Significance
Dominant GATA4 mutations cause congenital heart defects in humans, but the mechanistic basis whereby Gata4 haploinsufficiency causes disease is unknown. We demonstrate that Gata4 is required in a subset of cardiac progenitor cells called the “second heart field” for cardiac septation. Furthermore, we identified two distinct pathways downstream of Gata4, phosphatase and tensin homolog (Pten)-modulated cell-cycle transition and Hedgehog signaling, which appear to act independently. Restoration of either Pten-mediated cell-cycle transition or Hedgehog signaling restored cardiac septation in Gata4-mutant mice, suggesting that defects in both pathways are required for disease causation. This work generates a working model for understanding the molecular basis of human congenital heart disease caused by dominant transcription factor mutations.
Keywords: Gata4, ASDs, Hedgehog signaling, second heart field, cell cycle
Abstract
GATA4, an essential cardiogenic transcription factor, provides a model for dominant transcription factor mutations in human disease. Dominant GATA4 mutations cause congenital heart disease (CHD), specifically atrial and atrioventricular septal defects (ASDs and AVSDs). We found that second heart field (SHF)-specific Gata4 heterozygote embryos recapitulated the AVSDs observed in germline Gata4 heterozygote embryos. A proliferation defect of SHF atrial septum progenitors and hypoplasia of the dorsal mesenchymal protrusion, rather than anlage of the atrioventricular septum, were observed in this model. Knockdown of the cell-cycle repressor phosphatase and tensin homolog (Pten) restored cell-cycle progression and rescued the AVSDs. Gata4 mutants also demonstrated Hedgehog (Hh) signaling defects. Gata4 acts directly upstream of Hh components: Gata4 activated a cis-regulatory element at Gli1 in vitro and occupied the element in vivo. Remarkably, SHF-specific constitutive Hh signaling activation rescued AVSDs in Gata4 SHF-specific heterozygous knockout embryos. Pten expression was unchanged in Smoothened mutants, and Hh pathway genes were unchanged in Pten mutants, suggesting pathway independence. Thus, both the cell-cycle and Hh-signaling defects caused by dominant Gata4 mutations were required for CHD pathogenesis, suggesting a combinatorial model of disease causation by transcription factor haploinsufficiency.
Gata4, a member of the GATA family of zinc finger transcription factors, is an essential cardiogenic transcriptional regulator implicated in many aspects of cardiac development and function (1–15). Human genetic studies have implicated dominant GATA4 mutations in atrial septal defects (ASDs), including atrioventricular (AV) septal defects (AVSDs), in human individuals and families (2, 16–21). Gata4 is a transcriptional activator of genes essential for cardiac function. Although important Gata4 transcriptional targets in the heart have been identified, such as Nppa, α-MHC, α-CA, B-type natriuretic peptide (BNP), Mef2c, and Cyclin D2 (1, 7, 22), none has been linked to cardiac structural defects. Therefore, the Gata4-driven regulatory networks that are essential for atrial septation remain unknown.
The developmental ontogeny of ASDs has undergone recent revision. ASD is a broad designation for congenital heart disease (CHD) including multiple distinct anatomic defects of the atrial septum. Development of the atrial septum includes contributions from distinct lineages including the AV endocardial cushions, the primary atrial septum (PAS), and the dorsal mesenchymal protrusion (DMP) (23–29). Deficiency of the DMP results in primum ASD, a type of AVSD. Recent work has demonstrated that atrial septation is dependent on the second heart field (SHF), a group of cardiac progenitors that make late additions to the heart (24, 29–32). For example, genetic inducible fate mapping (GIFM) has shown that the entire atrial septum, including the DMP, derives from the SHF (32). Furthermore, the molecular requirement for the Hedgehog (Hh) and BMP signaling pathways and the Tbx5 cardiogenic transcription factor for AV septation reside in the SHF (32–38). These observations lay the groundwork for investigating the molecular pathways required for atrial septum formation in SHF cardiac progenitor cells.
We investigated the lineage-specific requirement for Gata4 in atrial septation and found that SHF-specific heterozygote Gata4 knockout recapitulated the AVSDs observed in germline heterozygote Gata4 knockouts in mice. Gata4 deletion in the SHF caused a failure of DMP formation and cell-cycle progression defects that were consistent with disrupted Cdk4/Cyclin D2 expression. AVSDs caused by heterozygous SHF Gata4 knockout could be rescued by knockdown of the cell-cycle repressor phosphatase and tensin homolog (Pten), which restored expression of Cdk4 and cell-cycle progression. Heterozygous Gata4 deletion also caused SHF Hh signaling defects. Hh signaling markers were diminished in SHF-specific Gata4 heterozygous embryos, and Gata4 interacted with the obligate Hh receptor gene Smoothened (Smo) in vivo. A Gata4-driven enhancer was identified at Gli1, a modulator and target of Hh signaling. Furthermore, AVSDs caused by SHF-specific Gata4 heterozygosity were rescued by constitutive activation of Hh signaling in the SHF. These observations define two independent pathways disrupted by heterozygous Gata4 knockout. Restoration of either pathway rescues atrial septation in Gata4 heterozygous embryos, suggesting that disruption of both pathways is required for ASD pathogenesis in this model of dominant transcription factor mutation.
Results
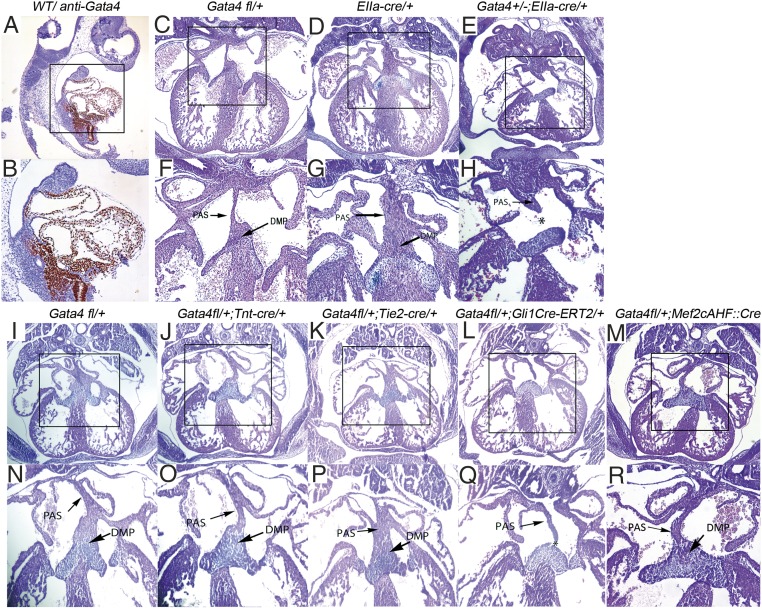
Previous work has demonstrated Gata4 expression in the heart, including the dorsal mesocardium, myocardium, and endocardium, during AV septation (1, 3, 8, 39–42). Gata4 is also expressed in the SHF of human embryos from Carnegie stage 10–16 (39, 43). We examined Gata4 protein expression in embryonic day (E)9.5 wild-type mouse embryos by immunohistochemistry (IHC) staining. As expected, strong Gata4 expression was observed in the heart, including the atrium, ventricles, and outflow tract (Fig. 1A). Strong Gata4 expression also was observed in the dorsal mesocardium and SHF mesenchyme behind the heart (Fig. 1A, B). The density of Gata4-expressing cells appeared to be higher in the posterior SHF (pSHF) than in the anterior SHF (aSHF) (Fig. 1B). These observations were confirmed by Gata4 transcript levels analyzed by RNA-sequencing (RNA-seq) from aSHF, pSHF, and heart, each microdissected from wild-type mouse embryos at E9.5 (Table 1) (44). Gata4 expression, represented by fragments per kilobase of transcript per million mapped reads (FPKM) values, was high in the heart (37.43 ± 6.31) and the pSHF (18.86 ± 4.09) and was lower in the aSHF (4.00 ± 3.12) (Table 1). Expression of the control SHF marker genes Fgf8, Isl1, Tbx1, and Gli1 was substantially higher in the SHF than in the heart (Table 1). Furthermore, FPKM counts for Tbx5 were greatly enriched in the heart and pSHF as compared to the aSHF in accordance with historical in situ hybridization experiments (35, 43, 45–47). These results indicated strong Gata4 expression in the pSHF.
Fig. 1.
Gata4 is required in the atrial septum progenitor of pSHF for atrial septation. (A and B) IHC staining of Gata4 in wild-type mouse embryos at E9.5. (C–R) Histology of Gata4 transgenic mouse embryo hearts at E13.5. Asterisks indicate missing structures of atrial septation. B, F–H, and N–R show the boxed areas in A, C–E, and I–M, respectively, at higher magnification. (Magnification: 40× in A, C–E, and I–M; 100× in B, F–H, and N–R.)
Table 1.
Expression of selected genes in heart, aSHF, and pSHF of mouse embryos at E9.5 measured by RNA-seq (n = 6)
| Gene | Heart | aSHF | pSHF |
| Gata4 | 37.43 ± 6.31a | 4.00 ± 3.12b | 18.86 ± 4.09c |
| Tbx5 | 24.77 ± 2.21a | 0.91 ± 0.76b | 12.28 ± 1.98c |
| Fgf8 | 2.12 ± 1.16a | 6.94 ± 2.36b | 3.87 ± 1.76b |
| Tbx1 | 0.39 ± 0.17a | 14.12 ± 1.64b | 9.20 ± 2.12c |
| Gli1 | 1.32 ± 0.39a | 14.76 ± 4.52b | 16.26 ± 4.26b |
| Isl1 | 3.71 ± 1.21a | 22.84 ± 3.78b | 17.44 ± 4.01b |
| Pten | 13.06 ± 0.79a | 14.92 ± 1.16a | 14.88 ± 0.70a |
| Adipoq | 0.18 ± 0.12a | 0.00 ± 0.02a | 0.02 ± 0.04a |
Mouse embryos were microdissected at E9.5 as described in Materials and Methods. The significant difference in the expression of each gene in heart, aSHF, and pSHF was tested by ANOVA analysis. Different letters denote a significant difference between different tissues (a vs. b, a vs. c, b vs. c; P < 0.05, n = 6). Adipoq, adiponectin.
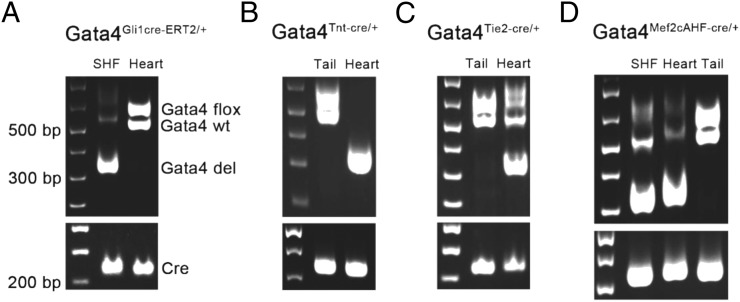
To determine the lineage requirement for Gata4 in AV septation, we analyzed mouse embryos with lineage-specific, heterozygous deletion of Gata4 in the germline, myocardium, endocardium, or SHF. We used a Gata4 floxed allele with loxP sites flanking the second exon, encoding the first coding exon essential for Gata4 function (8), and converted it into lineage-specific knockouts with a series of murine lineage-specific Cre lines. Tissue-specific knockdown of Gata4 was confirmed by gel electrophoresis (Fig. S1). This Gata4 floxed allele resulted in a 20% reduction in Gata4 protein level before Cre excision (6, 8). We converted a single Gata4fl/+ into a germline knockout using EIIaCre (48). Although Gata4fl/+ (n = 13) and EIIaCre/+ (n = 12) embryos demonstrated normal cardiac anatomy, including complete atrial septation at E13.5 (Fig. 1 C, D, F, and G), 39% (7/18) of Gata4+/−; EIIaCre/+ embryos demonstrated primum ASDs with absence of the DMP (P = 0.0053 vs. Gata4fl/+ or EIIaCre/+) (Fig. 1 E and H and Table 2). This observation is consistent with previous reports implicating germline heterozygous Gata4 deletion in ASDs (6). We examined Gata4 heterozygosity in the myocardium by combining cardiomyocyte-specific Tnt:Cre (49) with Gata4fl/+ (Fig. 1 I and N and Table 2). Normal atrial septation was observed in all TntCre/+; Gata4fl/+ (12/12) and littermate control Gata4fl/+ (13/13) embryos at E13.5 (P = 1) (Fig. 1 J and O). We examined Gata4 haploinsufficiency in the endocardium by combining endocardial-specific Tie2:Cre (34, 50) with Gata4fl/+ (Fig. 1 K and P and Table 2). Normal atrial septation was observed in all Tie2Cre/+; Gata4fl/+ mutant embryos (23/23) and littermate control Gata4fl/+ embryos (13/13) at E13.5 (P = 1) (Fig. 1 I and M). These results demonstrated that Gata4 haploinsufficiency in the myocardium or endocardium supported normal atrial septation in a mixed genetic background (Materials and Methods).
Fig. S1.
Confirmation of tissue-specific knockdown of Gata4 by gel electrophoresis. (A) DNA was extracted from SHF and heart tissues of Gata4fl/+;Gli1Cre-ERT2/+ mice and was tested for Cre-mediated knockdown of Gata4. (B) DNA was extracted from tail and heart tissues of Gata4fl/+;TntCre/+ mice and was tested for Cre-mediated knockdown of Gata4. (C) DNA was extracted from tail and heart tissues of Gata4fl/+;Tie2Cre/+ mice and was tested for Cre-mediated knockdown of Gata4. (D) DNA was extracted from SHF, heart, and tail of Gata4fl/+;Mef2cCre/+ mice and was tested for Cre-mediated knockdown of Gata4. The following primers were used: Cre forward: 5′-TCGACCAGGTTCGTTC ACTCATGG-3′; Cre reverse: 5′-CAGGCTAAGTGCCTTCTCTACACC-3′; Gata4-WT/flox forward: 5′-ACCCTGGAAGAC ACCCCAATCTCGG-3′; Gata4-del forward: 5′-TGTCATTCTTCGCTGGAGCCGC-3′; Gata4 reverse: 5′-TCCATGAGAC CCCAGAGTGTGCCTGA-3′. The size of Cre product is ∼220 bp. The Gata4 wild type and Gata4-flox products are ∼510 bp and 530 bp in size, respectively. The size of the Gata4-del product is ∼300 bp.
Table 2.
Incidence of ASDs in Gata4-mutant embryos
| Genotype | ASD | Total | Type | Vs. littermate control | P value |
| Conditional mutation of Gata4 | |||||
| Gata4+/−;EllaCre/+ | 7 | 18 | All primum | vs. EIIacre/+ (0/13) | 0.0053 |
| Gata4fl/+;TntCre/+ | 0 | 12 | — | vs. Gata4fl/+ (0/13) | 1 |
| Gata4fl/+; TieCre/+ | 0 | 23 | — | vs. Gata4fl/+ (0/13) | 1 |
| Gata4fl/+;Gli1Cre-ERT2/+ (TM at E7.5 and E8.5) | 13 | 21 | All primum | vs. Gata4fl/+ (0/15) | <0.0001 |
| Gata4fl/+;Gli1Cre-ERT2/+ (TM at E8.5 and E9.5) | 2 | 9 | All primum | vs. Gata4fl/+ (0/10) | 0.0675 |
| 0.1053* | |||||
| Gata4fl/+; Mef2cAHF::Cre | 1 | 22 | All primum | vs. Gata4fl/+ (0/15) | 1 |
| Tbx5–Gata4 compound mutant embryos | |||||
| Tbx5fl/+;Gata4fl/+; Mef2cAHF::Cre | 3 | 9 | 1 primum | vs. Gata4fl/+;Mef2cAHF::Cre (0/13) | 0.0125 |
| 2 common atrium with CCAVC | 0.0273* | ||||
| vs. Tbx5fl/+;Mef2cAHF::Cre (0/13) | 0.0125 | ||||
| 0.0273* | |||||
| Pten–Gata4 compound mutant embryos | |||||
| Ptenfl/+;Gata4fl/+;Gli1Cre-ERT2/+ | 1 | 10 | All primum | vs. Gata4fl/+;Gli1Cre-ERT2/+ (4/6) | 0.0179 |
| 0.0179* | |||||
| vs. Ptenfl/+;Gli1Cre-ERT2/+ (0/18) | 0.1719 | ||||
| Smo – Gata4 compound mutant embryos | |||||
| Smofl/+;Gata4fl/+; Mef2cAHF::Cre | 5 | 15 | All common atrium | vs. Gata4fl/+;Mef2cAHF::Cre (0/9) | 0.0258 |
| 0.0590* | |||||
| vs. Smofl/+;Mef2cAHF::Cre (0/12) | 0.0134 | ||||
| 0.0235* | |||||
| Gata4fl/+,SmoM2fl/+,Gli1Cre-ERT2/+ | 1 | 10 | All primum | vs. SmoM2fl/+;Gli1Cre-ERT2/+ (0/7) | 0.3719 |
| vs. Gata4fl/+;Gli1Cre-ERT2/+ (13/21) | 0.0106 | ||||
| 0.0045* | |||||
ASDs were evaluated by the integrity and completion of the DMP and the AV cushion. Incidence of ASDs was evaluated in Gata4-mutant embryos at E13.5.
If the χ2 P value was significant within the range (0.01 < P value < 0.1), a second P value was reported using the one-tailed Fisher exact test.
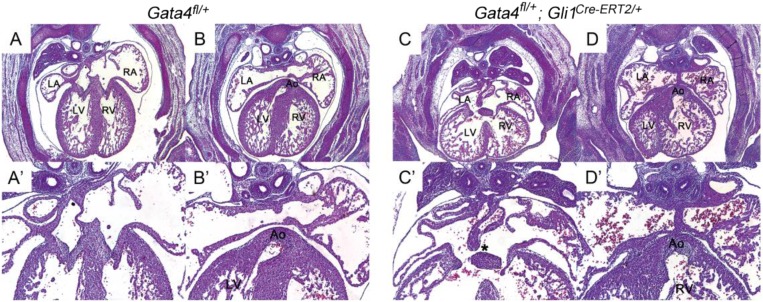
We hypothesized that Gata4 was required in the SHF for atrial septation (32, 34). We combined Gli1Cre-ERT2/+, expressed in SHF Hh signal-receiving cells, with Gata4fl/+ to generate heterozygote SHF-specific Gata4-knockout embryos (35). CreERT2 was activated by tamoxifen (TM) administration at E7.5 and E8.5 or at E8.5 and E9.5 in Gli1Cre-ERT2/+;Gata4fl/+ embryos, a regime previously used to implicate Hh signaling in the SHF for AV septation (32). Primum ASDs were observed in 62% (13/21) of Gli1Cre-ERT2/+;Gata4fl/+ embryos with TM administration at E7.5 and E8.5 but not in littermate control Gata4fl/+ embryos (0/15; P < 0.0001) or Gli1Cre-ERT2/+ embryos (0/15; P < 0.0001) at E13.5 (compare Fig. 1 I and N with Fig. 1 L and Q and Table 1). Gli1Cre-ERT2/+;Gata4fl/+ embryos also displayed a double-outlet right ventricle (DORV) with ventricular septal defect (11/18, 83%) (Fig. S2) and thinner myocardium (Fig. 1 and Fig. S2). Primum ASD in Gli1Cre-ERT2/+;Gata4fl/+ embryos administered TM at E8.5 and E9.5 occurred at a much lower frequency (2/9) that was not statistically different from that in littermate control Gata4fl/+ embryos (0/10, P = 0.0675). These findings implicated heterozygous Gata4 knockout in the SHF for AV septation, with temporal requirements mirroring those previously described for Hh signaling (32).
Fig. S2.
Gata4 haploinsufficiency in the Hh-receiving cells caused ASD and DORV. Gata4fl/+ and Gata4fl/+;Gli1Cre-ERT2/+ mouse embryos were collected at E14.5 and were sectioned and stained with H&E. (A and A′) Left atrium and right atrium were well separated in Gata4fl/+ embryos. (B and B′) The aorta was connected correctly to the left ventricle. (C and C′) Primum ASD was observed in Gata4fl/+;Gli1Cre-ERT2/+ mutant embryos, as indicated by the asterisk. (D and D′) DORV was also found in Gata4fl/+;Gli1Cre-ERT2/+ embryos, as shown by the connection of aorta to the right ventricle. Ao, aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. (Magnification: 40× in A–D; 100× in A′–D′.)
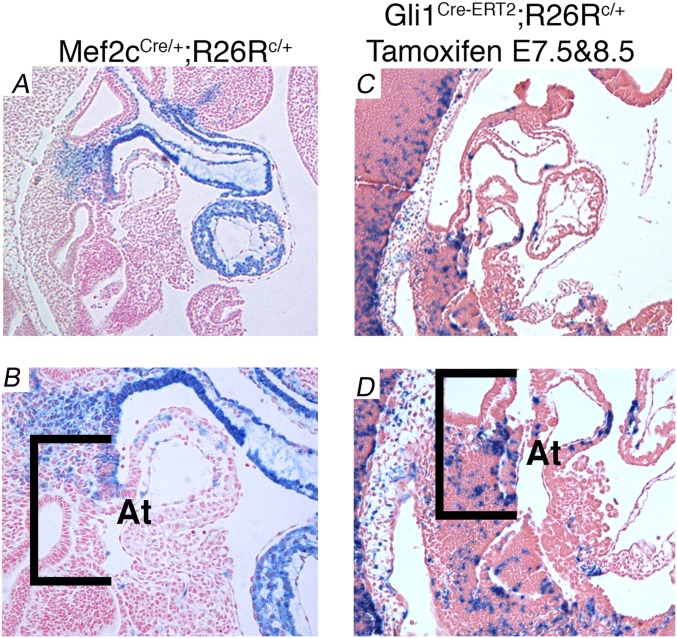
Interestingly, AVSDs were not observed in SHF-specific Gata4 heterozygous mice generated with an alternate SHF-expressing Cre line, Mef2cAHF. Neither Mef2cAHF::Cre Gata4fl/+ embryos (1/22) (Fig. 1 M and R) nor littermate control Gata4fl/+ embryos (0/15) demonstrated AVSDs at E13.5 (Fig. 1 I and N and Table 2). Thus, AVSD penetrance was high using Gli1CreERT2 but not using Mef2cAHF::Cre. We compared SHF Cre expression directly in these lines and observed distinct SHF Cre expression domains. Cre-dependent lacZ expression from the R26R locus in Mef2cAHF::Cre;R26R embryos was truncated at an anterior position and did not include the posterior DMP compared with Cre expression from Gli1CreERT2;R26R embryos at E10.5 (Fig. S3). This observation suggested that the pSHF expression domain of Gli1CreERT2 compared with Mef2cAHF::Cre (34) was necessary for AVSD causation by Gata4 heterozygosity.
Fig. S3.
Broader pSHF Cre expression is seen Gli1CreERT2 embryos than in Mef2cAHF::Cre embryos. Sagittal sections at E10.0 showing β-gal activity in Mef2cAHF::Cre;R26Rfl/+ embryos (A and B) and Gli1Cre-ERT2/+;R26Rfl/+ embryos (C and D) treated with TM at E7.5 and 8.5. β-Gal activity extends more posteriorly in the Gli1Cre-ERT2/+;R26Rfl/+ embryos than in the Mef2cAHF::Cre;R26Rfl/+ embryos. Brackets indicate the posterior SHF with the DMP as the posterior margin. At, atrium.
Gata4 Is Required for DMP Development.
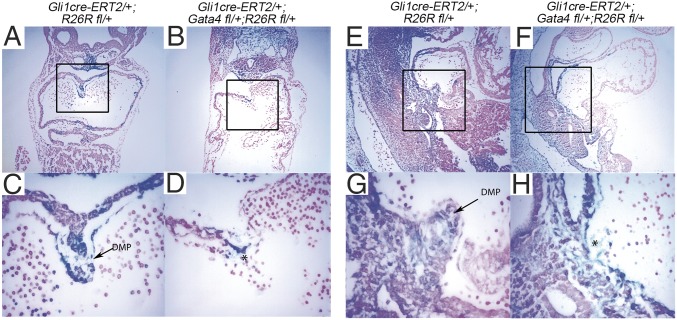
We examined the requirement for Gata4 in the SHF for DMP development using GIFM (51). We previously demonstrated that the DMP is derived from Hh signal-receiving atrial septum progenitors, marked in R26R;Gli1Cre-ERT2/+ embryos by TM administration at E7.5 and E8.5 and evaluated by β-gal expression at E10.5 (32). Gata4 heterozygous deletion combined with Hh-specific GIFM in Gli1Cre-ERT2/+;Gata4fl/+;R26Rfl/+ embryos treated with TM at E7.5 and E8.5 caused a hypoplastic or missing DMP, with qualitatively fewer marked cells (Fig. 2 B, D, F, and H) than in control Gli1Cre-ERT2/+;R26Rfl/+ embryos (Fig. 2 A, C, E, and G) at E10.5. These results demonstrated the SHF requirement for Gata4 in DMP development and suggested an intersection between Gata4 and Hh signaling.
Fig. 2.
Gata4 is required for DMP development. The Hh-receiving cardiac fate map was abnormal in Gata4-mutant embryos. Hh-receiving cells were marked by β-gal expression in Gli1Cre-ERT2/+;R26Rfl/+ embryos at E7.5 and E8.5 by TM administration and were analyzed by lacZ staining at E10.5. A marked hypoplastic or missing DMP was observed in Gli1Cre-ERT2/+;Gata4fl/+;R26Rfl/+ embryos (C, D, G, and H) compared with the Gli1Cre-ERT2/+;R26Rfl/+ embryos (A, B, E, and F). C, D, G, and H show the boxed areas in A, B, E, and F, respectively, at higher magnification. (Magnification: 100× in A, B, E, and F; 400× in C, D, G, and H.)
Gata4 Regulates Cell-Cycle Progression of Cardiac Progenitors in the pSHF.
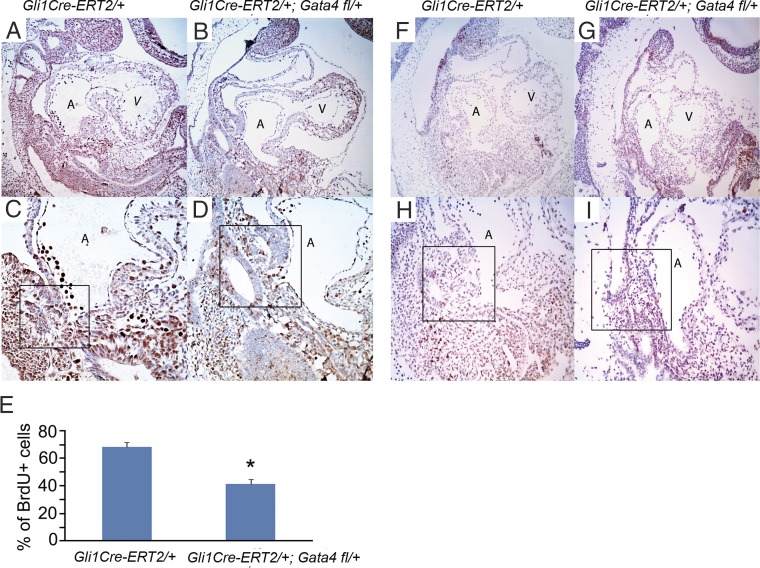
We hypothesized that the requirement for Gata4 for DMP development reflected a requirement for SHF cell proliferation. We assessed proliferation by BrdU incorporation and found that Gata4 was required for normal proliferation of Hh-receiving cells. Gli1Cre-ERT2/+;Gata4fl/+ embryos treated with TM at E7.5 and 8.5 demonstrated 39.1% fewer BrdU-positive SHF cells than control Gata4fl/+ embryos at E9.5. (41.6 ± 3.36% vs. 68.2 ± 3.43%; P = 0.002) (compare Fig. 3C with Fig. 3 D and E). In contrast, we observed no Gata4 dependence on SHF apoptosis. We assessed cell death by TUNEL staining and observed no differences in the pSHF between Gli1Cre-ERT2/+;Gata4fl/+ mutant and Gata4fl/+ littermate control embryos (Fig. 3 F–I). Together, these findings define a requirement for Gata4 in the proliferation but not in the survival of pSHF cardiac progenitors.
Fig. 3.
Gata4 is required for the proliferation but not for the survival of atrial septum progenitors. (A–D) BrdU staining was performed in Gli1Cre-ERT2/+;Gata4fl/+ embryos (B and D) and Gli1Cre-ERT2/+ embryos (A and C) at E9.5. (E) The percent of cardiac progenitors that incorporated BrdU in the pSHF and the DMP region (typical regions are shown as the boxed areas in C, D, H, and I) was calculated in a total of 500 cells, counted in five serial sections. Data are presented as mean ± SEM, n = 3 or 4; *P < 0.05. (F–I) TUNEL staining was performed in Gli1Cre-ERT2/+;Gata4fl/+ embryos (G and I) and Gli1Cre-ERT2/+ littermate control embryos (F and H) at E9.5. (Magnification: 100× in A, B, F, and G; 200× in C, D, H, and I.) A, atrium; V, ventricle.
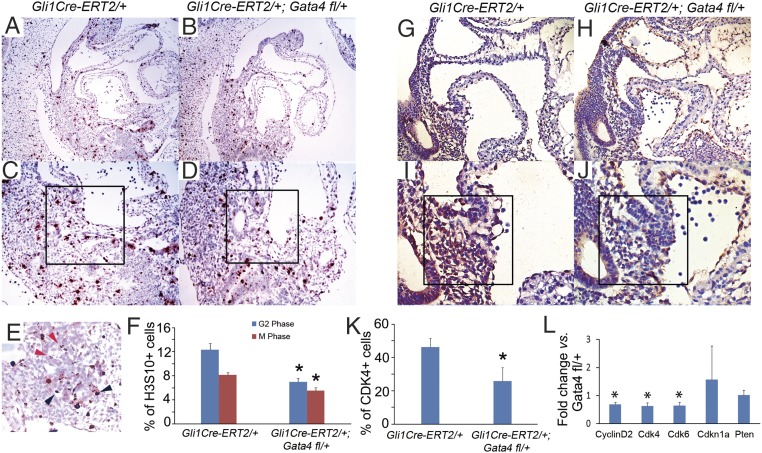
We analyzed the role of Gata4 in SHF cell-cycle progression. We identified cells in specific phases of the cell cycle by IHC for the mitotic marker phosphohistone H3 (H3S10) [punctate in G2 phase (Fig. 4E, black arrowheads) and homogeneous in M phase (Fig. 4E, red arrowheads] (35, 52) and the G1-S phase marker Cdk4. We observed a 43.5% reduction of cells in G2 phase (6.99 ± 0.59% vs.12.35 ± 1.06%, P = 0.03) and a 32.1% reduction of cells in M phase (5.52 ± 0.52% vs. 8.13 ± 0.41%, P = 0.04) (Fig. 4F) in the pSHF of Gli1Cre-ERT2/+;Gata4fl/+ embryos (Fig. 4 B and D) compared with Gata4fl/+ embryos (Fig. 4 A and C) at E10.5. We also observed a 44.5% reduction of cells in G1-S, marked by Cdk4, in the pSHF of Gli1Cre-ERT2/+;Gata4fl/+ embryos (Fig. 4 H, J, and K) compared with Gata4fl/+ littermate controls (25.7 ± 8.21% vs. 46.3 ± 5.26%, P = 0.028) (Fig. 4 G, I, and K). We investigated the molecular basis of the requirement for Gata4 for cell-cycle progression by examining the pSHF expression of Cdk4, Cdk6, Cdk2, Cyclin D2, Cdkn1a, and Pten, which are responsible for G1-S phase transition. We observed that the expression of Cdk4, Cdk6, and Cyclin D2 was decreased in the pSHF of Gli1Cre-ERT2/+;Gata4fl/+ embryos by 34.6, 34.5, and 30.7%, respectively, compared with Gata4fl/+ littermate controls at E9.5 by real-time PCR (0.693 ± 0.074, P = 0.008; 0.642 ± 0.112, P = 0.007; and 0.645 ± 0.125, P = 0.022, respectively) (Fig. 4L). These results establish Gata4 as a driver of cell-cycle progression in SHF cardiac progenitors in addition to its known role in the cell-cycle progression of cardiomyocytes (1).
Fig. 4.
Gata4 regulates cell-cycle progression of pSHF. (A–D) IHC staining for H3S10 was performed in the pSHF of the Gata4Gli1Cre-ERT2/+ embryos (B and D) and in Gli1Cre-ERT2/+ embryos (A and C) at E9.5. (E) The black arrowheads indicate typical cells in M phase; the red arrowheads indicate typical cells in G2 phase. (F) The percentage of H3S10+ cardiac progenitors was calculated in 500 cells in the pSHF and the DMP region (typical regions are shown as the boxed areas in C and D) counted in five serial sections. Data are presented as mean ± SEM, n = 3 or 4; *P < 0.05. (G–J) IHC staining for Cdk4 was performed in Gli1Cre-ERT2/+;Gata4fl/+ embryos (H and J) and in Gli1Cre-ERT2/+ embryos (G and I) at E9.5. (K) The percentage of Cdk4+ cardiac progenitors was calculated in 500 cells in the pSHF and the DMP region (typical regions are shown as the boxed areas in I and J) counted in five serial sections. Data are presented as mean ± SEM, n = 3 or 4; *P < 0.05. (L) Expression levels of cell-cycle–related genes in the pSHF of mouse embryos at E9.5 were measured by real-time PCR. Data are presented as mean ± SEM, n = 3; *P < 0.05. (Magnification: 100× in A, and B; 200× in C, and D; 400× in E; 150× in G and H; 300× in I and J.)
Genetically Targeted Disruption of PTEN Expression in Atrial Septum Progenitors Rescued ASDs in Gata4-Mutant Embryos.
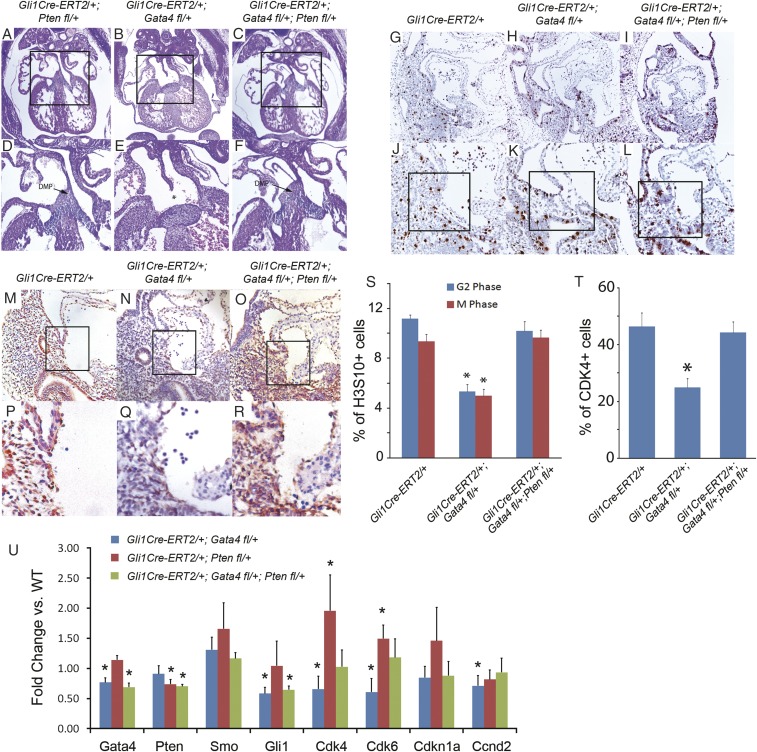
Pten is a tumor suppressor and is a well-established negative regulator of cell-cycle progression (53, 54) and Cyclin D expression (55–57). RNA-seq indicated that Pten was expressed extensively in heart and SHF (Table 2). We therefore hypothesized that a normal Gata4 and Pten balance may be required for proper SHF cell-cycle control and, furthermore, that reduction of Pten activity may rescue AVSDs caused by decreased Gata4 activity in the SHF. We tested this hypothesis by combining conditional dominant Gata4 knockout with dominant Pten knockout in the SHF using Gli1Cre:ERT2. In control Gli1Cre-ERT2/+;Ptenfl/+ embryos treated with TM at E7.5 and E8.5, normal atrial septation was always observed at E14.5 (18/18) (Fig. 5 A and D). In Gli1Cre-ERT2/+;Gata4fl/+;Ptenfl/+ embryos, conditional Pten haploinsufficiency rescued AVSDs observed in littermate Gli1Cre-ERT2/+;Gata4fl/+ embryos (1/10 vs. 4/6 AVSDs, P = 0.0179 (χ2 test) or 0.0288 (one-tailed Fisher exact test) (compare Fig. 5 C and F with Fig. 5 B and E and Table 2).
Fig. 5.
Genetically targeted disruption of Pten expression in atrial septum progenitors rescued ASDs in Gata4-mutant embryos. (A–F) Histology of Gata4 transgenic mouse embryo hearts at E13.5. Asterisks indicate missing structures of atrial septation. D–F show the boxed areas in A–C at greater magnification. (Magnification: 40× in A–C; 100× in D–F.) (G–L) IHC staining for H3S10 was performed on Gli1Cre-ERT2/+;Gata4fl/+;Ptenfl/+ (I and L), Gli1Cre-ERT2/+;Gata4fl/+ (H and K), and Gli1Cre-ERT2/+ (G and J) embryos at E9.5. (Magnification: 100× in G–I; 200× in J–L.) (M–R) IHC staining for H3S10 was performed on Gli1Cre-ERT2/+;Gata4fl/+;Ptenfl/+ (O and R), Gli1Cre-ERT2/+;Gata4fl/+ (N and Q), and Gli1Cre-ERT2/+ (M and P) embryos at E9.5. P–R show the boxed areas in M–O, respectively, at higher magnification. (Magnification: 200× in M–O; 400× in P–R.) (S and T) The percentage of H3S10+ (S) or Cdk4+ (T) cardiac progenitors was calculated in 500 cells in the pSHF and the DMP region (typical regions are shown as the boxed areas in J−L) counted in five serial sections. Data are presented as mean ± SEM, n = 3; *P < 0.05. (U) Gene expression in the pSHF of Gli1Cre-ERT2/+;Gata4fl/+, Gli1Cre-ERT2/+;Ptenfl/+, or Gli1Cre-ERT2/+;Gata4fl/+;Ptenfl/+ embryos was measured by real-time PCR and was compared with the expression in wild-type embryos. *P < 0.05.
We tested whether Pten haploinsufficiency restored cell-cycle progression as a mechanism for rescue of AVSDs caused by dominant Gata4 knockout. We analyzed the percentage of G2- and M-phase cells in the pSHF in the presence or absence of Pten haploinsufficiency in Gli1Cre-ERT2/+;Gata4fl/+ embryos at E9.5 by IHC staining of H3S10 (Fig. 5 G–L and S). The G2-phase and M-phase cell-cycle defects in Gli1Cre-ERT2/+;Gata4fl/+ embryos were rescued in Gli1Cre-ERT2/+;Gata4fl/+;Ptenfl/+ embryos (for G2 phase, 5.33 ± 0.58% vs. 10.2 ± 0.76%, P = 0.001; for M phase, 5 ± 0.5% vs. 9.67 ± 0.58%, P = 0.001) (Fig. 5S) and cell-cycle parameters were restored to those observed in control Gli1Cre-ERT2/+ embryos (11.2 ± 0.29% for G2 phase, P = 0.074; 9.33 ± 0.58% for M phase, P = 0.422) (Fig. 5S). We further observed that the defects in cell-cycle gene expression observed in Gata4-mutant embryos (Fig. 5 M–R and T) were rescued by decreased Pten dose. IHC staining for Cdk4 expression in pSHF of Gli1Cre-ERT2/+;Gata4fl/+;Ptenfl/+ embryos (44.3 ± 3.85%) (Fig. 5 O and R) was normalized to levels observed in control Gli1Cre-ERT2/+ embryos (46.3 ± 4.80%, P = 0.66) (Fig. 5 M and P). We next analyzed the pSHF RNA expression of cell-cycle regulators in Gli1Cre-ERT2/+;Gata4fl/+, Gli1Cre-ERT2/+;Ptenfl/+, or Gli1Cre-ERT2/+;Gata4fl/+;Ptenfl/+ embryos at E9.5. As expected, we observed decreased expression of Gata4, Cdk6, Cdk4, and Ccnd2 in the pSHF of Gli1Cre-ERT2/+;Gata4fl/+ embryos and decreased expression of Pten but significantly higher expression of Cdk6 and Cdk4 in the pSHF of Gli1Cre-ERT2/+;Ptenfl/+ embryos (Fig. 5U). Compound heterozygous knockout of Gata4 and Pten restored the SHF expression of Cdk6 and Cdk4 to normal levels observed in control Gata4fl/+ embryos, rescuing the expression decrement caused by heterozygous loss of Gata4 alone (Fig. 5U). Together these findings implicate a Gata4/Pten balance in cell-cycle control in the SHF for AV septation.
Gata4 Interacts with Tbx5 in AV Septation.
Because Gata4 interacts genetically with Tbx5 for normal cardiac morphogenesis (3), and each is independently required in the SHF for AV septation (Fig. 1) (35), we hypothesized that these transcription factor genes may interact genetically in the SHF. We created SHF-specific Gata4 and Tbx5 haploinsufficiency using the SHF-specific Mef2cAHF::Cre BAC transgenic mouse line (Tbx5fl/+;Gata4fl/+;Mef2cAHF::Cre). Tbx5fl/+;Gata4fl/+;Mef2cAHF::Cre embryos had a higher incidence of AVSDs than seen in littermate single Gata4fl/+;Mef2cAHF::Cre or Tbx5fl/+;Mef2cAHF::Cre embryos at E13.5 (3/9, 0/13, and 0/13, respectively) (Table 2) (Tbx5fl/+;Gata4fl/+;Mef2cAHF::Cre vs. Gata4fl/+;Mef2cAHF::Cre, P = 0.0125 by χ2 test or 0.0273 by one-tailed Fisher exact test; Tbx5fl/+;Gata4fl/+;Mef2cAHF::Cre vs. Tbx5fl/+;Mef2cAHF::Cre, P = 0.0125 by χ2 test or 0.0273 by one-tailed Fisher exact test). Furthermore, we observed more severe AVSDs [including a common atrium and complete common AV canal (CCAVC) (Fig. 6 A–C and A′–C′ and Table 2)] in compound Tbx5fl/+;Gata4fl/+;Mef2cAHF::Cre embryos than in single heterozygotes. Therefore, Gata4 and Tbx5 interact genetically in the SHF or in SHF-derived cells for AV septation.
Fig. 6.
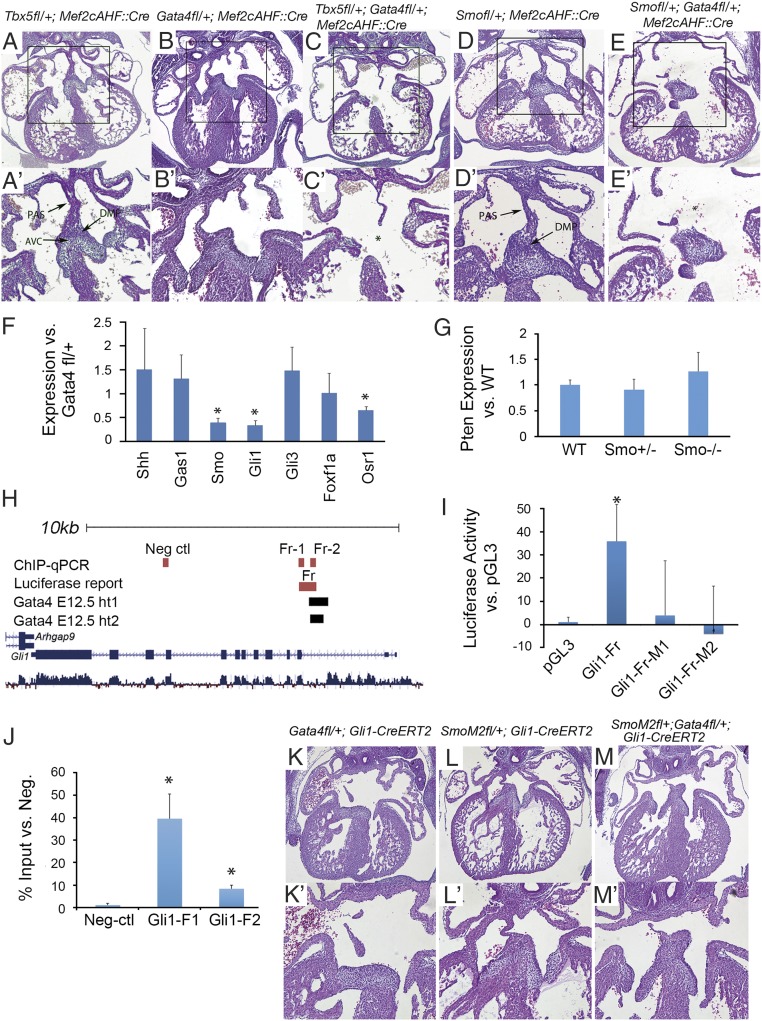
Gata4 interacts with Hh signaling in AV septation. (A–E) Histology of Gata4 transgenic mouse embryo hearts at E14.5. Asterisks indicate missing structures of atrial septation. A′–E′ show the boxed areas in A–E at higher magnification. (Magnification: 40× in A–E; 100× in A′–E′.) (F) Gene expression in the pSHF of Gli1Cre-ERT2/+;Gata4fl/fl embryos was measured by real-time PCR and was compared with gene expression in Gata4fl/+ embryos. (G) Gene expression in the pSHF of Smo+/− or Smo−/− embryos was measured by real-time PCR and was compared with the gene expression in wild-type embryos. (H) Schematic of the mouse Gli1 genomic locus including Gata4-binding regions and the cloned genomic fragments used for Gata4 regulation assays (luciferase reporter assay and ChIP-qPCR). (I) Gata4-stimulated firefly luciferase expression is seen in wild-type Gli1-Fr but not in mutated Gli1-Fr-M1 and Gli1-Fr-M2 fragments. Results are presented as mean ± SEM; n = 3; *P < 0.05 compared with pGL3. (J) Enrichment of Gata4-responsive Gli1 genomic fragments in the SHF by Gata4 ChIP-qPCR. Results are presented as mean ± SEM; n = 3; * P < 0.05. (K–M) Histology of Gata4 transgenic mouse embryo hearts at E14.5. Asterisks indicate missing structures of atrial septation. (Magnification 40× in K–M; 100× in K′–M′.)
Gata4 Interacts with Hh signaling in AV Septation.
The requirement of Gata4 in Hh-receiving cells for AV septation (Fig. 1) suggested that Gata4 and Hh signaling may interact genetically in the SHF for atrial septation. We tested this hypothesis by creating SHF compound heterozygous knockouts of Gata4 and Smo using Mef2c-Cre, encoding the obligate Hh receptor (Smofl/+;Gata4fl/+;Mef2cAHF::Cre). No ASDs or AVSDs were observed in Smofl/+;Mef2cAHF::Cre embryos (0/12) (Fig. 6 D and D′) or in Gata4fl/+;Mef2cAHF::Cre embryos (0/9) (Fig. 6 B and B′) at E13.5. In contrast, severe AVSDs, including common atrium and CCAVC, were observed in 33% (5/15) of Smofl/+;Gata4fl/+;Mef2cAHF::Cre embryos at E13.5 [P = 0.0258 (χ2 test) or 0.0590 (Fishers exact test) vs. Gata4fl/+;Mef2cAHF::Cre and P = 0.0125 (χ2 test) or 0.0273 (Fishers exact test) vs. Smofl/+;Mef2cAHF::Cre)] (Fig. 6 E and E′ and Table 2). These results suggested a genetic interaction between Gata4 and the Hh signaling pathway for AV septation.
We tested the hypothesis that Gata4 was required for SHF Hh signaling by evaluating the expression of the known Hh signaling targets Gli1 and Osr1 and the Hh signaling components Shh, Smo, Gli3, and Gas1. Gata4 expression in the pSHF of Gli1Cre-ERT2/+;Gata4fl/fl embryos was significantly decreased as compared with Gata4fl/+ embryos (0.446 ± 0.098, P = 0.013), as expected. Expression of Gli1, Osr1, and Smo was significantly reduced in the pSHF of Gli1Cre-ERT2/+;Gata4fl/fl embryos compared with control Gata4fl/+ embryos as determined by real-time PCR at E9.5 (0.338 ± 0.105, P = 0.0004; 0.440 ± 0.059, P = 0.0008; and 0.654 ± 0.076, P = 0.0067, respectively) (Fig. 6F). Other Hh pathway genes that are not known direct Hh targets, such as Shh and Gli3, were unchanged. These results suggested that Gata4 heterozygous knockout caused quantitative Hh signaling defects.
We asked if Pten expression was dependent on Hh signaling in pSHF. Pten expression was unaltered in the pSHF of Smo heterozygote or Smo-knockout mouse embryos at E9.5 compared with that in wild-type control littermates (Fig. 6G). Reciprocally, we asked if Hh pathway gene expression was dependent on Pten activity. We observed that the expression of neither Gli1 nor Smo was altered by Pten heterozygous knockout (Fig. 5U). The data suggested that Pten expression was independent of Hh signaling and that Hh signaling pathway gene expression is independent of Pten activity.
We hypothesized that Gata4 may regulate Hh signaling components directly. We bioinformatically interrogated the Smo, Osr1, and Gli1 loci for potential Gata4-responsive elements. We used the overlap of evolutionary conservation and Gata4 occupancy in HL-1 cells or embryonic mouse hearts (58, 59) and identified conserved Gata4-binding sites within the second intron of Gli1 (Fig. 6H and Table 3). We tested the conserved region including the Gata4-binding sites for cis-regulatory activity by luciferase reporter assay. Gata4 expression significantly transactivated firefly luciferase expression from this genomic construct in HEK293 cells (Fig. 6I). Mutant constructs, each ablating one Gata4-binding site, failed to activate luciferase expression (Fig. 6I). We next assessed whether Gata4 physically occupied the Gata4-responsive region at Gli1 in the SHF in vivo during atrial septum progenitor specification at E9.5. ChIP-quantitative PCR (qPCR) was performed for Gata4 using microdissected SHF tissues of wild-type mouse embryos at E9.5. We observed significant enrichment of the Gata4-dependent enhancer at Gli1 but not of control fragments from neighboring upstream or downstream genomic regions (Fig. 6J and Table 3). Together, these results place Gata4 upstream of Gli1, an effector of Hh signaling activation, in the SHF.
Table 3.
Genomic regions with Gata4-binding sites assessed by luciferase reporter assay and ChIP-qPCR
| Gene name | Luciferase assay | ChIP | |||||
| Genomic fragment | Locus | Luciferase results | Gata4-binding sites in subcloned fragments | Genomic fragment | Locus | ChIP results, % of input | |
| Gli1 | Gli1-Fr | chr10:126775570–126776129 | 42.1 ± 16.2, P = 0.0478 | chr10:126775655–126775660 | Gli1-Fr1 | chr10:126775576–126775736 | 0.44 ± 0.12, P = 0.0039 |
| chr10:126776103–126776108 | |||||||
| Gli-Fr-M1 | 6.3 ± 25.8, P = 0.4711 | chr10:126776103–126776108* | Gli1-Fr2 | chr10:126775984–126776129 | 0.09 ± 0.01, P = 0.0163 | ||
| Gli1-Fr-M2 | −6.3 ± 22.3, P = 0.4270 | chr10:126775655–126775660* | Negative control | chr10:126771190–126771322 | 0.01 ± 0.01 | ||
All genomic coordinates are shown in mouse genome build mm9.
This site was deleted in the mutated subclone of the Gata4 fragment.
To elucidate the in vivo hierarchy between Gata4 and Hh signaling, we combined conditional dominant Gata4 knockout in Hh-receiving cells with Gli1:Cre-dependent expression of SmoM2, a constitutively activated Smo mutant (60). In control Gli1Cre-ERT2/+;R26-SmoM2fl/+ embryos, normal septation was observed at E14.5 (7/7) (Fig. 6 L and L′). In contrast to the Gli1Cre-ERT2/+;Gata4fl/+ embryos (13/21) that showed AVSDs, only 1/10 of Gata4fl/+;Gli1Cre-ERT2/+;R26-SmoM2fl/+ embryos showed ASVDs, indicating significant rescue by R26-SmoM2 (P = 0.0106 by χ2 test or P = 0.0045 by one-tailed Fisher exact test) (compare Fig. 6 M and M′ with Fig. 6 K and K′ and Table 2). Rescue of AVSDs in Gata4-mutant embryos by constitutive Hh signaling was consistent with the molecular data placing Gata4 upstream of Hh signaling in SHF atrial septum progenitors.
Discussion
Dominant mutations in the essential cardiogenic transcription factors including Gata4, Tbx5, and Nkx2-5 cause human atrial and AVSDs; however, the gene-regulatory networks required for atrial septation downstream of these essential transcription factors have remained unidentified. Here we have implicated Gata4 in the SHF for atrial/AV septation and have defined genetic interactions between Gata4 and both Hh signaling and Pten for cardiac septation. Our study provides genetic, cellular, and molecular evidence supporting a dosage-sensitive requirement for Gata4 in SHF progenitors. Support for a SHF role of Gata4 in atrial septation includes the observation that Gata4 is required in Hh signal-receiving SHF progenitor cells. Use of the TM-inducible Gli1CreERT2 allowed the temporal requirement for Gata4 to be ascertained: Gli1-CreERT2 activation by TM treatment at E7.5 and E8.5 caused penetrant AVSDs, but TM treatment at E8.5 and E9.5 did not. This result suggests that Gata4 is required in SHF Hh-receiving progenitors for AV septation but not at a later time in SHF-derived myocardium. This temporal requirement mirrors that previously observed for Hh signaling in the SHF (32). Together, these observations suggest an essential role for Gata4 in SHF Hh-receiving cardiac progenitors for atrial septation. This work joins our previous work implicating Tbx5 and Hh signaling and the work of others implicating Wnt and Bmp signaling in the SHF for AV septation, providing support for the importance of SHF pathways in AVSD causation (32, 34–36, 61, 62). These studies imply that disrupted SHF gene regulatory networks play an important role in the ontogeny of AVSDs and should be considered by groups working to identify the genetic causation of AVSDs in humans.
Our observations do not preclude a requirement for Gata4 in myocardium or endocardium in atrial septation, despite the absence of defects in embryos with conditional dominant Gata4 knockout generated by myocardial-specific Tnt:Cre or endocardial-specific Tie2:Cre. In fact, previous studies have identified interactions between Gata4 and other genes essential for cardiac septation in other lineages. For example, lineage-specific Gata4 and Tbx5 heterozygosity in endocardium (63) or myocardium (4) caused ASDs and AVSDs, respectively, demonstrating a combinatorial requirement for Gata4 and Tbx5 in these lineages. Gata4 is highly expressed in the SHF in a pattern overlapping with Tbx5 (13, 39), and we observed that Gata4 and Tbx5 interact genetically in the SHF for atrial septation (Fig. 6). GATA4 and TBX5 interact physically to activate gene expression synergistically in the heart (2), and multiple Gata4 transcriptional targets have been identified in the heart (1, 4, 5, 7, 22, 64). Recent studies have indicated that GATA4 and TBX5 are essential for each other’s transcriptional fidelity in the control of cardiac gene regulatory networks in mouse and human models (65, 66). Determining GATA4 and TBX5 coregulated targets in the SHF may identify genes essential for normal atrial septum morphogenesis.
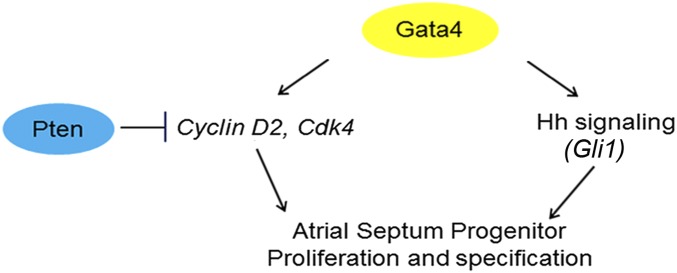
Gata4 directly regulates the transcription of cell-cycle genes in the myocardium (1, 67). We observed that Cyclin D2, Cdk4, and Cdk6 are Gata4 dependent in the SHF. Diminished expression of cell-cycle control genes in the SHF was a plausible molecular mechanism for AVSD causation in Gata4 mutants. In support of this hypothesis, SHF-specific knockdown of Pten, a tumor suppressor that negatively regulates Cdk4 and Cdk6, rescued atrial septation in Gata4 mutants. These observations support a model in which Gata4 drives SHF progenitor proliferation to generate a progenitor pool capable of supporting atrial septum morphogenesis. The Pten rescue experiments describe a balance between SHF Gata4 and Pten dose required for proper expansion of atrial septum progenitors, integrating cardiogenic transcription factor and cell-cycle regulatory genes in an SHF pathway for AV septation (Fig. 7).
Fig. 7.
Model of Gata4 transcriptional regulation in atrial septation. Gata4 acts upstream of both Hh signaling and the Pten/Cdk4/Cyclin D2 pathways. These pathways appear to be independent, and rescue of either pathway prevents CHDs in Gata4 heterozygote knockouts, supporting a model in which combined deficiency of both pathways is required for CHD pathogenesis.
We provide evidence that Gata4 acts upstream of Hh signaling in atrial septation (Fig. 7). Gata4 and Smo interact genetically, and, remarkably, SHF-specific constitutive Hh signaling rescued AVSDs in Gata4-mutant embryos (Fig. 6). Genetic rescue suggested a Gata4–Hh signaling hierarchy, which we identified molecularly: Gata4 mutants have diminished SHF Hh signaling, because Hh signaling components, including the known Hh target genes Gli1 and Osr1, demonstrated Gata4-dependent pSHF expression. Furthermore, we identified Gata4-responsive cis-regulatory elements at Gli1 that were occupied by GATA4 in vivo, providing evidence that Gli1 is a direct GATA4 SHF target. Together these observations support a model in which Gata4 is required upstream of SHF Hh signaling for atrial septation (Fig. 7).
The mechanism whereby dominant transcription factor mutations cause developmental phenotypes remains largely unanswered. We have linked Gata4 to two distinct molecular pathways in vivo, Pten-modulated cell cycle and Hh signaling. Our prior work suggested that Hh signaling was required for SHF patterning but not for proliferation for AV septation (33), and transcriptional profiling for SHF Hh-dependent gene expression did not uncover genes required for proliferation (44). Furthermore, Pten expression in not altered in Hh pathway mutants, and, reciprocally, Hh pathway gene expression is not altered in Pten mutants, suggesting that these pathways function independently. Interestingly, ASDs caused by Gata4 heterozygous knockout were rescued by restoring cell-cycle transition (using Pten knockdown; Fig. 5) or by constitutive Hh signaling (Fig. 6). Because restoration of either pathway restored normal cardiac morphogenesis and prevented CHD, we concluded that both the cell-cycle and Hh signaling decrements were required for Gata4-dependent CHD. Together these observations suggest that deficiency of multiple, discrete target pathways downstream of Gata4 are required in combination for CHD pathogenesis caused by dominant Gata4 transcription factor mutations, suggesting a combinatorial model for disease causation by transcription factor haploinsufficiency.
Materials and Methods
Mouse Lines.
All mouse experiments were performed in a mixed B6/129/SvEv background. The Gata4fl/fl mouse line was a kind gift from the laboratory of William Pu, Harvard University, Cambridge, MA. Tbx5fl/+, Gli1CreERT2/+, Mef2cAHF::Cre, Tie2Cre/+, and Smofl/+ mouse lines were obtained from the I.P.M. laboratory. The TnTCre/+ mouse line was from the laboratory of Yiping Chen, Tulane University, New Orleans. Generation and genotyping of the Gata4fl/fl, Tbx5fl/+, Gli1CreERT2/+, Tie2Cre/+, TnTCre/+, and Mef2cAHF::Cre mouse lines have been reported (8, 35, 49, 50, 68–70). Ptenfl/+ and EIIacre/+ mouse lines were purchased from the Jackson Laboratory. Mouse experiments were performed according to a protocol reviewed and approved by the Institutional Animal Care and Use Committee of the University of North Dakota, in compliance with the US Public Health Service Policy on the Humane Care and Use of Laboratory Animals.
TM Administration and X-Gal Staining.
TM-induced activation of CreERT2 was accomplished by oral gavage with two doses of TM (75 mg/kg) at E7.5 and E8.5 (32). X-gal staining of embryos was performed as described (32).
IHC.
Standard procedures were used for histology and IHC. IHC was performed using the antibodies rabbit anti-mouse p-Histone-H3 (H3S10) (Abcam) and rabbit anti-mouse CDK4 (Abcam). For colorimetric staining, slides were incubated with rabbit ImmPress reagent (Vector Labs), developed using a 3,3′-diaminobenzidine-tetrahydrochloride (DAB) substrate kit (Vector Labs), and counterstained with hematoxylin. For BrdU incorporation, pregnant mice were given 100 mg BrdU/kg body weight in 10 mg/mL solutions at E9.0 in two doses, 3 h and 6 h, respectively, before the animals were killed. BrdU staining was performed using a BrdU In-Situ Detection Kit (EMD Millipore). For TUNEL staining, an ApopTag Plus Peroxidase in-Situ Apoptosis Detection Kit was used (EMD Millipore).
Microdissection of pSHF and RNA Extraction.
To obtain the pSHF splanchnic mesoderm for use in real-time qPCR, E9.5 embryos were dissected as described previously (35, 44). The heart, aSHF, and pSHF were collected separately in RNAlater RNA Stabilization Reagent (QIAGEN) and then were stored at −20 °C until genotyping was completed.
Real-Time PCR.
Total RNA was extracted from the pSHF regions of mouse embryo hearts using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. Two hundred nanograms of total RNA was reverse transcribed using a SuperScript III Reverse Transcriptase kit from Invitrogen. The qPCR was performed using a Power SYBR Green PCR Master Mix from Applied Biosystems. Results were analyzed using the ΔΔCt method with GAPDH as a normalization control.
Luciferase Reporter Assay.
Amplified promoter regions as noted in Results were cloned into pGL3 Basic vector (Promega) modified to include a minimal TA promoter. Gata4 was cloned into a pcDNA3 vector. Transient transfections were performed in HEK293T cells using FuGENE HD (Promega) according to the manufacturer’s instructions. Total cell lysates were prepared 48 h posttransfection, and luciferase activity was assessed using the Promega Dual Luciferase Reporter kit (Promega) according to the manufacturer’s protocol.
Site-Directed Mutagenesis.
Mutant reporter vectors were generated by deleting Gata4-binding sites using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies). The following primers were used: Gli1-del1 forward (5′-GGAACGAAACAGAGAATGACAGTTTCAGGC-3′), reverse (5′-GCCTGAAACTGTCATTCTCTGTTTCGTTCC-3′) and Gli1-del2 forward (5′-CCTCGTTTCAGTCCACTGGTAGGGCCAG-3′), reverse (5′-CTGGCCCTACCAGTGGACTGAAACGAGG-3′). Detailed site information is listed in Table 3.
ChIP.
E9.5 embryos were microdissected in cold PBS containing Protease Inhibitor Mixture (Roche) to isolate the primitive heart region. Approximately 20 tissues were pooled as one sample. Tissues were cross-linked with 1% formaldehyde for 15 min at room temperature and terminated with glycine. After PBS washes, tissues were dissociated by shaking at 37 °C for 1–2 h at 100 rpm in collagenase type II (Gibco) solution. Sonication was performed using a Covaris S220 sonicator to generate fragments with an average size of 600 bp. Samples were incubated with Gata4 antibody (sc-1237 X; Santa Cruz) overnight at 4 °C and then were incubated with Dynabeads Protein G (Life Technologies) for 2 h, washed, and reverse cross-linked.
Acknowledgments
This project was supported by NIH Grants NIH-1R15HL117238 (to L.X.) and R01 HL092153 and R01 HL124836 (to I.P.M.), by Grants 5P20RR016471-12 and 8 P20 GM103442-12 from the National Center for Research Resources (to L.X. and K.Z.), by American Heart Association Grants 13SDG14650009 (to L.X.) and 15GRNT25700195 (to K.Z.), and by Established Investigator Award 13EIA14690081 (to I.P.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605137114/-/DCSupplemental.
References
- 1.Rojas A, et al. GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium. Mol Cell Biol. 2008;28(17):5420–5431. doi: 10.1128/MCB.00717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg V, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424(6947):443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 3.Maitra M, et al. Interaction of Gata4 and Gata6 with Tbx5 is critical for normal cardiac development. Dev Biol. 2009;326(2):368–377. doi: 10.1016/j.ydbio.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra C, Chang SW, Basu M, Huang N, Garg V. Disruption of myocardial Gata4 and Tbx5 results in defects in cardiomyocyte proliferation and atrioventricular septation. Hum Mol Genet. 2014;23(19):5025–5035. doi: 10.1093/hmg/ddu215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra C, et al. Congenital heart disease-causing Gata4 mutation displays functional deficits in vivo. PLoS Genet. 2012;8(5):e1002690. doi: 10.1371/journal.pgen.1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajagopal SK, et al. Spectrum of heart disease associated with murine and human GATA4 mutation. J Mol Cell Cardiol. 2007;43(6):677–685. doi: 10.1016/j.yjmcc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131(16):3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- 8.Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275(1):235–244. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg EM, et al. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115(6):1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisping E, et al. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci USA. 2006;103(39):14471–14476. doi: 10.1073/pnas.0602543103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera-Feliciano J, et al. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133(18):3607–3618. doi: 10.1242/dev.02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi S, et al. Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. FASEB J. 2006;20(6):800–802. doi: 10.1096/fj.05-5426fje. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CT, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11(8):1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 14.Ip HS, Wilson DB, Heikinheimo M, Leiden JM, Parmacek MS. The GATA-4 transcription factor transactivates the cardiac-specific troponin C promoter-enhancer in non-muscle cells. Adv Exp Med Biol. 1995;382:117–124. doi: 10.1007/978-1-4615-1893-8_13. [DOI] [PubMed] [Google Scholar]
- 15.Ip HS, et al. The GATA-4 transcription factor transactivates the cardiac muscle-specific troponin C promoter-enhancer in nonmuscle cells. Mol Cell Biol. 1994;14(11):7517–7526. doi: 10.1128/mcb.14.11.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basson CT, et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15(1):30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 17.Li QY, et al. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet. 1997;15(1):21–29. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- 18.Schott JJ, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281(5373):108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- 19.Sarkozy A, et al. Spectrum of atrial septal defects associated with mutations of NKX2.5 and GATA4 transcription factors. J Med Genet. 2005;42(2):e16. doi: 10.1136/jmg.2004.026740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirayama-Yamada K, et al. Phenotypes with GATA4 or NKX2.5 mutations in familial atrial septal defect. Am J Med Genet A. 2005;135(1):47–52. doi: 10.1002/ajmg.a.30684. [DOI] [PubMed] [Google Scholar]
- 21.Okubo A, et al. A novel GATA4 mutation completely segregated with atrial septal defect in a large Japanese family. J Med Genet. 2004;41(7):e97. doi: 10.1136/jmg.2004.018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morin S, Charron F, Robitaille L, Nemer M. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J. 2000;19(9):2046–2055. doi: 10.1093/emboj/19.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30(11):630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn. 2008;237(10):2804–2819. doi: 10.1002/dvdy.21725. [DOI] [PubMed] [Google Scholar]
- 25.Webb S, Brown NA, Anderson RH. Formation of the atrioventricular septal structures in the normal mouse. Circ Res. 1998;82(6):645–656. doi: 10.1161/01.res.82.6.645. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105(6):781–792. [PubMed] [Google Scholar]
- 27.Lan Y, Liu H, Ovitt CE, Jiang R. Generation of Osr1 conditional mutant mice. Genesis. 2011;49(5):419–422. doi: 10.1002/dvg.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wessels A, et al. Atrial development in the human heart: An immunohistochemical study with emphasis on the role of mesenchymal tissues. Anat Rec. 2000;259(3):288–300. doi: 10.1002/1097-0185(20000701)259:3<288::AID-AR60>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Snarr BS, Wirrig EE, Phelps AL, Trusk TC, Wessels A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn. 2007;236(5):1287–1294. doi: 10.1002/dvdy.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snarr BS, et al. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res. 2007;101(10):971–974. doi: 10.1161/CIRCRESAHA.107.162206. [DOI] [PubMed] [Google Scholar]
- 31.Mommersteeg MT, et al. Two distinct pools of mesenchyme contribute to the development of the atrial septum. Circ Res. 2006;99(4):351–353. doi: 10.1161/01.RES.0000238360.33284.a0. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. sonic hedgehog is required in pulmonary endoderm for atrial septation. Development. 2009;136(10):1761–1770. doi: 10.1242/dev.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin L, Bu L, Cai CL, Zhang X, Evans S. Isl1 is upstream of sonic hedgehog in a pathway required for cardiac morphogenesis. Dev Biol. 2006;295(2):756–763. doi: 10.1016/j.ydbio.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 34.Goddeeris MM, et al. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135(10):1887–1895. doi: 10.1242/dev.016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie L, et al. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell. 2012;23(2):280–291. doi: 10.1016/j.devcel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briggs LE, et al. Expression of the BMP receptor Alk3 in the second heart field is essential for development of the dorsal mesenchymal protrusion and atrioventricular septation. Circ Res. 2013;112(11):1420–1432. doi: 10.1161/CIRCRESAHA.112.300821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L, et al. Tbx5 and Osr1 interact to regulate posterior second heart field cell cycle progression for cardiac septation. J Mol Cell Cardiol. 2015;85:1–12. doi: 10.1016/j.yjmcc.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang KK, et al. Gene network and familial analyses uncover a gene network involving Tbx5/Osr1/Pcsk6 interaction in the second heart field for atrial septation. Hum Mol Genet. 2016;25(6):1140–1151. doi: 10.1093/hmg/ddv636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang YP, et al. Second heart field and the development of the outflow tract in human embryonic heart. Dev Growth Differ. 2013;55(3):359–367. doi: 10.1111/dgd.12050. [DOI] [PubMed] [Google Scholar]
- 40.Moskowitz IP, et al. Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development. [corrected] Proc Natl Acad Sci USA. 2011;108(10):4006–4011. doi: 10.1073/pnas.1019025108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci USA. 2004;101(34):12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojas A, et al. Gata4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development. 2005;132(15):3405–3417. doi: 10.1242/dev.01913. [DOI] [PubMed] [Google Scholar]
- 43.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11(8):1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann AD, et al. Foxf genes integrate tbx5 and hedgehog pathways in the second heart field for cardiac septation. PLoS Genet. 2014;10(10):e1004604. doi: 10.1371/journal.pgen.1004604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz RJ, Olson EN. Building the heart piece by piece: Modularity of cis-elements regulating Nkx2-5 transcription. Development. 1999;126(19):4187–4192. doi: 10.1242/dev.126.19.4187. [DOI] [PubMed] [Google Scholar]
- 46.Bruneau BG. The developing heart and congenital heart defects: A make or break situation. Clin Genet. 2003;63(4):252–261. doi: 10.1034/j.1399-0004.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- 47.Bruneau BG, et al. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev Biol. 1999;211(1):100–108. doi: 10.1006/dbio.1999.9298. [DOI] [PubMed] [Google Scholar]
- 48.Lakso M, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93(12):5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiao K, et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17(19):2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kisanuki YY, et al. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230(2):230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 51.Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: Establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn. 2006;235(9):2376–2385. doi: 10.1002/dvdy.20884. [DOI] [PubMed] [Google Scholar]
- 52.Crosio C, et al. Mitotic phosphorylation of histone H3: Spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22(3):874–885. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 54.Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94(21):11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diao L, Chen YG. PTEN, a general negative regulator of cyclin D expression. Cell Res. 2007;17(4):291–292. doi: 10.1038/cr.2007.24. [DOI] [PubMed] [Google Scholar]
- 56.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100(4):387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 57.Huang W, Chang HY, Fei T, Wu H, Chen YG. GSK3 beta mediates suppression of cyclin D2 expression by tumor suppressor PTEN. Oncogene. 2007;26(17):2471–2482. doi: 10.1038/sj.onc.1210033. [DOI] [PubMed] [Google Scholar]
- 58.He A, et al. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat Commun. 2014;5:4907. doi: 10.1038/ncomms5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He A, Kong SW, Ma Q, Pu WT. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc Natl Acad Sci USA. 2011;108(14):5632–5637. doi: 10.1073/pnas.1016959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mao J, et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 2006;66(20):10171–10178. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Briggs LE, Kakarla J, Wessels A. The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation. 2012;84(1):117–130. doi: 10.1016/j.diff.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian Y, et al. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell. 2010;18(2):275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nadeau M, et al. An endocardial pathway involving Tbx5, Gata4, and Nos3 required for atrial septum formation. Proc Natl Acad Sci USA. 2010;107(45):19356–19361. doi: 10.1073/pnas.0914888107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamak A, Latinkic BV, Dali R, Temsah R, Nemer M. Cyclin D2 is a GATA4 cofactor in cardiogenesis. Proc Natl Acad Sci USA. 2014;111(4):1415–1420. doi: 10.1073/pnas.1312993111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luna-Zurita L, et al. Complex interdependence regulates heterotypic transcription factor distribution and coordinates cardiogenesis. Cell. 2016;164(5):999–1014. doi: 10.1016/j.cell.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ang YS, et al. Disease model of GATA4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell. 2016;167(7):1734–1749 e1722. doi: 10.1016/j.cell.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakajima K, et al. Coordinated regulation of differentiation and proliferation of embryonic cardiomyocytes by a jumonji (Jarid2)-cyclin D1 pathway. Development. 2011;138(9):1771–1782. doi: 10.1242/dev.059295. [DOI] [PubMed] [Google Scholar]
- 68.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287(1):134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 69.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118(4):505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 70.Bruneau BG, et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106(6):709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]