Significance
Secondary progressive multiple sclerosis (SPMS) inflicts severe and irreversible disability on the affected individuals. Astrocytes are thought to play a central role in the pathogenesis of SPMS. Here, we demonstrate that Sphingosine-1-receptor (S1PR) modulation suppresses pathogenic astrocyte activation and disease progression in an animal model of SPMS. Using functional in vitro assays, we defined direct effects of S1PR modulation on murine and human astrocytes, as well as astrocyte-mediated effects on microglia and proinflammatory monocytes. Finally, in unbiased transcriptome-wide studies on human astrocytes, we identified candidate targets for the modulation of astrocyte function in SPMS. Collectively, this study sheds light on the pathogenesis of SPMS and evaluates the therapeutic value of S1PR modulation in an animal model of SPMS.
Keywords: multiple sclerosis, sphingolipid metabolism, astrocytes, EAE, secondary progression
Abstract
Multiple sclerosis (MS) is an autoimmune inflammatory demyelinating disease of the CNS that causes disability in young adults as a result of the irreversible accumulation of neurological deficits. Although there are potent disease-modifying agents for its initial relapsing-remitting phase, these therapies show limited efficacy in secondary progressive MS (SPMS). Thus, there is an unmet clinical need for the identification of disease mechanisms and potential therapeutic approaches for SPMS. Here, we show that the sphingosine 1-phosphate receptor (S1PR) modulator fingolimod (FTY720) ameliorated chronic progressive experimental autoimmune encephalomyelitis in nonobese diabetic mice, an experimental model that resembles several aspects of SPMS, including neurodegeneration and disease progression driven by the innate immune response in the CNS. Indeed, S1PR modulation by FTY720 in murine and human astrocytes suppressed neurodegeneration-promoting mechanisms mediated by astrocytes, microglia, and CNS-infiltrating proinflammatory monocytes. Genome-wide studies showed that FTY720 suppresses transcriptional programs associated with the promotion of disease progression by astrocytes. The study of the molecular mechanisms controlling these transcriptional modules may open new avenues for the development of therapeutic strategies for progressive MS.
Multiple sclerosis (MS) is a chronic autoimmune disease of the CNS that, in most patients, initially presents with a relapsing-remitting course. This relapsing-remitting stage is often followed by a secondary progressive phase characterized by the progressive and irreversible accumulation of neurological deficits. The available therapeutic approaches for relapsing-remitting MS (RRMS) show limited efficacy in secondary progressive MS (SPMS), reflecting our insufficient understanding of the pathologic mechanisms that drive disease progression in SPMS and primary progressive MS (1). Recent findings, however, suggest that the innate immune response in the CNS promotes disease progression in MS. Indeed, astrocytes (the most abundant cell population in the mammalian CNS), microglia, and proinflammatory monocytes are thought to promote neurodegeneration, demyelination, and scar formation (1–6). However, therapeutic strategies targeting these cell types remain elusive to date.
Sphingosine 1-phosphate (S1P) is a sphingosine-containing lipid generated from ceramide, which binds G protein-coupled receptors [Sphingosine 1-phospate receptors (S1PRs) 1–5] and modulates the proliferation and trafficking of several cell types, including immune cells. Consequently, S1PRs are considered candidate therapeutic targets for inflammatory diseases, including MS, psoriasis, asthma, and polyneuritis, and also for hematologic and solid tumors, ischemic stroke, and wound healing (7–12).
FTY720 (fingolimod) is a modulator of S1P receptors 1, 3, 4, and 5 with therapeutic effects on RRMS (13–18). The therapeutic effects of FTY720 in RRMS are thought to result mainly from the internalization of S1PR1 in T and B cells, blocking lymphocyte egress from lymph nodes and consequently limiting their recruitment to the CNS (19). FTY720 has also been shown to modulate proinflammatory pathways in B and T cells (19–22). In addition to these effects of FTY720 on the peripheral immune system, phosphorylated FTY720 crosses the blood-brain barrier (BBB) and is thus capable of interacting with CNS-resident cell populations (19, 22, 23).
In vitro observations suggest direct effects of FTY720 on astrocyte biology, neurodegeneration, and remyelination, which impact mechanisms of disease pathogenesis relevant for the progressive stages of MS (20, 22–28). Indeed, animal studies using acute models of RRMS suggest that FTY720 modulates the activity of CNS-resident cell populations (28–31). However, limited information is available on the effects of S1PR modulation on SPMS and its experimental models of CNS chronic inflammation and progressive neurodegeneration. Thus, we investigated the effects of FTY720 on the chronic progressive model of experimental autoimmune encephalomyelitis (EAE) in nonobese diabetic (NOD) mice, which resembles several aspects of SPMS (32). We found that FTY720 ameliorates EAE in NOD mice and decreases the production of proinflammatory and neurotoxic mediators by mouse and human astrocytes. These findings identify potential targets for the modulation of local CNS inflammation driven by astrocytes, microglia, and inflammatory monocytes in SPMS.
Results
FTY720 Ameliorates Chronic Progressive EAE in NOD Mice.
EAE induced in NOD mice by immunization with myelin oligodendrocyte glycoprotein 35–55 (MOG35–55) peptide recapitulates several features of SPMS, including the progressive accumulation of neurodegeneration and axonal loss and the chronic activation of the innate immune system in the CNS (3, 33, 34). Thus, to assess the therapeutic potential of S1P receptor modulators in SPMS, we evaluated the effects of FTY720 on NOD EAE.
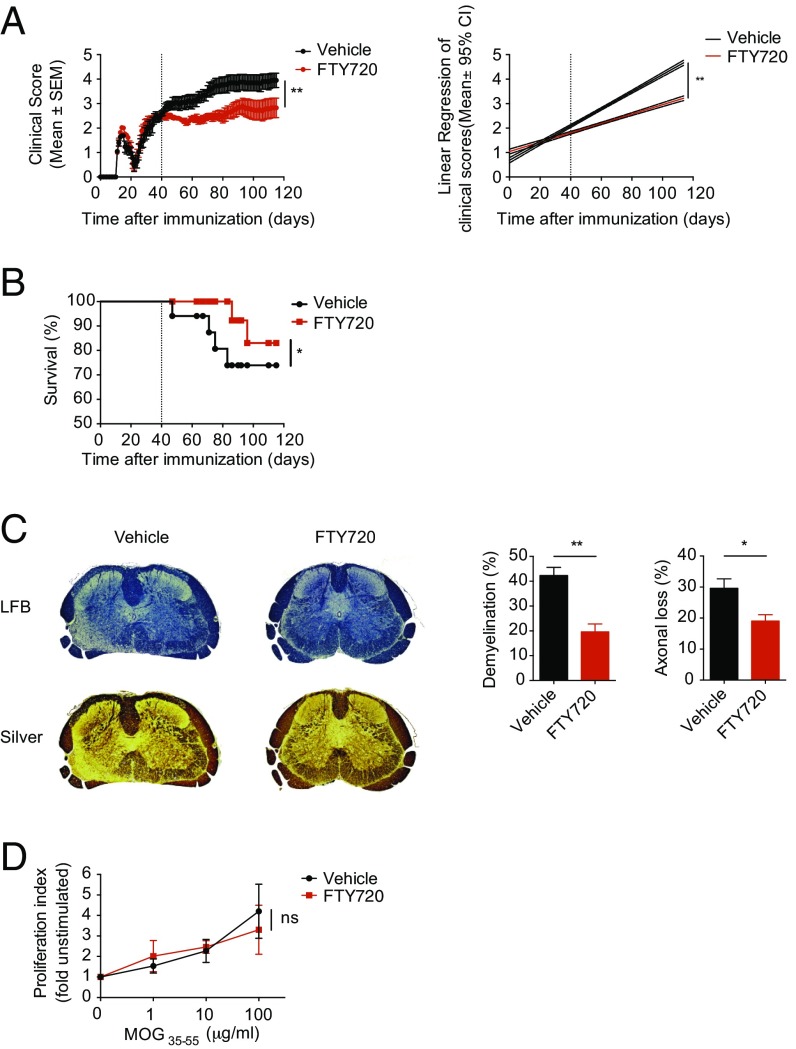
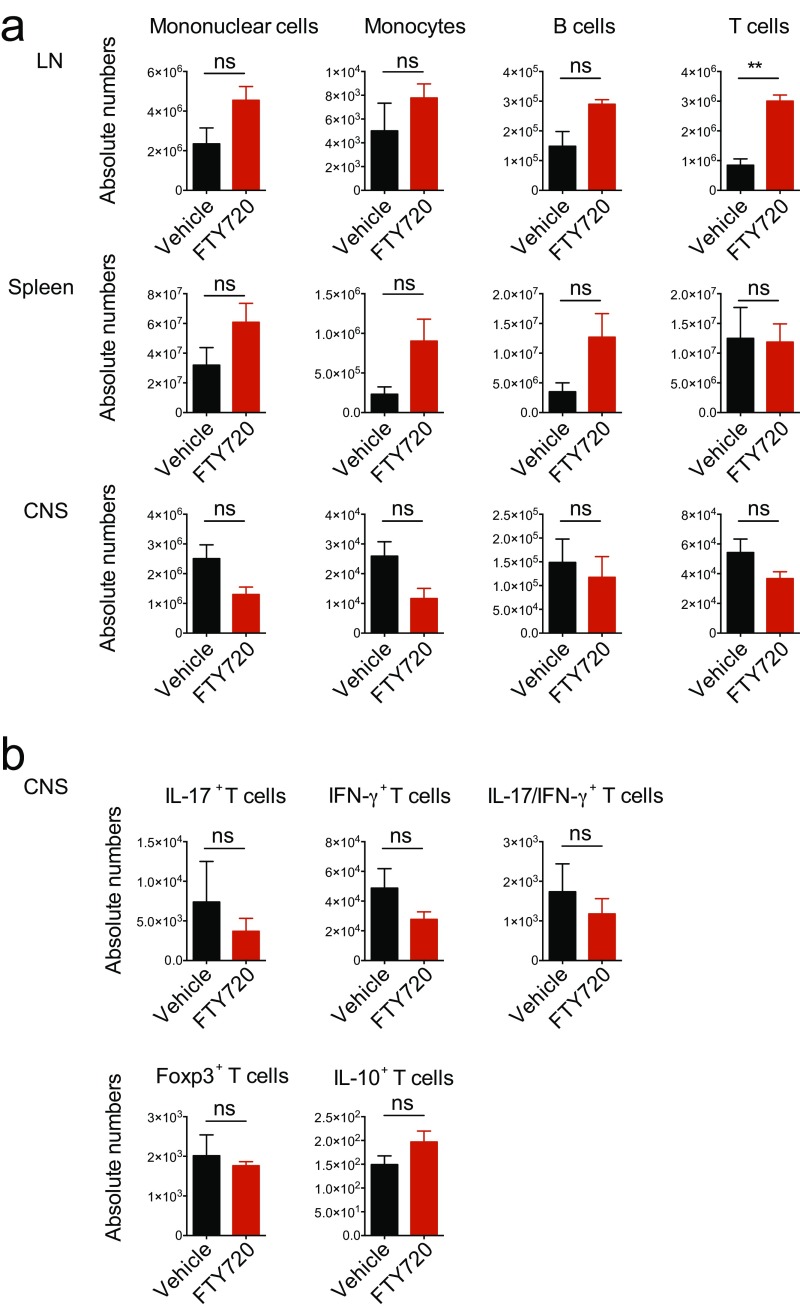
Following immunization with MOG35–55 in complete Freund’s adjuvant (CFA) and pertussis toxin administration, NOD mice initially develop an acute neurological event, which is followed by a chronic progressive phase that starts at approximately day 25 (Fig. 1A). Thus, we initiated treatment with daily doses of FTY720 (0.3 mg/kg body weight) or vehicle 40 d after NOD EAE induction and monitored disease development until day 120. FTY720 ameliorated NOD EAE during the progressive phase, as indicated by the reduction of clinical scores and mortality (Fig. 1 A and B). The beneficial effects of FTY720 in NOD EAE were also reflected in decreased demyelination and axonal loss (Fig. 1C). However, the amelioration of NOD EAE by FTY720 was not linked to alterations in the recall T-cell response to MOG35–55, or with changes in peripheral or CNS immune cell counts (Fig. 1D and Fig. S1). Thus, FTY720 ameliorated progressive NOD EAE without significant effects on the peripheral T-cell response.
Fig. 1.
FTY ameliorates chronic progressive EAE in NOD mice. EAE was induced in NOD/ShiLtJ mice, which were treated with daily i.p. injections of FTY720 or vehicle in the secondary progressive phase of the disease starting from day 40 after disease induction. (A) Clinical scores (Left) and linear regression analysis (Right) of mice under treatment with FTY720 or vehicle (n = 10 mice per group; two-way ANOVA). (B) Kaplan–Meier survival analysis of mice in the experiment described in A by two-way ANOVA. (C) Histologic examination of transversal lumbar spinal cord sections isolated from FTY720- or vehicle-treated mice at day 120. (Left) representative sections stained for Luxol fast blue (LFB) for demyelination or Bielschowsky’s Silver stain (silver) for axonal loss. Representative of three sections of three mice. (Right) Quantification of demyelination and axonal loss in FTY720- or vehicle-treated mice (Student’s t test). (D) Proliferation assay from splenocytes isolated on day 120 of the experiment (n = 5; two-way ANOVA). Throughout, data are mean ± SEM and representative of two independent experiments (*P < 0.05 and **P < 0.01; ns, not significant).
Fig. S1.
Quantification of immune cell subtypes in FTY720- or vehicle-treated NOD EAE mice. EAE was induced in NOD/ShiLtJ mice, which were treated with daily i.p. injections of FTY720 or vehicle in the secondary progressive phase of the disease starting from day 40 after disease induction. (A) Absolute numbers of mononuclear cells, CD11b+CD45highLy6Chigh proinflammatory monocytes, CD19+ B cells, and CD3+CD4+ T cells at day 120 after disease induction in lymph nodes (LN), spleen, and CNS as determined by FACS analysis. (B) Absolute numbers of CNS CD3+CD4+ T cells positive for IL-17, IFN-γ, Foxp3, and IL-10. Data are mean ± SEM and representative of two independent experiments. P values were derived by Student’s t test (**P< 0.01; ns, not significant).
FTY720 Reduces Pathogenic CNS Innate Immune Activation.
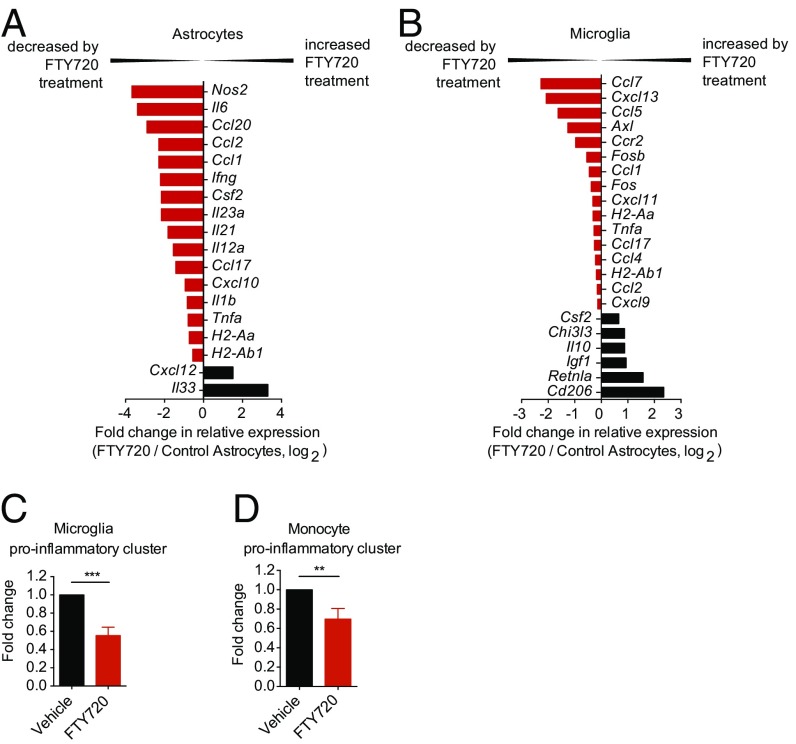
The CNS innate immune response plays a central role in the progressive phase of NOD EAE (1, 3, 5, 6, 35–39). Thus, we analyzed the transcriptional profile of astrocytes, microglia, and proinflammatory monocytes isolated from vehicle- and FTY720-treated mice 120 d after EAE induction using custom-made Nanostring nCounter arrays (Table S1) (2). FTY720 administration down-regulated the expression of proinflammatory cytokines and chemokines in astrocytes, including Il6, chemokine (C-C motif) ligand 2 (Ccl2), Ccl20, Ifng, Il23a, C-X-C motif chemokine 10 (Cxcl10), and Il1b, among others (Fig. 2A). Importantly, neurotoxic mediators such as Tnfa and Nos2 as well as factors governing proinflammatory macrophage polarization, including colony stimulating factor 2 (Csf2) and Il12a, showed diminished expression under FTY720 treatment. Conversely, the expression of anti-inflammatory factors such as Cxcl12 (40) and Il33 (41, 42) was up-regulated by FTY720 (Fig. 2A).
Table S1.
Custom-Made NanoString nCounter code set
| NanoString array | ||||||
| Ahr | Chi3l3 | Fgf2 | Il18 | Mbp | Sirt2 | Traf3ip2 |
| Arg1 | Ciita | Fos | Il18r1 | Mcam | Sirt3 | Tubb5 |
| Arnt | Cltc | Fosb | Il18rap | Megf10 | Sirt4 | Tyro3 |
| Axl | Cntf | Fosl1 | Il1b | Meig1 | Sirt5 | Vcam1 |
| B2m | Csf1 | Fosl2 | Il2 | Mertk | Sirt6 | Vegfa |
| B4galt6 | Csf2 | Foxj1 | Il21 | Mmp11 | Sirt7 | Vim |
| Bach1 | Csf3 | Gapdh | Il21r | Mmp12 | Slc1a2 | Traf3ip2 |
| BAG3 | Cspg4 | Glul | Il23a | Mmp2 | Socs3 | Tubb5 |
| Bcan | Ctla4 | Glycam1 | Il27 | Mmp3 | Sox8 | Tyro3 |
| Bcl2 | Cx3cl1 | Gusb | Il27ra | Mmp9 | Spp1 | Vcam1 |
| Bcl3 | Cx3cr1 | H2-Aa | Il33 | Cd206 | Sra1 | Vegfa |
| Beclin1 | Cxcl10 | H2-Ab1 | Il4 | Msx1 | Stat1 | Vim |
| Ccl1 | Cxcl11 | H2-Ea | Il4ra | Msx2 | Stat2 | |
| Ccl17 | Cxcl12 | Helz2 | Il5 | cMyc | Stat3 | |
| Ccl19 | Cxcl13 | Hif1a | Il6 | Ncan | Stat4 | |
| Ccl2 | Cxcl14 | Hmox1 | Il7r | Nfe2l2 | Stat5a | |
| Ccl20 | Cxcl15 | Hprt1 | Irf1 | Nfil3 | Stat6 | |
| Ccl3 | Cxcl16 | Hsp90 | Irf2 | Ngf | Tbk1 | |
| Ccl4 | Cxcl2 | Icam1 | Irf3 | Nos2 | Tgfb1 | |
| Ccl5 | Cxcl3 | Ifih1 | Irf4 | Nqo1 | Tgfb2 | |
| Ccl7 | Cxcl9 | Ifnar1 | Irf5 | Nr1d1 | Tgfb3 | |
| Ccl8 | Cxcl8 | Ifnb1 | Irf6 | Cd73 | Timp1 | |
| Ccr2 | Cxcr4 | Ifng | Irf7 | Ntf3 | Tiparp | |
| Ccr5 | Cyp1a1 | Igf1 | Irf8 | Ntf4 | Tlr1 | |
| Cd14 | Cyp1b1 | Il10 | Irf9 | Pdgfa | Tlr11 | |
| Cd163 | Ddx58 | Il10ra | Itga7 | Pdgfb | Tlr12 | |
| Cd209 | Dhx58 | Il11 | Itgam | Pgk1 | Tlr2 | |
| Cd24a | Ebi3 | Il12a | Itgax | Ptgs1 | Tlr3 | |
| Cd36 | Emr1 | Il12b | Keap1 | Ptgs2 | Tlr4 | |
| Cd38 | Entpd1 | Il12rb1 | Lif | Rela | Tlr5 | |
| Cd40 | Era | Il12rb2 | Lifr | Relb | Tlr6 | |
| Cd80 | Esrra | Il13 | Ly6c1 | Retnla | Tlr7 | |
| Cd83 | Esrrb | Il15 | Ly6g | Runx1 | Tlr8 | |
| Cd86 | Fas | Il15ra | Maf | Sele | Tlr9 | |
| Brunol4 | Fasl | Il17ra | Marco | Sirt1 | Tnfa | |
Fig. 2.
FTY720 modulates activation of astrocytes, microglia, and proinflammatory monocytes. Astrocytes, microglia, and proinflammatory monocytes were isolated by FACS sorting at day 120 of EAE, and RNA was subjected to custom-made nCounter Nanostring arrays. (A and B) Fold change in mRNA expression of the indicated genes from sorted astrocytes (A) and microglia (B) from FTY720- or vehicle-treated mice at day 120 of EAE as determined by NanoString analysis [fold change in relative expression as determined by log2(FTY720/vehicle)]. Data are representative of two independent experiments of pooled astrocytes and microglia from three mice per group. (B and C) NanoString analysis of proinflammatory gene clusters (Table S2) from sorted microglia (C) and Ly6C1hi proinflammatory monocytes (D). Data are ratio of count numbers of cells from FTY720-treated to vehicle-treated mice, and are representative of two independent experiments of pooled microglia and Ly6Chi proinflammatory monocytes with three mice per group. Data are mean ± SEM (**P < 0.01 and ***P < 0.001, Student’s t test).
Table S2.
Proinflammatory gene cluster used for NanoString analyses
| Proinflammatory gene cluster | ||||
| Ccl4 | Cd83 | H2-ab | Il15 | Il23a |
| Ccl5 | Cd86 | H2-Ea | Il15ra | Irf5 |
| Ccl8 | Cxcl9 | Marco | Il12a | Nos2 |
| Ccl19 | Cxcl10 | Icam | Il12b | Ptgs2 |
| Ccl20 | Cxcl11 | Il1b | Il12ra | Socs3 |
| Cd40 | Cxcl13 | Il6 | Il18 | Stat1 |
| Cd80 | H2-Aa | Il7r | Il18r | Tnfa |
Microglia and CNS-infiltrating proinflammatory monocytes are thought to play an important role in the pathogenesis of SPMS and the progressive phase of NOD EAE (36, 37, 43, 44). We thus analyzed the transcriptional profile of microglia and CNS-infiltrating CD11b+CD45+Ly6Chi proinflammatory monocytes in vehicle- and FTY720-treated mice. We detected decreased expression of proinflammatory gene clusters associated with NOD EAE pathology (2, 3) in microglia and CNS-infiltrating proinflammatory monocytes (Fig. 2 B–D). Of note, the down-regulation of proinflammatory gene expression was stronger in microglia than in monocytes. Moreover, microglia displayed strong up-regulation of factors expressed in alternatively activated microglia such as Csf2, Chi3l3, and Cd206 in FTY720-treated animals (Fig. 2B). In summary, FTY720 decreased the expression of proinflammatory, chemoattractant, and neurotoxic molecules thought to mediate the pathogenic role of astrocytes, microglia, and CNS-infiltrating monocytes in MS and NOD EAE.
Astrocytic S1PR Controls Astrocyte Neurotoxicity and Monocyte Recruitment and Activation.
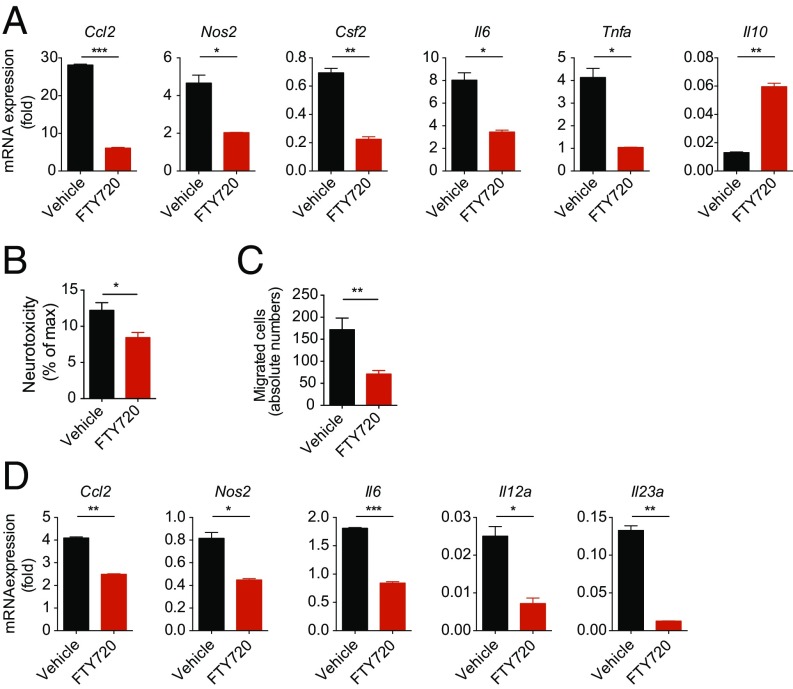
S1PR1 expression in astrocytes contributes to the protective effects of FTY720 in acute EAE (22), but the mechanisms involved are not completely understood. We detected decreased expression of proinflammatory chemokines (Ccl2), cytokines (Csf2, Il6, Tnfa), neurotoxic (Nos2, Tnfa), and macrophage polarizing factors (Csf2) in highly purified primary astrocyte cultures (Fig. S2) activated in the presence of FTY720 in vitro. Conversely, FTY720 up-regulated the expression of neuroprotective Il10 (Fig. 3A). Thus, S1PR modulation in astrocytes affects the expression of mediators thought to promote disease progression in MS and NOD EAE.
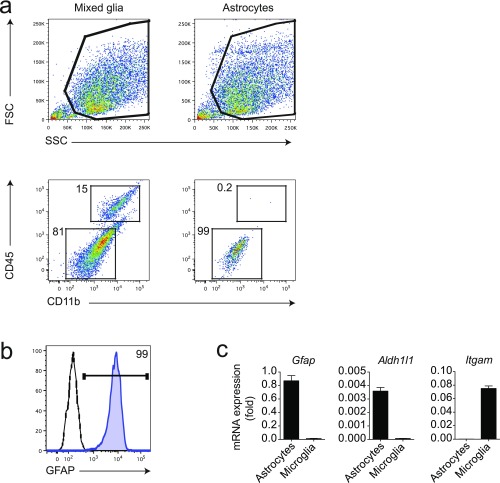
Fig. S2.
Purity control of in vitro astrocyte cultures. (A) FACS staining of mixed glial cultures and highly pure astrocyte cultures for CD11b and CD45. Numbers indicate percentages in respective gates. (B) Intracellular staining of astrocyte cultures for GFAP. Numbers indicate percentages in respective gate. (C) qPCR analysis for indicated genes of astrocyte and microglia cultures. Data are mean ± SEM and are representative for routine purity controls as performed for each astrocyte culture used in the experiments outlined.
Fig. 3.
FTY720 modulates proinflammatory activation of astrocytes. Primary cultures of murine astrocytes were activated with LPS and treated with FTY720 or vehicle and analyzed for gene expression, neurotoxic and chemotaxic mediator production, as well as monocyte polarizing properties. (A) RNA expression of indicated genes from astrocytes isolated 24 h after activation and treatment from three biological replicates. (B) Neurotoxicity assay with supernatants of activated astrocyte cultures treated with FTY720 or vehicle. (C) Migration assays with supernatants from activated FTY720- or vehicle-treated astrocytes using CD11b+Ly6C1hi monocytes as migrating cells. (D) qPCR analysis for expression of the indicated genes from sorted CD11b+Ly6C1hi proinflammatory monocytes that were cocultured with activated FTY720- or vehicle-treated astrocytes and reisolated thereafter for RNA analysis. Throughout, data are mean ± SEM and representative of three independent experiments with three biological replicates. P values were derived by Student’s t test (*P < 0.05, **P < 0.01, and ***P < 0.001).
Astrocytes display neurotoxic activities in the context of chronic CNS inflammation and also induce and amplify pathogenic activities in microglia and monocytes recruited to the CNS (40, 45–51). Thus, to evaluate the relevance to disease pathogenesis of the effects of FTY720 on the transcriptional program of astrocytes, we analyzed the effects of FTY720 on the neurotoxic potential of astrocytes and on their ability to control migration and monocyte polarization (2).
Astrocytes promote neuronal death through the production of TNF-α, glutamate, lactate, and reactive oxygen species, among others (1, 6, 49). Thus, we used a cell-based assay to evaluate the effects of FTY720 on astrocyte-driven neurotoxicity. In this assay, we activated astrocytes in vitro in the presence of FTY720 or vehicle for 24 h and then added new culture medium after extensive washings. Following culture for additional 48 h, astrocyte-conditioned medium (ACM) was collected. To evaluate the neurotoxic activity of ACMs, we exposed neuronal cells to the ACMs and monitored neuronal death by quantifying lactate dehydrogenase (LDH) release. FTY720 treatment led to a small but significant reduction of ACM neurotoxic activity (Fig. 3B).
During MS and EAE, proinflammatory monocytes are recruited to the CNS, where they play a central role in promoting neurodegeneration (2, 36, 37, 39). Thus, chemotactic factors produced by astrocytes play an important role in promoting disease progression in MS (40, 52, 53). To address the effects of FTY720 on the recruitment of Ly6Chi proinflammatory monocytes by astrocytes we used an in vitro transwell migration assay. We found that FTY720 decreased Ly6Chi inflammatory monocyte recruitment by ACM (Fig. 3C).
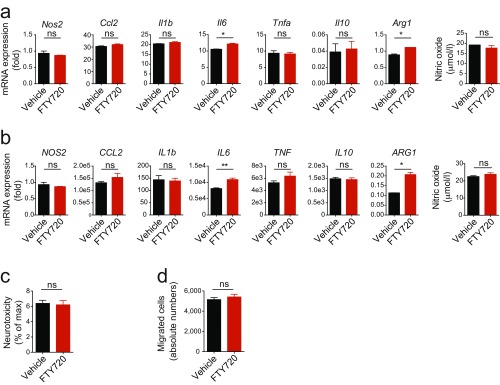
Astrocytes also modulate myeloid cell activation and polarization in the CNS (1, 54). To test the effects of FTY720 on the control of monocytes by astrocytes, we activated astrocytes in the presence of FTY720 or vehicle for 24 h, washed them extensively, and established cocultures with sorted Ly6Chi inflammatory monocytes. Following coculture for 24 h, the monocytes were reisolated and their transcriptional profile was analyzed. Treatment of astrocytes with FTY720 decreased the expression of proinflammatory cytokines, chemokines, and neurotoxic molecules in monocytes (Fig. 3D). Mouse and human activated microglia, however, did not exhibit significant changes in the expression of proinflammatory cytokines or neurotoxic mediators upon exposure to FTY720 (Fig. S3 A and B). Also, neurotoxicity and induction of migration was not altered in supernatants of activated microglia upon treatment with FTY720 (Fig. S3 C and D).
Fig. S3.
Exposure of mouse and human microglia to FTY720 does not dampen proinflammatory activation. Mouse and human microglia were activated and treated with FTY720 or vehicle. (A and B, Left) qPCR analysis of indicated genes expressed in activated murine (A) or human (B) microglia under FTY720 or vehicle treatment 24 h after activation. (A and B, Right) Measurement of NO content in supernatant of FTY720- or vehicle-treated microglia (n = 3 biological replicates). Data are mean ± SEM and representative of three independent experiments. (C and D) Assessment of neurotoxicity (C) and migration induction (D) in supernatants from activated murine microglia under treatment with FTY720 or vehicle. Data are mean ± SEM and representative of two independent experiments. P values were derived by Student’s t test (ns, not significant).
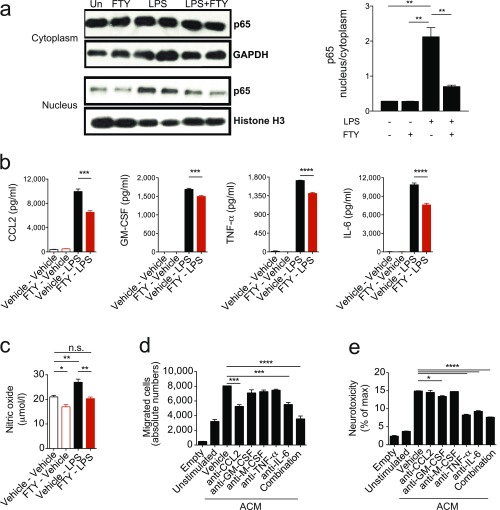
FTY720 treatment led to a significant suppression of NF-κB p65 nuclear translocation in activated astrocytes (Fig. S4A), concomitant with decreased production of the proinflammatory and neurotoxic mediators IL-6, TNF-α, GM-CSF, CCL2, and nitric oxide (NO; Fig. S4 B and C). Indeed, in blocking experiments, we identified CCL2 and IL-6 as the active components in ACM driving monocyte migration in vitro, whereas TNF-α, GM-CSF, and IL-6 mediated astrocyte neurotoxic activity (Fig. S4 D and E).
Fig. S4.
FTY720 dampens proinflammatory activation of astrocytes. Astrocytes were activated with LPS or vehicle in the presence or absence of FTY720. (A) Western blot analysis for NF-κB (p65; Left) and quantification of the ratio of nuclear to cytoplasmic fraction of NF-κB p65 (Right) of astrocytes stimulated as indicated. (B and C) Quantification of active components in ACM generated from astrocytes treated as indicated by ELISA and colorimetric analysis (NO). (D and E) Migration and neurotoxicity assays using ACM from astrocytes treated as indicated in the presence of individual or combined blocking antibodies. Data are mean ± SEM and are representative of three independent experiments. P values were derived by one-way ANOVA followed by Tukey’s multiple-comparisons test (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns, not significant).
Collectively, these studies suggest that S1PR modulation in astrocytes by FTY720 contributes to the amelioration of chronic progressive NOD EAE.
FTY720 Modulates Activation of Human Astrocytes.
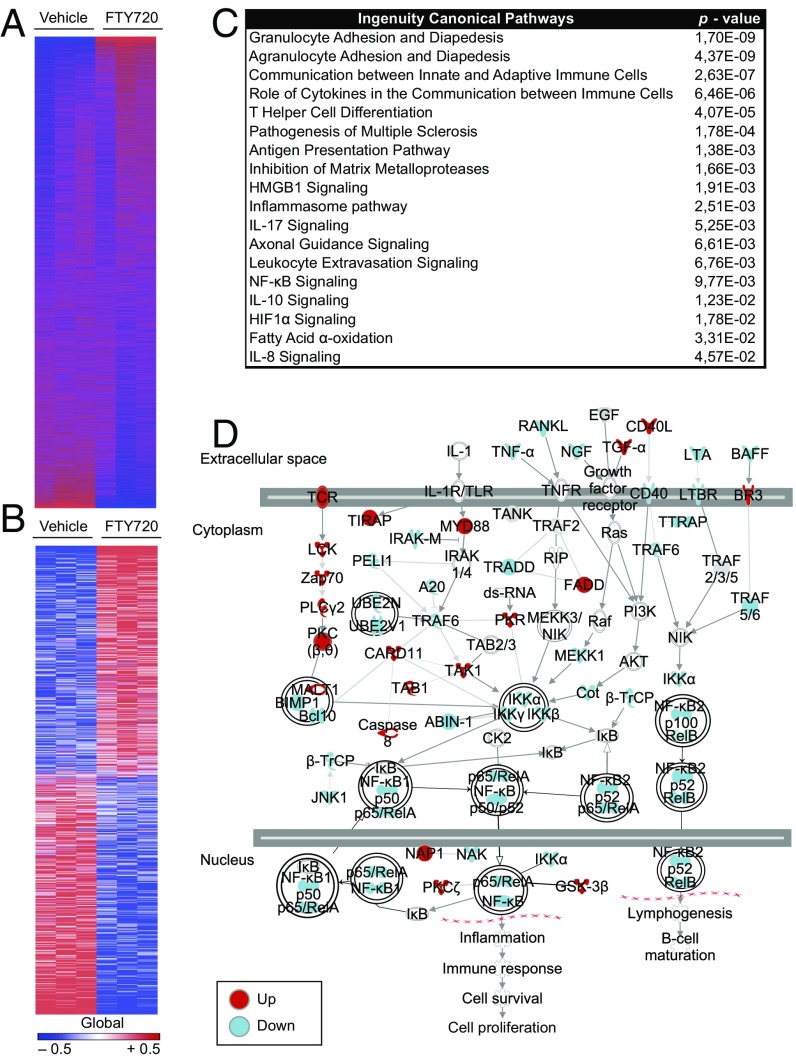
To further investigate the relevance of S1PR modulation in astrocytes to its potential effects on MS, we analyzed the genome-wide transcriptional response of human astrocytes activated in vitro in the presence of FTY720. We identified 1,221 transcripts that were differentially regulated under FTY720 treatment (Fig. 4 A and B). Ingenuity pathway analysis determined that FTY720 modulates the expression of transcriptional modules associated with migratory pathways, antigen presentation, inflammasome activation, axonal guidance, and fatty acid α-oxidation (Fig. 4C). Interestingly, NF-κB signaling was significantly altered by FTY720 in human astrocytes (Fig. 4C), recapitulating our findings of decreased NF-κB p65 activation in mouse astrocytes in vitro (Fig. S4A). NF-κB plays a central role in the control of astrocyte activities that promote inflammation and neurodegeneration (2, 40). Thus, we performed an in-depth computational analysis of the NF-κB pathway, which confirmed that FTY720 down-modulates NF-κB signaling in human astrocytes (activation z-score, −1.89; Fig. 4D).
Fig. 4.
Unbiased analyses reveal down-modulatory effects of FTY720 on human astrocytes. Primary human fetal astrocytes were activated with IL-1β in the presence or absence of FTY720, and gene expression was assessed by Affymetrix arrays. (A) Heat map of 16,799 expressed (detected at level 0.1 in at least two of three samples) and 1,221 differentially regulated (B) genes (signal:noise ratio) of activated FTY720- or vehicle-treated human primary astrocytes as assessed by Affymetrix assay. Data represent three replicates of three independent astrocyte cultures. Gene expression levels are row-centered and log2-transformed, and saturated at levels −0.5 and +0.5 for visualization satisfying a false discovery rate (FDR) < 0.1. (C) Ingenuity pathway analysis of the transcriptional profile of activated human primary astrocytes under treatment with FTY720 or vehicle. (D) Ingenuity pathway analysis diagram of NF-κB signaling pathways comparing FTY720 vs. vehicle treatment. Colors code for up- and down-regulation of individual members in red (up) and blue (down).
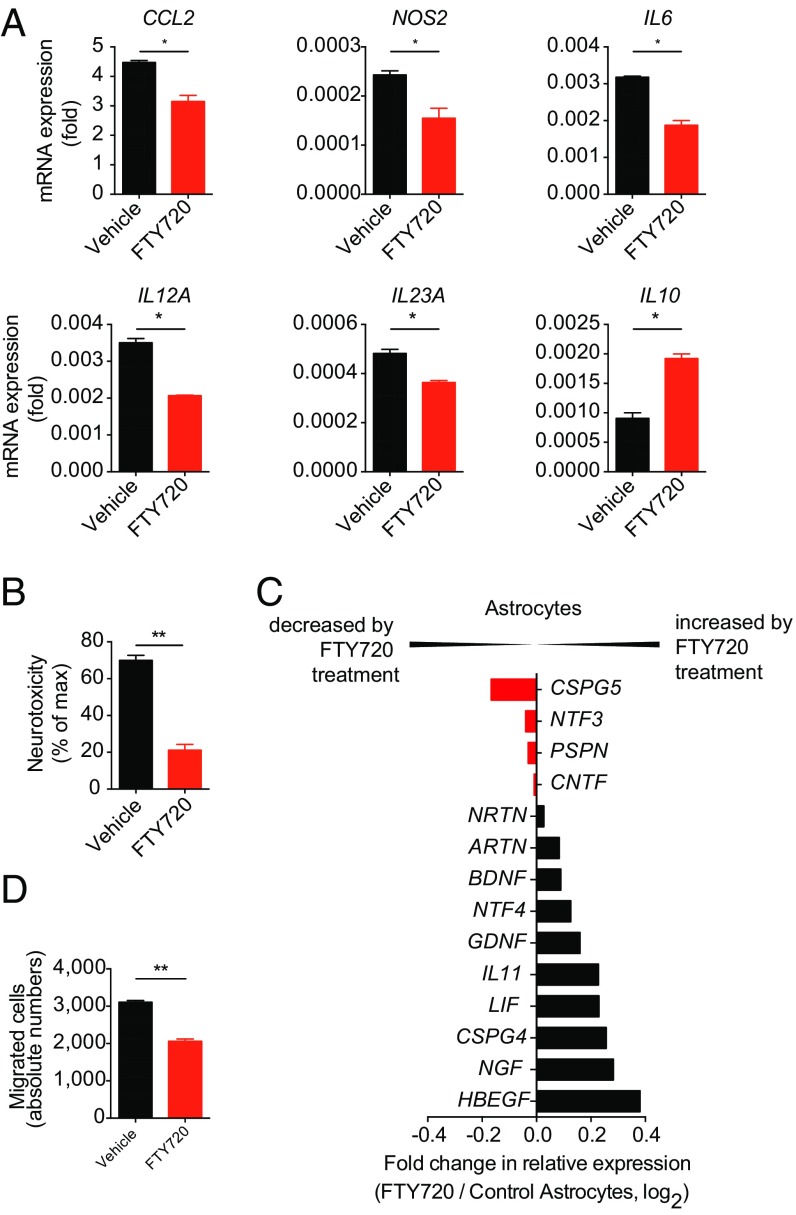
To evaluate the functional relevance of our transcriptional analyses, we validated the expression of selected transcripts on independent samples and examined the functional effects of FTY720 on human astrocytes. FTY720 decreased the expression of proinflammatory and neurotoxic mediators, including cytokines, chemokines, and degenerative mediators, while increasing anti-inflammatory IL10 in human astrocytes, as determined in quantitative PCR (qPCR) analyses from separate sets of samples (Fig. 5A). Moreover, ACMs from FTY720-treated human astrocytes exhibited a marked reduction in neurotoxic and chemotactic properties, concomitant with an increased production of neuroprotective mediators (Fig. 5 B–D). Human microglia, however, did not show a significant response to exposure to FTY720 (Fig. S3B).
Fig. 5.
FTY720 mitigates proinflammatory and neurotoxic properties of human astrocytes. Human fetal astrocytes were activated and treated with FTY720 or vehicle. (A) qPCR analysis of indicated genes expressed in activated astrocytes under FTY720 or vehicle treatment 24 h after activation (n = 3 biological replicates). (B) Neurotoxicity assay with supernatants of activated human primary astrocytes treated with FTY720 or vehicle (n = 3 biological replicates). (C) Fold change in mRNA expression of the indicated genes in human activated astrocytes treated with FTY720 or vehicle as determined by Affymetrix arrays [fold change in relative expression as determined by log2(FTY720/vehicle)]. (D) Migration assays with supernatants from activated FTY720- or vehicle-treated human primary astrocytes using CD11b+Ly6C1hi proinflammatory monocytes as migrating cells (n = 3 biological replicates). Throughout, data are mean ± SEM and representative of three independent experiments. P values were derived by Student’s t test (*P < 0.05, **P < 0.01, and ***P < 0.001).
Collectively, these findings reveal an overall neuroprotective effect of FTY720 on human astrocytes mediated, at least in part, by the suppression of NF-κB signaling. In addition, these findings highlight targets of FTY720 on astrocytes, which may guide novel potential therapeutic interventions for the modulation of astrocyte function during progressive MS.
Discussion
The immunosuppressive and disease-ameliorating effects of FTY720 in RRMS are mostly attributed to the blockade of inflammatory cell migration into the CNS (19, 28). Phosphorylated FTY720, however, crosses the BBB and acts on CNS-resident cells, including astrocytes (22, 24, 26). The effects of FTY720 in astrocytes, neurons, and oligodendrocytes have been previously documented in vitro (26, 27, 55, 56). Given the beneficial effects of FTY720 on organotypic cultures in vitro and its BBB permeability (27, 56), FTY720 has the potential to modulate the local innate immune response in the CNS, thought to contribute to disease progression in MS (1, 6, 49). However, the effects of FTY720 on chronic CNS-innate immune responses in vivo, as well as their relevance to the pathogenesis of disease progression in MS, are still unknown.
Our studies highlight the anti-inflammatory and neuroprotective effects of S1PR modulation on mouse and human astrocytes at transcriptional and functional levels. Moreover, we evaluated the biological relevance of these observations in the NOD EAE model (32). In the chronic phase of this EAE model, disease progression is driven by the pathogenic activity of astrocytes, microglia, and inflammatory monocytes, without a major contribution of the adaptive immune system (e.g., T cells), thus recapitulating several aspects of SPMS (3, 32, 57, 58). Our data show that FTY720 ameliorates progressive CNS inflammation and neurodegeneration, most likely as a result of its CNS-intrinsic effects on astrocytes, microglia, and proinflammatory monocytes. Of note, our in vitro data on microglia point to an indirect effect of FTY720 on microglia mediated by the modulation of astrocyte-derived factors that influence microglia polarization and pathogenic activities.
Five isoforms of S1P receptors are expressed by immune and nonimmune cells relevant to autoimmune diseases, stroke, and cancer (11, 12, 59–62). Thus, S1P receptors constitute attractive targets for therapeutic intervention. Indeed, S1PR modulation by FTY720 is efficacious in the treatment of RRMS (13, 14). S1PR modulation did not reach the primary endpoints in the INFORMS (oral fingolimod in primary progressive multiple sclerosis) trial in primary progressive MS (63), but ameliorated MRI parameters of disease activity (63). BAF312 (siponimod) is a BBB-permeable modulator of S1P1 and S1P5 receptors effective in slowing down demyelination in vitro (64–66). The safety and therapeutic efficacy of BAF312 on RRMS has been recently demonstrated in terms of clinical and MRI parameters in the phase II BOLD (BAF312 on MRI lesion given once daily) study (67). The therapeutic effects of BAF312 on SPMS are currently being investigated in the EXPAND (exploring the efficacy and safety of siponimod in patients with secondary progress multiple sclerosis) trial. Based on the effects of FTY720 on the CNS-innate immune response during NOD EAE, S1P receptor modulation by BAF312 is likely to modulate local CNS inflammation in SPMS. Indeed, preliminary results of the EXPAND trial suggest a protective effect of BAF312 on 3-mo confirmed disease progression in SPMS (68). However, side-by-side comparisons of the effects of FTY720 and BAF312 on adaptive and innate immune cells are needed to determine whether the structural differences existing between these compounds affect their biologic activities on specific cell populations relevant to SPMS. Indeed, it is worth noting that, whereas FTY720 modulates S1P1, S1P3, S1P4, and S1P5 receptors, BAF312 modulates S1P1 and S1P5 receptors only.
FTY720 is also reported to modulate sphingolipid metabolism, which is thought to promote disease progression in NOD EAE and MS (3, 19, 23, 69, 70). Indeed, we showed that inhibitors of B4GALT6 and lactosyl ceramide production suppress disease progression in NOD EAE by arresting local CNS-innate immunity and neurodegeneration (3). The arrest of NOD EAE obtained with B4GALT6 inhibitors is stronger than the amelioration obtained with FTY720, thus calling for caution as to the therapeutic potency of FTY720 and its analog BAF312 in SPMS. Moreover, these findings suggest that the beneficial effects of FTY720 on NOD EAE may reflect its moderate inhibitory effects on sphingolipid metabolism.
Finally, our unbiased genome-wide analyses identify additional potential targets for the therapeutic modulation of astrocyte function and the CNS-innate immune response, which may guide the development of novel therapeutics for the treatment of progressive MS.
Materials and Methods
Mice.
Female NOD/ShiLtJ mice and 1–3-d-old pups from C57BL/6J mice were obtained from the Jackson Laboratory and were kept in a pathogen-free facility at the Harvard Institutes of Medicine. All experiments were carried out in accordance with guidelines prescribed by the institutional animal care and use committee at Harvard Medical School.
EAE Induction and Treatment.
EAE was induced in 8-wk-old mice by s.c. immunization with 150 µg MOG35–55 peptide emulsified in CFA (Difco Laboratories) per mouse, followed by administration of 200 ng pertussis toxin (List Biological Laboratories) on days 0 and 2 as described previously (2, 3). Starting from day 40 after disease induction, mice were treaty daily with i.p. injections of FTY720 0.3 mg/kg body weight or vehicle, respectively.
Generation of Astrocyte- and Microglia-Conditioned Medium for Migration and Neurotoxicity Assays.
In vitro astrocyte and microglia cultures from WT pups were treated with LPS (100 ng/mL) in the presence of FTY720 (1 µg/mL) or vehicle for 24 h, extensively washed, and supplemented with fresh culture medium. Forty-eight hours later, supernatants were spun down and kept for migration and neurotoxicity assays at −80 °C.
Monocyte Migration Assay.
Splenic monocytes were purified from WT mice by CD11b beads (Miltenyi) and sorted for F4/80+SSClowLy6C1hi (SSC, sideways scatter). These monocytes were seeded in the upper chamber of a 24-well cell culture insert, with a 5-μm pore size (Corning), containing astrocyte- or microglia-conditioned medium (as detailed earlier). Migrating monocytes in the lower chamber were quantified after 3 h by FACS.
Neurotoxicity Assay.
N2A neuronal cells (CCL-131, American Type Culture Collection) were grown in 96-well plates and preactivated with mouse IFN-γ (100 ng/mL; R&D Systems) for 24 h. Thereafter, medium was replaced, after extensive washing with PBS solution, with astrocyte- or microglia-conditioned medium. Cytotoxicity was measured by using LDH release (CytoTox 96 Nonradioactive Cytotoxicity Assay; Promega) after 24 h as suggested by the manufacturer’s protocol.
Monocyte Polarization Assays.
Primary astrocyte cultures were activated in the presence of FTY720 or vehicle for 24 h. Thereafter, activation medium was removed and primed astrocytes were washed extensively. CD11b+CD45+Ly6Chi proinflammatory monocytes were FACS-sorted from spleens of naive WT mice and cocultured with primed astrocytes. After 24 h, monocytes were reisolated and RNA was isolated, transcribed, and subjected to qPCR analysis.
EAE clinical scoring, mouse and human primary astrocyte and microglia cultures, isolation of cells from adult mouse CNS, subcellular fractionation and immunoblot analysis, T-cell proliferation, flow cytometry staining and acquisition, ELISA, detection of NO, histology, nCounter gene expression, qPCR, Affymetrix gene assay, heat-map generation, and Ingenuity pathway and statistical analysis are provided in SI Materials and Methods.
SI Materials and Methods
EAE Clinical Scoring and Agents.
Clinical signs of EAE were assessed as follows: 0, no signs of disease; 1, loss of tone in the tail; 2, hindlimb paresis; 3, hindlimb paralysis; 4, tetraplegia; and 5, moribund. All agents were purchased from Sigma-Aldrich.
Isolation of Cells from Adult Mouse CNS.
Mononuclear cells were isolated from the CNS as previously described, and astrocytes, monocytes, and microglia were sorted as described before (2, 3). Isolated CNS cells were stained with fluorochrome-conjugated antibody to CD11b (M1/70, 1:100), CD45 (90, 1:100), Ly6C1 (HK1.4, 1:100), CD105 (N418, 1:100), CD140a (APA5, 1:100), CD11c (N418, 1:100), F4/80 (BM8, 1:50), O4 (O4, 1:10; Miltenyi Biotec), and CD19 (eBio1D3, 1:100). All antibodies were from eBioscience or BD Pharmingen unless stated otherwise. Microglia were sorted as CD11b+ cells with low CD45 expression and low LY6C1 (CD11b+CD45lowLy6C1low), and inflammatory monocytes were considered as CD45hiCD11b+Ly6C1hi. Astrocytes were sorted as CD11blowCD45low Ly6C1lowCD105lowCD140alowCD11blowF4/80lowO4lowCD19low after the exclusion of lymphocytes, microglia, oligodendrocytes, and monocytes. Sorted astrocytes were >85% GFAP+ as determined by FACS analysis and by qPCR analysis of the expression of the astrocyte markers Gfap, Aldh1l1, and Aqp4.
Mouse Primary Astrocyte and Microglia Cultures.
Cerebral cortices from neonatal C57BL/6J mice aged 1–3 d were dissected, stripped of their meninges, digested with 0.25% trypsin–EDTA and DNase I (1 mg/mL) for 15 min, and dispersed to single-cell level by passing through a cell strainer (70 µm). The cell suspension was then cultured at 37 °C in humidified 5% (vol/vol) CO2, 95% (vol/vol) air on poly-l-lysine (Sigma)-coated 175-cm2 cell culture flasks. Medium was replaced every 4–5 d. After 7–10 d, cells reached confluence, and astrocytes were isolated by mild trypsinization with trypsin–EDTA (0.06%) as previously described (2). Microglia were subcultured separately after mild trypsinization as previously described (3) and used for further experiments. Cells were >95% astrocytes as determined by staining with GFAP or glutamate aspartate transporter (GLAST), with less than 5% contamination of CD11b+ microglia cells (Fig. 2). After the isolation procedure, cells were further plated as required for the specific experiments. Astrocyte and microglia cultures were activated with LPS 100 ng/mL (Sigma) with FTY720 1 µg/mL or vehicle. Unless otherwise indicated, RNA analysis was done 24 h after the start of treatment.
Human Primary Astrocytes and Microglia.
Human fetal astrocytes and microglia were isolated as previously described (2, 3) from human CNS tissue from fetuses at 17–23 wk of gestation that were obtained from the Human Fetal Tissue Repository (Albert Einstein College of Medicine) following Canadian Institutes of Health Research–approved guidelines. Primary human astrocytes were treated with poly(I:C) (10 mg/mL) with or without FTY720 (1 µg/mL). After 24 h, total RNA was isolated, transcribed, and subjected to qPCR.
Subcellular Fractionation and Immunoblot Analysis.
In vitro astrocyte cultures were treated as indicated in specific experiments; subcellular fractions were generated by using a Cell Fractionation kit (Cell Signaling), and 10 μg of nuclear and cytoplasmic fractions were separated by 4–12% Bis-Tris NuPAGE gels (Invitrogen) and transferred onto PVDF membranes (Millipore). Primary antibodies (Abs) used were rabbit anti-GAPDH mAb (14C10; Cell Signaling), anti-histone H3 rabbit polyclonal Ab (EMD Millipore), and anti–NF-κB p65 rabbit mAb (D14E12; Cell Signaling), followed by HRP-linked goat anti-rabbit IgG Ab (7074S; Cell Signaling). All antibodies were used at a dilution of 1:1,000. Blots were developed by using the SuperSignal West Femto Maximum Sensitivity kit (Thermo Scientific–Life Technologies). Data quantification was done by using ImageJ software (version 1.48; National Institutes of Health), and specific signals were normalized to GAPDH (cytoplasm) or histone 3 (nucleus).
T-Cell Proliferation.
Splenocytes were cultured in X-VIVO 15 medium (Lonza) and were plated for 72 h at a density of 5 × 105 cells per well, with increasing concentrations of MOG35–55 peptide. During the final 16 h, cells were pulsed with 1 Ci [3H]thymidine (PerkinElmer), followed by collection on glass fiber filters and analysis of incorporated [3H]thymidine in a β-counter (1450 MicroBeta TriLux; PerkinElmer).
Flow Cytometry Staining and Acquisition.
Mononuclear-cell suspensions were prepared as previously described (2). Antibodies for flow cytometry were purchased from eBioscience or BD Pharmingen and used at a concentration of 1:100 unless recommended otherwise by the manufacturer. Cells were then analyzed on a LSRII or MACSQuant flow cytometer (BD Biosciences and Miltenyi Biotec, respectively).
ELISA.
Tissue culture supernatants from astrocytes and microglia were generated as described previously and subjected to ELISA by using commercial kits purchased from R&D Systems as described previously (3).
Detection of NO.
Detection of NO in tissue culture supernatants was performed by using an NO detection kit (cat. no. ADI-917-020; Enzo) as suggested by the manufacturer.
Histology.
Transverse sections from lumbar spinal cords of EAE mice at the end of the experiment were fixed in 4% paraformaldehyde, pH 7.4, embedded in paraffin and cut at 10-µm thickness. Sections were stained with H&E, Luxol fast blue, or silver nitrate as described in the manufacturer’s protocol (Sigma Aldrich). Images were acquired by using a Zeiss Axioskop2 plus microscope with AxioCam HRC and AxioVision software, and were analyzed by using ImageJ software (71).
nCounter Gene Expression.
Total RNA (100 ng) was hybridized with reporter and capture probes in custom-made astrocyte-targeted nCounter gene expression code sets (Table S1) according to the manufacturer’s instructions (NanoString Technologies). Data were analyzed by using nSolver Analysis software.
qPCR.
RNA was extracted with an RNeasy kit (Qiagen), and cDNA was prepared and used for qPCR with the results normalized to Gapdh levels. All primers and probes were from Applied Biosystems as follows: mouse, Ccl2, Mm00441242_m1; Csf2, Mm01290062_m1; Gapdh, Mm99999915_g1; Il10, Mm00439614_m1; Il12a, Mm00434165_m1; Il23a, Mm01160011_g1; Il6, Mm00446190_m1; Nos2, Mm00440502_m1; Tnf, Mm00443258_m1; Gfap, Mm01253033_m1; Aldh1a1, Mm00657317_m1; Itgam, Mm00434455_m1; and human, CCL2, Hs00234140_m1; IL6, Hs00985639_m1; NOS2, Hs01075529_m1; IL10, Hs00961622_m1; IL12A, Hs01073447_m1; and IL23A, Hs00900828_g1.
Affymetrix Gene Assay, Heat-Map Generation, and Pathway Analysis.
Cultured fetal human astrocytes were exposed to IL-1β plus FTY720 or IL-1β plus vehicle and were screened by using Affymetrix GeneChip Human Gene 2.0 ST Array Screen. Microarray data were analyzed using Genomics Suite software (Partek), Bioconductor, and R statistical software. The Affy package was used for background correction, quantile normalization, and gene expression calculations. Differentially regulated genes were assessed with the Limma package, and FDR control based on the Benjamini–Hochberg procedure was used to correct for multiple testing. Heat maps were generated by using Gene-E program.
Ingenuity Pathway Analysis.
To determine significant pathways, differentially expressed genes that passed FDR < 0.1 for vehicle vs. FTY treatment were uploaded and analyzed by using the Ingenuity Pathway Analysis (IPA) tool. P values were calculated by using Fisher’s exact test.
Network Analysis.
The NF-κB network diagram was generated by using IPA. The activation status of a pathway was predicted by using IPA Upstream Regulator tool by an activation z-score. The z-score was calculated by using the formula z = (Σiwixi)/(√Σiwi2), where wi is the weight of gene i and xi is the number of activating and inhibiting predictions of gene i. Positive z-score indicated up-regulated pathway whereas negative z-score indicated down-regulated pathway as the overall effect.
Statistical Analysis.
Statistical analyses were performed with Prism software (GraphPad) by using the statistical tests indicated in the individual figure legends. No samples were excluded. The investigators were blinded to the treatment of mice in individual experiments. P values of <0.05 were considered significant. All error bars represent SEM or SD as noted in the individual figure legends. Unless otherwise stated, three independent experiments were used for all assays, and displayed figures are representative.
Acknowledgments
We thank Howard L. Weiner for his suggestions. This work was supported by National Institutes of Health Grants AI075285 and AI093903 (to F.J.Q.), National Multiple Sclerosis Society Grants RG4111A1 and JF2161-A-5 (to F.J.Q.), Novartis educational grants (to F.J.Q. and J.A.), a Canadian Institutes of Health Research Industry grant (to J.A.), Mallinckrodt Pharmaceuticals Educational Grant A219074 (to V.R.), and German Research Foundation Fellowship DFG RO4866 1/1.
Footnotes
Conflict of interest statement: These studies were funded in part by a Novartis grant (to F.J.Q. and J.A.).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615413114/-/DCSupplemental.
References
- 1.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 2.Rothhammer V, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayo L, et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014;20(10):1147–1156. doi: 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butovsky O, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savarin C, et al. Astrocyte response to IFN-γ limits IL-6-mediated microglia activation and progressive autoimmune encephalomyelitis. J Neuroinflammation. 2015;12(1):79. doi: 10.1186/s12974-015-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 7.Tiper IV, East JE, Subrahmanyam PB, Webb TJ. Sphingosine 1-phosphate signaling impacts lymphocyte migration, inflammation and infection. Pathog Dis. 2016;74(6):ftw063. doi: 10.1093/femspd/ftw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garris CS, Blaho VA, Hla T, Han MH. Sphingosine-1-phosphate receptor 1 signalling in T cells: Trafficking and beyond. Immunology. 2014;142(3):347–353. doi: 10.1111/imm.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510(7503):58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: Therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158(5):1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White C, Alshaker H, Cooper C, Winkler M, Pchejetski D. The emerging role of FTY720 (fingolimod) in cancer treatment. Oncotarget. 2016;7(17):23106–23127. doi: 10.18632/oncotarget.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Sphingosine-1-phosphate signaling in immune cells and inflammation: Roles and therapeutic potential. Mediators Inflamm. 2016;2016:8606878. doi: 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabresi PA, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 14.Kappos L, et al. FREEDOMS Study Group A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 15.Hohlfeld R, Barkhof F, Polman C. Future clinical challenges in multiple sclerosis: Relevance to sphingosine 1-phosphate receptor modulator therapy. Neurology. 2011;76(8) Suppl 3:S28–S37. doi: 10.1212/WNL.0b013e31820db40f. [DOI] [PubMed] [Google Scholar]
- 16.Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology. 2011;76(8) suppl 3:S3–S8. doi: 10.1212/WNL.0b013e31820d5ec1. [DOI] [PubMed] [Google Scholar]
- 17.Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A. Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology. 2011;76(8) suppl 3:S20–S27. doi: 10.1212/WNL.0b013e31820db341. [DOI] [PubMed] [Google Scholar]
- 18.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33(2):91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CW, Choi JW, Chun J. Neurological S1P signaling as an emerging mechanism of action of oral FTY720 (fingolimod) in multiple sclerosis. Arch Pharm Res. 2010;33(10):1567–1574. doi: 10.1007/s12272-010-1008-5. [DOI] [PubMed] [Google Scholar]
- 20.Brunkhorst R, Vutukuri R, Pfeilschifter W. Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front Cell Neurosci. 2014;8:283. doi: 10.3389/fncel.2014.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willis MA, Cohen JA. Fingolimod therapy for multiple sclerosis. Semin Neurol. 2013;33(1):37–44. doi: 10.1055/s-0033-1343794. [DOI] [PubMed] [Google Scholar]
- 22.Choi JW, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108(2):751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, et al. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. FASEB J. 2011;25(5):1509–1518. doi: 10.1096/fj.10-173203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo E, et al. Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol. 2014;76(3):325–337. doi: 10.1002/ana.24217. [DOI] [PubMed] [Google Scholar]
- 25.Groves A, Kihara Y, Chun J. Fingolimod: Direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci. 2013;328(1-2):9–18. doi: 10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, et al. Dual effects of daily FTY720 on human astrocytes in vitro: Relevance for neuroinflammation. J Neuroinflammation. 2013;10:41. doi: 10.1186/1742-2094-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miron VE, et al. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol. 2010;176(6):2682–2694. doi: 10.2353/ajpath.2010.091234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imeri F, et al. Sphingosine kinase 2 deficient mice exhibit reduced experimental autoimmune encephalomyelitis: Resistance to FTY720 but not ST-968 treatments. Neuropharmacology. 2016;105:341–350. doi: 10.1016/j.neuropharm.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Foster CA, et al. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: Expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol. 2009;19(2):254–266. doi: 10.1111/j.1750-3639.2008.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anthony DC, Sibson NR, Losey P, Meier DP, Leppert D. Investigation of immune and CNS-mediated effects of fingolimod in the focal delayed-type hypersensitivity multiple sclerosis model. Neuropharmacology. 2014;79:534–541. doi: 10.1016/j.neuropharm.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 31.di Nuzzo L, Orlando R, Nasca C, Nicoletti F. Molecular pharmacodynamics of new oral drugs used in the treatment of multiple sclerosis. Drug Des Devel Ther. 2014;8:555–568. doi: 10.2147/DDDT.S52428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons SB, Pierson ER, Lee SY, Goverman JM. Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 2013;34(8):410–422. doi: 10.1016/j.it.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ignatius Arokia Doss PM, Roy AP, Wang A, Anderson AC, Rangachari M. The non-obese diabetic mouse strain as a model to study CD8(+) T cell function in relapsing and progressive multiple sclerosis. Front Immunol. 2015;6:541. doi: 10.3389/fimmu.2015.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dang PT, Bui Q, D’Souza CS, Orian JM. Modelling MS: Chronic-relapsing EAE in the NOD/Lt mouse strain. Curr Top Behav Neurosci. 2015;26:143–177. doi: 10.1007/7854_2015_378. [DOI] [PubMed] [Google Scholar]
- 35.Moreno M, et al. Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J Neurosci. 2014;34(24):8175–8185. doi: 10.1523/JNEUROSCI.1137-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14(9):1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 37.Mildner A, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132(pt 9):2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 38.Kim RY, et al. Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014;274(1-2):53–61. doi: 10.1016/j.jneuroim.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldmann T, et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci. 2013;16(11):1618–1626. doi: 10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- 40.Rothhammer V, Quintana FJ. Control of autoimmune CNS inflammation by astrocytes. Semin Immunopathol. 2015;37(6):625–638. doi: 10.1007/s00281-015-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H, et al. Interleukin-33 is released in spinal cord and suppresses experimental autoimmune encephalomyelitis in mice. Neuroscience. 2015;308:157–168. doi: 10.1016/j.neuroscience.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Jiang HR, et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42(7):1804–1814. doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- 43.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193(6):713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192(7):1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16(5):249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olabarria M, Putilina M, Riemer EC, Goldman JE. Astrocyte pathology in Alexander disease causes a marked inflammatory environment. Acta Neuropathol. 2015;130(4):469–486. doi: 10.1007/s00401-015-1469-1. [DOI] [PubMed] [Google Scholar]
- 47.Chung WS, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol. 2015;7(9):a020370. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundgaard I, Osório MJ, Kress BT, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neuroscience. 2014;276:161–173. doi: 10.1016/j.neuroscience.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lassmann H. Mechanisms of white matter damage in multiple sclerosis. Glia. 2014;62(11):1816–1830. doi: 10.1002/glia.22597. [DOI] [PubMed] [Google Scholar]
- 50.Linne ML, Jalonen TO. Astrocyte-neuron interactions: From experimental research-based models to translational medicine. Prog Mol Biol Transl Sci. 2014;123:191–217. doi: 10.1016/B978-0-12-397897-4.00005-X. [DOI] [PubMed] [Google Scholar]
- 51.Brosnan CF, Raine CS. The astrocyte in multiple sclerosis revisited. Glia. 2013;61(4):453–465. doi: 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- 52.Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8(11):602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopes Pinheiro MA, et al. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta. 2016;1862(3):461–471. doi: 10.1016/j.bbadis.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Sheridan GK, Murphy KJ. Neuron-glia crosstalk in health and disease: Fractalkine and CX3CR1 take centre stage. Open Biol. 2013;3(12):130181. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen S, et al. Effect of FTY720-phosphate on the expression of inflammation-associated molecules in astrocytes in vitro. Mol Med Rep. 2015;12(4):6171–6177. doi: 10.3892/mmr.2015.4120. [DOI] [PubMed] [Google Scholar]
- 56.Sheridan GK, Dev KK. S1P1 receptor subtype inhibits demyelination and regulates chemokine release in cerebellar slice cultures. Glia. 2012;60(3):382–392. doi: 10.1002/glia.22272. [DOI] [PubMed] [Google Scholar]
- 57.Basso AS, et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J Clin Invest. 2008;118(4):1532–1543. doi: 10.1172/JCI33464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farez MF, et al. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol. 2009;10(9):958–964. doi: 10.1038/ni.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farez MF, Correale J. Sphingosine 1-phosphate signaling in astrocytes: Implications for progressive multiple sclerosis. J Neurol Sci. 2016;361:60–65. doi: 10.1016/j.jns.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 60.Healy LM, Antel JP. Sphingosine-1-phosphate receptors in the central nervous and immune systems. Curr Drug Targets. 2016;17(16):1841–1850. doi: 10.2174/1389450116666151001112710. [DOI] [PubMed] [Google Scholar]
- 61.Nazari M, Keshavarz S, Rafati A, Namavar MR, Haghani M. Fingolimod (FTY720) improves hippocampal synaptic plasticity and memory deficit in rats following focal cerebral ischemia. Brain Res Bull. 2016;124:95–102. doi: 10.1016/j.brainresbull.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Tsai HC, Han MH. Sphingosine-1-phosphate (S1P) and S1P signaling pathway: Therapeutic targets in autoimmunity and inflammation. Drugs. 2016;76(11):1067–1079. doi: 10.1007/s40265-016-0603-2. [DOI] [PubMed] [Google Scholar]
- 63.Lublin F, et al. INFORMS study investigators Oral fingolimod in primary progressive multiple sclerosis (INFORMS): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075–1084. doi: 10.1016/S0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- 64.O’Sullivan C, Schubart A, Mir AK, Dev KK. The dual S1PR1/S1PR5 drug BAF312 (siponimod) attenuates demyelination in organotypic slice cultures. J Neuroinflammation. 2016;13:31. doi: 10.1186/s12974-016-0494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan S, et al. Discovery of BAF312 (siponimod), a potent and selective S1P receptor modulator. ACS Med Chem Lett. 2013;4(3):333–337. doi: 10.1021/ml300396r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Briard E, Rudolph B, Desrayaud S, Krauser JA, Auberson YP. MS565: A SPECT tracer for evaluating the brain penetration of BAF312 (siponimod) Chem Med Chem. 2015;10(6):1008–1018. doi: 10.1002/cmdc.201500115. [DOI] [PubMed] [Google Scholar]
- 67.Kappos L, et al. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: Dose-blinded, randomized extension of the phase 2 bold study. JAMA Neurol. 2016;73(9):1089–1098. doi: 10.1001/jamaneurol.2016.1451. [DOI] [PubMed] [Google Scholar]
- 68.Novartis Media Relations 2016 Novartis announces positive phase III results showing efficacy of BAF312 in patients with secondary progressive MS. Available at https://www.novartis.com/news/media-releases/novartis-announces-positive-phase-iii-results-showing-efficacy-baf312-patients. Accessed September 1, 2016.
- 69.Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125(4):1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asle-Rousta M, Kolahdooz Z, Oryan S, Ahmadiani A, Dargahi L. FTY720 (fingolimod) attenuates beta-amyloid peptide (Aβ42)-induced impairment of spatial learning and memory in rats. J Mol Neurosci. 2013;50(3):524–532. doi: 10.1007/s12031-013-9979-6. [DOI] [PubMed] [Google Scholar]
- 71.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]