Summary
The B7-CD28 family of ligands and receptors play important roles in T cell costimulation and coinhibition. Phylogenetically they can be divided into three groups. The recent discovery of the new molecules [B7-H3 (CD276), B7x (B7-H4/B7S1) and HHLA2 (B7H7/B7-H5)/TMIGD2 (IGPR-1/CD28H)] of the group III has expanded therapeutic possibilities for the treatment of human diseases. In this review, we describe the discovery, structure and function of B7-H3, B7x, HHLA2 and TMIGD2 in immune regulation. We also discuss their roles in important pathological states such as cancers, autoimmune diseases, transplantation, and infection. Various immunotherapeutical approaches are emerging including antagonistic monoclonal antibodies and agonistic fusion proteins to inhibit or potentiate these molecules and pathways in cancers and autoimmune diseases.
Keywords: HHLA2, TMIGD2, B7x, B7-H3, immune checkpoint, immunotherapy
Introduction
In the past two decades there has been a major advancement in understanding the functions of the immune system and one of the foremost achievements is the development of new immune checkpoint inhibitors. Checkpoint molecules are now sufficiently numerous such that they can be divided into families. The B7-CD28 family can be phylogenetically divided into three groups (1, 2) - Group I consisting of B7-1/B7-2/CD28/CTLA4 and B7h/ICOS; group II containing PD-L1/PD-L2/PD-1; group III including B7-H3 (CD276), B7x (B7-H4/B7S1) and HHLA2 (B7H7/B7-H5)/TMIGD2 (IGPR-1/CD28H). The B7-1/B7-2/CD28/CTLA-4 pathway is important in modulating central immune tolerance while PD-L1/PD-L2/PD-1, B7-H3, B7x and HHLA2 are important in peripheral immune regulation. The discovery, understanding and therapeutic manipulation of the CTLA4 and PD-1/PD-L1 pathways have led to important therapeutic advances in cancer immunotherapy in patients and have led to improved patient cures and survival (3, 4). In this review, we will focus on the advances of the new immune checkpoints in the third group of the B7 family namely B7-H3, B7x, HHLA2 and TMIGD2.
B7-H3
B7 homolog 3 protein (B7-H3) also known as CD276 is an immune checkpoint molecule that belongs to the B7-CD28 family and was discovered in 2001 (5). This molecule has been associated with costimulatory as well as coinhibitory functions in regulating T cell responses. B7-H3 is encoded on chromosome 15 in humans (5) but in mice it is located on chromosome 9 (6). B7-H3 is universally expressed across various species from teleost fish to mammals, thus it is one of the most evolutionarily conserved B7 family members (7).
It is a 316 amino acid type I transmembrane glycoprotein with a molecular weight of 45–66 kDa. The extracellular domain is composed of two identical pairs of the immunoglobulin constant (IgC) and variable (IgV) domain in humans due to exon duplication (four IgB7-H3) while it consists of a single pair in mice (two IgB7-H3). The intracytoplasmic tail is short and has no known signaling motif (6, 8, 9). The B7-H3 receptor has not yet been identified (10, 11).
Structure
Murine B7-H3 (mB7-H3) possesses an ectodomain composed of a single IgV domain and a consecutive IgC domain (1, 5). The structure of the ectodomain, and elucidation of the detailed biophysical properties of the molecule, provides the models for mapping the possible receptor binding sites and functional analysis (11).
The crystal structure of mB7-H3 construct, including both IgV and IgC domains, was determined to the resolution of 3 Å with the space group P6122. The ectodomain of mB7-H3 was produced by Drosophila S2 cells and purified as a glycosylated monomer. However, mB7-H3 crystallized as an unusual dimeric form, resulting from the mutual swap of the IgV domain G strands between two adjacent molecules (Figure 1). The connection segment of the F and G strands adopts an extended “beta strand-like” conformation rather than the classical FG loop conformation of the IgV domains. Consequently, the crystal structure of the mB7-H3 IgV domain is formed by a “back sheet” (ABED strands) and a “front sheet” (C”C’CFG* strands), of which the G* strand is contributed by the neighboring mB7-H3 IgV domain. The mB7-H3 C-terminal IgC domain (residues 140–239) located on the C-terminus of the ectodomain adopts the classical IgC folding with sheets ABED and CFG (12).
Figure 1. Structures of B7-H3, B7x and PD-L1.
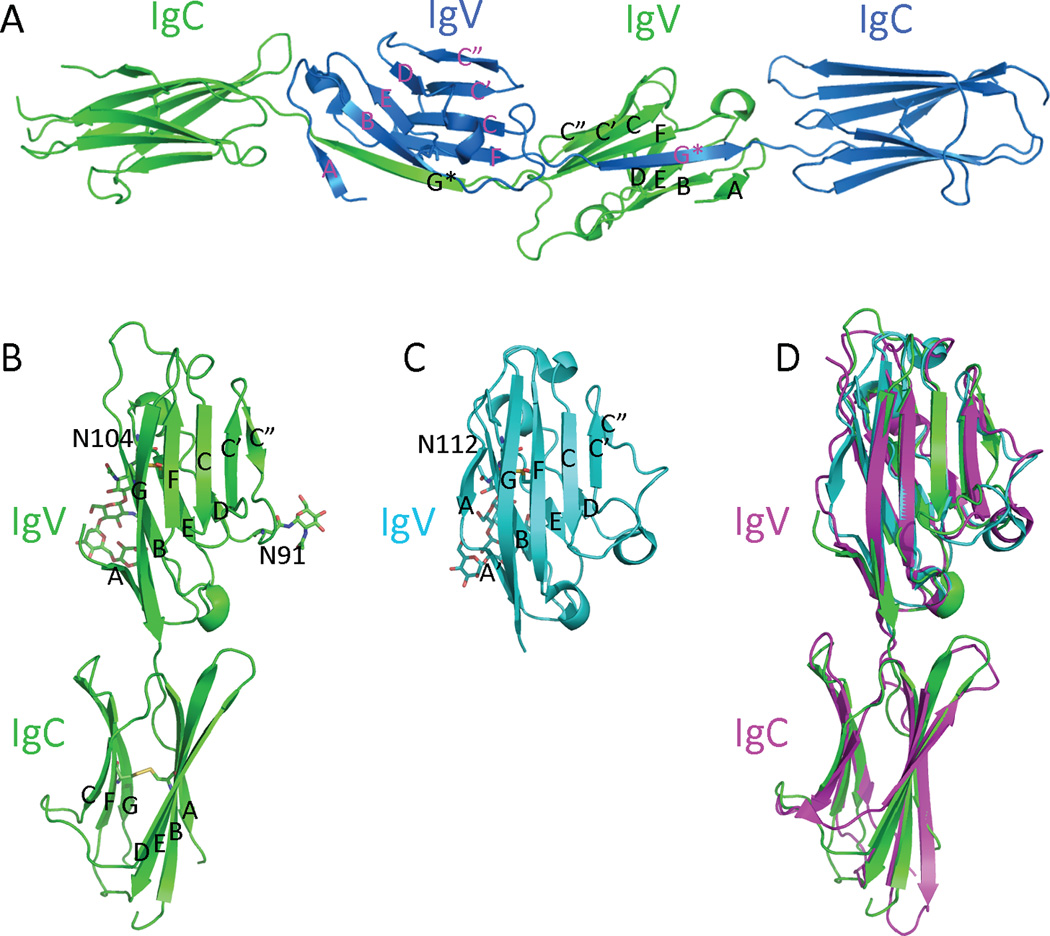
(A) Overall structure of the dimeric mB7H3 in the crystal (PDB entry 4I0K). The strands from each monomeric mB7H3 are colored and labeled differently. (B) The generated model of monomeric mB7H3 based on the crystal structure from PDB entry 4I0K. (C) The structure of the hB7x IgV domain (PDB entry 4GOS). (D) Superimposition of the monomeric mB7H3, hB7x and hPD-L1 (PD-L1 is from A chain of the PDB entry 3BIK). The disulfide bonds, carbohydrates and the connecting Asn residues are shown as sticks.
Upon days of storage at high concentration at 4°C, monomeric mB7-H3 gradually forms a stable dimer in a concentration-dependent manner (11). Consistent with the crystal structure of mB7-H3, of which the IgV FG loop residues adopts an unusual extended strand-like conformation, replacements f the residues by other residues in the FG loop perturb the dimerization, suggesting that the observed G loops exchanging in the crystal structure contributes to the dimerization of mB7-H3 in solution. Interestingly, both monomeric and dimeric mB7-H3 showed consistent abilities to inhibit T cell proliferation with no significant difference. Thus dimerization of mB7-H3 alone does not change the inhibitory function of the molecule (11).
A canonical monomeric model of mB7-H3 was generated based on the crystal structure of the dimeric mB7-H3 (13). Two potential N-glycosylation sites (residues Asn91 and Asn104) are predicted on the IgV domain of mB7-H3 based on the sequence of the molecule. Significant electron density was observed near Asn91 and was identified as a single N-acetyl glucosamine (NAG). More electro density was observed in adjacent to residue Asn104 and was interpreted as two NAG and two mannose sugar residues. Both glycosylation residues and sugars are located in the “back sheet” of mB7H3 IgV domain (Figure 1). A chimera mB7-H3 mutant, of which the whole FG loop (residues 126–129; sequences: IQDF) was replaced to the cognate sequences from human PD-L1 (sequences: YGGA), completely lost the mB7-H3-mediated inhibitory activity. Surprisingly, alanine scanning targeted the residues on the “front sheet” of mB7-H3 IgV domain did not significantly change the inhibitory activity of mB7H3 as compared to the wild type mB7H3 (11). These results indicate the individual mutation of the residues on the “front sheet” to alanine may not be sufficient to disrupt the receptor recognition, whereas replacement of the FG loop residues is significant enough to disrupt mB7-H3 function. This also demonstrates the FG loop is important for mB7-H3-mediated inhibition.
Expression
B7-H3 mRNA is widely expressed on many tissues such as the heart, thymus, prostate, testis, uterus, placenta, spleen, liver, pancreas, small intestine and colon (14) (Table 1). Despite its broad mRNA expression, there appears to be a tightly regulated posttranscriptional control mechanism as protein expression is limited in steady state and maintained at low levels. The protein is constitutively expressed on non-immune resting fibroblasts, endothelial cells, osteoblasts and amniotic fluid stem cells (15). Its expression can be induced on immune cells such as T cells, natural killer (NK) cells and antigen-presenting cells (APCs) including dendritic cells (DCs) and macrophages (8, 16, 17). B7-H3 protein overexpression in tumor tissue was highly correlated with decreased expression of miR-29 as compared to normal tissues, and B7-H3 protein level could be modulated through manipulating miR-29 level in cultured cell lines, suggesting that a microRNA regulatory mechanism is involved in its differential expression (18). In vitro its expression, specifically on DCs, has been noted in co-culture with regulatory T (Treg) cells (19), Interferon-gamma (IFN-γ), lipopolysaccharide (LPS) or anti-CD40 stimulation (17, 20). B7-H3 is upregulated on monocytes and monocyte derived DCs after LPS stimulation or cytokine induced differentiation respectively (21) while it is detected on NK cells and B cells following ionomycin stimulation (8, 15). The limited expression of B7-H3 in normal tissues is consistent with the expression of other members of the B7 family.
Table 1.
Expression of B7-H3, B7x and HHLA2 in tissues and cancers
| Expression | B7-H3 | B7x | HHLA2 |
|---|---|---|---|
| Immune cells and tissues | |||
| Dendritic cells | Strong (8, 21); Induced (17, 19) |
Weak (1); Induced (71) |
Negative (2); Induced (100) |
| Monocytes | Weak (21) | Induced (71) | Strong (2) |
| Macrophages | - | Weak (1) | Induced (100) |
| NK cells | Induced (8) | - | - |
| B cells | Negative (21); Induced (8) |
Weak (1); Induced (71) |
Induced (2) |
| T cells | Negative (21); Induced (8) |
Weak (1); Induced (71) |
Negative (2) |
| Osteoblasts | Strong (25) | Weak (110) | - |
| Tissues | Heart, Thymus, Prostate, Testis, Uterus, Placenta, Spleen, Liver, Pancreas, Small intestine and Colon (14) |
Lung, Testis, Prostate, Pancreas (1) |
Placenta, Colon, Breast, Small Intestine, Kidney, Gallbladder (103) |
| Cancers | |||
| Endothelial cells | Strong (111) | Strong (94) | - |
| Hepatocellular carcinoma |
93.8% (33) | 68.67% (HBV related) (112) |
40% (103) |
| Pancreatic cancer | 93.7% (39) | 61.9% (39) | 50% (103) |
| Colorectal cancer | C-86%, S-77%, N-27% (31) C-62%, N-30% (36) |
48.21% (113) | 37.5% (103) |
| Esophageal cancer | NS (114) | 95.5% (115) | 20% (103) |
| Renal cell carcinoma | 19%, E-98% (clear cell) (45) |
59.1%, E-81.5% (94) | 33.33% (103) |
| Bladder cancer | 58.6% (116) | 49% (117) | 40% (103) |
| Ovarian carcinoma | 93% (37) E-44% (37) |
100% (37) 85% (80) |
50% (103) |
| Endometrial cancer | 75.7% (34) | 100% primary (118) 96% metastatic (118) |
0% (103) |
| Cervical cancer | 72.22% (48) | 80.56% (48) | 0% (103) |
| Breast cancer | 90.60% (43) 80.55% (55) |
95.4% primary, 97.6% metastatic (119) |
56% TNBC (103) 70% (103) |
| Prostate cancer | 93% (40) 100% (41) |
99% (40) | 33.33% (103) |
| Osteosarcoma | 91.8% (35) | 70.19% (110) | 68% (120) |
| Oral squamous cell carcinoma |
74.75% (38) | NS (121) | - |
| Lung cancer | 69.5% (47) 37.1% (46) |
43% (46) 31% (80) |
66.67% (103) 66% (106) |
| Glioma | NS (49) | NS (82) | - |
| Melanoma | NS (44) | 0% (80) | 55.56% (103) |
| Thyroid | - | 95.3% (122) | 66.67% (103) |
NS: Not specified
%: % of tumor expressing molecule
Weak/Strong: IHC staining of molecule
Function
Our appreciation of the roles of B7-H3 is evolving and the context of its expression is central to further defining the importance of this molecule. Its significance in innate and adaptive immunity is complex and still needs further elucidation.
T cell
B7-H3 has been shown to have costimulatory and co-inhibitory activities in various studies. The initial study which led to the discovery of B7-H3 showed that B7-H3 increased proliferation of CD4 and CD8 T cells in the presence of anti-CD3 antibody. Moreover, B7-H3 played an important role in the generation of IFN-γ during activation of T cells (5). In murine cancer models B7-H3 has been shown to activate tumor specific cytotoxic T lymphocytes (CTL) and hence potentiate antitumor immunity (22). These studies showed that mB7-H3 can function as a costimulatory molecule.
Several studies show that B7-H3 predominantly causes T cell coinhibition. Similar T cell proliferation assays showed that B7-H3 inhibited proliferation of CD4 and CD8 T cell in a dose dependent manner and also reduced IL-2 and IFN-γ (17). This inhibitory effect of B7-H3 was overcome by CD28 mediated costimulation. The expression of B7-H3 on APCs decreases T cell proliferation. In mouse models of airway inflammation, B7-H3 downregulated Th1 but not Th2 responses, and B7-H3 gene knock-out mice developed severe airway disease than the wild type (WT). In a mouse model of experimental autoimmune encephalitis (EAE), B7-H3 deficient mice or blocking B7-H3 through antibodies resulted in earlier onset and worse disease respectively through enhanced Th1 responses (20). B7-H3, by modulating or inhibiting NFAT (nuclear factor of activated T cells), NF-κβ and AP-1, can regulate T cell receptor (TCR) mediated gene transcription and hence cause T cell co-inhibition (22). These studies comprehensively show that B7-H3 causes T cell co-inhibition. These effects of T cell costimulation or coinhibition by the same molecule could be due to multiple receptors, presence of other costimulatory or coinhibitory molecules or the presence of different immune cells/cytokines leading to modulation of B7-H3 function.
NK Cell
B7-H3 has been shown to inhibit NK cell activity in tissue cultures and hence decreases NK cell function and therefore there should be a receptor on NK cells for B7-H3 (23). In glioma, higher grades are associated with a higher percentage of B7-H3 expression (24). This study also showed that both soluble B7-H3 and cell bound B7-H3 were able to inhibit NK cell mediated lysis. In an in vivo model of B7-H3 silenced glioma cell lines, tumor formation was present but there was a marked decrease in the ability to form metastatic deposits when compared to control animals. This is important in tumor immunity, since when tumor cells lose HLA Class I molecules, and hence escape cytolytic T cell clearance they now become susceptible to NK cell mediated lysis (Figure 2).
Figure 2. The third group of the B7-CD28 immune checkpoint family in the human cancer microenvironment.
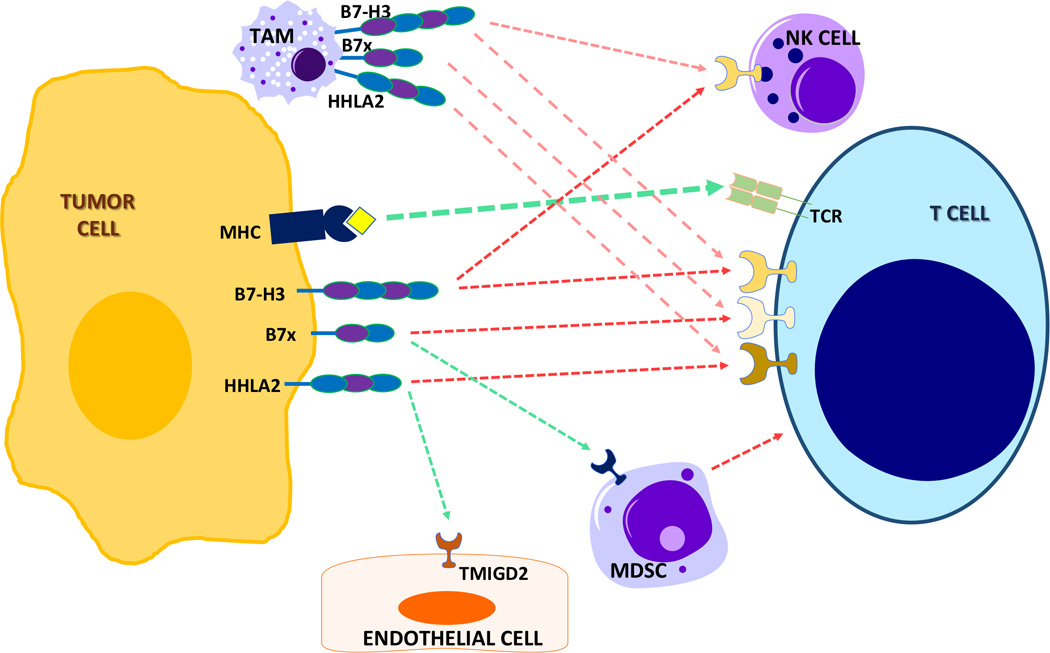
B7-H3 dampens (red broken line) NK cells and T cells, and may reprogram metabolism in cancer cells. B7x inhibits T cells and potentiates (green broken line) myeloid derived suppressor cells (MDSC). HHLA2 restricts T cells and may promote angiogenesis through its receptor TMIGD2 on endothelial cells. Receptors for B7x and B7-H3 molecules have not yet been identified.
Other cells
B7-H3 also promotes osteoblast differentiation and bone mineralization. Despite having no gross skeletal abnormalities, the B7-H3 knockout mice displayed a lower bone mineral density in cortical bones compared with wild type controls. These results show that B7-H3 is another factor in the growing interface between bone and the immune system (25).
B7-H3 in pathological states
Infection
Soluble B7-H3 is elevated in patients with sepsis when compared with healthy persons and is even higher in patients who died from sepsis (26). Whether soluble B7H3 represents a predictive or prognostic factor for sepsis is unclear as this was a small study and other confounding factors could have influenced the reported results. In patients with sepsis, serum B7-H3 levels correlate with increased levels of Tumor Necrosis Factor α (TNF-α) and IL-6. TNF-α is the most potent inducer of B7-H3 release from monocytes. B7-H3 alone was unable to cause cytokine release from murine macrophages but it strongly augmented LPS induced NF-κβ activation in a Toll-like receptor (TLR) 2- and TLR4-dependent manner. (26). Similarly, B7-H3 substantially augmented pro-inflammatory cytokine production in the development of pneumococcal meningitis in a murine model (27). Thus B7-H3 potentiates the inflammatory response in sepsis in murine models. Its levels are higher in humans during sepsis but its significance remains uncertain.
Transplant
Allogeneic bone marrow transplant (BMT) is performed for hematological malignancies and achieves a cure; however, graft-versus-host disease (GVHD) after allogeneic transplant remains a major cause of death in transplant recipients. B7-H3 is upregulated in the colon, liver and lung in mice and in the intestine of GVHD patients. B7-H3 knockout recipients as well as recipients of B7-H3 knockout donor T cells had accelerated GVHD lethality. The increased GVHD lethality is a result of increased T-cell proliferation, colon inflammatory cytokines, and intestinal permeability. However, these T cells lacking B7-H3 are capable of providing graft-versus-leukemia (GVL) effects that are advantageous. Therefore, the B7-H3 pathway acts as a suppressor of acute GVHD. Approaches to increase B7-H3 expression soon after BMT to reduce GVHD and to decrease B7-H3 later post-BMT via the development of potent antagonistic B7-H3 monoclonal antibodies (mAbs) to enhance donor lymphocyte infusion-mediated GVL effect may benefit transplant recipients (28).
In solid organ transplants an early study showed B7-H3 had a costimulatory role in the alloimmune response. Its expression by cells promotes T cell-mediated immune responses and subsequently mediates the rejection of acute and chronic cardiac and islet cell allografts (29). However, more recent results are consistent with an inhibitory function of B7-H3. Its signaling prolongs the survival of a fully MHC-mismatched cardiac model and promotes a shift towards a Th2 milieu (30). Further studies are needed to clarify its role in solid organ transplants.
Cancer
Aberrant expression of B7-H3 in various human malignancies including melanoma, glioma, leukemia, lung, pancreatic, renal, colorectal, ovarian, breast, gastric and endometrial cancers was detected in 60–93% of the tumor tissue, while limited expression is seen on normal healthy tissues (15). B7-H3 staining by immunohistochemistry (IHC) is present on the membrane, cytoplasm, nucleus or vasculature of tumors. A study of more than 700 colorectal cancer patients showed cytoplasmic/membrane, stromal and nuclear expression of B7-H3 in 86%, 77% and 27% of the samples respectively (31). The molecular mechanisms that regulate its expression and specific functions remain unclear with both favorable and adverse outcomes associated with its expression in human tissues and mouse models (16, 32).
B7-H3 expression in hepatocellular carcinoma (33), endometrial carcinoma (34), osteosarcoma (35), colon cancer (36), ovarian carcinoma (37) and oral squamous cell carcinoma (38) is associated with a shortened overall survival (OS). Its expression was also associated with disease progression or advanced stage in pancreatic cancer (39), prostate cancer (40–42), breast cancer (43), melanoma (44), clear cell renal cell carcinoma (45), oral squamous cell carcinoma (38) and non-small cell lung cancer (46, 47). Similarly, studies have also found a direct correlation with increased B7-H3 expression and tumor size, tumor grade and rate of recurrence in humans (31, 48, 49). Thus there is ample evidence for the association of B7-H3 with poor prognosis in human cancers.
Although B7-H3 expressing cells are able to evade tumor immunity the exact mechanisms are not yet known. Upregulation of B7-H3 is associated with impaired T cell stimulatory function (19, 50), suppressed NK mediated cell lysis (24), increased IL-10 secretion (43), decreased IL-12 (51), modulation of the Jak/Stat pathway (52) which contribute to immune suppression and evasion by tumors.
There are few early studies that associate B7-H3 expression with favorable clinical outcomes in cancer. In 102 patients with gastric cancer and 68 patients with pancreatic cancer 58.8% and 88.2% expressed B7-H3 respectively. In both these studies, high levels of B7-H3 expression were associated with a significantly better prognosis (53, 54). Its expression was correlated with a higher number of tumor-infiltrating CD8 T-cells in the pancreatic cancer patients, while in breast cancer B7-H3 suppressed tumor growth by inhibiting VEGF expression (55). B7-H3 slowed tumor growth mediated by CD8 T cells and 50% had complete regression in mouse models of lymphoma and mastocytoma (56, 57). In HCC mouse models multiple distant tumor nodules regressed and tumor angiogenesis was inhibited (58, 59). In colon cancer survival time was prolonged, and tumor size as well as the occurrence of metastases was reduced (60, 61). Ablation of B7-H3 resulted in increased tumor burden in a spontaneous prostate cancer model (62). These studies show that a high level of B7-H3 may be beneficial for anti-tumor immune responses.
There are several potential explanations for B7-H3’s complex and conflicting immunomodulatory activity: 1) B7-H3 interacts with both inhibitory and stimulatory receptors; 2) it has different affinities for several receptors; 3) it has other aberrant isoforms or splice variants; and 4) genetic polymorphisms or differential glycosylation patterns may exist for the B7-H3 gene and protein (16).
Immunotherapy targeting B7-H3
Recent advances in immunotherapy targeting the B7-CD28 family members, CTLA-4/B7-1/B7-2 and PD-1/PD-L1 have been clinically successful and caused tumor regression (63–65). Therapies targeting the B7-CD28 pathway are related to blocking the coinhibitory signal between immune cells-immune cells and/or immune cells-tumor cells as well as the potentiation of the costimulatory signals. Progress in developing immunotherapies targeted at B7-H3 is deterred by the conflicting evidence of the role of this molecule in tumorigenesis. In addition, until the receptor is identified therapy can only be targeted towards the molecule itself. Clinical trials with B7-H3 targeted immunotherapy are underway showing some promise. There are multiple modalities by which this molecule can be targeted as described below (15).
Blocking mAbs such as nivolumab and ipilimumab have been effective and are now widely used clinically. Similar antibodies to B7-H3 are currently unavailable clinically and the ability of these antibodies to neutralize the signaling pathway remains unknown. Until the exact role of B7-H3 in specific tumors is defined, the development of functional blocking antibodies to B7-H3 will be a challenge.
Enoblituzumab (MGA271), a mAb against B7-H3 with potent antitumor activity through antibody-dependent cell-mediated cytotoxicity (ADCC), is being explored. This antibody showed tumor growth inhibition in B7-H3 expressing renal and bladder carcinoma xenografts (66). There is currently an ongoing phase I trial being conducted in patients with refractory B7-H3 expressing tumors or vasculature (trial NCT01391143). The preliminary results of the dose escalation study show that it is well tolerated and has anti-tumor activity by increasing the T cell repertoire clonality in patients (http://www.macrogenics.com/enoblituzumab-anti-B7-H3/).
Similarly, an antibody drug conjugate with 8H9, a mAb radiolabeled to iodine-131 showed clinical success as salvage therapy in patients with metastatic central nervous system tumor called neuroblastoma. The patients received compartmental intrathecal antibody-based radioimmunotherapy and 83.3% of patients had prolonged survival (67). Currently, a Phase I clinical trials with radiolabeled 8H9 are ongoing in patients with peritoneal cancers, gliomas, and advanced CNS cancers (NCT01099644, NCT01502917, and NCT00089245). Recently a humanized affinity-matured potent form of this antibody (hu8H9) has been developed that binds to the FG loop of B7-H3, a region critical to its immunologic function (68).
Synergistic therapy with a B7-H3 mAb and chemotherapy has met with preclinical success. In a murine pancreatic cancer model, B7-H3 blockade with the mAb MJ18 along with gemcitabine showed a synergistic anti-tumor effect without overt toxicity (69). Two other preclinical animal studies have shown that silencing B7-H3 as well as utilizing conventional chemotherapy with cytarabine or paclitaxel led to 80% tumor reduction in murine models of histiocytic lymphoma-derived human cell line U937 and human breast cancer line respectively compared to wild type models (52, 70). In both studies, silencing B7-H3 significantly enhanced tumor cell chemosensititvity and drug-induced apoptosis. Hence this provides a rationale for the potential synergistic effects between B7-H3 blockade and chemotherapy for treatment of cancers.
The majority of B7-H3 is expressed on tumor cells and vasculature while the other checkpoint inhibitors are expressed on immune cells, normal cells and tumor cells. Such phase I clinical trials are under way to explore the safety of enoblituzumab in combination with either anti-CTLA4 (ipilimumab) or anti–PD-1 (pembrolizumab) in patients with refractory cancer (NCT02381314 and NCT02475213)(15). This difference can be highly advantageous for combination therapy that can generate local responses through tumor specific B7-H3 targeting, with additional checkpoint blockade enhancing antitumor immunity.
Summary of B7-H3
B7-H3 is predominantly a T cell coinhibitory molecule with a partial costimulatory function reported in some studies. It is a type I transmembrane protein with four extracellular domains due to exon duplication in humans. B7-H3 potently inhibits T cell and NK cell functions and also has a role in bone development. The receptor for B7-H3 has not yet been characterized but is presumed to be on T and NK cells. In human cancers, consistent with the function of B7-H3, most studies show that its expression is associated with poor prognosis while a few studies show that its expression is associated with a better prognosis. Currently antibodies targeting B7-H3 through an ADCC mechanism and B7-H3 antibody drug conjugates are in Phase 1 clinical trials in humans and the initial results are encouraging.
B7x
B7x (B7-H4, B7-S1) belongs to the Immunoglobulin superfamily and the B7 family of ligands. It was discovered by us and others using bioinformatic analyses in search for proteins with homology to other members of the B7 family (1, 71, 72). The human B7x consists of 282 amino acids and shares an 87% amino acid identity with mouse B7x which shows that this molecule is conserved from an evolutionary standpoint. It shares varying degrees of identity with human - B7-1 (12%), B7-2 (13%), B7h (16%), PD-L1 (18%), PD-L2 (18%), and B7-H3 (24%) (1). Human B7x is located on chromosome 1p12/13.1. This is a type 1 Transmembrane protein with a signal peptide in the N terminus, an extracellular domain with IgV and IgC like domains with four conserved cysteine residues and seven sites for N linked glycosylation.
Structure of B7x
The crystal structure of the human B7x (hB7x) IgV domain was determined to the resolution of 1.59 Å with the space group P43212. The hB7x IgV domain was expressed by Drosophila S2 cells and the purified protein crystallized as a monomeric form with each asymmetric unit containing one hB7x molecule (73). The structure of hB7x IgV adopts a typical IgV organization (12), which is characterized by “β sandwich” folding formed by a “back sheet” (ABED strands) and a “front sheet” (C”C’CFGA’ strands) stabilized by a disulfide bond between B and F strand (formed by Cys56 and Cys130). A significant electron density, which could be fitted by five sugar residues of a branched glycan, is well defined near Asn112, corresponding to the glycosylation site at Asn112 as predicted by hB7x sequences. Notably, the large observed branched glycan covers a significant area of the “back sheet” surface (Figure 1). Similarly, a glycosylation site at the homologue position (Asn104 of B7H3) has been observed for the mB7H3 ectodomain (11).
Crystal structure of hB7x provides a model for epitope mapping, which may further improve the efficacy of antibodies targeting hB7x. For example, 1H3 is an IgG1 mAb against B7x, which has been proved to inhibit tumor nodule formation and prolong the survival of mice inoculated with hB7x expressing CT26 tumors (74). The Fab of 1H3 is effective in blocking the B7x-mediated T cell coinhibition, suggesting 1H3 is a functional neutralizing antibody. Mutagenesis study showed that BC loop (residues Ile62 and Lys63) of the “back sheet” and FG loop (residue Ser135) of the “front sheet” define the minimal footprint for hB7x:1H3 interaction interface (73). In combination of these data, it is very likely that the B7x receptor binds to the B7x IgV domain, which overlaps with the 1H3 binding interface.
Superimposition of the monomeric model of mB7H3 with human PD-L1 structure (from PDB entry 3BIK chain A) shows a similar organization with an overall Cα RMSD (root-mean-square deviation) ~2.7 Å (Figure 1). In addition, superimposition of hB7x IgV domain with human PD-L1 IgV domain resulted in even smaller Cα RMSD ~1.3 Å, with the most apparent differences in the loop regions (Figure 1). These indicate that overall structures of B7H3 and B7x resemble the other well-identified B7 family members. Most of the characterized B7 family members apply the “front sheet” of IgV domains to engage the receptors. For example, B7-1, B7-2, PD-L1 and PD-L2 all bind to the corresponding receptors through the “front sheet” of IgV domains (75–79). Considering the obvious carbohydrates modifications of both B7-H3 and B7x on the “back sheet” of the IgV domains as identified in the crystal structures, it is very likely that B7-H3 and B7x may interact with their receptors through the “front sheet” of IgV domains, although more concrete evidence is required to support this hypothesis.
Expression
B7x mRNA in immune cells is expressed by professional APC, bone marrow derived dendritic cells, peritoneal macrophages, splenic CD11c+ dendritic cells and splenic B cells (1, 80). B7x mRNA can be detected by PCR in most tissues but it is highly expressed in nonlymphoid organs like the lung, testis and pancreas (1) (Table 1). Despite the high level of mRNA expression in most tissues, there is no detectable B7x protein in most healthy tissues (80). Low levels of B7x expression were found in genital tract, lung, pancreas and kidney but were absent in other normal somatic tissues (81).
The functional mechanisms of upregulation of B7x in glioma cells were explored(82). IL-6 potently upregulated B7x expression in glioma cell lines. Stimulation of IL-6 mediated phosphorylation of STAT3 upregulated B7-H4 promoter activity, which was abrogated by knockdown of STAT3 with shRNA. This shows that STAT3 upregulates B7x transcription via its promoter and represents one mechanism of B7x regulation (82).
Function
T cells
B7x negatively regulates T cell responses. B7x decreases T cell proliferation, which is a hallmark of T cell activation, in a dose dependent fashion by decreasing IL-2 (72) and by inducing cell cycle arrest in T cells (a non-IL-2 dependent mechanism) (80). This is similar to CTLA-4 which can also inhibit TCR induced T cell proliferation by cell cycle arrest (83). JunB which regulates IL2 gene transcription after T cell activation is decreased after B7x stimulation and this could be one mechanism of decreasing IL-2 production by B7x (72). B7x inhibits T cell growth, cytokine secretion and cytolytic activity against allogeneic antigens in vivo (80). The inhibitory effects of B7x could only be partially reversed by costimulation through the CD28 signaling pathway. B7x blocks the differentiation of naïve CD4 T cells to effector Th1 or Th17 cells by blocking IFN-γ and IL-17 production (84) (Figure 2).In all these studies it has been shown that B7x interacts with a receptor on activated T cells which is distinct form CD28, CTLA-4, ICOS and PD-1 (1).
Myeloid derived suppressor cells
Myeloid derived suppressor cells are immature cells of the myeloid origin, which are mainly present in tumor and have a potent ability to suppress T cell proliferation and cytokine production (85). MDSC are divided into granulocytic (g-MDSC) and monocytic MDSC. In a B7x knockout mouse model g-MDSC was the predominant population to infiltrate a tumor (86). This g-MDSC make up 60% of the tumor immune cells in wild type mice while their composition is only 20% in B7x knockout mice, suggesting the presence of B7x promotes expansion of MDSC. Moreover, B7x binds to g-MDSC indicating that MDSC has a receptor for B7x, there may be a distinct receptor on the MDSC different from the T cells.
B7x in pathological states
Autoimmune diseases and infection
B7x has been shown to dampen T cell responses. To demonstrate the role of B7x in infections, the effects of Streptococcus pneumonia infection in B7x knockout mice were studied (87). In the B7x knockout mouse model, the severity of S. pneumonia infection was markedly reduced when compared to wild type mice. Control of infection in B7x knockout mice was associated with a marked increase in activated CD4 and CD8 T cells and fewer neutrophils in lungs, whereas the susceptible WT mice had higher neutrophils with decreased CD4 and CD8 T cells. This suggests that B7x plays an important role in the dampening of immune responses to infection.
The role of B7x in the induction and maintenance of autoimmune diseases was studied in an EAE model. In vivo blockage of B7x with B7x antibodies during the T cell priming phase led to an increase in the severity of the disease (20). Similarly, EAE was severe in B7x deficient when compared to WT mice due to the expansion of Th1 and Th17 cells during EAE induction without changing the regulatory T cell population leading to severe disease (88).
CD8 T cell induced diabetes in a B7x deficient and B7x overexpressing mouse models was used to study the roles of B7x in peripheral tissues. Mice lacking B7x developed a severe form of diabetes and transfer of antigen specific CD8 T cells to B7x overexpressing mice did not lead to diabetes. These studies showed that the CD8 T cells migrated to the pancreas of the B7x overexpressing mice, but their proliferation and thereby tissue destruction is markedly reduced in the presence of B7x (89).
The effects of B7x in an autoimmune kidney disease mouse model showed that B7x is expressed by several different kidney cells including tubular cells, podocytes and glomerular epithelial cells at a low level. This expression is rapidly induced in the tubular cells after stimulation with LPS (90). B7x knockout mice have an enhanced humoral immune response and develope severe renal injury after passive administration of antibodies against glomerular antigens. Correspondingly the macrophages in the spleen of B7x knockout mice were polarized to an inflammatory M1 phenotype. In accord with these studies, high levels of serum B7x were found in the sera of patients with rheumatoid arthritis and correlated with the disease severity score (91). All these studies show that B7x regulates the induction and severity of autoimmune diseases and plays an important role in peripheral tolerance.
Cancer
Aberrant expression of B7x has been detected in many human cancers which include – breast, lung, ovary, uterus, kidney and others (92). B7x is rapidly lost in vitro cultures and hence most human (93) and mouse tumor cell lines are B7x negative (73). Several human studies have studied the expression of B7x in cancers and its prognostic role in these malignancies. A few studies are described in detail below.
Prostate cancer
Expression of B7x and correlation with clinical features were analyzed in a large study of 948 patients with localized prostate cancer treated with a pelvic lymph node dissection and radical retropubic prostatectomy (40). B7x was highly expressed in the majority of prostate cancer with a strong intensity in 15% of cancer specimens. Strong intensity of staining for B7x in tumors was significantly associated with extracapsular extension, seminal vesicle invasion, and metastatic disease. These patients were also more likely to develop a biochemical recurrence (HR 1.38; 95% C.I. 0.94–2.02; p= 0.10), clinical recurrence (HR 2.22; 95% C.I. 1.27–3.87; p= 0.005) and had a higher probability of death from prostate cancer (HR 2.71; 95% C.I. 1.04–7.02; p= 0.04). Hence B7x is highly expressed in prostate cancer and its strong expression in the tumor is associated with poor prognostic factors.
Breast cancer
Similar to prostate cancer B7x expression was detected in 95 –97% of primary and metastatic breast cancers. The staining intensity was much higher in invasive ductal carcinoma than in normal breast epithelium (81). Even though B7x expression was associated with negative progesterone receptor status and history of neoadjuvant chemotherapy these factors are not necessarily predictive of a poor outcome in breast cancer.
Renal cancer
In 259 renal cell carcinoma (RCC) patients treated with nephrectomy, 59% tumor specimens exhibited B7-H4 staining (94). Tumor cell B7-H4 expression was associated with adverse clinical and pathologic features, including constitutional symptoms, tumor necrosis, and advanced tumor size, stage, and grade. Patients with tumors expressing B7-H4 were three times more likely to die from their RCC (risk ratio = 3.05; 95% confidence interval = 1.51–6.14; P = 0.002). Interestingly 81.5% specimens exhibited tumor vasculature endothelial B7-H4 expression, whereas only 6.5% of normal adjacent renal tissue vessels exhibited endothelial B7-H4 staining. Similarly, serum B7x was higher in 53 RCC patients (14.4 ng/mL) when compared to 18 control patients (2.7 ng/mL) (95). Median levels were significantly higher for patients with a tumor thrombus, positive lymph nodes, and distant metastases at nephrectomy. The median B7x levels also were progressively higher with increasing tumor-node-metastasis stage. These findings suggest that B7x is highly expressed in renal cell carcinoma, is associated with poor prognostic features and since it is expressed in the vascular endothelium, whether this is involved in angiogenesis or metastases needs to be determined.
Glioma
In glioma, a CNS tumor, higher grades of tumor are associated with higher B7x mRNA and protein expression when compared with normal brain tissue (82). Similarly, increasing levels of soluble B7x was also found in CSF with increasing grades of glioma. CD133+ glioma initiating cells mediated expression of B7x on macrophages through IL-6 and IL-10 secretion. This results in a polarization of the macrophages towards a tumor promoting or a M2 phenotype, inhibition of T-cell proliferation and cytokine production, and led to reduced cytotoxicity of T cells. In a xenograft model of NOD/SCID mice, suppression of B7x leads to T-cell activation and tumor regression in mice. These experiments prove that B7x is an important checkpoint molecule in the development of glioma.
Based on these studies, B7x is expressed in most cancers and its expression is associated with a poor prognosis. Targeting B7x in human cancers is an attractive option for cancer immunotherapy.
Immunotherapy targeting B7x
Cancer
Anti-B7x therapy has been tested in mouse models in the form of anti-B7x single chain fragment variables (scFv), ADCC and functional antibodies, antibody drug conjugates and chimeric antigen receptor (CAR) T cell-B7x therapy. An anti–B7x antibody was generated to study the effects of inhibiting B7x in mouse cancer models. This antibody functions both as a blocking antibody to inhibit function and kill through an ADCC mechanism and since B7x is evolutionally conserved the antibody also inhibits human B7x. A colon carcinoma CT26 cell line which stably expressed B7x and induced experimental lung metastasis was tested with anti-B7x treatment(73). The average number of metastasis was higher along with a poor survival in the B7x/CT26 injected mice than in the CT26 group. Treatment with Anti-B7x antibody (1H3 clone) significantly lowered pulmonary metastases and increased survival in the B7x/CT26 model. The anti-B7x antibody treated mice had increased infiltration of antigen specific cytotoxic T cell and NK cells in the lungs and decreased CD4 or CD8 T cells of the exhausted phenotype. Similarly, the proportion of MDSC infiltrating the tumor and cytokines including VEGF and TGF-β was also reduced. As expected, the B7x antibodies had minimal off target effects.
Single chain fragment variables are recombinant antibodies with single antigen binding domains and have the advantages of small size and versatility for in vivo targeting and binding to conjugates. Anti-B7x scFv fragments decreased the growth of ovarian tumors. These antibodies also stimulate tumor antigen specific T cell activation when tumor immune responses exist prior to therapy (93).
A CAR T cell-B7x therapy was tested in a murine ovarian cancer model. It was very effective against human ovarian tumor xenografts but also had delayed lethal toxicity (96). Post mortem analysis showed that the normal tissues showed increased expression of B7x protein. This late toxicity may be overcome by means of a switch receptor/chimera or by using suicidal gene therapy before the delayed onset of normal organ toxicity which is distinct from the anti-tumor effects. These therapies represent different approaches to target B7x in the tumor microenvironment.
Autoimmune disease
In contrast to cancers where inhibition of B7x is beneficial, potentiation of B7x induced immune suppression has been shown to suppress autoimmune diseases. A non-obese diabetic (NOD) mouse model was used to study the effects of B7x in development of type 1 diabetes (97). NOD mice were injected with B7x immunoglobulin fusion protein (B7x-Ig) at 3 weeks, before the onset of diabetes. Early treatment of NOD mice with B7x-Ig resulted in a later development of diabetes. It did not prevent the onset of inflammation but rather decreased the amount of islet cell destruction by modulating the immune infiltrate. This is due to a decreased Th1 response mediated by upregulation of T regulatory cells in the pancreas while this did not happen systemically in the blood. In addition B7x-Ig treated mice had decreased numbers of Th17 cells and hence decreased levels of Th17 associated cytokine and an increase in IFN-γ which potently inhibits Th17 cells (98). The treatment down regulates the Th17 pathway which is important in the development of autoimmune diseases (99).
A similar approach was used to demonstrate that B7x-Ig effectively ameliorated EAE in mice by decreasing CD4 T cells and by increasing the number and function of T regulatory cells (84). Specifically it blocked IFN-γ and IL-17 production and hence blocks differentiation of naïve CD4 T cells to either a Th1 or Th17 phenotype. This could be used as therapeutic model to potentiate the B7x pathway for human demyelinating disorders like multiple sclerosis.
As previously described, a mouse nephritis model was used to demonstrate that B7x decreases autoimmune kidney injury (90). Following nephrotoxic kidney injury, B7x-Ig inhibits the expansion of CD4 and CD8 T cells and restricts macrophage polarization to a M1 phenotype. The M1 phenotype is likely to cause autoimmune diseases in experimental models. B7x-Ig treatment also decreases the levels of various cytokines involved in autoimmune nephritis namely - CXCL13, IRF5, ICOS-L and TGF-β. All these studies show that therapeutic potentiation of B7x pathway in humans with preexisting autoimmune diseases or in those with a high risk of developing autoimmune disease is likely to be beneficial.
Summary of B7x
B7x is a T cell coinhibitory molecule with a 15 –25% homology to other members of the B7 family. B7x is a type 1 transmembrane protein with two extracellular domains. B7x inhibits T cell function through IL2 dependent and independent mechanisms and inhibits proliferation of T cells by causing cell cycle arrest. One mechanism of induction of B7x is through IL-6. IL-6 phosphorylates STAT3 which in turn binds to the B7x promoter and upregulates B7x. The receptor for B7x has yet to be unidentified but is distinct from the other members of the B7 family. Even though B7x mRNA is expressed in multiple tissues, B7x protein is rarely detected in normal tissues. Multiple in vivo studies have shown that B7x is important for induction and maintenance of peripheral tolerance in tissues and hence plays an important role in autoimmunity. Stimulation of the B7x pathway has been shown to prevent or inhibit the development of autoimmune diseases in mouse models and hence potentiation of this pathway could be a therapeutic target for human autoimmune diseases. B7x protein is widely expressed in various cancers and its expression is associated with poor prognostic features in human cancers. B7x has multiple mechanisms of tumorigenesis – decreasing the effector anti-tumor T cells in the tumor, increasing angiogenic factors like VEGF, TGF-B and activating MDSCs. Blocking B7x in cancer through anti B7x antibodies, CAR T cell or anti-B7x scFV fragments have yielded good results in in vivo cancer models. Hence B7x represents an attractive therapeutic target in the treatment of cancers and autoimmune diseases.
HHLA2
We and others recently identified HHLA2 as a new member of the B7 family (2, 100, 101). It is a type I transmembrane molecule with three extracellular Ig domains (2). This family usually has one IgV and one IgC extracellular domain (B7-1, B7-2, ICOS-L, PD-L1, PD-L2, and B7x) with an intracytoplasmic tail. B7-H3 is the only member which has a tandem copy of the IgV-IgC extracellular domain resulting in 4 extracellular domains. The difference in structure with respect to function is currently unknown. The differences could be related to bidirectional signaling of this family or the binding conformations could generate a binding site for another molecule, which could be related to generating a new receptor at the site of their binding (78).
Evolution
HHLA2 is an acronym for Human Endogenous retro virus-H Long terminal repeat-associating 2. This gene was discovered when a systematic search for human retroviral long terminal repeats (LTR) was performed as these LTR’s can possess enhancer, promoter or polyadenylation functions and one such gene identified was HHLA2 (102). The LTR provides the primary polyadenylation signal and hence this integration is important for this gene to be read and translated to mRNA. The LTR of the HHLA2 locus has been integrated into the primate lineage since it is found only in gorilla, chimpanzee and humans of the New World. HHLA2 is not expressed in mice but it is expressed in higher primates. The integration of the LTR sequence in higher primates could account for the significant differences in gene expression between the different species.
Expression
HHLA2 is constitutively expressed on human monocytes and induced on B cells after stimulation with IFN-γ (2) (Table 1). Most normal tissues do not express HHLA2 except the placenta, intestines, kidney, gallbladder and breast (103). The placenta is a site of immune privilege and other coinhibitory molecules namely PD-L1, B7-H3 and B7-x are also highly expressed by this organ.
Function
Since HHLA2 belongs to the B7 family, it is hypothesized that HHLA2 regulates T cell function. HHLA2 immunoglobulin fusion protein (HHLA2-Ig) binds to T cells (resting and activated) and other immune cells demonstrating that there are constitutive receptors on the cell surface. HHLA2-Ig is able to decrease both CD4 and CD8 T cell proliferation when incubated with anti-CD3. Functionally incubating T cells with HHLA2-Ig decreases the production of several cytokines including IFN-γ, TNF-α, IL-5, IL-10, IL-13, IL-17A, and IL-22 (2). HHLA2 also inhibits IL-2 secretion by T cells in a dose dependent manner (104). These experiments demonstrate that HHLA2 inhibits T cell proliferation and function. HHLA2 also functions as a costimulatory molecule and increases cytokine production (100). It is not uncommon for members of the B7 family to have dual functions depending on the immune milieu, receptor engagement or blockade or interaction with different receptors (105) (Figure 2). Overall these studies demonstrate that HHLA2 predominantly functions as a T cell coinhibitory molecule.
HHLA2 in human cancers
HHLA2 is expressed in a majority of tumor specimens of the breast, lung, thyroid, melanoma, ovary, and pancreas (103). The localization of the protein is both membranous and cytoplasmic in tumor cells. Even though HHLA2 is a transmembrane protein, this type of distribution is not uncommon as this could be due to structural differences in staining vs shuttling of the protein between the cytoplasm and the membrane. HHLA2 protein is also expressed in a lower percentage of other cancers such as liver, bladder, colon, prostate, kidney, and esophagus (103). The prognostic significance of such expression was determined in three cancers – breast, osteosarcoma and lung.
Breast cancer
Triple negative breast cancer (TNBC) is an aggressive subtype of breast cancer when compared to estrogen receptor positive and Her2 positive breast cancer. In a retrospective study, 50 patients with local or locally advanced breast cancer were identified and their clinical characteristics collected. All these patients underwent surgery as the primary treatment followed by chemotherapy or radiotherapy or both. TNBC with a high expression of HHLA2 was associated with lymph node positivity and higher stage at the time of diagnosis, indicating more aggressive disease when compared with tumors with low expression of HHLA2. By analyzing the cBio portal to assess for HHLA2 gene alterations, we found that HHLA2 was altered in 32% of the basal subtype and the vast majority of HHLA2 copy number variations in basal breast cancers were amplifications or gains (>95%). This suggests that one mechanism of upregulation of HHLA2 protein in human cancers could be copy-number gain (103).
Lung cancer
Tissue microarrays (TMAs) from stage I to III non-small cell lung cancer (NSCLC) specimens from patients undergoing lung cancer resection was constructed from 392 NSCLCs in the discovery cohort tumor tissues and the TMAs in the separate validation cohort consisted of 287 NSCLC cases. Scoring of tumor infiltrating lymphocytes (TILs) as absent, low (1–30%) or high (>30%) was performed in the same slides which were used for HHLA2 staining (106). HHLA2 was expressed in 66% of NSCLC while it was not expressed in normal lung tissue including alveolar type 1 and type II cells, endothelial cells and smooth muscle cells in blood vessels. In both the discovery and validation cohort HHLA2 expression was significantly overexpressed in the EGFR mutant cohort than the wild type (89 vs. 69% p=0.01). In the validation but not the discovery cohort, HHLA2 positivity was associated with a higher TIL score (86 vs. 67% p=0.03). In the multivariate analysis, a higher TIL score and presence of EGFR mutation were independently associated with HHLA2 expression in lung cancer (106). Lung cancer is an immunogenic tumor and has variable expression of tumor immune checkpoints. Immunotherapy against PD-1/PD-L1 has been shown to improve survival in patients with disseminated lung cancer. The caveat to this is that only 20% of patients and those who express PD-L1 respond to PD-1/PD-L1 checkpoint blockade. This suggests that other checkpoints like HHLA2 may be more important in the cancers that do not express PD-L1 or in tumors that escape PD-1/PD-L1 blockade (107).
Osteosarcoma
Osteosarcoma is another primary malignancy of the bone with a less than 20% 5 year survival rate and the prognostic role of HHLA2 was recently evaluated (108). In this study, HHLA2 was expressed in metastatic disease more than in primary disease and the differences was statistically significant (93% vs 53%, p=0.02). Higher levels of HHLA2 expression in the tumor (25 vs 50%) portended a worse overall survival. Together these studies suggest that HHLA2, by functioning as a T cell co-inhibitory molecule in the tumor microenvironment, could provide a survival advantage for the tumor and hence its expression is associated with a worse prognosis.
Summary of HHLA2
HHLA2 is a newly discovered T cell immune checkpoint molecule that belongs to the B7 family of ligands. It predominantly functions to inhibit T cell proliferation and T cell cytokine responses. HHLA2 is expressed on few normal tissues but it is expressed in various human cancers. High expression of HHLA2 in human cancer of lung, breast, and osteosarcoma is associated with worse prognostic features.
TMIGD2
We and others recently discovered TMIGD2 (Transmembrane and Immunoglobulin Domain Containing 2) as one of the receptors for HHLA2 which is a 31 kDa protein and is present on chromosome 19q13.3 (100, 103). Sequence analysis showed that TMIGD2, the immunoglobulin-containing and proline-rich receptor-1 (IGPR-1), and CD28 homolog (CD28H) are the same molecule. TMIGD2 (IGPR) was originally identified as an adhesion molecule involved in angiogenesis (109).
TMIGD2 is an Ig superfamily member with an extracellular IgV-like domain, a transmembrane region, and a cytoplasmic tail. The extracellular region has two possible glycosylation sites while the cytoplasmic tail contains tyrosine residues and a proline rich domain. It shares greater than 10% amino acid identity with other B7 family receptors namely -CD28, CTLA, ICOS and PD-1 (100). Similar to HHLA2, TMIGD2 is absent in mouse and rat while it is found in higher primates (109).
TMIGD2 is a membranous glycoprotein which is confirmed by immunofluorescence microscopy. TMIGD2 appears to be in a glycosylated form in the thymus, placenta, heart, small intestine, skin, and kidney with a molecular weight of 55 kDa while in the skeletal muscle, brain, colon, lung, and ovary TMIGD2 seems to be unglycosylated with an apparent molecular weight of 35 kDa (109).
TMIGD2 on its initial discovery as IGPR in cell culture models has been shown to have multiple functions -promote angiogenesis by promoting capillary tube formation of endothelial cells, increases actin filament formation and alters cellular morphology, causes focal adhesion of cells and inhibits cellular migration (97). TMIGD2 can associate with the SH3 domain containing signaling protein SPIN90 and may play a role in the regulation of angiogenesis.
TMIGD2 seems to be preferentially expressed more on lymphoid organs by PCR (100). TMIGD2 is widely expressed on naïve T cells as well as dendritic cells, monocytes, and B cells. Naïve CD4 and CD8 αβT cell expressed TMIGD2 while γδT cells and T regulatory cells were negative. However TMIGD2 is rapidly lost on activation of T cells. TMIGD2 is expressed on naïve T cells but is rapidly lost after activation, this is unlikely to be the receptor through which HHLA2 results in a T cell coinhibitory function in human cancers. Further research into the expression and role of TMIGD2 protein are needed.
Conclusions
The B7-CD28 family of ligands and receptors play very important roles in regulating the immune response in normal and pathological states including infection, autoimmunity and cancer. Blocking the CTLA-4 and the PD-1/PD-L1 axis has directly improved patient survival in metastatic cancer and has underscored the importance of immune checkpoints in cancer. Our understanding of the third group of B7-CD28 members namely B7-H3, B7x, HHLA2 and TMIGD2 has markedly improved, but several questions remain. How do these different immune checkpoints work in tandem? How do we measure the level of T cell costimulation or T cell coinhibition rather than viewing it as an ‘on-off’ state with respect to immune checkpoints? Can the same molecule deliver costimulatory and coinhibitory signals depending on the milieu, interacting receptor, and existence of ligand in more than one state? Some of the challenges with the third group have been: receptor identification, absence of reliable commercial antibodies and lack of expression of HHLA2 and TMIGD2 in mice. Nevertheless, several in vivo and in vitro studies presented in this review underscore the importance of these new ligands and receptors. Continued research and an improved understanding of these pathways can lead to successful therapeutic applications in human autoimmune diseases and cancer.
Acknowledgments
MJ is supported by AMC grant UM1CA121947. Research in the Zang lab is supported by NIH R01CA175495 and R01DK100525, DOD Established Investigator Idea Development Award PC131008, Pfizer CTI, Hengrui Medicine Co., and Irma T. Hirschl/Monique Weill-Caulier Trust (to X.Zang).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100(18):10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A. 2013;110(24):9879–9884. doi: 10.1073/pnas.1303524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinai JM, Janakiram M, Chen F, Chen W, Kaplan M, Zang X. New immunotherapies targeting the PD-1 pathway. Trends Pharmacol Sci. 2015;36(9):587–595. doi: 10.1016/j.tips.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janakiram M, Pareek V, Cheng H, Narasimhulu DM, Zang X. Immune checkpoint blockade in human cancer therapy: lung cancer and hematologic malignancies. Immunotherapy. 2016;8(7):809–819. doi: 10.2217/imt-2016-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 6.Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168(12):6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Fu F, Gu W, Yan R, Zhang G, Shen Z, et al. Origination of new immunological functions in the costimulatory molecule B7-H3: the role of exon duplication in evolution of the immune system. PLoS One. 2011;6(9):e24751. doi: 10.1371/journal.pone.0024751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172(4):2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 9.Duan H, Huang M. Genome-wide identification and evolutionary analysis of B7-H3. Int J Data Min Bioinform. 2012;6(3):292–303. doi: 10.1504/ijdmb.2012.049247. [DOI] [PubMed] [Google Scholar]
- 10.Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39(7):1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vigdorovich V, Ramagopal UA, Lazar-Molnar E, Sylvestre E, Lee JS, Hofmeyer KA, et al. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure. 2013;21(5):707–717. doi: 10.1016/j.str.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun PD, Boyington JC. Overview of protein folds in the immune system. Current protocols in immunology / edited by John E Coligan [et al]. 2001;Appendix 1:Appendix 1N. doi: 10.1002/0471142735.ima01ns44. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Fuentes N, Zhai J, Fiser A. ArchPRED: a template based loop structure prediction server. Nucleic Acids Res. 2006;34(Web Server issue):W173–W176. doi: 10.1093/nar/gkl113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nygren MK, Tekle C, Ingebrigtsen VA, Fodstad O. B7-H3 and its relevance in cancer; immunological and non-immunological perspectives. Front Biosci. 2011;3:989–993. doi: 10.2741/e304. [DOI] [PubMed] [Google Scholar]
- 15.Picarda E, Ohaegbulam KC, Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res. 2016;22(14):3425–3431. doi: 10.1158/1078-0432.CCR-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer. 2014;134(12):2764–2771. doi: 10.1002/ijc.28474. [DOI] [PubMed] [Google Scholar]
- 17.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69(15):6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahnke K, Ring S, Johnson TS, Schallenberg S, Schonfeld K, Storn V, et al. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: role of B7-H3 expression and antigen presentation. Eur J Immunol. 2007;37(8):2117–2126. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- 20.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Dong Q, Xu Y, Yu G, Zhang X. B7-H3: another molecule marker for Mo-DCs? Cell Mol Immunol. 2005;2(4):307–311. [PubMed] [Google Scholar]
- 22.Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci U S A. 2008;105(30):10277–10278. doi: 10.1073/pnas.0805458105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101(34):12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemke D, Pfenning PN, Sahm F, Klein AC, Kempf T, Warnken U, et al. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin Cancer Res. 2012;18(1):105–117. doi: 10.1158/1078-0432.CCR-11-0880. [DOI] [PubMed] [Google Scholar]
- 25.Suh WK, Wang SX, Jheon AH, Moreno L, Yoshinaga SK, Ganss B, et al. The immune regulatory protein B7-H3 promotes osteoblast differentiation and bone mineralization. Proc Natl Acad Sci U S A. 2004;101(35):12969–12973. doi: 10.1073/pnas.0405259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang G, Wang J, Kelly J, Gu G, Hou J, Zhou Y, et al. B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol. 2010;185(6):3677–3684. doi: 10.4049/jimmunol.0904020. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Quinn EM, Ni H, Wang J, Blankson S, Redmond HP, et al. B7-H3 participates in the development of experimental pneumococcal meningitis by augmentation of the inflammatory response via a TLR2-dependent mechanism. J Immunol. 2012;189(1):347–355. doi: 10.4049/jimmunol.1103715. [DOI] [PubMed] [Google Scholar]
- 28.Veenstra RG, Flynn R, Kreymborg K, McDonald-Hyman C, Saha A, Taylor PA, et al. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood. 2015;125(21):3335–3346. doi: 10.1182/blood-2014-09-603357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Fraser CC, Kikly K, Wells AD, Han R, Coyle AJ, et al. B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol. 2005;35(2):428–438. doi: 10.1002/eji.200425518. [DOI] [PubMed] [Google Scholar]
- 30.Ueno T, Yeung MY, McGrath M, Yang S, Zaman N, Snawder B, et al. Intact B7-H3 signaling promotes allograft prolongation through preferential suppression of Th1 effector responses. Eur J Immunol. 2012;42(9):2343–2353. doi: 10.1002/eji.201242501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingebrigtsen VA, Boye K, Nesland JM, Nesbakken A, Flatmark K, Fodstad O. B7-H3 expression in colorectal cancer: associations with clinicopathological parameters and patient outcome. BMC Cancer. 2014;14:602. doi: 10.1186/1471-2407-14-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loos M, Hedderich DM, Friess H, Kleeff J. B7-h3 and its role in antitumor immunity. Clin Dev Immunol. 2010;2010:683875. doi: 10.1155/2010/683875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi Y, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61(11):2171–2182. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunner A, Hinterholzer S, Riss P, Heinze G, Brustmann H. Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol. 2012;124(1):105–111. doi: 10.1016/j.ygyno.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, et al. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One. 2013;8(8):e70689. doi: 10.1371/journal.pone.0070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingebrigtsen VA, Boye K, Tekle C, Nesland JM, Flatmark K, Fodstad O. B7-H3 expression in colorectal cancer: nuclear localization strongly predicts poor outcome in colon cancer. Int J Cancer. 2012;131(11):2528–2536. doi: 10.1002/ijc.27566. [DOI] [PubMed] [Google Scholar]
- 37.Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23(8):1104–1112. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JT, Chen CH, Ku KL, Hsiao M, Chiang CP, Hsu TL, et al. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc Natl Acad Sci U S A. 2015;112(42):13057–13062. doi: 10.1073/pnas.1516991112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Sun J, Zhao H, Zhu D, Zhi Q, Song S, et al. The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. Onco Targets Ther. 2014;7:1465–1472. doi: 10.2147/OTT.S66809. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104(49):19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67(16):7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 42.Yuan H, Wei X, Zhang G, Li C, Zhang X, Hou J. B7-H3 over expression in prostate cancer promotes tumor cell progression. J Urol. 2011;186(3):1093–1099. doi: 10.1016/j.juro.2011.04.103. [DOI] [PubMed] [Google Scholar]
- 43.Liu C, Liu J, Wang J, Liu Y, Zhang F, Lin W, et al. B7-H3 expression in ductal and lobular breast cancer and its association with IL-10. Mol Med Rep. 2013;7(1):134–138. doi: 10.3892/mmr.2012.1158. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Chong KK, Nakamura Y, Nguyen L, Huang SK, Kuo C, et al. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J Invest Dermatol. 2013;133(8):2050–2058. doi: 10.1038/jid.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin X, Zhang H, Ye D, Dai B, Zhu Y, Shi G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. Onco Targets Ther. 2013;6:1667–1673. doi: 10.2147/OTT.S53565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53(2):143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Mao Y, Li W, Chen K, Xie Y, Liu Q, Yao M, et al. B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer. Oncotarget. 2015;6(5):3452–3461. doi: 10.18632/oncotarget.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C, Zhou L, Chang X, Pang X, Zhang H, Zhang S. B7-H3, B7-H4, Foxp3 and IL-2 expression in cervical cancer: Associations with patient outcome and clinical significance. Oncol Rep. 2016;35(4):2183–2190. doi: 10.3892/or.2016.4607. [DOI] [PubMed] [Google Scholar]
- 49.Baral A, Ye HX, Jiang PC, Yao Y, Mao Y. B7-H3 and B7-H1 expression in cerebral spinal fluid and tumor tissue correlates with the malignancy grade of glioma patients. Oncol Lett. 2014;8(3):1195–1201. doi: 10.3892/ol.2014.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C, Shen Y, Qu QX, Chen XQ, Zhang XG, Huang JA. Induced expression of B7-H3 on the lung cancer cells and macrophages suppresses T-cell mediating anti-tumor immune response. Exp Cell Res. 2013;319(1):96–102. doi: 10.1016/j.yexcr.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Schneider T, Hoffmann H, Dienemann H, Schnabel PA, Enk AH, Ring S, et al. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J Thorac Oncol. 2011;6(7):1162–1168. doi: 10.1097/JTO.0b013e31821c421d. [DOI] [PubMed] [Google Scholar]
- 52.Liu H, Tekle C, Chen YW, Kristian A, Zhao Y, Zhou M, et al. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol Cancer Ther. 2011;10(6):960–971. doi: 10.1158/1535-7163.MCT-11-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer. 2009;9:463. doi: 10.1186/1471-2407-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, et al. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12(3):457–459. doi: 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun J, Guo YD, Li XN, Zhang YQ, Gu L, Wu PP, et al. B7-H3 expression in breast cancer and upregulation of VEGF through gene silence. Onco Targets Ther. 2014;7:1979–1986. doi: 10.2147/OTT.S63424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun X, Vale M, Leung E, Kanwar JR, Gupta R, Krissansen GW. Mouse B7-H3 induces antitumor immunity. Gene Ther. 2003;10(20):1728–1734. doi: 10.1038/sj.gt.3302070. [DOI] [PubMed] [Google Scholar]
- 57.Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, et al. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. 2004;173(9):5445–5450. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- 58.Luo L, Qiao H, Meng F, Dong X, Zhou B, Jiang H, et al. Arsenic trioxide synergizes with B7H3-mediated immunotherapy to eradicate hepatocellular carcinomas. Int J Cancer. 2006;118(7):1823–1830. doi: 10.1002/ijc.21557. [DOI] [PubMed] [Google Scholar]
- 59.Ma L, Luo L, Qiao H, Dong X, Pan S, Jiang H, et al. Complete eradication of hepatocellular carcinomas by combined vasostatin gene therapy and B7H3-mediated immunotherapy. J Hepatol. 2007;46(1):98–106. doi: 10.1016/j.jhep.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Lupu CM, Eisenbach C, Kuefner MA, Schmidt J, Lupu AD, Stremmel W, et al. An orthotopic colon cancer model for studying the B7-H3 antitumor effect in vivo. J Gastrointest Surg. 2006;10(5):635–645. doi: 10.1007/BF03239969. [DOI] [PubMed] [Google Scholar]
- 61.Lupu CM, Eisenbach C, Lupu AD, Kuefner MA, Hoyler B, Stremmel W, et al. Adenoviral B7-H3 therapy induces tumor specific immune responses and reduces secondary metastasis in a murine model of colon cancer. Oncol Rep. 2007;18(3):745–748. [PubMed] [Google Scholar]
- 62.Kreymborg K, Haak S, Murali R, Wei J, Waitz R, Gasteiger G, et al. Ablation of B7-H3 but Not B7-H4 Results in Highly Increased Tumor Burden in a Murine Model of Spontaneous Prostate Cancer. Cancer Immunol Res. 2015;3(8):849–854. doi: 10.1158/2326-6066.CIR-15-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18(14):3834–3845. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 67.Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97(3):409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo HF, et al. Humanized Affinity-matured Monoclonal Antibody 8H9 Has Potent Antitumor Activity and Binds to FG Loop of Tumor Antigen B7-H3. J Biol Chem. 2015;290(50):30018–30029. doi: 10.1074/jbc.M115.679852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamato I, Sho M, Nomi T, Akahori T, Shimada K, Hotta K, et al. Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer. 2009;101(10):1709–1716. doi: 10.1038/sj.bjc.6605375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, Wang J, Wang Y, Dong F, Zhu M, Wan W, et al. B7-H3 silencing by RNAi inhibits tumor progression and enhances chemosensitivity in U937 cells. Onco Targets Ther. 2015;8:1721–1733. doi: 10.2147/OTT.S85272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 72.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18(6):863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 73.Jeon H, Vigdorovich V, Garrett-Thomson SC, Janakiram M, Ramagopal UA, Abadi YM, et al. Structure and cancer immunotherapy of the B7 family member B7x. Cell Rep. 2014;9(3):1089–1098. doi: 10.1016/j.celrep.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeon H, Ohaegbulam KC, Abadi YM, Zang X. B7x and myeloid-derived suppressor cells in the tumor microenvironment: A tale of two cities. Oncoimmunology. 2013;2(7):e24744. doi: 10.4161/onci.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410(6828):604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 76.Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410(6828):608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 77.Lazar-Molnar E, Yan Q, Cao E, Ramagopal U, Nathenson SG, Almo SC. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci U S A. 2008;105(30):10483–10488. doi: 10.1073/pnas.0804453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin DY-w, Tanaka Y, Iwasaki M, Gittis AG, Su H-P, Mikami B, et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2008;105(8):3011–3016. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zak KM, Kitel R, Przetocka S, Golik P, Guzik K, Musielak B, et al. Structure of the Complex of Human Programmed Death 1, PD-1, and Its Ligand PD-L1. Structure. 2015;23(12):2341–2348. doi: 10.1016/j.str.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171(9):4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 81.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, et al. B7-H4 Is Highly Expressed in Ductal and Lobular Breast Cancer. Clin Cancer Res. 2005;11(5):1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 82.Yao Y, Ye H, Qi Z, Mo L, Yue Q, Baral A, et al. B7-H4(B7x)-Mediated Cross-talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients. Clin Cancer Res. 2016;22(11):2778–2790. doi: 10.1158/1078-0432.CCR-15-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183(6):2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Podojil JR, Liu LN, Marshall SA, Chiang M-Y, Goings GE, Chen L, et al. B7-H4Ig inhibits mouse and human T-cell function and treats EAE via IL-10/Treg-dependent mechanisms. J Autoimmun. 2013;44:71–81. doi: 10.1016/j.jaut.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Youn J-I, Gabrilovich DI. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40(11):2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abadi YM, Jeon H, Ohaegbulam KC, Scandiuzzi L, Ghosh K, Hofmeyer KA, et al. Host b7x promotes pulmonary metastasis of breast cancer. J Immunol. 2013;190(7):3806–3814. doi: 10.4049/jimmunol.1202439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hofmeyer KA, Scandiuzzi L, Ghosh K, Pirofski LA, Zang X. Tissue-expressed B7x affects the immune response to and outcome of lethal pulmonary infection. J Immunol. 2012;189(6):3054–3063. doi: 10.4049/jimmunol.1200701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei J, Loke P, Zang X, Allison JP. Tissue-specific expression of B7x protects from CD4 T cell-mediated autoimmunity. J Exp Med. 2011;208(8):1683–1694. doi: 10.1084/jem.20100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JS, Scandiuzzi L, Ray A, Wei J, Hofmeyer KA, Abadi YM, et al. B7x in the periphery abrogates pancreas-specific damage mediated by self-reactive CD8 T cells. J Immunol. 2012;189(8):4165–4174. doi: 10.4049/jimmunol.1201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pawar RD, Goilav B, Xia Y, Herlitz L, Doerner J, Chalmers S, et al. B7x/B7-H4 modulates the adaptive immune response and ameliorates renal injury in antibody-mediated nephritis. Clin Exper Immunol. 2015;179(2):329–343. doi: 10.1111/cei.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Azuma T, Zhu G, Xu H, Rietz AC, Drake CG, Matteson EL, et al. Potential role of decoy B7-H4 in the pathogenesis of rheumatoid arthritis: a mouse model informed by clinical data. PLoS Med. 2009;6(10):e1000166. doi: 10.1371/journal.pmed.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zang X, Allison JP. The B7 Family and Cancer Therapy: Costimulation and Coinhibition. Clin Cancer Res. 2007;13(18):5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 93.Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, et al. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73(15):4820–4829. doi: 10.1158/0008-5472.CAN-12-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thompson RH, Zang X, Lohse CM, Leibovich BC, Slovin SF, Reuter VE, et al. Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer Res. 2008;68(15):6054–6058. doi: 10.1158/0008-5472.CAN-08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith JB, Lanitis E, Dangaj D, Buza E, Poussin M, Stashwick C, et al. Tumor regression and delayed onset toxicity following B7-H4 CAR T cell Therapy. Mol Ther. 2016 Jul 21; doi: 10.1038/mt.2016.149. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X, Hao J, Metzger DL, Mui A, Ao Z, Akhoundsadegh N, et al. Early Treatment of NOD Mice With B7-H4 Reduces the Incidence of Autoimmune Diabetes. Diabetes. 2011;60(12):3246–3255. doi: 10.2337/db11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee IF, Wang X, Hao J, Akhoundsadegh N, Chen L, Liu L, et al. B7-H4.Ig inhibits the development of Type 1 diabetes by regulating Th17 cells in NOD mice. Cell Immunol. 2013;282(1):1–8. doi: 10.1016/j.cellimm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 99.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58(6):1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, et al. B7-H5 costimulates human T cells via CD28H. Nat Commun. 2013;4:2043. doi: 10.1038/ncomms3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flajnik MF, Tlapakova T, Criscitiello MF, Krylov V, Ohta Y. Evolution of the B7 family: co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7's historical relationship with the MHC. Immunogenetics. 2012;64(8):571–590. doi: 10.1007/s00251-012-0616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mager DL, Hunter DG, Schertzer M, Freeman JD. Endogenous retroviruses provide the primary polyadenylation signal for two new human genes (HHLA2 and HHLA3) Genomics. 1999;59(3):255–263. doi: 10.1006/geno.1999.5877. [DOI] [PubMed] [Google Scholar]
- 103.Janakiram M, Chinai JM, Fineberg S, Fiser A, Montagna C, Medavarapu R, et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin Cancer Res. 2015;21(10):2359–2366. doi: 10.1158/1078-0432.CCR-14-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Manick B, Wu G, Hao R. Biofunctions of three new B7 family members (IRM7P.486) J Immunol. 2014;192(1 Supplement):126.11. [Google Scholar]
- 105.Xiao Y, Freeman GJ. A new B7:CD28 family checkpoint target for cancer immunotherapy: HHLA2. Clin Cancer Res. 2015;21(10):2201–2203. doi: 10.1158/1078-0432.CCR-14-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng H, Janakiram M, Borczuk A, Lin J, Qiu W, Liu H, et al. HHLA2, a new immune checkpoint member of the B7 family, is widely expressed in human lung cancer and associated with EGFR mutational status. Clin Cancer Res. 2016 Aug 23; doi: 10.1158/1078-0432.CCR-15-3071. pii: clincanres.3071.2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schalper KA, Carvajal-Hausdorf D, McLaughlin J, Altan M, Velcheti V, Gaule P, et al. Differential expression and significance of PD-L1, IDO-1 and B7-H4 in human lung cancer. Clin Cancer Res. 2016 Jul 20; doi: 10.1158/1078-0432.CCR-16-0150. pii: clincanres.0150.2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep. 2016;6:31154. doi: 10.1038/srep31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rahimi N, Rezazadeh K, Mahoney JE, Hartsough E, Meyer RD. Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Mol Biol Cell. 2012;23(9):1646–1656. doi: 10.1091/mbc.E11-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dong Q, Ma X. B7-H4 expression is associated with tumor progression and prognosis in patients with osteosarcoma. Biomed Res Int. 2015;2015:156432. doi: 10.1155/2015/156432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kraan J, van den Broek P, Verhoef C, Grunhagen DJ, Taal W, Gratama JW, et al. Endothelial CD276 (B7-H3) expression is increased in human malignancies and distinguishes between normal and tumour-derived circulating endothelial cells. Br J Cancer. 2014;111(1):149–156. doi: 10.1038/bjc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong B, Qian Y, Zhang H, Sang YW, Cheng LF, Wang Q, et al. Expression of B7-H4 and hepatitis B virus X in hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2016;22(18):4538–4546. doi: 10.3748/wjg.v22.i18.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao LW, Li C, Zhang RL, Xue HG, Zhang FX, Zhang F, et al. B7-H1 and B7-H4 expression in colorectal carcinoma: correlation with tumor FOXP3(+) regulatory T-cell infiltration. Acta Histochem. 2014;116(7):1163–1168. doi: 10.1016/j.acthis.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 114.Chen L, Chen J, Xu B, Wang Q, Zhou W, Zhang G, et al. B7-H3 expression associates with tumor invasion and patient's poor survival in human esophageal cancer. Am J Transl Res. 2015;7(12):2646–2660. [PMC free article] [PubMed] [Google Scholar]
- 115.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, et al. B7-H4 expression associates with cancer progression and predicts patient's survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60(7):1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xylinas E, Robinson BD, Kluth LA, Volkmer BG, Hautmann R, Kufer R, et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol. 2014;40(1):121–127. doi: 10.1016/j.ejso.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 117.Liu WH, Chen YY, Zhu SX, Li YN, Xu YP, Wu XJ, et al. B7-H4 expression in bladder urothelial carcinoma and immune escape mechanisms. Oncol Lett. 2014;8(6):2527–2534. doi: 10.3892/ol.2014.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu J, Liu Y, Wang W, Wang C, Che Y. Expression of immune checkpoint molecules in endometrial carcinoma. Exp Ther Med. 2015;10(5):1947–1952. doi: 10.3892/etm.2015.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, et al. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11(5):1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 120.Koirala P, Roth ME, Gill J, Chinai JM, Ewart MR, Piperdi S, et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep. 2016;6:31154. doi: 10.1038/srep31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu L, Deng WW, Yu GT, Mao L, Bu LL, Ma SR, et al. B7-H4 expression indicates poor prognosis of oral squamous cell carcinoma. Cancer Immunol Immunother. 2016;65(9):1035–1045. doi: 10.1007/s00262-016-1867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu J, Chu BF, Yang YP, Zhang SL, Zhuang M, Lu WJ, et al. B7-H4 expression is associated with cancer progression and predicts patient survival in human thyroid cancer. Asian Pac J Cancer Prev. 2013;14(5):3011–3015. doi: 10.7314/apjcp.2013.14.5.3011. [DOI] [PubMed] [Google Scholar]


