peptide-stimulated cells. On unstimulated cells, Gag CM9 tetramer was used to identify SIV Gag-specific CD8 T cells. On stimulated cells, intracellular IFNγ was used to identify SIV Gag-specific CD8 T cells.
SIV-Specific CXCR5+ CD8 T Cells Expand in LNs Following Pathogenic SIV Infection and at Higher Levels in Animals That Exhibited Superior Viral Control.
To understand the contribution of SIV-specific CXCR5+ CD8 T cells to viral control during chronic SIV infection, we determined the magnitude of Gag CM9 tetramer+ CD8 T cells (Tet+) and their coexpression of CXCR5 in the LNs during the acute (week 2) and chronic (week 24) phases of SIV infection in a group of Mamu A*01+-vaccinated RMs that exhibited a varying degree of viral control (Fig. 1 and Fig. S1A). During the acute phase, the CXCR5+ Tet+ cells were readily detectable in the LNs; however, the magnitude of these cells was quite variable and ranged from 0.01% (limit of detection) to 6% (Fig. 1 A and B). At this time, the magnitude of CXCR5+ Tet+ cells (geometric mean of 0.32%) was about 14 times lower compared with CXCR5– Tet+ CD8 T cells (geometric mean of 4.4%) (Fig. 1B). During the chronic phase, the magnitude of CXCR5– Tet+ cells decreased by 2.5-fold (geometric mean of 1.7%) compared with acute phase. However, we observed a twofold increase in the magnitude of CXCR5+ Tet+ cells (geometric mean of 0.75%) that was associated with a fourfold increase in the proportion of Tet+ cells expressing CXCR5 during the chronic phase (24%) compared with the acute phase (6%) (Fig. 1C). Similar to LNs, about 5% of the Tet+ cells in the blood expressed CXCR5 during the acute phase, and this expression increased to 10% during the chronic phase (Fig. 1C). In addition, we observed a direct correlation between the magnitude of CXCR5+ Tet+ cells in the LNs and the proportion of Tet+ cells expressing CXCR5 in the blood during chronic phase (Fig. S1B). These results clearly demonstrated that the SIV-specific CXCR5+ CD8 T cells expand in LNs and blood following pathogenic SIV infection in vaccinated macaques.
We next investigated the relationship between the magnitude of total, CXCR5+, and CXCR5– Tet+ cells in the LNs and plasma viremia during the chronic phase (Fig. 1D). Impressively, the frequency of CXCR5+ Tet+ CD8 T cells in the LNs showed a strong inverse association with plasma viremia, and this association was not evident with CXCR5– Tet+ CD8 T cells. The magnitude of total Tet+ cells also showed an inverse association with plasma viremia; however, this association was much weaker than the association observed with CXCR5+ Tet+ cells. This association between CXCR5+ CD8 T cells and viral control was not influenced by a specific vaccine group (Fig. S1C). In a limited number of animals, we also observed a strong inverse association between the magnitude of CXCR5+ Tet+ CD8 T cells and multiply spliced SIV TatRev RNA levels in GC–Tfh in LNs (Fig. 1E). A similar inverse association was also observed with cell-associated viral DNA within the Tfh subset (P = 0.003, r = –0.85; Fig. S1D). We also observed a similar association with total Tet+ CD8 T cells but did not with CXCR5– Tet+ CD8 T cells. Collectively, these data demonstrated that the magnitude of CXCR5+ Tet+ CD8 T cells within the LNs potentially contribute to both control of plasma viremia and viral replication within the GC–Tfh during pathogenic SIV infection. These data highlight an important role for a SIV-specific CXCR5+ follicular CD8 T-cell subset in control of viral replication during chronic SIV infection.
SIV-Specific CD8 T Cells Localize to GCs in SIV-Infected Low-VL RMs.
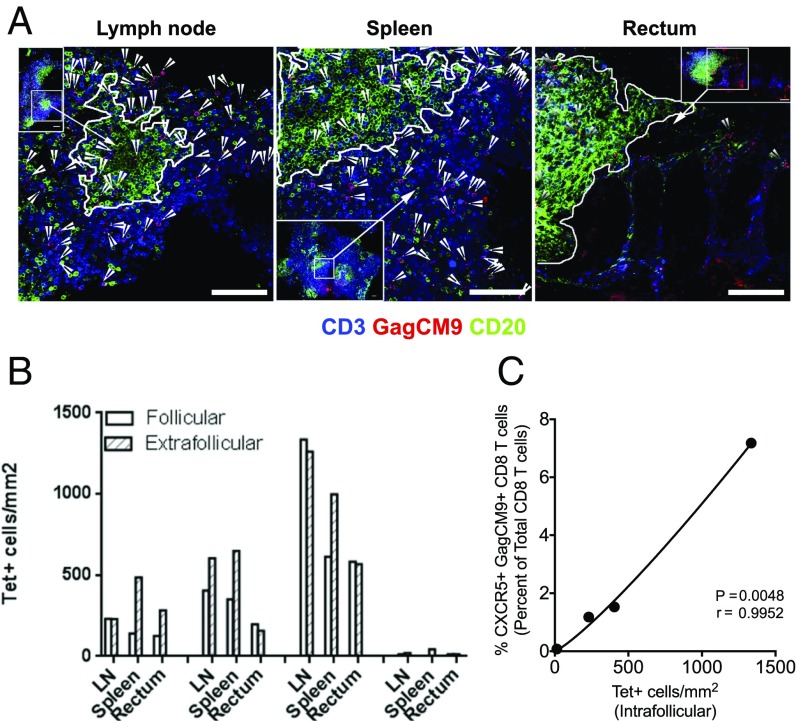
We next investigated the presence and distribution of Tet+ CD8 T cells in the B-cell follicles and extrafollicular regions of LNs, spleen, and rectum of vaccinated and SIV-infected RM with low VL. We carried out in situ Gag CM9 tetramer staining using samples obtained from four vaccinated animals with low VL using antibodies to CD3 and CD20 (the marker used to delineate GC when identifying Tet+ CD8 T cells in situ) (16–18) and Gag CM9 Tetramer. We observed the presence of CD3+ Tet+ cells within both the extrafollicular as well as intrafollicular regions of the sampled lymphoid tissue (Fig. 2A and Fig. S2). The density of Tet+ cells in the follicular and extrafollicular regions was either comparable or marginally higher in the latter region (Fig. 2B). We observed a strong correlation between the frequency of CXCR5+ Tet+ CD8 T cells in the LNs defined by flow cytometry and the density of Tet+ CD8 T cells in GCs determined by in situ tetramer analysis (Fig. 2C). Unfortunately, we could not perform the in situ tetramer analysis on samples obtained from high-VL RMs due to the lack of fresh tissue, which is a requirement for this assay. However, a previous study had demonstrated a relatively lower ratio of Tet+ cells in the follicular region compared with extrafollicular region in RMs with high VL (17). However, using frozen sections, we performed in situ immunofluorescence staining on LN sections of three low-VL and three high-VL RMs to study the localization of total CD8 T cells and found the presence of CD8 T cells in the follicles primarily in the low-VL RMs but not high-VL RMs (Fig. S3A). To confirm that the GC resident CD8 T cells are positive for CXCR5, we stained sections for CD8, CXCR5, and IgD and found that some of the CD8 T cells localized in GC are positive for CXCR5 (Fig. S3B). Taken together, these data demonstrate that antiviral CXCR5+ CD8 T cells in vaccinated RMs with low VL localize to GC regions of lymphoid follicles and this may provide them with an immunological advantage to target and eliminate virus-infected Tfh cells.
Fig. 2.
CXCR5+ CD8 T cells are localized in the GCs of vaccinated low-VL RMs. (A) Representative in situ tetramer staining of Gag CM9 (red), CD20 (green), and CD3 (blue) of LN, spleen, and rectal tissue sections from a SIV low-VL RM showing the presence of Gag CM9 tetramer+ cells in the GC. The confocal images were collected with a 20× objective and each scale bar indicates 100 μm. Arrowheads indicate tetramer+ cells. (B) Density of follicular and extrafollicular tetramer+ cells in LN, spleen, and rectum (n = 4) of four low-VL RMs. (C) Correlation between the frequency of CXCR5+ Gag CM9+ CD8 T cells (% of CD8) measured by flow cytometry and absolute number of follicular Gag CM9+ CD8 T cells measured by in situ tetramer staining (n = 4).
Fig. S2.
Representative in situ tetramer staining images of MLN, spleen, and rectal tissue sections from SIV+-vaccinated controller RMs (n = 3) showing the presence of Gag CM9 tetramer+ cells in lymphoid aggregates. The confocal images were collected with a 20× objective and each scale bar indicates 100 μm. Arrowheads indicate follicular tetramer+ cells.
Fig. S3.
(A) Representative immunofluorescence staining for CD8 (green), IgD (blue), and PD-1 (red) of LN sections from SIV+ noncontroller (n = 3) and SIV+ vaccine controller (n = 3) RMs at week 24 post-SIV infection. (B) Representative immunofluorescence staining of CXCR5 (red), CD8 (green), and IgD (blue) of a LN section from a vaccinated low-VL RM showing the presence of intrafollicular CD8 T cells coexpressing CXCR5. (C) Granzyme B transfer to Tfh cells for the experiment described in Fig. 3D. The confocal images were collected with a 20× objective and each scale bar indicates 100 μm.
SIV-Specific CXCR5+ CD8 T Cells Express Cytolytic Molecules and Limit Expansion of SIV Antigen-Pulsed Tfh in Vitro.
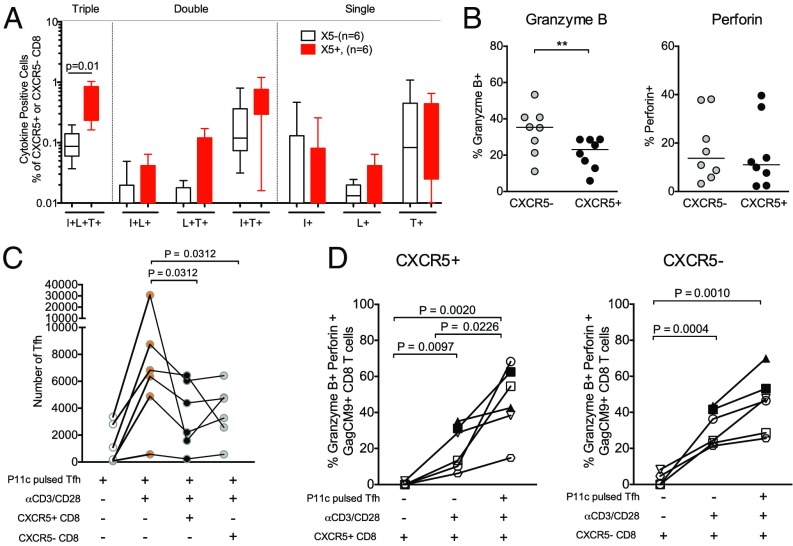
To understand the functional quality of CXCR5+ and CXCR5– SIV-specific CD8 T cells, we characterized their ability to coproduce cytokines IFNγ, TNF-α, and IL-2 in response to stimulation with SIV Gag CM9 peptide (Fig. 3). The frequency of CD8 T cells coproducing all three cytokines (TP) was greater (P = 0.01) in the CXCR5+ subset compared with the CXCR5– subset (Fig. 3A and Fig. S4A). All of these TPs also showed degranulation as evidenced by CD107a expression on the cell surface. These results demonstrated that CXCR5+ cells retain a polyfunctional advantage to CXCR5– CD8 T cells for triple cytokine production, a measure that has been shown to be associated with enhanced control of human chronic viral infections (6, 29).
Fig. 3.
CXCR5+ SIV-specific CD8 T cells express cytolytic molecules and limit SIV antigen-pulsed Tfh expansion in vitro. (A) The frequency of cytokine coexpressing cells in response to P11c (Gag CM9) peptide stimulation. I, IFN-γ; L, IL-2; T, TNF-α. Scatter plots show the median. (B) Expression of granzyme B and perforin on CXCR5+ and CXCR5– Gag CM9 tetramer+ CD8 T cells at week 24 postinfection in the LNs (n = 8). (C) Tfh cell-limited expansion assay showing sorted GC–Tfh cells pulsed with P11c peptide and cultured alone, driven to expand with αCD3/CD28 stimulation with or without autologous sorted CXCR5+ and CXCR5– CD8 T cells from six Mamu A01+ SIV-infected low-VL RMs. (D) Expression of granzyme B and perforin on sorted CXCR5+ and CXCR5– CD8 T cells following a 5-d in vitro Tfh expansion assay (n = 6). **P < 0.01.
Fig. S4.
(A) Representative FACS plots showing TNF-α, IFN-γ, and IL-2 production by CXCR5+ and CXCR5– CD8 T cells in response to Gag CM9 stimulation at week 24 post-SIV infection in the blood of an SIV-infected low-VL RM in response to P11c (Gag CM9) peptide stimulation. (B) Representative FACS plots showing the expression of granzyme B and perforin on CXCR5+ and CXCR5– Gag CM9 tetramer+ CD8 T cells at week 24 postinfection in the LNs. (C) Tfh cell-limited expansion assay showing sorted unpulsed GC–Tfh cells as a control cultured alone and driven to expand with αCD3/CD28 stimulation with or without autologous sorted CXCR5+ and CXCR5– CD8 T cells from six Mamu A01+ SIV-infected low-VL RMs. (D) Representative FACS plots showing the coexpression of granzyme B and perforin on sorted CXCR5+ and CXCR5– Gag CM9+ CD8 T cells following a 5-d in vitro Tfh-limited expansion assay. (E) Representative FACS plots showing the expression of granzyme B on Tfh cells cultured with sorted CXCR5+ and CXCR5– CD8 T cells in the Tfh-limited expansion assay. (F) Plots showing the expression of PD-1 between SIV-specific CXCR5+ and CXCR5– CD8 T cells. CXCR5+ CD8 T cells tend to express higher levels of PD-1 in LNs at week 24 post-SIV infection.
We next characterized the expression of molecules associated with cytolytic function in CXCR5+ and CXCR5– SIV-specific CD8 T cells from the LNs post-SIV infection (Fig. 3B and Fig. S4B). These analyses revealed that a significant fraction of CXCR5+ Tet+ cells express cytolytic molecules granzyme B and perforin; however, granzyme B was marginally lower in CXCR5+ cells compared with CXCR5– CD8 T cells. These results suggested that CXCR5+ CD8 T cells may have the potential to eliminate SIV-infected GC–Tfh cells in vivo. To address this, we sorted Tfh cells from the LNs of six Mamu A*01+ SIV-infected low-VL RMs and stimulated them with anti-CD3 and anti-CD28 antibodies for 5 d in the absence and presence of sorted autologous CXCR5+ or CXCR5– CD8 T cells (Fig. 3C). Tfh cells were additionally pulsed with P11C peptide so they could serve as ideal target cells for the responding CXCR5+ and CXCR5– Tet+ cells present in the coculture. At the end of 5 d, we determined the number of Tfh cells that remained after stimulation with or without CXCR5+ and CXCR5– CD8 T cells. As can be seen in Fig. 3C, the number of Tfh cells increased significantly following anti-CD3/CD28 stimulation in the absence of CD8 T cells. However, inclusion of either CXCR5+ or CXCR5– CD8 T cells in the culture reduced the number of Tfh cells significantly in five out of six animals, demonstrating that both CXCR5+ and CXCR5– cells are capable of limiting the expansion of SIV antigen-pulsed Tfh in vitro. This decrease was not evident when we used unpulsed Tfhs as target cells in four out of five animals tested (Fig. S4C). However, for one animal (#1), we observed a decrease in the number of both pulsed Tfhs and unpulsed Tfhs, but the decrease was much greater for pulsed Tfhs. It is not unexpected to see this decrease in expansion of unpulsed Tfhs in the presence of CD8 T cells, as we are using Tfhs from SIV-infected animals and it is likely that a fraction of these Tfhs are infected with SIV and thus can serve as targets.
To understand the mechanism involved in limiting the expansion of TCR-stimulated Tfh cells, we studied the expression of the cytolytic molecules granzyme B and perforin on responding SIV-specific CD8 T cells and observed a significant increase in the expression of these effector molecules on both CXCR5+ and CXCR5– CD8 T cells (Fig. 3D and Fig. S4D). The increase in coexpression of granzyme B and perforin was higher in the presence of peptide-pulsed target Tfh cells. In addition, to further investigate the mechanism behind the diminished TCR-driven expansion of Tfh cells in vitro, we assessed the transfer of granzyme B to the target Tfh cells when cocultured with the CXCR5+ and CXCR5– CD8 T-cell subsets. We observed a higher frequency of granzyme B+ Tfhs when CXCR5+ or CXCR5– CD8 T cells were included in the culture (Fig. S3C and Fig. S4E), suggesting the cytolytic function of CXCR5+ and CXCR5– CD8 T cells in the culture. Collectively these results demonstrate that both CXCR5+ and CXCR5– CD8 T cells can limit the expansion of antigen-pulsed Tfhs in vitro potentially through cytolytic molecules. However, it is important to note that, in vivo, only CXCR5+ CD8 T cells but not CXCR5– CD8 T cells will likely gain access to the Tfh population due to the expression of CXCR5 and that CXCR5+ CD8 T cells are more polyfunctional than CXCR5– CD8 T cells.
SIV-Specific CXCR5+ CD8 T Cells Share Features of Tfh and Th2 Cells.
To elucidate the differences in cellular processes and functional states between the CXCR5+ and CXCR5– CD8 T-cell subsets during chronic SIV/HIV infection, we performed global gene expression microarray analysis on sorted SIV-specific CXCR5+ and CXCR5– CD8 T cells from SIV-infected RMs with low VL (Fig. 4). For this analysis, we sorted cells from either SIVmac251-infected or SIVE660-infected animals. Interestingly, the CXCR5+ CD8 T cells revealed a distinct gene expression pattern compared with CXCR5– CD8 T cells. Unlike the CXCR5– CD8 T cells, the CXCR5+ CD8 T cells showed higher signal intensities of genes associated with Tfh CD4 T cells, such as the master transcription factor Bcl-6, NFATC1, NFATC2, CD200, and CTLA-4, as well as markers associated with Th2 CD4 T cells, such as IL-4R (CD124), CCR4, STAT6, and IL-10 (Fig. 4A). The signal intensities for effector molecules typically observed in cytotoxic CD8 T cells such as granzyme A, B, and K were lower on SIV-specific CXCR5+ CD8 T cells compared with their CXCR5– counterparts, confirming our ex vivo phenotypic analysis of these effector molecules on the subsets (Fig. 3B). Thus, it is not surprising that CXCR5+ SIV-specific CD8 T cells express lower levels of cytolytic molecules ex vivo, as these cells retain a unique profile similar to Tfh cells and data have shown that overexpression of Bcl-6 in CD8 T cells results in reduced granzyme B expression (30). However, it is important to note that CXCR5+ CD8 T cells rapidly up-regulate expression of some of these genes following stimulation (Fig. 3D); thus, the activation state and exposure to antigen may strongly influence the effector state of CXCR5+ SIV-specific CD8 T cells. Further studies need to be conducted to understand what drives these differential phenotypes in these antiviral CXCR5+ CD8 T cells.
Fig. 4.
Global gene expression analysis revealed distinct gene expression profile for CXCR5+ SIV-specific CD8 T cells. (A) Microarray analysis of sorted CXCR5+ and CXCR5– Gag CM9 tetramer+ CD8 T cells from the LNs of six vaccinated SIV-infected low-VL RMs. The color intensity for heat maps represents expression by CXCR5+ CD8 vs. CXCR5– CD8 T cells. (B) Representative histogram plots and scatter plots showing the expression of the indicated markers on naïve CD8 T cells (CD95–, CD45RA+), CXCR5– and CXCR5+ Gag CM9 tetramer+ CD8 T cells, and Tfh cells. *P < 0.05.
CXCR5+ CD8 T cells also showed higher signal intensities for molecules associated with costimulation/antigen presentation such as CD40, CD83, 41BBL, and MAMU-DRA (Fig. 4A) and inhibitory receptors CD200 and SPRY2 but lower intensities for inhibitory receptors CD160 and CD244 (Fig. 4A). The functional consequence of the expression of these molecules is yet to be determined. Additionally, CXCR5+ CD8 T cells showed higher signal intensities for the antiapoptotic gene Bcl-2 and lower intensity for the proapoptotic genes annexin1 and -2, suggestive of their better survival potential during chronic SIV infection compared with CXCR5– CD8 T cells (Fig. 4A).
The expression of transcriptional regulators such as TCF4, TCF24, SOX4, CULT1, MEF2C, and CDCA7 was also higher in CXCR5+ CD8 T cells. However, the expression of ID2, the transcription factor associated with terminal effectors (31), was lower in CXCR5+ CD8 T cells, suggesting that the CXCR5+ CD8 T cells have transcriptional signatures associated with memory precursor cells that survive and give rise to the pool of long-lived memory CD8 T cells. In addition, CXCR5+ CD8 T cells showed higher expression of chemokines such as CCL5 and CXCL10 and cytokines IL-2 and CSF-1 compared with CXCR5– CD8 T cells. We further confirmed the expression of some of these genes on a protein level using flow cytometry (Fig. 4B). We also measured the level of PD-1 expression and, consistent with the LCMV model, found that CXCR5+ CD8 T cells express marginally higher levels of PD-1 compared with CXCR5– CD8 T cells (Fig. S4F). Collectively, these results demonstrate that SIV-specific CXCR5+ CD8 T cells possess a unique gene expression pattern that is distinct compared with SIV-specific CXCR5– CD8 T cells.
Induction of CXCR5+ CD8 T Cells Can Be Enhanced in Vitro.
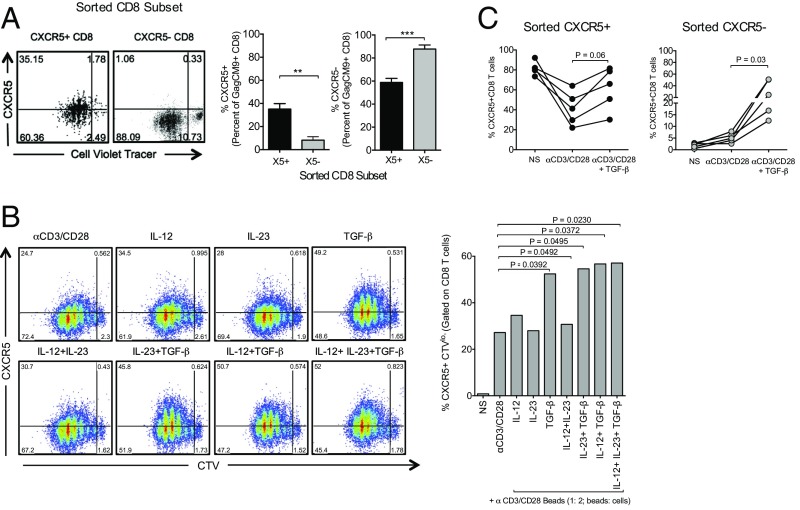
In an effort to understand the modulation of CXCR5 expression following TCR-driven CD8 T-cell proliferation, we sorted CXCR5+ and CXCR5– CD8 T cells from the LNs of chronically infected RMs and stimulated them in vitro with anti-CD3/CD28 (Fig. 5A and Fig. S5). Both CXCR5+ and CXCR5– CD8 T cells proliferated equally, but interestingly the CXCR5+ CD8 T cells differentiated into both CXCR5+ and CXCR5– CD8 T-cell subsets. In contrast, CXCR5– CD8 T cells largely remained CXCR5– (Fig. S5A). This was also true for Gag CM9 Tet+ CD8 T cells in the culture (Fig. 5A). Therefore, CXCR5+ CD8 T cells may potentially gain access to the GC through increased expression of the follicular homing receptor CXCR5 and, then once activated and in response to antigen, can differentiate into a population of CXCR5– CD8 T cells retaining an effector CD8 T-cell profile. One question that remains is what signals may drive the induction or expression of CXCR5 on these SIV-specific CD8 T cells. A recent study found that TGF-β was critical in providing the initial signals required for Tfh differentiation (32). Therefore, we cultured anti-CD3/CD28–stimulated PBMCs in vitro in the presence of TGF-β and found that TGF-β can also significantly increase the expression of CXCR5 on proliferating RM CD8 T cells (Fig. 5B). TGF-β in combination with IL-12 and IL-23 induced higher CXCR5 expression compared with cells stimulated with IL-12 and IL-23 alone. Similar analysis on sorted CXCR5+ and CXCR5– CD8 T cells confirmed that TGF-β could enhance CXCR5 expression on CXCR5– CD8 T cells as well (Fig. 5C).
Fig. 5.
Induction of CXCR5+ CD8 T cells can be enhanced in vitro. (A) CXCR5 expression on purified CXCR5+ or CXCR5– Gag CM9 tetramer+ CD8 T cells on day 5 following stimulation with anti-CD3 and anti-CD28 antibodies. (B) CXCR5 expression on total CD8 T cells on day 5 following stimulation with anti-CD3 and anti-CD28 antibodies in the presence of indicated cytokines (n = 3). (C) CXCR5 expression on sorted CXCR5+ and CXCR5– CD8 T cells on day 3 following stimulation with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β (n = 3). **P < 0.01, ***P < 0.001.
Fig. S5.
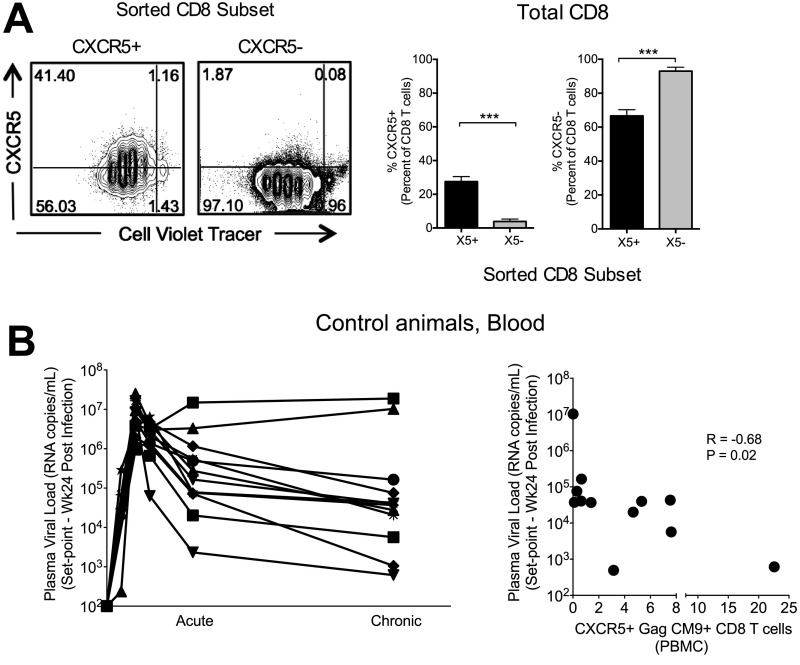
(A) Representative FACS plots showing CXCR5 expression on purified total CXCR5+ or CXCR5– CD8 T cells following a 5-d proliferation assay with anti-CD3 and anti-CD28 antibodies (Left) and the frequency of CXCR5+ and CXCR5– CD8 T cells (% of CD8) (Right). (B) Temporal plasma VL (Left) and association between set point viremia and CXCR5 expression on SIV Gag CM9 tetramer+ CD8 T cells in blood at week 24 postinfection (chronic) in a group of unvaccinated SIV251-infected rhesus macaques. ***P < 0.001.
Discussion
In this study, we focused on investigating the dynamics of follicular homing antiviral CD8 T cells during chronic SIV infection in vaccinated and unvaccinated RMs that showed a varying degree of SIV control. Our findings revealed the rapid expansion of a subset of highly functional antiviral CXCR5+ follicular CD8 T cells with potential to migrate to B-cell follicles/GCs in the LNs and possess a unique phenotype in RMs. Importantly, the expansion of SIV-specific CXCR5+ CD8 T cells in the blood and LNs was associated with vaccine-mediated control of pathogenic SIV infection, suggesting that it is important to generate these cells by vaccination to enhance control of chronic immunodeficiency virus infections.
In this study, we focused our analyses on SIV-specific CD8 T-cell responses in Mamu A*01 animals. We chose to use Mamu A*01 animals because CXCR5 expression is down-regulated following peptide stimulation (Fig. S1E), and the use of Mamu A*01 animals allowed us to use the immunodominant Gag CM9 tetramer to measure the majority of SIV-specific CD8 T cell response without stimulation. In addition, we note that even in unvaccinated SIV-infected animals with varying degrees of viral control, higher CXCR5 expression on Gag CM9 Tet+ CD8 T cells in blood is associated with improved viral control during the chronic phase, highlighting their contribution to enhanced viral control (Fig. S5B).
Our results showed that SIV-specific CXCR5+ CD8 T cells can restrict expansion of SIV antigen-pulsed Tfhs from SIV-infected macaques in vitro, supporting the potential for these cells to limit Tfh expansion in vivo. However, the mechanisms by which these cells are able to restrict Tfh expansion require further investigation. In the current study, several lines of evidence suggested that CXCR5+ CD8 T cells may potentially limit the expansion of antigen-pulsed Tfh through perforin- and granzyme B-dependent mechanisms, as these cells strongly up-regulated these molecules in culture and transferred granzyme B to Tfh. Our attempts to perform a CD8 T-cell-killing assay ex vivo were not successful, as this assay requires a large number of sorted SIV-specific CXCR5+ CD8 T cells.
It is interesting to note that SIV-specific CXCR5+ CD8 T cells express transcripts/proteins associated with Tfh and Th2 cells. This raises the possibility that cytokines and signals that shape a T-helper differentiation program may also help differentiation of CXCR5+ CD8 T cells. We did find that TGF-β, which has been shown to promote Tfh differentiation in human cells, also enhanced CXCR5 expression on CD8 T cells (discussed further below). Another interesting finding was that the CXCR5+ CD8 T cells gave rise to both CXCR5+ and CXCR5– CD8 T cells when stimulated through TCR, supporting their self-renewal and stem cell-like properties. These data also suggest a supporting role that CXCR5+ CD8 T cells may potentially play in regulating an extrafollicular effector CXCR5– CD8 T-cell response during chronic HIV/SIV infection.
It is very important to understand the mechanisms underlying the induction of CXCR5+ CD8 T cells by vaccination and during infection to develop immune-based strategies that can substantially reduce the HIV reservoir and generate effective control of viral replication in the absence of ART. Our in vitro studies showed that TGF-β may play a significant role in inducing these cells. Previous studies showed that TGF-β levels increase significantly following SIV (33) and HIV infections (34). Moreover, pathogenic SIV infection in rhesus macaques is characterized by activation of the smad7 pathway, an inhibitor of the TGF-β1 signaling cascade, whereas nonpathogenic SIV infection in African green monkeys is characterized by prolonged smad4 signaling, a mediator that enhances TGF-β1 signaling, and an absence of smad7 signaling (33). These data suggest that immunoregulatory mechanisms that occur very early during acute infection may potentially influence the phenotypic outcome of antiviral CD8 T cells. Further investigation into early immune signatures will help to address what mechanisms influence the in vivo generation of these CXCR5+ antiviral CD8 T cells. These in vitro studies suggest that the TGF-β pathway could be modulated in vivo to augment induction of antigen-specific CXCR5+ CD8 T cells.
The recently described follicular CXCR5+ CD8 T cells from chronic LCMV and HIV infections (21, 22) have been shown to express transcription factor BCL-6 similar to what we observed in our SIV-specific CXCR5+ CD8 T cells, and selectively entered into B-cell follicles eradicating both infected Tfh and B cells (21). LCMV-specific CXCR5+ CD8 T cells described by Im et al. (23) express TCF-1 and act as stem cells during LCMV infection. Interestingly, these cells reside in the T-cell zones and not in follicles. Further investigation into early immune signatures will help to address what mechanisms influence the in vivo generation of these CXCR5+ antiviral CD8 T cells, but our data in combination with these recently published studies highlight the importance of CXCR5+ CD8 T cells and underscore the need to develop immune-based strategies capable of generating these cells in vivo as a means of targeting and eliminating HIV-infected CD4 T cells that contribute to ongoing viral production and persistence.
In conclusion, our results show a population of SIV-specific CXCR5+ CD8 T cells induced early after infection with potential to migrate to the GC within lymphoid sites and contribute to control of pathogenic SIV. The potential for these cells to infiltrate sites of ongoing viral replication and viral persistence and the ability to induce these cells by vaccination provide a tremendous opportunity to develop and optimize therapeutic strategies that can successfully target and reduce the viral reservoir in lymphoid tissues. Our findings have important implications for functional cure efforts for HIV.
Materials and Methods
Immunizations and Infections.
Young adult (1.5–4.5 y) male Indian rhesus macaques (Macca mulatta) from the Yerkes breeding colony were cared for under the guidelines established by the Animal Welfare Act and the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals (35) using protocols approved by the Emory University Institutional Animal Care and Use Committee. All RMs sampled were unvaccinated or vaccinated with DNA/MVA SIV vaccine (DM). Vaccination consisted of two DNA primes on weeks 0 and 8 and two MVA boosts on weeks 16 and 24. Both DNA and MVA immunogens expressed SIV239 Gag, Pol, and Env, as described previously (36). Vaccinated animals either received unadjuvanted DNA/MVA vaccine (36), CD40L adjuvanted DNA/MVA vaccine (described previously in ref. 36), or unadjuvanted DNA/MVA vaccine with rapamycin. Rapamycin was administered intramuscularly from day –3 to day 28 during each of the DNA and MVA immunizations. The dose of Rapamycin on day 0 of each cycle was 50 μg/kg and was adjusted to reach a serum concentration of 5–15 ng/mL. This resulted in the use at a dose between 10 and 50 μg/kg. All animals were challenged intrarectally weekly with SIVmac251 starting 21–24 wk after the final MVA immunization with a dose of 647 TCID50 (1.25 × 107 copies of viral RNA) until they were infected. All animals were infected by seven challenges under these conditions. N. Miller, NIH, Bethesda, MD, provided the challenge stock.
Phenotyping and Intracellular Cytokine Staining.
Phenotying and intracellular cytokine staining were performed as described previously (37). SI Materials and Methods for details.
Immunofluorescence Staining and in Situ Tetramer Staining.
These procedures were performed as described previously (17). SI Materials and Methods for details.
Microarray Analysis.
SI Materials and Methods for details. Microarray results have been deposited in the Gene Expression Omnibus database (accession no. GSE74751). The data can be accessed using the following link: www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=epmdikuulfmbhmj&acc=GSE74751.
Statistical Analysis.
Statistical analyses were performed using Prism (version 5.0d; GraphPad Software Inc.). Statistical significance (P values) was obtained using nonparametric Mann–Whitney test (for comparisons between groups/subsets), nonparametric paired t test (for comparisons between subsets within the same animal), or Spearman rank test (for correlations). Statistical analyses of cytokine coexpression profiles were performed by partial permutation tests using SPICE software (NIAID, NIH) as previously described (15). For microarray analysis, background adjustment, normalization, and median polish summarization of CEL files were performed with the robust multichip average (RMA) algorithm using the affy Bioconductor package. The quality of hybridized chips was assessed after normalization by examining their NUSE and RLE plots. Downstream analyses were performed using Partek Genomics Suite software version 6.5 (Partek Inc.). P values for the contrasts between CXCR5+ and CXCR5– CD8 T cells in the low-VL RMs were determined by paired t test. To be consistent, we used robust nonparametric tests that do not depend on assumptions of data distribution. A two-sided P value with less than 0.05 was considered as significant. P values were not corrected for multiple comparisons.
SI Materials and Methods
Antibodies.
The following antibodies were used: CXCR5-PE (clone MU5UBEE; eBioscience), CD3-PerCP (Clone SP-34–2; BD Biosciences), CD20-BV650 (Clone 2H7; Biolegend), CD28-PeCy5 (Clone CD28.2; Biolegend), CD95- PE-TR (clone DX2; BD Biosciences), CD279-BV421 (PD-1; Clone EH12.2H7; Biolegend), CD8-V500 (Clone SK1; BD Biosciences), Streptavidin-APC–conjugated P11c tetramer (kindly provided by the R.A. laboratory at Emory University), Ki-67-Pe-Cy7 and Alexa700 (Clone B56; BD Biosciences), CD4-BV650 (Clone OKT4; Biolegend), Granzyme B-Alexa700 (Clone GB11), Perforin-FITC (Clones Pf-80/164; BD Biosciences), CD45RA-PeCy7 (Clone 5H9; BD Biosciences), CCR7-FITC and PE-CF594 (Clone 3D1; BD Biosciences) IL-2-BV605 (Clone MQ1-17H12; Biolegend), IFNγ-Alexa700 (Clone B27; BD Biosciences), TNF-α-PE-CF594 (Clone MAb11; BD Biosciences), CD152-PE-CF594 (CTLA4; Clone BNI3; BD Biosciences), CD83-PeCy5 (Clone HB15e; BD Biosciences), CD200-PeCy7 (Clone MRC OX-104; BD Biosciences), and Bcl-6- PE-CF594 (Clone K112-91; BD Biosciences).
Phenotyping and Intracellular Cytokine Staining.
Phenotyping.
Mononuclear cells were stained with LIVE/DEAD Near-IR Dead Cell at room temperature for 15 min in PBS to stain for dead cells. Cells were then washed with FACS wash, stained on the surface using antibodies specific to respective cell markers, and then treated with 1× BD FACS lysing solution for 10 min at room temperature, permeabilized with 1× BD permeablizing solution for 8 min at room temperature, washed with FACS wash, stained intracellularly using antibodies specific to the respective intracellular markers; washed 2× with FACS wash, and assessed by flow cytometry.
Intracellular cytokine staining.
Mononucelar cells were isolated from the blood, axillary lymph nodes, and rectal tissue, and flow cytometry analysis was performed as described previously (37). Stimulations were conducted in the presence of anti-CD28 antibody and anti-CD49d antibody (1 μg/mL; BD Pharmingen). One million cells were stimulated with either 1 μg/mL of pooled peptides spanning the entire SIV Gag protein (single pool of 125 peptides with each peptide at a concentration of 1.0 µg/mL; NIH AIDS Research and Reference Reagent Program catalog number 6204), 0.1 μg/mL of P11c peptide (gag sequence CTPYDINQM;), or 80 ng/mL of PMA and 1 µg/mL of ionomycin as a positive control. Brefeldin A (5 μg/mL; Sigma) and GolgiStop (0.5 μL/mL; BD Pharmingen) were then added to the cells after 2 h of stimulation, and incubation was continued for 4 h at 37 °C in the presence of 5% CO2. At the end of stimulation, cells were washed once with FACS wash [PBS containing 2% (vol/vol) FBS and 0.25% of sodium azide] and surface stained with anti-CD8, anti-CD4, and LIVE/DEAD Near-IR Dead Cell stain (Life Technologies) at room temperature for 20 min. Cells were then fixed with cytofix/cytoperm (BD Pharmingen) for 20 min at 4 °C and washed with Perm wash (BD Pharmingen). Cells were then incubated for 30 min at 4 °C with antibodies specific to IL-2, IFN-γ, TNF-α, CD107a, and CD3; washed once with Perm wash and once with FACS wash; and resuspended in PBS containing 1% formalin. Cells were acquired on LSR-Fortessa with four lasers (205, 288, 532, and 633 nm) and analyzed using the FlowJo software (Treestar Inc.). At least 50,000 events were acquired for each sample.
Immunofluorescence Staining and in Situ Tetramer Staining.
Immunofluorescence staining.
Freshly isolated lymph node and splenic tissues were snap-frozen in Tissue-Tek OCT. We fixed 0.5-μm sections for 20 min in 100% acetone at –20 °C. Sections were then stained overnight at 4 °C with the following primary antibodies: anti-IgD at 25 µg/mL (Southern Biotech), anti-CD8 5 µg/mL (Abcam, ab4055), anti-CXCR5 at 12.5 μg/mL (eBiosciences, MU5BEE), and anti–PD-1 at 2.5 µg/mL (BioLegend, EH12.2H7). After incubation with primary antibody, sections were washed in PBS for 3 min. Sections were then incubated with the following secondary antibodies: donkey anti-goat IgG Alexa488 at 2 µg/mL (Life Technologies) and donkey anti-rabbit Alexa 546 at 2 µg/mL (Life Technologies) for 2 h at room temperature. Sections were then counterstained with DAPI and mounted in Prolong Anti-Fade Gold reagent and imaged. For detection of CXCR5, staining was carried out on unfixed tissue overnight at 4 °C. After staining with primary antibody, sections were fixed for 20 min at room temperature in 4% (vol/vol) paraformaldehyde. Secondary antibody staining was then carried out as stated above. All images were acquired and analyzed with a Nikon Eclipse 80i microscope using a 20× objective.
In situ tetramer staining combined with immunohistochemistry.
Fresh tissues (lymph node, spleen, and rectum) were sectioned with a compresstome as described (38), and in situ tetramer staining combined with immunohistochemistry, confocal image acquisition, and quantitative image analysis was performed as described previously. We used FITC-labeled Mamu-A*001:01 tetramers loaded with SIV Gag CM9 (181–189) (CTPYDINQM) peptide or with an irrelevant negative control peptide FLP (FLPSDYFPSV) from the hepatitis B viral core protein and antibodies including rabbit anti-FITC (AbDSerotec), rat anti-human CD3 (clone CD3- 12; Thermofisher), mouse anti-human CD20 (Novacastra clone L26; Leica Microsystems), Cy3-conjugated goat anti-rabbit (Jackson ImmunoResearch), Alexa 488-conjugated goat anti-mouse (Molecular Probes), Cy5-conjugated goat anti-rat (Jackson ImmunoResearch), and Dylight 649-conjugated goat anti-human IgM (Jackson ImmunoResearch). For each lymph node and spleen, we analyzed an average of eight follicles (range of 6–12). An average of 1.22 mm2 (range, 0.53–1.91 mm2) was evaluated for lymph node and 1.54 mm2 of tissue (range, 1.49–1.58 mm2) for spleen.
Cell Sorting.
Mononuclear cells isolated from the lymph node were processed and stained with anti-CD3, anti-CD4, anti-CD8, anti-CD279 (PD-1), anti-CXCR5, anti-CD95, and/or P11C tetramer for 25 min at 25 °C and then either CD95+ PD-1negCXCR5+, PD-1posCXCR5+, PD-1negCXCR5+, and PD-1hiCXCR5hi CD4 T-cell populations or CD8+, GagCM9+ CXCR5+, and – CD8 T cells sorted using FACSAriaII (BD). In all sorting experiments, the grade of purity on the sorted cells was >93%.
In Vitro Tfh-Limiting Expansion Assay.
Mononuclear cells isolated from the lymph node of SIV-infected Mamu A*01+ SIV controller RM were processed; stained with Live/Dead IR, anti-CD3, anti-CD4, anti-CD8, anti-CD95, and anti-CXCR5 antibodies; and sorted for CD95+ CD8 T cells and CD95+ CXCR5hi CD4 T cells (Tfh cells) using FACS AriaII (BD). Tfh cells were then pulsed with P11c peptide for 1 h at 37 °C at a concentration of 0.1 μg/mL and washed. CXCR5+ and CXCR5– CD8 T cells were cocultured with unpulsed or pulsed Tfh cells at a 2:1 ratio of CD8 T cells to Tfh cells with no stimulation or anti-CD3/CD28 stimulation at one bead to two cells (Miltenyi Biotech) for 5 d. Cells were then harvested and analyzed using flow cytometry.
Induction of CXCR5 Using Cytokines in Vitro.
PBMCs were stained with Cell Trace Violet (5 μM; Life Technologies) and stimulated with anti-CD3/CD28 at a one bead to two cells ratio (Miltenyi Biotech) for 5 d in the presence of IL-12 (1 ng/mL; eBiosciences), IL-23 (25 ng/mL; eBiosciences), or TGF-β (5 ng/mL; eBiosciences). Cells were harvested; stained with Live/Dead IR, anti-CD3, anti-CD4, anti-CD8, and anti-CXCR5; and analyzed using flow cytometry. Lymph node cells were stained with anti-CD3, anti-CD4, anti-CD8, Live/Dead Fixable Aqua (Life Technologies), anti-CD20, and anti-CXCR5. CXCR5+ and CXCR5– CD8 T cells (CD3+, CD4–, CD20–) were sorted using FACSAriaII (BD) and stimulated with anti-CD3/CD28 at a one bead to two cells ratio (Miltenyi Biotech) and cultured for 4 d in the presence of TGF-β (5 ng/mL). Cells were harvested on day 4 and stained with anti-CD3, anti-CD4, anti-CD8, Live/Dead Aqua, and anti-CXCR5. Cells were then harvested and analyzed using flow cytometry.
SIV Tat/Rev Multiply-Spliced RNA Quantification.
SIV Tat/Rev msRNA were quantified using an ultrasensitive nested RT-PCR. RNA was extracted using the allprep DNA/RNA mini kit (Qiagen) including on-column DNase I treatment, and RNA quantity was measured using Nanodrop analysis. RNA samples (7.5–98 µg) were reverse transcribed and preamplified using the SuperScript III Platinum One-step quantitative RT-PCR (qRT-PCR) kit (Invitrogen). Reactions were performed in a volume of 10 µL with 250 nM of each primer (Tat-Fwd1 and Rev-Rev1) using the following thermal profile: 50 °C for 15 min, 95 °C for 2 min followed by 18 cycles of 95 °C for 15 s, and 60 °C for 4 min. The PCR products were diluted 1:5 in water, and 2 µL of the dilution was used as template for the Tat/Rev seminested quantitative PCR (qPCR) reactions performed in duplicate. Final reactions were performed in 20 µL and contained 1× TaqMan Universal Master Mix II (Applied Biosystems), 500 nM of each primer (Tat-Fwd2 and Rev-Rev1), and 150 nM of the SIV probe. The real-time PCR was carried out in Applied Biosystems 7500 using the following cycling conditions: 50 °C for 2 min, 95 °C for 10 min, 45 cycles of 95 °C for 15 s, 60 °C for 1 min, and 72 °C for 1 s. Serial dilutions of a Tat/Rev plasmid were used for the quantification. Results were normalized to CD4 RNA expression measured by qRT-PCR (adapted from ref. 39). Briefly, cDNA was synthesized using SuperScriptIII first stand synthesis kit (Invitrogen) and random hexamers. qPCR was performed in duplicate in 30 µL, containing 1× TaqMan Universal Master Mix II (Applied Biosystems), 200 nM of each CD4-Fwd and CD4-Rev primers, and 150 nM of the CD4 probe. The real-time PCR was carried out in Applied Biosystems 7500 following the cycling conditions described above. Primer sequences for PCR were as follows: SIV-Tat-Fwd1, 5′-TGA GCA ATC ACG AAA GAG AAG AAG AAC TC-3′; SIV-Tat-Fwd2, 5′-GCT AAG GCT AAT ACA TCT TCT GCA TCA AAC-3′; Rev-Rev1, 5′-TCC ACC GTC TCT TTC TTT GCC-3′; SIVprobe, 5′-FAM-CTC TGG TTG GCA GTG CCG GGT C-3IABkFQ-3′; CD4-Fwd, 5′-ACA TCG TGG TGC TAG CTT TCC AGA-3′; CD4probe, 5′-FAM-AGG CCT CCA GCA CAG TCT ATA AGA AAG AGG-TAMRA-3′; and CD4-Rev, 5′-AAG TGT AAA GGC GAG TGG GAA GGA-3′.
Microarray Analysis.
Briefly, sorted cells from lymph nodes were lysed in 350 μL of Qiagen RLT buffer. Total RNA extraction was then performed using the RNeasy micro kit (Qiagen) according to manufacturer’s specifications with on-column DNase I digestion to remove Genomic DNA. RNA integrity was assessed by Agilent Bioanalyzer (Agilent Technologies) capillary electrophoresis on a RNA Pico chip. All samples had an RNA Integrity Number (RIN) score of 8.5 or higher. For each individual sample, cDNA synthesis and amplification was performed using the NuGEN Ovation Pico WTA V2 system (NuGEN). Briefly, 500 pg of total RNA was used for cDNA synthesis followed by whole transcriptome amplification by NuGEN’s Ribo-SPIA technology. The Ribo-SPIA technology uses DNA/RNA chimeric primers to amplify cDNA isothermally maintaining the stoichiometry of the input RNA. The amplified single-stranded DNA was purified using AMpure XP beads (Beckman). Qualitative and quantitative analyses were performed on the Bioanalyzer and NanoDrop, respectively, to assess the size distribution of the amplified DNA and quantity. We used 4.5 µg of the amplified DNA for biotinylation and fragmentation using the NuGEN Ovation Encore Biotin Module (NuGEN). All samples were hybridized to Affymetrix GeneChip Rhesus Macaque Genome Arrays (Affymetrix), which contains over 52,000 individual probe sets that assay over 47,000 transcripts. The probe arrays were washed, stained, and scanned as described in the Affymetrix GeneChip Expression Analysis Technical Manual (media.affymetrix.com/support/downloads/manuals/expression_analysis_technical_manual.pdf). CEL files were extracted from the raw scanned images using the Affymetrix GeneChip command console software. Quality control metrics were monitored on the Affymetrix Expression console software; discordant arrays were excluded from further downstream analyses.
Acknowledgments
We thank the Yerkes Division of Research Resources and veterinary staff for animal care and procedures, the Emory Flow Cytometry core for cell sorting, Emory Center For Aids Research Virology Core for VL assays, and the NIH AIDS Research and Reference Reagent Program for the provision of peptides. This work was supported by National Institutes of Health Grants R36 AI112787, P01 AI88575, and U19 AI109633 (to R.R.A.) and AI096966 (to P.J.S.); Yerkes National Primate Research Center Base Grant P51 RR00165; and Emory Center for AIDS Research Grant P30 AI050409.
Footnotes
Conflict of interest statement: R.R.A. is a coinventor of DNA/MVA vaccine technology, and Emory University licensed this technology to Geovax Inc.
Data deposition: Microarray results have been deposited in the Gene Expression Omnibus database, www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=epmdikuulfmbhmj&acc=GSE74751 (accession no. GSE74751).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621418114/-/DCSupplemental.
References
- 1.Matano T, et al. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72(1):164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz JE, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 3.Jin X, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189(6):991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migueles SA, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3(11):1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 6.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koup RA, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68(7):4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndhlovu ZM, et al. Magnitude and kinetics of CD8+ T cell activation during hyperacute HIV infection impact viral set point. Immunity. 2015;43(3):591–604. doi: 10.1016/j.immuni.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barouch DH, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290(5491):486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 10.Amara RR, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292(5514):69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457(7225):87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantaleo G, et al. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88(21):9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210(1):143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mylvaganam GH, et al. Diminished viral control during simian immunodeficiency virus infection is associated with aberrant PD-1hi CD4 T cell enrichment in the lymphoid follicles of the rectal mucosa. J Immunol. 2014;193(9):4527–4536. doi: 10.4049/jimmunol.1401222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connick E, et al. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol. 2007;178(11):6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- 17.Connick E, et al. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol. 2014;193(11):5613–5625. doi: 10.4049/jimmunol.1401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukazawa Y, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21(2):132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Förster R, et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87(6):1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 20.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192(11):1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong YA, et al. CXCR5+ follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17(10):1187–1196. doi: 10.1038/ni.3543. [DOI] [PubMed] [Google Scholar]
- 22.He R, et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature. 2016;537(7620):412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 23.Im SJ, et al. Defining CD8(+) T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrovas C, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122(9):3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122(9):3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol. 2012;188(7):3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velu V, et al. Induction of Th1-biased T follicular helper (Tfh) cells in lymphoid tissues during chronic simian immunodeficiency virus infection defines functionally distinct germinal center Tfh cells. J Immunol. 2016;197(5):1832–1842. doi: 10.4049/jimmunol.1600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quigley MF, Gonzalez VD, Granath A, Andersson J, Sandberg JK. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol. 2007;37(12):3352–3362. doi: 10.1002/eji.200636746. [DOI] [PubMed] [Google Scholar]
- 29.Kannanganat S, et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol. 2007;81(21):12071–12076. doi: 10.1128/JVI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida K, et al. Bcl6 controls granzyme B expression in effector CD8+ T cells. Eur J Immunol. 2006;36(12):3146–3156. doi: 10.1002/eji.200636165. [DOI] [PubMed] [Google Scholar]
- 31.Yang CY, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol. 2011;12(12):1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt N, et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15(9):856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ploquin MJ, et al. Distinct expression profiles of TGF-beta1 signaling mediators in pathogenic SIVmac and non-pathogenic SIVagm infections. Retrovirology. 2006;3:37. doi: 10.1186/1742-4690-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poli G, et al. Transforming growth factor beta suppresses human immunodeficiency virus expression and replication in infected cells of the monocyte/macrophage lineage. J Exp Med. 1991;173(3):589–597. doi: 10.1084/jem.173.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Committee on Care and Use of Laboratory Animals 1996. Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 36.Kwa S, et al. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus (SIV) vaccine enhances protection against neutralization-resistant mucosal SIV infection. J Virol. 2015;89(8):4690–4695. doi: 10.1128/JVI.03527-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velu V, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelaal HM, et al. Comparison of Vibratome and Compresstome sectioning of fresh primate lymphoid and genital tissues for in situ MHC-tetramer and immunofluorescence staining. Biol Proced Online. 2015;17(1):2. doi: 10.1186/s12575-014-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kline C, et al. Persistence of viral reservoirs in multiple tissues after antiretroviral therapy suppression in a macaque RT-SHIV model. PLoS One. 2013;8(12):e84275. doi: 10.1371/journal.pone.0084275. [DOI] [PMC free article] [PubMed] [Google Scholar]