Abstract
Background
There have been significant advancements in lower extremity reconstruction over the last several decades, and the plastic surgeon’s armamentarium has grown to include free muscle and fasciocutaneous flaps along with local perforator and propeller flaps. While we have found a use for a variety of techniques for lower extremity reconstruction, the free gracilis has been our workhorse flap due to the ease of harvest, reliability, and low donor site morbidity.
Methods
This is a retrospective review of a single surgeon’s series of free gracilis flaps utilized for lower extremity reconstruction. Demographic information, comorbidities, outcomes and secondary procedures were analyzed.
Results
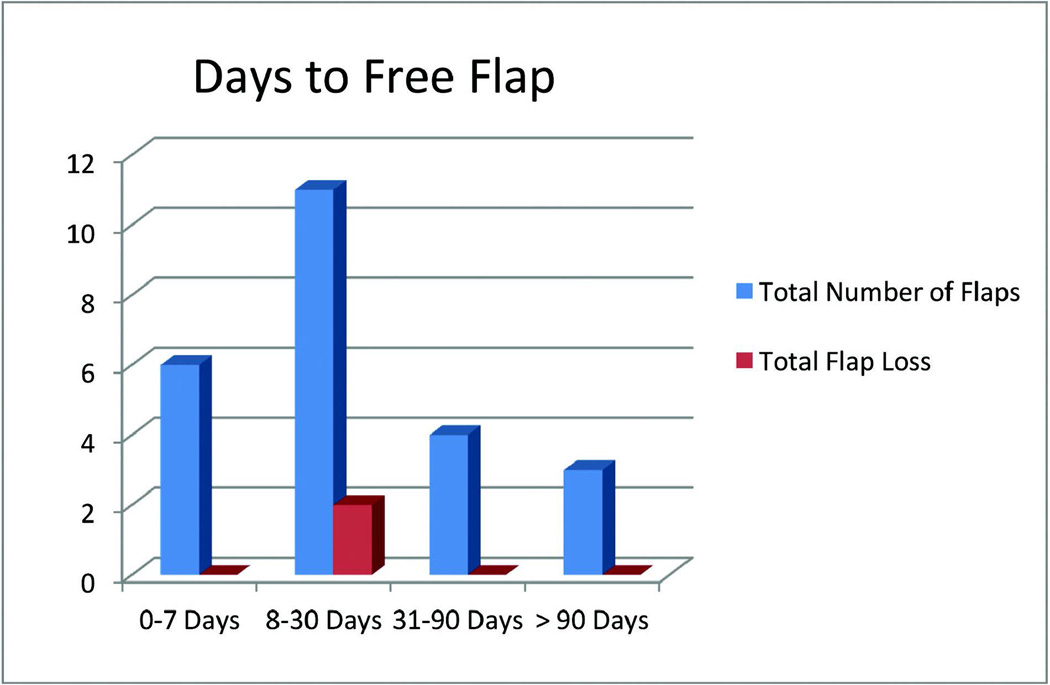
We identified 24 free gracilis flaps. The duration from injury to free flap coverage was 7 days or less in 6 patients, 8–30 days in 11 patients, 31–90 days in 4 patients, and > 90 days in 3 patients. There were 22 (92%) successful flaps and an overall limb salvage rate of 92%. There was one partial flap loss. Two flaps underwent incision and drainage in the operating room for infection. Two patients developed donor site hematomas. Four patients underwent secondary procedures for contouring. Our subset of pediatric patients had 100% flap survival and no secondary procedures at a mean 30 month follow up.
Conclusions
This study demonstrates the utility of the free gracilis flap in reconstruction of small to medium sized defects of the lower extremity. This flap has a high success rate and low donor site morbidity. Atrophy of the denervated muscle over time allows for good shoe fit, often obviating the need for secondary contouring procedures.
Keywords: lower extremity reconstruction, gracilis muscle flap, free gracilis
Introduction
The foundation for lower extremity reconstruction with free tissue transfer was established in the 1980s and early 1990s and was deemed safe and effective in lower extremity salvage, securing the role of free tissue transfer in the armamentarium of plastic surgeons 1–8. Muscle flaps such as the latissimus dorsi and the rectus abdominus, the initial workhorse flaps, remained staples of lower extremity reconstruction while newer fasciocutaneous flaps such as the anterolateral thigh (ALT) flap were developed. As surgeons became comfortable harvesting the ALT, this flap was found to be reliable and effective in covering open fractures in the lower extremity 9–11. While the introduction of locoregional propeller and perforator flaps over the last decade has expanded reconstructive options in the lower extremity, free tissue transfer with the ALT flap has remained the predominant technique for coverage of complex lower extremity defects as it avoids the zone of injury, which often limits the utilization of locoregional flap coverage 12–14. However, despite its popularity, the ALT flap is not without limitations in the coverage of lower extremity defects. Specifically, in the treatment of small and medium sized defects of the lower extremity, the ALT flap often requires multiple secondary operations for flap debulking, as it does not atrophy over time 15. Furthermore, for reconstruction of foot and ankle defects, the bulk of the ALT flap impairs aesthetic outcomes and hinders shoe and orthotic fitting which is a significant limitation for return to functional activity 15.
Given these limitations with the ALT flap in select patients, it is our institutional preference to utilize the gracilis muscle flap for reconstruction of small to medium sized defects of the lower extremity in adult and pediatric patients. The gracilis muscle flap has many of the same advantages as the ALT flap, while providing additional benefits in lower extremity reconstruction. Similar to the ALT flap, the gracilis can be harvested in a 2-team approach on the ipsilateral leg, therefore obviating the need for draping surgical sites on the abdomen or back and intraoperative position changes. As the gracilis donor site morbidity is low and the upper extremity and trunk are left undisturbed, ambulating with a crutch is not a problem in the postoperative period 16–19. Furthermore, unlike the fasciocutaneous ALT flap, the gracilis is a deneravated muscle flap that will atrophy over time, potentially improving flap contour and aesthetic outcomes without requiring multiple secondary procedures. This is especially important in the pediatric population, where patient growth puts a high demand on flap contour.
While the gracilis muscle flap has been well described from an anatomic standpoint, the utility of this flap in lower extremity reconstruction is underreported in the literature as it is often considered a secondary or tertiary option 12,14,19,20. Furthermore, there is a paucity of data on long-term outcomes and revision rates following free gracilis muscle flap reconstruction of lower extremity defects, with reports including pediatric patients specifically lacking in the literature. The objective of this study is to demonstrate the safety and efficacy of the free gracilis muscle flap as a primary option in reconstructing small and medium sized lower extremity defects in the adult and pediatric population. In addition, we demonstrate the ability of the gracilis flap to contour to defects over time, and evaluate the need for secondary debulking or aesthetic procedures following initial reconstruction.
Methods
Following institutional review board approval, a retrospective review was performed to identify all patients who underwent free tissue transfer for lower extremity reconstruction over an 11-year period from January 2001 to January 2012. A single surgeon (THHT) performed all surgeries. To identify patients, we searched our database for the CPT code 15756: free muscle or mycocutaneous flap with microvascular anastomosis. Demographic information, medical comorbidities, mechanism of injury, details of the wound, preoperative imaging, recipient vessel selection, complications and flap survival were recorded. Flap survival was broken down into partial and complete flap loss. Secondary reconstructive procedures and revision operations were also identified and tabulated.
Results
The study population included 24 patients who underwent 24 free gracilis flaps. There were 17 (71%) men and 7 (29%) women in the study group. The ages ranged from 5–70 with a mean of 37 (SD ± 20). Relevant patient comorbidities are listed in Table 1. Four patients reported current tobacco use, three patients had diabetes, and one patient was obese (body-mass index > 30 kg/m2). Average follow up time was 29 months. The mechanism of injury for 50% of patients was motor vehicle collisions, and motor cycle or all-terrain vehicle accidents. The remaining injuries were related to falls from height, crush injuries, and ballistic injury. There was one case of free tissue transfer for lower extremity salvage after an infected total knee arthroplasty, and one case was related to infection due to underlying diabetes (Table 2). There were 2 proximal third, 2 middle third, and 9 distal third defects. There were also 7 ankle and 4 foot wounds treated with free tissue transfer. Of the patients with traumatic injuries, 7 had Gustilo grade IIIB injuries and 1 had a Gustilo grade IIIa injury. The mean defect size was 81 cm2 with a range of 12–260 cm2 (SD ± 65 cm2) (Table 2).
Table 1.
Summary of patient characteristics and comorbidities
| Number | N = 24 | Percent |
|---|---|---|
| Age (years) (standard deviation) |
37 (20) | |
| Sex | ||
| Male | 17 | 71 |
| Female | 7 | 29 |
| Follow up (months) (mean ± SD) |
29 ± 35 | |
| Comorbidites | ||
| Diabetes | 3 | 12 |
| Tobacco Use | 4 | 17 |
| Obesity | 1 | 4 |
| Coronary artery disease | 2 | 8 |
| Hypertension | 4 | 17 |
Table 2.
Summary of wound characteristics and mechanism
| Number | N = 24 | Percent |
|---|---|---|
| Location | ||
| Proximal third | 2 | 8 |
| Middle third | 2 | 8 |
| Lower third | 9 | 38 |
| Ankle | 7 | 29 |
| Foot | 4 | 17 |
|
Size (cm2)(standard deviation) |
||
| Mechanism | ||
| Acute trauma | ||
| MVC/MCC/ATV | 12 | 50 |
| Fall from height | 4 | 17 |
| Crush injury | 3 | 12 |
| Ballistic injury | 1 | 4 |
| Other (dog bite, lawn mower) |
2 | 8 |
| Non-trauma | ||
| Post-surgical chronic wound | 1 | 4 |
| Diabetic wound | 1 | 4 |
MVC = motor vehicle collision, MCC = motorcycle collision, ATV = all-terrain vehicle
In regards to preoperative imaging, 11 patients did not undergo any imaging, 11 patients had formal angiography to evaluate lower extremity vessels, and 2 patients had a CT angiogram for vessel evaluation. Both CT angiograms were read as normal; however, 6 of the formal angiograms were abnormal: the anterior tibial artery was occluded in 2 patients, the posterior tibial artery was occluded in 2 patients, and a combination of vessels were occluded in the remaining 2 patients, leaving those patients with a leg dependent on a single vessel. In all cases where there was an occluded vessel, the distal end of the damaged vessel was used for the end-to-end anastomosis. The anterior tibial artery was used as a recipient vessel in 6 cases, the posterior tibial artery in 14 cases, branches off the geniculate arteries in 2 cases, and the dorsalis pedis and medial plantar in 1 case each (Table 3). We used an end-to-end anastomosis in all cases. The mean warm-ischemia time was 70 minutes. The duration from injury to free flap coverage was 7 days or less in six patients, 8–30 days in eleven patients, 31–90 days in four patients, and > 90 days in three patients.
Table 3.
Imaging and findings along with artery used for anastomosis
| Number | N = 24 | Percent |
|---|---|---|
| Imaging | ||
| No imaging | 11 | 46 |
| Formal angiogram | 11 | 46 |
| Abnormal | 6 | 25 |
| CT angiogram | 2 | 8 |
| Abnormal | 0 | 0 |
| Vessel(s) occluded | ||
| Anterior tibial artery | 2 | 8 |
| Posterior tibial artery | 2 | 8 |
| 2 vessels occluded | 2 | 8 |
| Artery used for anastomosis | ||
| Anterior tibial artery | 6 | 25 |
| Posterior tibial artery | 14 | 58 |
| Other (genicular, dorsalis pedis, medial plantar) |
4 | 17 |
Overall, there were 22 (92%) successful flaps. There were two (8%) total flap losses. The flap losses occurred in flaps performed 8 and 14 days after injury (Figure 2). Of the flaps that were ultimately successful, there was 1 partial flap loss. In regards to complications, 2 patients (8%) underwent incision and drainage in the operating room for flap infections, 2 patients (8%) developed donor site hematomas which were drained in the operating room, 4 patients (17%) eventually underwent bone-grafting procedures for non-union, and 5 patients (21%) had problems with chronic osteomyelitis that resulted in eventual hardware removal. Revisions were infrequent, there was 1 case of complete skin graft loss requiring a repeat skin grafting procedure, and 4 patients underwent flap debulking and contouring after the flap had healed (Table 4). On comparison to published reports in the literature on lower extremity reconstruction using flaps other than the gracilis, our limb salvage rate, flap failure rate, and complication rates are all consistent with accepted standards (Table 5).
Figure 2.
Days to free flap after injury with number of flaps lost in each group
Table 4.
Complications and secondary procedures
| Number | N = 24 | Percent |
|---|---|---|
| Flap loss | ||
| Total flap loss | 2 | 8 |
| Partial flap loss | 1 | 4 |
| Amputation | 2 | 8 |
| Complications | ||
| Infection at flap site | 2 | 8 |
| Skin graft loss | 1 | 4 |
| Non-union | 4 | 17 |
| Donor site | ||
| Hematoma | 2 | 8 |
| Secondary procedures | ||
| Flap debulking | 4 | 17 |
Table 5.
Comparison of outcomes in this study to the published literature on microsurgical free flap reconstruction of lower extremity wounds
| Our Study | Published Literature* | |
|---|---|---|
| Outcomes | Percent (%) | Percent (%) |
| Limb Salvage | 92% | 94% |
| Flap Loss | ||
| Total flap loss | 8% | 6% |
| Partial flap loss | 4% | 6% |
| Complications | ||
| Wound Complications | 8% | 7% |
| Hematoma | 8% | 4% |
Represents data from meta-analysis on microsurgical free flap reconstruction of lower extremity wounds (Reference #14).
Our overall lower extremity salvage rate was 92%, as there were 2 (8%) amputations; one early and one late. The patient undergoing lower extremity salvage after total knee arthroplasty ultimately underwent an above the knee amputation 8 days after partial loss of the gracilis flap left the knee extensor mechanism exposed. The second amputation occurred 2 years after the original free flap in the patient with a distal tibial wound after a fall from height. This patient had a total flap loss followed by a reverse sural artery flap as a salvage procedure. This was complicated by chronic osteomyelitis necessitating the eventual below the knee amputation. All patients with limb salvage were able to ambulate independently at time of last follow-up (mean 29 months).
There was a subset of 4 pediatric patients with an average age of 9 years (range 5–13). There were no medical comorbidities in this group. Two patients presented with a crush injury, 1 after an ATV accident and the other after a crush/avulsion from a lawnmower. One patient had wound coverage within 7 days, while the other three fell in the 8–30 day category. Flaps were covered with a skin graft at the initial operation. All flaps survived with no complications. There was no donor site morbidity reported. At a mean follow up time of 30 months (SD±16), no one required a secondary operation for orthotic or shoe fitting (Table 5)and all pediatric patients had the ability to ambulate independently without gait abnormalities.
Discussion
This study represents an institutional review of lower extremity reconstruction using a free gracilis muscle flap over an 11-year period. In our practice, we preferentially choose the gracilis muscle flap for small and medium sized defects of the lower extremity. The technique of gracilis harvest has been well described 19, 20. The harvest of this particular muscle flap is straightforward and technically feasible as there is a reliable vascular pedicle approximately 6–7 cm in length coming off the medial circumflex femoral artery. With proximal dissection, a vessel caliber of 2 mm can be obtained 19. The muscle is innervated by a branch of the obturator nerve and can be harvested as a free functioning muscle flap if needed 21,22. The gracilis flap is approximately 30 cm in length, and its average width is 4–5 cm 19. In most cases of lower extremity reconstruction with a free gracilis flap, the muscle is covered with a split thickness skin graft at the initial operation. This allows for improved wound care while preserving the ability to monitor the flap.
Our flap loss rate of 8% is in line with published reports of free flap survival in lower extremity reconstruction 12,18,19,23. It is important to acknowledge that while we lost only 2 flaps completely, our 1 partial flap loss led to exposure of critical structures despite the majority of the flap surviving. This patient had already been through multiple procedures to salvage a total knee arthroplasty. After the partial flap loss with exposure of the knee extensor mechanism, the surgical teams along with the patient opted for above the knee amputation. One of our flap losses occurred in a patient in a motor vehicle collision resulting in a Gustilo grade IIIB injury with an open tibia/fibula. The patient’s only significant medical issue was tobacco use. The patient had an external fixator applied to the lower extremity and underwent intramedullary nailing of the tibia along with ORIF of the lateral malleolus after two procedures for washout and debridement. A formal angiogram revealed an occluded anterior tibial and peroneal artery. The open wounds were prepared with a negative pressure dressing until free flap reconstruction occurred 8 days after the original injury. The arterial anastomosis was performed to the distal end of the anterior tibial artery and there were no intraoperative complications. The flap displayed signs of venous congestion on postoperative day 1, and went back to the OR emergently for evaluation. Ultimately, we used a vein graft to salvage the flap; however, the flap continued to have problems with venous drainage and was deemed non-viable. On postoperative day 8, the patient underwent debridement of the flap followed by integra placement and subsequent skin grafting. The limb was ultimately salvaged. The other total flap loss occurred in a patient that had a fall from height resulting in an open tibia and fibula fracture, along with a fracture of the talus. The patient had no medical comorbidities. The patient was treated with an external fixator while incision and drainage procedures were done to prepare the wound. The flap was performed 14 days after injury and there were no intraoperative complications. The flap was noted to have venous congestion on postoperative day 1 and underwent leech therapy. Ultimately, the flap was deemed non-viable. This patient had recipient site debridement and a reverse sural artery flap 2 weeks after the original flap. Unfortunately, this was complicated by chronic osteomyelitis and the patient underwent a below the knee amputation 2 years after the injury.
A recent meta-analysis on the subject of lower extremity reconstruction with free tissue transfer found an overall failure rate of 6%. This analysis revealed that the gracilis flap was the 3rd most utilized flap after the anterolateral thigh flap and latissimus dorsi flap 14. Our failure rate is consistent with this and other published reports 12,14,18. In a retrospective review of 119 free flaps in 114 patients, Fischer et al reported using the gracilis free flap in only 3 (2.5%) cases. The majority of the flaps used in their series were the anterolateral thigh flap (40%) and the rectus abdominus flap (18%). They reported that in 2 of the gracilis flaps there were intraoperative factors that led to major complications requiring reoperation but there was no total flap loss 12. Given that the free gracilis was only used in 3 cases, it is difficult to draw conclusions of safety and efficacy with regards to this specific flap. The limited reports on lower extremity reconstruction with the gracilis flap have suggested promising results. For example, Reddett and colleagues reviewed their experience with 50 gracilis flaps for lower extremity reconstruction, preferentially used in small and medium sized defects of the lower extremity, and identified ease of harvest, low donor site morbidity and the fact that the donor site does not interfere with crutch use as the reasons why they prefer this flap. These authors reported a 95% lower extremity salvage rate. In their series, smoking and Gustilo grade IIIC injuries were associated with lower flap survival 19. Additionally, in a series from Osiogo et al. focusing predominantly on the gracilis muscle flap for foot and ankle reconstruction in patients with diabetic wounds or infected wounds, there was only one flap failure (2%)18. Our experience corroborates and expands on these earlier reports. We echo these authors’ thoughts that the gracilis muscle flap is easy to harvest, allows for a 2 team approach on the ipsilateral leg and provides lower extremity salvage rates that are consistent with salvage rates reported for other flaps 18.
In regards to timing of wound coverage, our patients fell into 4 groups: 0–7 days, 8–30 days, 31–90 days and > 90 days after injury. The two total flap losses were done 8 days and 14 days after injury. The partial flap loss that resulted in the above the knee amputation was done 36 days after initial injury. The ideal timing of flap coverage for lower extremity wounds remains controversial. Recently, Hill et al. reviewed their series of 60 free flaps for lower extremity reconstruction to evaluate the effect of timing of wound coverage on outcomes. They found no difference in outcomes comparing patients undergoing reconstruction < 30 days, 31–90 days and > 90 days after injury and concluded that the historical data that suggested an advantage to early flap coverage (< 72 hours) may no longer be relevant given advances in wound care, debridement techniques, broad spectrum antibiotics and the advent of negative pressure dressings 24. We tend to agree with this conclusion. It is our practice to provide early wound coverage when possible, but it is reasonable to provide delayed wound coverage if physiologic or logistic issues make early coverage unsafe or impossible.
Our complication profile was in line with published reports 18,19. In complex lower extremity wounds, there are still challenges of non-union and osteomyelitis that come with open long bone fractures. Five of our cases were complicated by osteomyelitis. Four out of 5 of these patients had osteomyelitis noted in their records before flap coverage was performed. All of the flaps were done in a delayed fashion from 1 month to > 2 years after injury. Three of these patients had wounds resulting from a motor vehicle collision, 1 wound was from a dog bite and the other patient had an infected wound related to underlying diabetes.
Another potential advantage of the gracilis flap compared the ALT flap or the latissimus flap is the low rate of donor site complications. In our series, there were only 2 donor site complications; both were hematomas that were drained in then operating room. This low donor site complication rate is in line with the literature, which indicates that complications are rare and usually minor in nature. While it has been found that there is a small, measurable change in strength after gracilis harvest, we did not find this clinically; however, we did not use specific objective measures 17–20.
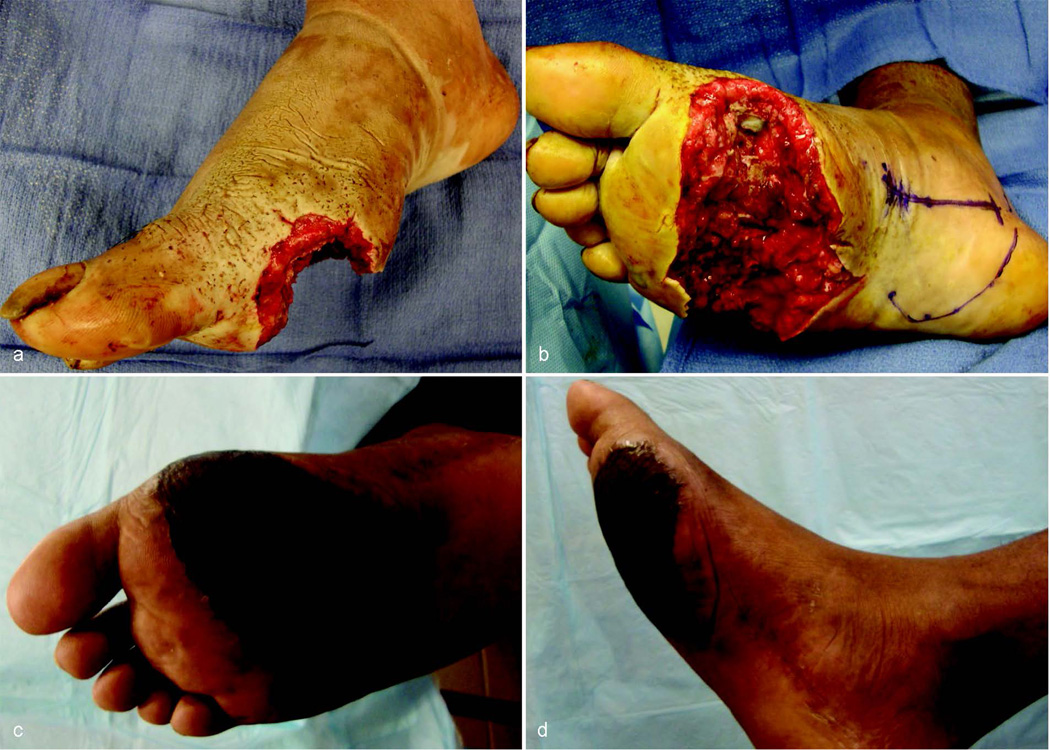
One of the major advantages of the gracilis free flap is the benefit inherent to a muscle-only flap; the muscle will atrophy over time and the contour will improve, often without secondary procedures. Recently, Nelson et al. reviewed their series of free flaps for lower extremity reconstruction identifying secondary procedures to improve esthetics. They found that approximately 20% of their patients underwent secondary procedures to improve the contour and esthetics of the free flap. The procedures included complex local tissue rearrangement or debulking, direct scar excision, liposuction and laser scar treatment 15. In our series, 4 patients (19%) out of the cohort that had successful lower extremity salvage ultimately underwent secondary debulking procedures. Two of the patients had wounds affecting the ankle, 1 had a calcaneal wound and the remaining patient had a distal tibia wound. All patients underwent revision procedures to help with esthetics to some degree, but more importantly, to assist with shoe fitting. Two of the patients had contouring procedures combined with hardware removal or revision, suggesting that they may not have underwent debulking if not for the need of another procedure. All patients had direct debulking with complex local tissue rearrangement along with revision skin grafting in one case. The debulking procedures were performed on average 14.5 months after the initial free flap. Fifteen patients (81%) that had successful lower extremity salvage with a viable free flap did not require or request secondary contouring surgery for esthetics or for shoe fitting. Eight of these patients (38%) had wounds of the ankle or foot. It is our experience that the free gracilis flap with skin graft experiences muscle atrophy over time, improving contour and providing durable soft tissue that fits well in a shoe. An example of this is shown in figures 1a–d. The patient in figure 1a–d had a shot-gun blast to the plantar foot resulting in a wound with exposed metatarsals. The patient did well after debridement and soft tissue coverage with a free gracilis muscle flap and a split thickness skin graft. After 8 months of follow up, the flap and skin graft are well healed with a functional and esthetic contour.
Figure 1.
a–d. This patient suffered a shot-gun blast to the foot. The patient had well healed and contoured flap 8 months after the original operation with no debulking procedures.
This study has limitations inherent to a retrospective review. There is no control group leaving conclusions to be drawn from comparisons to the current literature on the topic. A direct comparison between two flap types in lower extremity reconstruction remains challenging due to the extreme heterogeneity of patient comorbidities, mechanisms of injury, wound defects, and reconstructive options as well as the sample size needed to detect a meaningful difference between two reconstructive options. We performed a power analysis and if one considers a sample flap failure rate plus or minus 5.0% from the accepted flap failure rate for lower extremity flap reconstruction to be clinically significant, assuming a power level of 80% and an alpha of 0.05, we calculate that a study would need a minimum of n = 282 patients undergoing free flap procedures to detect a meaningful difference between two flap types in lower extremity reconstruction. Therefore, we compared our outcomes descriptively to published reports from the literature on lower extremity reconstruction. Our study is also limited by the lack of objective measures of function, esthetics and contour, which would have helped with determining the place of the free gracilis in reference to other flaps utilized for lower extremity reconstruction. We do not have length of hospital stay, which makes it difficult to identify the impact of complications on the hospital course and cost of free tissue transfer. In addition, due to the relatively small sample size, the use of rigorous statistics was precluded giving more of a qualitative analysis. Furthermore, the timing to surgery after injury was not standardized and the groupings were developed based on our outcomes and existing data. Despite these limitations, our experience demonstrates that, in the appropriate patient, the gracilis flap is a safe and effective flap that may have advantages over fasciocutaneous flaps or other muscle flaps from the abdomen or back. The gracilis be harvested from the ipsilateral leg, facilitating efficiency during the operation and as a muscle flap, as opposed to fasciocutaneous flaps, has the propensity to atrophy over time and potentially eliminate the need for secondary contouring procedures. In our four pediatric patients, the gracilis flap proved to be a good option with no donor site morbidity and no secondary operations required to date.
Conclusion
This data adds to the body of evidence that shows the free gracilis flap is a reasonable option when used to cover small to medium sized defects of the lower extremity. This is especially true when trauma to the lower extremity precludes the use of local tissue in a perforator style flap. The harvest is straightforward and there is a reliable neurovascular pedicle. Furthermore, the donor site morbidity is low, especially when compared to back and abdominal muscles as donors. An additional benefit of the free gracilis is the expected muscle atrophy, which can help with esthetics and contour in the critical area around the foot and ankle, reducing the need for secondary procedures and assisting with shoe fitting.
Table 6.
Pediatric subgroup demographics, mechanism and outcomes
| Number | N = 4 | Percent |
|---|---|---|
| Age (years) (range) | 9 (5–13) | |
| Sex | ||
| Male | 3 | 75 |
| Female | 1 | 25 |
| Follow up (months) (mean ± SD) |
30 ± 16 | |
| Mechanism | ||
| Crush | 2 | 50 |
| ATV accident | 1 | 25 |
| Lawnmower crush/avulsion | 1 | 25 |
| Time to wound coverage (days ± SD) |
11 ± 2 | |
| Flap loss | 0 | 0 |
| Complications | 0 | 0 |
| Donor site morbidity | 0 | 0 |
| Secondary procedures | 0 | 0 |
Contributor Information
Michael J Franco, Washington University School of Medicine, Division of Plastic and Reconstructive Surgery, 660 South Euclid, Campus Box 8238, St. Louis, MO 63110, Fran8207@gmail.com, (P) 856-981-2362, (F) 434-924-0782, Mailing address: 3 Cooper Plaza Suite 411, Camden, NJ 08103.
Michael C Nicoson, Washington University School of Medicine, Division of Plastic and Reconstructive Surgery, 660 South Euclid, Campus Box 8238, St. Louis, MO 63110.
Rajiv P Parikh, Washington University School of Medicine, Division of Plastic and Reconstructive Surgery, 660 South Euclid, Campus Box 8238, St. Louis, MO 63110.
Thomas H Tung, Washington University School of Medicine, Division of Plastic and Reconstructive Surgery, 660 South Euclid, Campus Box 8238, St. Louis, MO 63110.
References
- 1.Acland RD. Refinements in lower extremity free flap surgery. Clin Plast Surg. 1990;17:733–744. [PubMed] [Google Scholar]
- 2.Cordeiro PG, Neves RI, Hidalgo DA. The role of free tissue transfer following oncologic resection in the lower extremity. Ann Plast Surg. 1994;33:9–16. doi: 10.1097/00000637-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Serafin D, Voci VE. Reconstruction of the lower extremity. Microsurgical composite tissue transplantation. Clin Plast Surg. 1983;10:55–72. [PubMed] [Google Scholar]
- 4.Bibbo C, Nelson J, Fischer JP, Wu LC, Low DW, Mehta S, Kovach SJ, Levin LS. Lower Extremity Limb Salvage After Trauma: Versatility of the Anterolateral Thigh Free Flap. J Orthop Trauma. 2015;29:563–568. doi: 10.1097/BOT.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 5.Heller L, Levin LS. Lower extremity microsurgical reconstruction. Plast Reconstr Surg. 2001;108:1029–1041. doi: 10.1097/00006534-200109150-00036. quiz 1042. [DOI] [PubMed] [Google Scholar]
- 6.Medina ND, Kovach SJ, 3rd, Levin LS. An evidence-based approach to lower extremity acute trauma. Plast Reconstr Surg. 2011;127:926–931. doi: 10.1097/PRS.0b013e3182046a16. [DOI] [PubMed] [Google Scholar]
- 7.Ong YS, Levin LS. Lower limb salvage in trauma. Plast Reconstr Surg. 2010;125:582–588. doi: 10.1097/PRS.0b013e3181c82ed1. [DOI] [PubMed] [Google Scholar]
- 8.Zenn MR, Levin LS. Microvascular reconstruction of the lower extremity. Semin Surg Oncol. 2000;19:272–281. doi: 10.1002/1098-2388(200010/11)19:3<272::aid-ssu9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Engel H, Lin CH, Wei FC. Role of microsurgery in lower extremity reconstruction. Plast Reconstr Surg. 2011;127(Suppl 1):228S–238S. doi: 10.1097/PRS.0b013e3182008e12. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez ED, Bluebond-Langner R, Copeland C, Grim TN, Singh NK, Scalea T. Functional outcomes of posttraumatic lower limb salvage: a pilot study of anterolateral thigh perforator flaps versus muscle flaps. J Trauma. 2009;66:1311–1314. doi: 10.1097/TA.0b013e318187cc87. [DOI] [PubMed] [Google Scholar]
- 11.Yazar S, Lin CH, Lin YT, Ulusal AE, Wei FC. Outcome comparison between free muscle and free fasciocutaneous flaps for reconstruction of distal third and ankle traumatic open tibial fractures. Plast Reconstr Surg. 2006;117:2468–2475. doi: 10.1097/01.prs.0000224304.56885.c2. discussion 2476–7. [DOI] [PubMed] [Google Scholar]
- 12.Fischer JP, Wink JD, Nelson JA, Cleveland E, Grover R, Wu LC, Levin LS, Kovach SJ. A retrospective review of outcomes and flap selection in free tissue transfers for complex lower extremity reconstruction. J Reconstr Microsurg. 2013;29:407–416. doi: 10.1055/s-0033-1343952. [DOI] [PubMed] [Google Scholar]
- 13.Oh TS, Hallock G, Hong JP. Freestyle propeller flaps to reconstruct defects of the posterior trunk: a simple approach to a difficult problem. Ann Plast Surg. 2012;68:79–82. doi: 10.1097/SAP.0b013e3182157940. [DOI] [PubMed] [Google Scholar]
- 14.Xiong L, Gazyakan E, Kremer T, Hernekamp FJ, Harhaus L, Saint-Cyr M, Kneser U, Hirche C. Free flaps for reconstruction of soft tissue defects in lower extremity: A meta-analysis on microsurgical outcome and safety. Microsurgery. 2016 doi: 10.1002/micr.30020. [DOI] [PubMed] [Google Scholar]
- 15.Nelson JA, Fischer JP, Haddock NT, Mackay D, Wink JD, Newman AS, Levin LS, Kovach SJ. Striving for Normalcy after Lower Extremity Reconstruction with Free Tissue: The Role of Secondary Esthetic Refinements. J Reconstr Microsurg. 2016;32:101–108. doi: 10.1055/s-0035-1558986. [DOI] [PubMed] [Google Scholar]
- 16.Carr MM, Manktelow RT, Zuker RM. Gracilis donor site morbidity. Microsurgery. 1995;16:598–600. doi: 10.1002/micr.1920160904. [DOI] [PubMed] [Google Scholar]
- 17.Deutinger M, Kuzbari R, Paternostro-Sluga T, Quittan M, Zauner-Dungl A, Worseg A, Todoroff B, Holle J. Donor-site morbidity of the gracilis flap. Plast Reconstr Surg. 1995;95:1240–1244. doi: 10.1097/00006534-199506000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Osiogo FO, Lai CS, Wang WH, Chye YF, Lin SD. Retrospective review of free gracilis muscle flaps in the management of nonhealing diabetic foot ulceration. J Foot Ankle Surg. 2006;45:252–260. doi: 10.1053/j.jfas.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Redett RJ, Robertson BC, Chang B, Girotto J, Vaughan T. Limb salvage of lower-extremity wounds using free gracilis muscle reconstruction. Plast Reconstr Surg. 2000;106:1507–1513. doi: 10.1097/00006534-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Heckler FR. Gracilis myocutaneous and muscle flaps. Clin Plast Surg. 1980;7:27–44. [PubMed] [Google Scholar]
- 21.Harii K, Ohmori K, Torii S. Free gracilis muscle transplantation, with microneurovascular anastomoses for the treatment of facial paralysis. A preliminary report. Plast Reconstr Surg. 1976;57:133–143. doi: 10.1097/00006534-197602000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Manktelow RT, Zuker RM, McKee NH. Functioning free muscle transplantation. J Hand Surg Am. 1984;9A:32–39. doi: 10.1016/s0363-5023(84)80181-1. [DOI] [PubMed] [Google Scholar]
- 23.Meland NB, Fisher J, Irons GB, Wood MB, Cooney WP. Experience with 80 rectus abdominis free-tissue transfers. Plast Reconstr Surg. 1989;83:481–487. doi: 10.1097/00006534-198903000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Hill JB, Vogel JE, Sexton KW, Guillamondegui OD, Corral GA, Shack RB. Re-evaluating the paradigm of early free flap coverage in lower extremity trauma. Microsurgery. 2013;33:9–13. doi: 10.1002/micr.21994. [DOI] [PubMed] [Google Scholar]