Women are born with a complete complement of ovarian follicles for their reproductive lifetime. Each ovarian follicle contains a single oocyte, packaged in a region of the ovary that is as close to a metabolic desert as a functioning tissue can be and still survive. Little vasculature intercalates this outer cortex region, where a tough extracellular matrix holds the germ cell-somatic cell unit, the follicle, in its long-term suspended animation. The biological isolation of this million-follicle ovarian reserve is changed when individual follicles are activated through signaling mechanisms that are now known, but by factor(s) that have remained elusive. One of the key paracrine-acting factors that controls early follicle activity is Müllerian inhibiting substance/anti-Müllerian hormone (MIS/AMH), and its profound role on the entire ovarian reserve is elegantly described by Kano et al. (1) in PNAS.
MIS/AMH is a TGF-β superfamily member, first identified as a factor produced by male embryos, that blocks the development of female Müllerian ducts from the bipotential reproductive tract (2). Mutations in MIS/AMH in males can result in both male and female urogenital tracts that must be surgically resected. This powerful reproductive tract-regulating hormone has no known effect on adult male fertility; however, in the female, it takes on a completely different role that is necessary for follicle activation. The paper by Kano et al. (1) details this function using a gene therapy approach that produces chronic, systemic hormone resulting in complete contraception—in other words, absence of follicle activation (Fig. 1). This paper not only fills in a biological gap in knowledge but also provides evidence that the ligand could serve an important role as a reversible, nonsteroidal female contraceptive and as a fertoprotective, or fertility protective, neoadjuvant to protect the ovarian reserve of patients who have cancer (3). Particularly for the latter group of patients, and specifically for those patients who are prepubertal, these studies point the way toward a fast-track clinical trial that could profoundly improve the most devastating late effect of our most effective cancer treatments, sterilization.
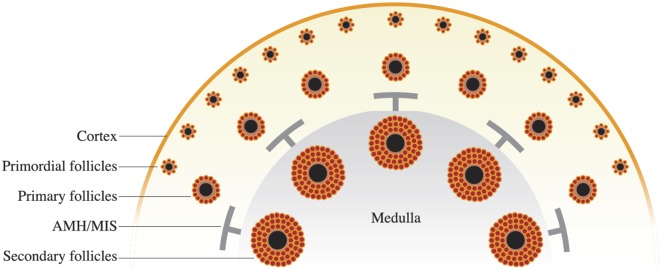
Fig. 1.
Ovary is organized into developmental zones, with the primordial follicles established at birth in the peripheral cortex region and growing follicles moving toward the less rigid medulla. Once secondary follicles are formed, they supply factors that limit subordinate follicle growth. One of those factors is MIS/AMH and when supplied in a chronic supraphysiological level, the follicle maturation process is blocked. This outcome could inform novel contraceptive development as well as provide a way to limit follicle activation during the time of life-preserving but sterilizing cancer treatment.
To understand the biology of MIS/AMH in controlling ovarian function, one must first appreciate the ovarian follicle hierarchy, which is established in utero. Although the testis takes a very short period to set up its stem cells and somatic cell compartments, the ovary initiates germ cell encapsulation into follicles over a much longer period of developmental time (4). It appears the reason for this timeline is to allow the first follicles the opportunity to progress, unmetered, through the primary and secondary stages of development (5). As they do so, they produce factors that influence (inhibit) the later formed follicles (Fig. 1). One of these global inhibiting factors is MIS/AMH. The million or so ovarian follicles that are present at birth are individually called “primordial follicles.” Primordial follicles include an immature, meiotic prophase I-arrested oocyte and eight to 10 very slowly mitotic squamous somatic cells. This unit can exist from birth to the age of 50 years or more, while maintaining the genetic integrity of the germ cell over a remarkable period. Although it is sometimes hard to imagine the five to six decades’ length of time one follicle may exist in the ovary, it is easy to appreciate that this organization ensures the timely and regular availability of ovarian follicles in each menstrual cycle period. Moreover, it allows a subset to be influenced by the pituitary hormone FSH; the follicle with the optimal FSH receptor level will now outcompete surrounding follicles for dominance. Thus, from the earliest stage of follicle activation to the time of ovulation, follicles are informing each other in a way that permits longevity and availability of the female gamete.
With this sequence of events in mind, Kano et al. (1) set out to exploit this biology and, using a gene delivery strategy, overexpressed AMH/MIS in young fertile animals and showed that follicle dynamics were halted at the primordial stage; fecundity studies confirmed that the animals were fully contracepted. They then removed the hormone, and the animals returned to normal fertility. There appeared to be no adverse effect of the drug on the animals as would be predicted by the relatively localized MIS receptor in the follicle pool.
The authors went further and examined whether the recombinant MIS protein could protect animals against the off-target effect of chemotherapeutics on the ovarian reserve. Fertoprotective (fertility protective) neoadjuvant therapies are urgently needed because the survival rate for young people with cancer has reached nearly 85% for some cancers (6, 7). This improved survival of the ovarian reserve means the fertility-related threats of the life-preserving treatments are no longer acceptable to many patients and their families. Because the profile of AMH/MIS as a drug is likely to be safe, particularly for the short-term treatment that would be predicted to accompany the most damaging chemotherapies, it is likely that patients and parents would welcome this “natural” hormonal protection of their gonadal function. Of course, an important question will be whether the oocytes that are protected from immediate apoptosis are healthy enough to support live healthy offspring. This experiment must still be performed, but the current results are supportive of treatment for those individuals who are not interested in fertility but do want to maintain their normal cycling ovarian hormone patterns for better bone and cardiovascular health.
Although the fundamental science and clinical opportunities have been advanced, we still do not know what activates a single primordial follicle at a given time, and we struggle to understand what constitutes a healthy egg. In the case of a fertoprotective therapy, the latter issue is critical because we may protect the oocyte from death but damage to the germline may persist, increasing the likelihood of birth defects. We also are learning that the stromal environment contributes to the health of follicles and that interfollicular regulators may not mitigate these stromal influences (8).
These issues are unresolved, but the overall message of the work is that important advances in fertility management are on the horizon. This message is good news for patients.
Acknowledgments
This work was supported by the Center for Reproductive Health After Disease (Grant P50HD076188) from the NIH National Center for Translational Research in Reproduction and Infertility.
Footnotes
The author declares no conflict of interest.
See companion article on page E1688.
References
- 1.Kano M, et al. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci USA. 2017;114:E1688–E1697. doi: 10.1073/pnas.1620729114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance: An update. Adv Exp Med Biol. 2002;511:25–38, discussion 38–40. doi: 10.1007/978-1-4615-0621-8_3. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff TK. Preserving fertility during cancer treatment. Nat Med. 2009;15(10):1124–1125. doi: 10.1038/nm1009-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin YT, Capel B. Cell fate commitment during mammalian sex determination. Curr Opin Genet Dev. 2015;32:144–152. doi: 10.1016/j.gde.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordeiro MH, et al. Geography of follicle formation in the embryonic mouse ovary impacts activation pattern during the first wave of folliculogenesis. Biol Reprod. 2015;93(4):88. doi: 10.1095/biolreprod.115.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong GT, Yasui Y, Robison LL. Reduction in late mortality after childhood cancer. N Engl J Med. 2016;375(3):290–292. doi: 10.1056/NEJMc1604184. [DOI] [PubMed] [Google Scholar]
- 7.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384(9950):1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briley SM, et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016;152(3):245–260. doi: 10.1530/REP-16-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]