Significance
High-affinity antibodies confer protective immunity against external antigens and are generated during germinal center (GC) reactions when B-lymphocytes, migrating between the dark zone (DZ) and light zone (LZ) of the GC, accumulate mutations in their immunoglobulin genes and are selected for high affinity to antigen. B cells move between DZ and LZ, guided by gradients of CXCL12 and CXCL13 chemokines. We show that immobilized CXCL12 is essential for the correct positioning of B-lymphocytes during the GC reaction and for the production of high-affinity antibodies. Our results provide fundamental insights into the role of surface-immobilized chemokines, molecules that regulate the migration of many cell types, thus controlling homeostasis in multiple tissues.
Keywords: CXCL12, humoral immune responses, germinal center reaction
Abstract
Chemokines control the migration of a large array of cells by binding to specific receptors on cell surfaces. The biological function of chemokines also depends on interactions between nonreceptor binding domains and proteoglycans, which mediate chemokine immobilization on cellular or extracellular surfaces and formation of fixed gradients. Chemokine gradients regulate synchronous cell motility and integrin-dependent cell adhesion. Of the various chemokines, CXCL12 has a unique structure because its receptor-binding domain is distinct and does not overlap with the immobilization domains. Although CXCL12 is known to be essential for the germinal center (GC) response, the role of its immobilization in biological functions has never been addressed. In this work, we investigated the unexplored paradigm of CXCL12 immobilization during the germinal center reaction, a fundamental process where cellular traffic is crucial for the quality of humoral immune responses. We show that the structure of murine germinal centers and the localization of GC B cells are impaired when CXCL12 is unable to bind to cellular or extracellular surfaces. In such mice, B cells carry fewer somatic mutations in Ig genes and are impaired in affinity maturation. Therefore, immobilization of CXCL12 is necessary for proper trafficking of B cells during GC reaction and for optimal humoral immune responses.
Chemokines control the migration of a large array of cells and, as a consequence, regulate cell function and homeostasis in many tissues (1). In particular, they regulate the migration and positioning of lymphocytes in secondary lymphoid organs (2). Besides specific signaling delivered by engagement of specific receptors on cell surfaces, the function of chemokines also depends on interactions between nonreceptor binding domains and the glycanic-glycosaminoglycan (GAG) moiety of proteoglycan, particularly heparan sulfate (HS), of the extracellular matrix and cell surfaces (3). This interaction results in immobilization of chemokines and allows the formation of fixed local gradients that, in in vitro models, regulate the synchronous coordination of cell motility (haptotaxis) and integrin-dependent cell adhesion (2). An immobilized, but not free, chemokine is a hallmark of cell signaling (4).
The importance of chemokine immobilization for their function has not been fully addressed, and its relevance has been difficult to evaluate in vivo, given the lack of information on the structure–function relationship of chemokine/HS interactions.
Of the various chemokines, C-X-C motif chemokine 12 (CXCL12) [also known as stromal-cell–derived factor 1 (SDF-1)] has unique structural characteristics because its binding domains, to the receptor C-X-C chemokine receptor type 4 (CXCR4) and to HS, are distinct and nonoverlapping, permitting the separation of their respective functions (5, 6). The interaction with proteoglycans is believed to contribute to CXCL12 activity by enabling the formation of local gradients essential for directed cellular migration (6–8). To investigate how GAG interactions regulate the functions of chemokines in vivo, we have previously developed a mouse strain carrying a mutated form of CXCL12 (CXCL12gagtm mice) where CXCL12/HS interactions are disabled (8). These mice show enhanced serum levels of free CXCL12 and an increased number of circulating leukocytes and CD34+ hematopoietic cells.
CXCL12 is an essential chemokine during development and is critical for the homeostatic regulation of leukocyte trafficking and tissue regeneration (1, 8–10). In addition, CXCL12 plays important roles in the germinal center (GC) reaction during immune responses and is involved in the reentry of long-lived plasma cells in the appropriate bone marrow (BM) niches (11–13).
Antibody responses against foreign antigens start at the T/B border of the follicles of peripheral lymphoid organs (spleen, lymph nodes, Peyer’s patches), through interactions between antigen-specific T and B cells (14). Activated B-lymphocytes migrate into the B-cell follicle where they proliferate extensively to form structures called GCs. Two histologically distinct areas are observed in the GC [the dark zone (DZ) and the light zone (LZ)] that depend on the response of GC B cells to opposing gradients of the chemokines CXCL12 (more expressed in the DZ) and CXCL13 (more expressed in the LZ) (11, 15). In the DZ, B cells (centroblasts) proliferate and express the enzyme activation-induced cytidine deaminase (AID), which mediates somatic hypermutation (SHM) of Ig genes (16, 17). In the LZ, where a network of follicular dendritic cells (FDCs) presenting antigen and follicular helper T cells (TFH) can be found, B cells (centrocytes) are selected into either the pool of recirculating memory B cells or the plasma cell compartment. Available evidence suggests that the GC reaction is maintained by reentry into the DZ of B cells selected in the LZ (18). In the DZ, cells undergo further rounds of proliferation and SHM (11, 19).
In this work, we investigated in vivo the unexplored role of CXCL12 chemokine immobilization during the GC reaction, a fundamental process where cellular traffic determines the quality of humoral immune responses. We studied the recruitment and distribution of B-lymphocytes in the GC, somatic hypermutation of Ig genes, and antibody affinity maturation in CXCL12gagtm mice, where CXCL12 is unable to bind to cellular or extracellular surfaces.
CXCL12gagtm mice have impaired germinal centers, with a majority of them showing poor DZ/LZ segregation. In the few GCs where a clear separation between DZ and LZ can be found, cells in the M phase of the cell cycle are found in the LZ, an aberrant localization for mitotic cells, usually restricted to the DZ compartment. These alterations ultimately result in reduced accumulation of somatic mutations in Ig genes and impaired affinity maturation. Thus, immobilization of CXCL12 is necessary for proper trafficking of B cells during GC reaction and for optimal humoral responses.
Results
Magnitude and Kinetics of Germinal Center Reaction in CXCL12gagtm Mice.
The original characterization of CXCL12gagtm mice showed that they develop normally but have increased numbers of circulating CD34+ cells (8). We analyzed B-cell subsets in BM and spleen of control and CXCL12gagtm mice and found that only the subset of BM mature recirculating B cells (B220+ IgMlow IgD+) was reduced (Fig. S1A). In particular, the frequency and number of BM plasma cells was comparable in both groups (Fig. S1B). In the spleen, all subpopulations were normally represented (Fig. S1 C and D).
Fig. S1.
B-cell subsets in the bone marrow and spleen of control and CXCL12gagtm mice. (A) Frequencies of Pro/Pre (B220+ CD43+ IgM− IgD−), immature (B220+ IgM+ IgDlow-neg), and mature B cells (B220+ IgMlow IgD+) in the bone marrow of control and CXCL12 mutant mice were evaluated by flow cytometry. The graph shows the percentage of each subpopulation among B220+ cells. Data are from two independent experiments with a total of 10 mice per group. Statistical significance was assessed by a two-way ANOVA test. (B, Left) Representative flow cytometry dot plots showing the percentage of CD138+ plasma cells in the BM of control (Upper) and CXCL12gagtm (Lower) mice. (Right) Number of antibody-secreting cells in the BM of control and mutant mice at 10 to 11 wk of age as determined by ELISPOT assays. Data are from one representative experiment with five mice per group out of two performed. (C) Frequency of splenic follicular (B220+ CD93− CD21low-neg IgMlow), transitional (B220+ CD93+), and marginal zone (B220+ CD93− CD21+ IgM+) B cells. The graph shows the percentage of each subpopulation among B220+ cells. (D) Frequency of CD138+ plasma cells in the spleen of control and CXCL12gagtm mice as evaluated by flow cytometry. Data from C and D are from two independent experiments with a total of 10 mice per group. (E) Histograms indicate FSC profiles of control (black) and CXCL12gagtm (green) LZ GC B cells from the gates shown in Fig. 1 A, Middle.
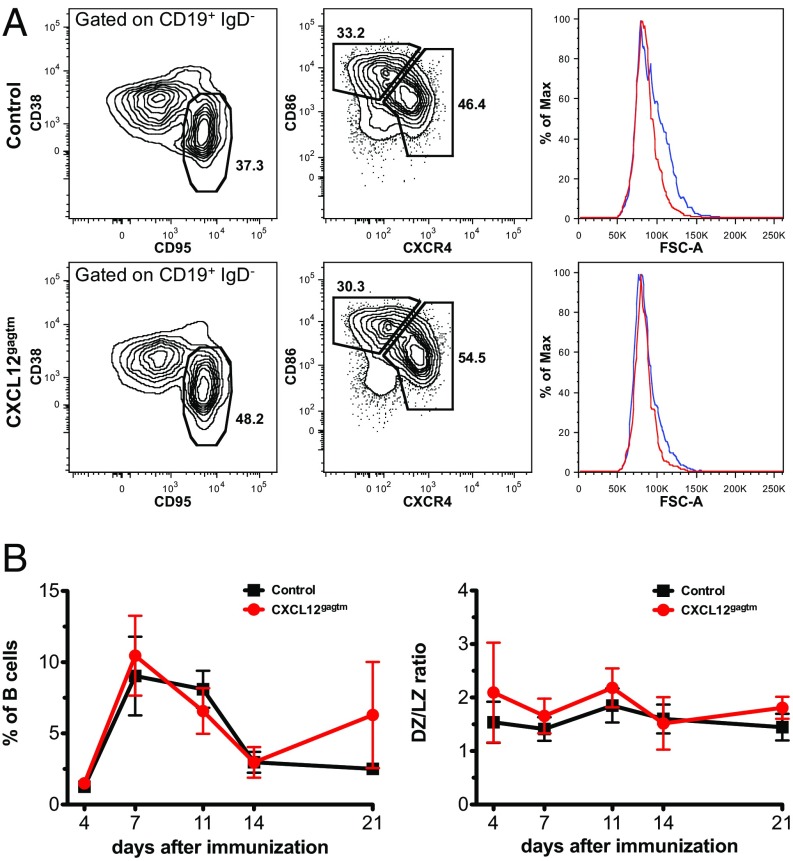
To investigate the magnitude and kinetics of the GC reaction, we immunized CXCL12gagtm mice with sheep red blood cells (SRBCs) and followed the splenic GC reaction using expression of CD19, IgD, CD38, CD95, CXCR4, and CD86 (19) to define GC, LZ and DZ B cells (Fig. 1A). Forward scatter was used to evaluate the relative size of LZ and DZ cells. DZ cells were slightly larger than LZ B cells in both groups of mice (Fig. 1A) whereas the size of the LZ cells was similar in control and mutant mice (Fig. S1E). No significant difference in the magnitude or kinetics of the GC reaction was observed (Fig. 1B, Left). Early in the response, GC B cells constituted 1.3% and 1.5% of B cells in control and mutant mice, respectively, and the peak of the response was on day 7 (9% and 10.5%, respectively), with a reduced GC reaction by day 21 (Fig. 1B, Left). DZ/LZ ratios were comparable at all time points studied (Fig. 1B, Right).
Fig. 1.
Magnitude and kinetics of germinal center reaction in CXCL12gagtm mice. (A) Flow cytometry analysis of splenic GC B cells. (Left) The percentage of GC B cells (CD38low CD95high) among CD19+ IgD− splenocytes from control (Upper) and CXCL12gagtm (Lower) mice 7 d after SRBC immunization. (Middle) Percentage of dark zone (CXCR4high CD86low) and light zone (CXCR4low CD86high) cells in the GC gate shown on the Left. (Right) Histograms indicate forward scatter (FSC) profiles of DZ (blue) and LZ (red) cells in the gates shown in Middle. Mean FSC intensity in control DZ = 89.3k (±2.6k), LZ = 83.6k (±1.9k) (n = 5, P = 0.0045); mean FSC in CXCL12gagtm DZ = 87.2k (±1.2k), LZ = 84.3k (±1.1k) (n = 5, P = 0.0037). Statistical significance determined by Student’s t test. (B) Kinetics of the splenic GC reaction after immunization with SRBC (Left), and DZ/LZ ratios for the corresponding time points (Right) in one representative experiment out of three performed. Data are from 3 to 11 mice per time point and are shown as mean ± SD.
Structure of GC and Analysis of B-Cell Size.
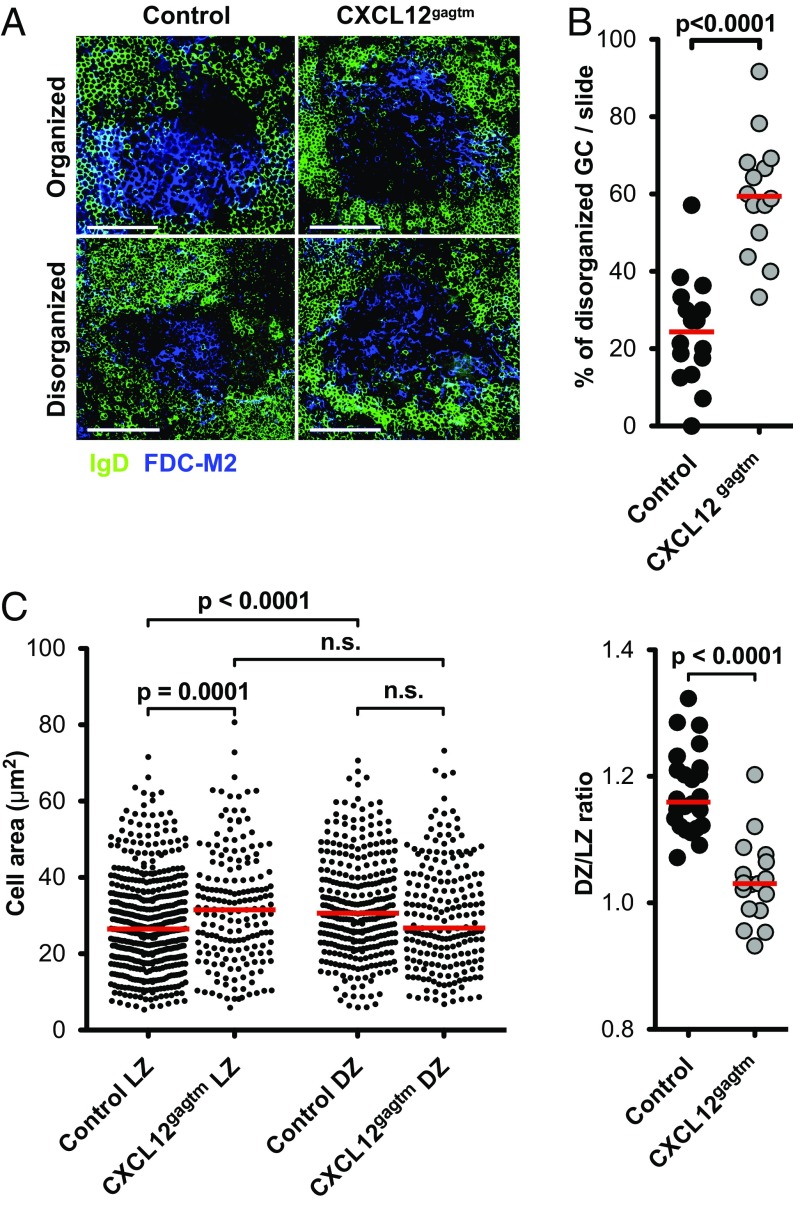
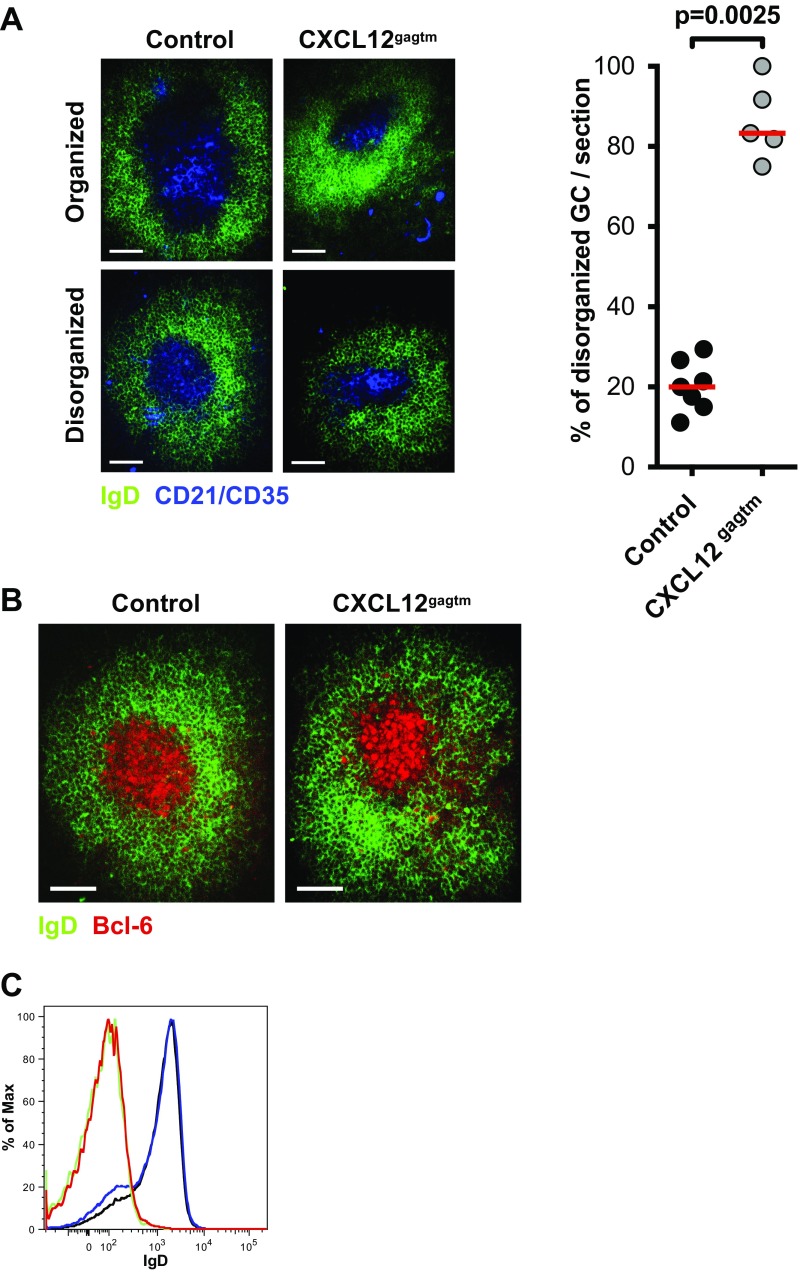
We further investigated the structure of GC and B-cell sizes in their native GC environment by focusing on the localization of centrocytes and centroblasts in the LZ and DZ. For this investigation, we performed histological analysis of splenic GCs at the peak of the response. Analysis of spleen sections of immunized animals revealed heterogeneity in GC structure, with two types of GC observed, based on IgD and FDC-M2 staining patterns of spleen sections: GCs where a clear discrimination between FDC-M2–positive (LZ) and FDC-M2–negative (DZ) GC areas could be seen (“organized” GC), and those where FDC-M2 staining was present throughout the entire GC, with no clear delineation of LZ and DZ (“disorganized” GC) (Fig. 2A). A significantly higher fraction of disorganized GCs was observed in CXCL12gagtm mice (Fig. 2B). Similar staining patterns were observed when we used antibodies specific for complement receptors 1 and 2 (CD21/CD35) to identify FDCs (Fig. S2A). In addition, the IgD-negative area in the follicles correctly delineates the GC, as determined by Bcl6 staining (Fig. S2B), and GC B cells in both control and CXCL12gagtm mice are IgD− (Fig. S2C). To quantify the size of the B cells in the LZ and DZ compartments, cell cross-section surface areas in the organized GCs were analyzed in detail. Using a semiautomated framework of ICY software (20) (Fig. S3A), individual B-cell surface areas of control and CXCL12gagtm GCs were measured (Fig. 2C, Left). This analysis revealed that, in control mice, the average area of the B cells was significantly larger in DZ than in LZ, in agreement with prior observations (21, 22). Our data also showed significant differences in the areas of the B cells found in the LZ of control and CXCL12gagtm mice. The surface area of LZ B cells in mutant mice was significantly larger than that in controls whereas no significant differences were found in DZ B cells. The observed cell size differences were not associated with different cellular densities in the LZ compartments because the numbers of B cells per 100 μm2 were similar in control and mutant mice (Fig. S3B). The differences were consistent in individual GCs and resulted in a significantly lower ratio of DZ/LZ surface areas in GCs from CXCL12gagtm animals (Fig. 2C, Right). Taken together, the analyses revealed important alterations in the GC organization and a significant increase in the surface area of LZ cells in the GC of CXCL12gagtm mice. Because cells with the DZ phenotype are generally larger than LZ phenotype cells (Figs. 1A and 2C) (21, 22), we hypothesized that this result could be due to the mispositioning of centroblasts, secondary to the disrupted binding of CXCL12 to HS in CXCL12gagtm mice.
Fig. 2.
Structure of GC and analysis of B-cell size. (A) Representative spleen sections showing organized and disorganized GCs 7 d after immunization with SRBC. Sections were stained with IgD (green) to identify the GC and FDC-M2 (blue) to delineate the LZ. In organized GCs, a clear discrimination between LZ and DZ areas can be made (Upper); in disorganized GCs, the discrimination is not evident (Lower). One representative GC of each type from eight control and seven CXCL12gagtm mice is shown. (Scale bars: 50 µm.) (B) Percentage of disorganized GCs per slide 7 d after immunization. Sixteen slides in eight control mice (202 GC), and 14 slides in seven CXCL12gagtm mice (219 GC) were analyzed blind. Red bars indicate the median values in each group. Statistical significance was determined by Mann-–Whitney test. (C, Left) Surface areas of individual B cells in the LZ and DZ of organized GC. Data from one representative experiment out of two performed. Each point corresponds to one cell in 15 control and 8 CXCL12gagtm GCs from four control and three CXCL12gagtm mice. Statistical significance determined by Student’s t test. (Right) Ratio between DZ/LZ mean surface areas calculated in individual GC. Data are pooled from two independent experiments with a total of 27 control and 17 CXCL12gagtm GCs from eight control and seven CXCL12gagtm mice. Red bars indicate the median values in each group. Statistical significance was determined by Mann-–Whitney test.
Fig. S2.
Structure of GC in control and CXCL12gagtm mice. (A, Left) Representative spleen sections showing organized and disorganized GCs 7 d after immunization with SRBC. Sections were stained with IgD (green) to identify the GC, and CD21/35 (blue) to delineate the LZ. In organized GCs, a clear discrimination between LZ and DZ areas can be made (Upper); in disorganized GCs, the discrimination is not evident (Lower). One representative GC of each type from three control and three CXCL12gagtm mice is shown. (Scale bars: 50 µm.) (Right) Percentage of disorganized GCs/slide 7 d after immunization. Seven sections in three control mice (107 GC) and five sections in three CXCL12gagtm mice (48 GC) were analyzed blind. Red bars indicate the median values in each group. Statistical significance was determined by Mann–Whitney test. (B) Spleen cryosections showing a representative GC from three control (Left) and three CXCL12gagtm (Right) mice on day 7 of SRBC immunization after staining with IgD (green) and Bcl-6 (red). (Scale bars: 50 µm.) (C) Histograms showing IgD expression in GC (CD19+ CD38− CD95+) and total splenic B cells (CD19+) in control and CXCL12gagtm mice from Fig. 1. Green line, control GC B cells; red line, CXCL12gagtm GC B cells; black line, control total splenic B cells; blue line, CXCL12gagtm total splenic B cells.
Fig. S3.
Strategy to analyze individual B-cell areas using ICY software. (A) The color images of spleen sections stained for IgD, FDC-M2, and B220 were split into separate color channels. The region of interest (ROI) for germinal center was delineated on the IgD channel (IgD− area in green), the ROI for the LZ was delineated on the FDC-M2 channel (FDC-M2+ area in green), and the ROI for DZ was defined by the LZ ROI minus the GC ROI. B-cell contours were analyzed inside LZ and DZ regions in the B220 channel using ICY software. (B) Cellular density in the GC LZ was calculated in 12 control (four mice) and 9 CXCL12gagtm (three mice) GCs and plotted as number of cells per 100 µm2. Statistical significance was determined by Mann–Whitney test.
Mitotic Cells Are Found in the LZ of GC in Mutant Mice.
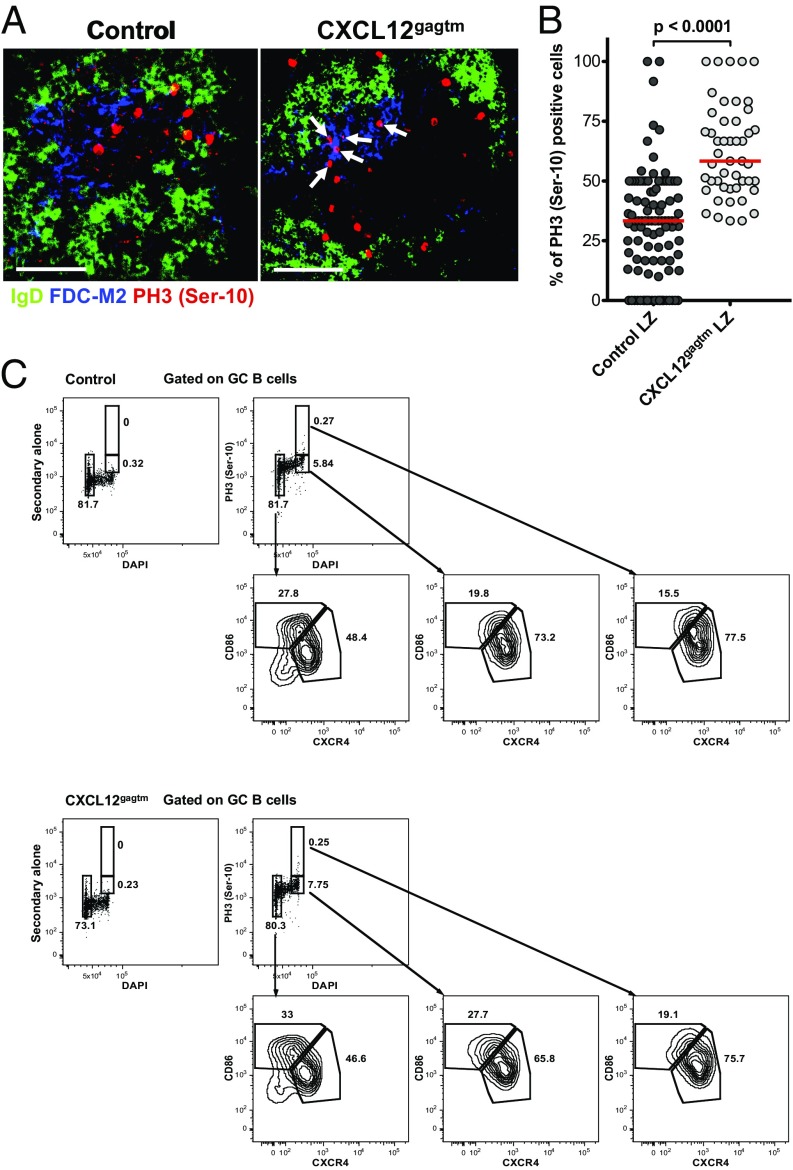
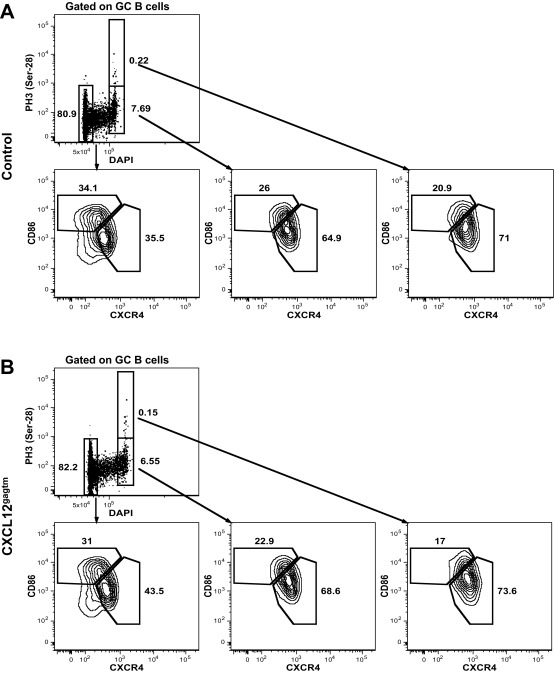
To determine whether the positioning of cells with a DZ phenotype is affected in CXCL12gagtm mice, we examined the sites in which GC B cells were undergoing mitosis. It is known that SHM and proliferation occur in the DZ and that cells in G2/M phase of the cell cycle display a DZ phenotype and locate to the DZ compartment (19). As the cells progress from late G2 into prophase, the histone H3 is phosphorylated at positions Ser-10 and Ser-28, with rapid dephosphorylation thereafter (23, 24). Thus, phosphorylated histone H3 is found exclusively in cells in mitosis. We therefore examined GCs for the expression of phospho-histone H3 (PH3), using an antibody specific for phosphorylated H3 at position Ser-10 to visualize the localization of mitotic B cells in GCs (25). In control mice, PH3 Ser-10+ cells were found mainly in the DZ compartment (Fig. 3 A, Left and B), confirming that mitosis occurs predominantly in that zone. In contrast, a significantly higher fraction of PH3 Ser-10+ cells were found in the LZ when GCs of CXCL12gagtm mice were analyzed (Fig. 3 A, Right and B). Analysis of 658 mitotic cells in 48 individual GCs from CXCL12gagtm animals revealed that PH3 Ser-10+ cells were distributed in nearly equal proportions between the DZ and LZ compartments (Fig. 3B).
Fig. 3.
Mitotic cells in the LZ of mutant mice. (A) Spleen sections 7 d after SRBC immunization. IgD (green), FDC-M2 (blue), phospho-histone H3 (red). Arrows point to PH3+ cells in the LZ. One representative GC from seven control and six CXCL12gagtm mice out of 152 organized GCs analyzed is shown. (Scale bars: 50 µm.) (B) The plot shows the proportion of total GC PH3 Ser-10+ B cells that are localized in the LZ compartment of organized GCs. PH3 Ser-10–positive GC B cells from A were counted in LZ and DZ compartments from 104 control (1,039 cells) and 48 CXCL12gagtm GCs (658 cells) and plotted as the percentage of LZ-localized PH3 Ser-10+ cells in individual organized GCs. Data pooled from two independent experiments. Red bars show the median; statistical significance was determined by Mann–Whitney test. (C) Splenic GC B cells from a pool of three control (Upper) and 3 CXCL12gagtm (Lower) mice 7 d after SRBC immunization were assessed for PH3 Ser-10, DNA content, CD86 and CXCR4 expression. Data are from one representative experiment out of two. Upper Left plots are negative controls with secondary antibody alone.
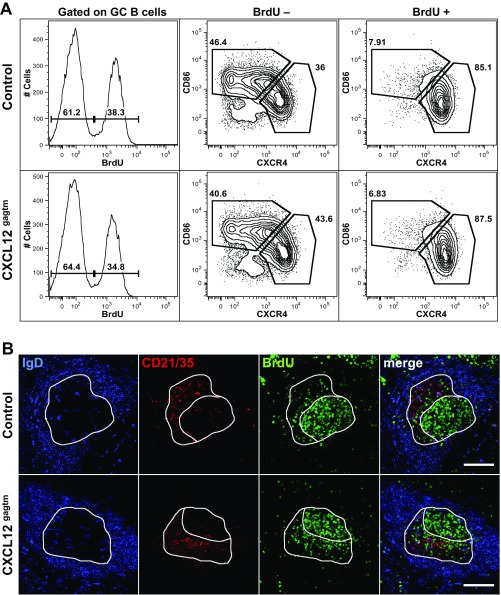
To examine the DZ or LZ phenotype of the PH3+ cells, we assessed the expression levels of CD86 and CXCR4 and DNA content by flow cytometry (Fig. 3C). DNA content measurement revealed that, among cells in G1 phase, the frequencies of LZ (33.0%) and DZ (46.6%) B cells were comparable with the frequencies observed in the total GC gate (30.3% LZ, 54.5% DZ) (Fig. 1A). Consistent with previous findings (19), cells in the G2/M phase of cell cycle constituted ∼8% of total GC B cells and were strongly enriched in cells displaying DZ phenotype in both control (73.2%) and mutant (65.8%) mice (Fig. 3C). This enrichment was even more marked among PH3 Ser-10+ cells and constituted 77.5% and 75.7% for control and CXCL12gagtm mice, respectively. The degree of enrichment for DZ cells among PH3 Ser-10+ GC B cells is likely to be even higher because, since DZ and LZ cells are not totally separated by CXCR4/CD86 staining, some DZ cells may fall in the LZ gate when this gate is defined on total GC cells. Similar results were obtained with an antibody specific for phosphorylated H3 at position Ser-28 (Fig. S4). We further analyzed the relationship between B-cell position and cell-cycle status by pulse labeling the dividing cells with 5-bromo-2′-deoxyuridine (BrdU), followed by flow cytometry and histological analysis. Five hours after BrdU injection, positive GC B cells locate to the DZ (26). We therefore examined the phenotype and localization of BrdU-labeled GC B cells 5 h after a single BrdU pulse. This analysis revealed that BrdU+ cells constituted ∼35% of total GC B cells and were strongly enriched in cells with a DZ phenotype, both in control and mutant mice (Fig. S5). Histological analysis of GCs revealed that, in control mice, BrdU+ cells were found mainly in the DZ compartment, as described (26), whereas, in mutant mice, a fraction of BrdU-labeled cells were found in the LZ (Fig. S5B). Thus, in CXCL12gagtm mice, some cells with a DZ phenotype, which include a fraction of PH3+ cells in mitosis and a fraction of BrdU+ cells, are mislocalized to the LZ.
Fig. S4.
Flow cytometry analysis of splenic GC B cells for the expression of phospho-histone H3 (Ser-28). Splenic GC B cells from the pool of three control (A) and three CXCL12gagtm (B) mice 7 d after SRBC immunization were assessed by flow cytometry for PH3 Ser-28, DNA content, CD86 and CXCR4 expression. Data are from one representative experiment out of two performed. Black boxes highlight the gates used to evaluate CD86 and CXCR4 expression.
Fig. S5.
Analysis of the light and dark zone BrdU-labeled GC B cells by flow cytometry and immunofluorescence. (A) Flow cytometry analysis of splenic GC B cells from control (Upper) and CXCL12gagtm (Lower) mice at day 7 of SRBC immunization and a 5-h pulse labeling with 2.5 mg of BrdU. Histograms on the Left show the frequency of BrdU+ GC B cells. Contour plots indicate the DZ/LZ phenotype in BrdU-negative (Middle) and BrdU-positive (Right) GC B cells. Immunized, non-BrdU–injected mice were used as negative controls for the BrdU staining. Data are from one representative mouse out of five control and four CXCL12gagtm mice analyzed. (B) Spleen cryosections, from control (Upper) and CXCL12gagtm (Lower) mice at day 7 of SRBC immunization and a 5-h pulse labeling with 2.5 mg of BrdU, were stained for IgD (blue), CD21/CD35 (red), and BrdU (green). Images depict the staining patterns for each channel separately and as merged image (Rightmost). White lines on the IgD channel delineate the borders of the GC (IgD− area). The CD21/35 channel was used to delineate LZ (CD21/35+ red area) and DZ (CD21/35− area). The distribution of the BrdU+ cells in the DZ and the LZ is shown on the BrdU channel. One representative GC from seven control and seven CXCL12gagtm mice analyzed in two independent experiments is shown. (Scale bars: 100 µm.)
Somatic Hypermutation and Affinity Maturation.
To determine whether lack of CXCL12 immobilization affects somatic mutation in GC B cells, we isolated λ1+ IgG1+ GC B cells from control and CXCL12gagtm mice on day 13 after immunization with NP-CGG and sequenced a region of 294 bp of the VH186.2 gene encompassing CDR1 and CDR2. NP-CGG elicits a strong humoral immune response dominated by λ1-expressing B cells carrying the heavy-chain gene VH186.2 (27, 28). Furthermore, a mutation in position 33 of the VH186.2 gene, resulting in the W33L amino acid substitution, confers a 10-fold increase in the affinity of the B cell receptor (BCR) for the hapten 4-hydroxy-3-nitrophenyl acetyl (NP) (29). Sequencing analysis of the VH186.2 gene in GC B cells revealed that the overall number of mutations per sequence was lower in CXCL12gagtm than in control GC B cells. The median number of mutations in control sequences was five, but only four in sequences from mutant mice, with nine sequences from the CXCL12gagtm GC showing no mutations (Fig. 4A). Furthermore, the fraction of clones carrying the W33L substitution, which confers high-affinity to NP, was also lower among CXCL12gagtm GC B cells (46.2%) than in control GC B cells (78.8%) (Fig. 4B). Overall, these results indicate that disrupted CXCL12 binding to HS affects the selection of high-affinity clones and the accumulation of somatic mutations in GC B cells.
Fig. 4.
Somatic hypermutation in GC B cells. (A) VH186.2 gene mutations in λ1+ IgG1+ GC B cells from control and CXCL12gagtm mice on day 13 after immunization with NP35-CGG. Data are grouped from two independent experiments, each including three control and three CXCL12gagtm mice. Results are presented as number of mutations per sequence. A total of 104 control and 80 CXCL12gagtm sequences were analyzed. Red bars indicate the median. Statistical significance determined by Mann–Whitney test. (B) Frequency of clones with the W33L substitution in the VH186.2 gene from A. Statistical significance determined by Fisher's exact test.
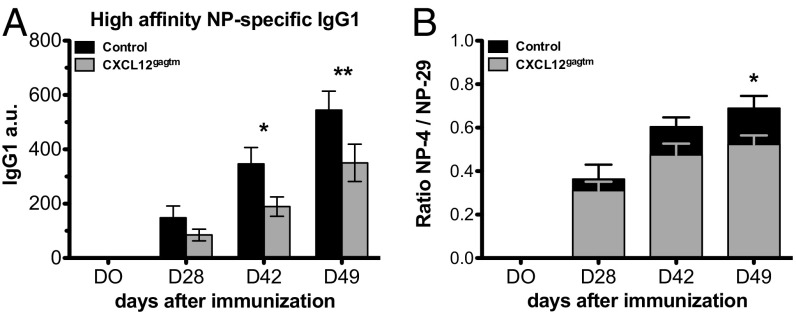
To explore the consequences of the reduced frequency of B cells with the W33L substitution in CXCL12gagtm mice, we studied affinity maturation. We determined the titer and the affinity of serum immunoglobulins after immunization with NP-CGG. As expected, the affinity of NP-specific antibodies was lower in the CXCL12gagtm mice, compared with controls (Fig. 5). Therefore, CXCL12 immobilization on HS is important for selection of high-affinity mutants, and, when the formation of a fixed CXCL12 gradient is prevented, affinity maturation is impaired and the humoral immune response is suboptimal.
Fig. 5.
Impaired affinity maturation in CXC12gagtm mice. (A) ELISA for high-affinity NP4-specific IgG1 in serum from control and CXC12gagtm mice at the indicated time points after immunization with NP35-CGG. (B) Ratio of NP4- and NP29-specific IgG1 titers. Data are from one representative experiment out of two and are presented as mean ± SEM from four control and five CXC12gagtm mice per time point. *P < 0.05; **P < 0.01 as determined by the two-way ANOVA test.
Discussion
Our results reveal the importance of CXCL12 immobilization in the quality of the humoral immune response. During the GC reaction, immobilized CXCL12 forms a fixed gradient with higher concentration of the chemokine in the DZ (11). Opposing gradients of CXCL12 and CXCL13 allow B cells to migrate between the DZ and the LZ, by alternating expression of CXCR4. B cells selected in the LZ for higher affinity to antigen return to the DZ where they undergo further rounds of proliferation and somatic mutation, before returning to the LZ for additional cycles of selection (19). Disruption of CXCL12 binding to HS prevents the establishment of the fixed gradient needed to direct cells selected in the LZ back to the DZ, thus impairing the mechanism of step-wise selection for cells carrying increasing affinity to antigen. As we show in this work, disrupted binding of CXCL12 to HS affects neither the magnitude nor kinetics of the GC reaction, nor the frequency of centroblasts in the GC. Our observations are consistent with reports where GC reaction was studied in CXCR4-deficient mice (11, 25) and suggest that the magnitude of the GC responses may depend on factors other than immobilized CXCL12.
Despite of the comparable magnitude and kinetics of the GC reaction, the structural organization of the splenic GC was significantly affected in mutant animals. The majority of GCs from CXC12gagtm mice showed disrupted organization with no evidence of LZ/DZ polarity. This observation cannot be explained by sectioning artifacts because, although some organized GCs could be sectioned entirely through the FDC-rich area such that they would appear as the disorganized GCs, the overall frequency of such occurrences is expected to be similar in control and mutant mice. Although, in control mice, we observed ∼24% of disorganized GCs, a fraction comparable with the previous large-scale confocal imaging studies in immunized mice (30), a significantly higher proportion of disorganized GCs were observed in the spleens of CXC12gagtm mice. The structure of these disorganized GCs resembled that observed in CXCR4 deficiency (11, 25), suggesting that B-cell responsiveness to immobilized, but not free, CXCL12 contributes to efficient organization of GCs.
It has been suggested that centroblasts residing in the DZ are larger than the LZ centrocytes (21). Although this morphological difference between the two types of the cells was recently questioned (19), our study clearly confirmed significant size differences of GC B cells in the different compartments of control mice. We also show that, in contrast to normal mice, the surface areas of LZ and DZ B cells in CXC12gagtm mice were comparable because of the increased size of B cells in the LZ, suggesting that the localization of larger centroblasts could be defective due to the disrupted binding of CXCL12 to HS. Indeed, analysis of the localization of mitotic cells in mutant mice revealed that they were equally distributed between the LZ and DZ compartments in splenic GCs. Although we also observed mitotic cells in the LZ of control GCs, their numbers were significantly lower (∼30% of all mitotic GC B cells in control compared with 62% in mutant GCs). Therefore, our studies provide quantitative measure and confirm previous observations (25) that some GC B cells undergo mitosis in the LZ, but at a significantly reduced frequency compared with DZ. They also show that disrupted CXCL12 binding to HS prevents efficient trafficking of B cells between the two compartments because the mislocalized cells maintain their morphological (cell size), phenotypic (CD86low, CXCR4high), and mitotic status. Our findings are compatible with the observation that the DZ and LZ programs are cell-intrinsic and position-independent (25).
The fine-tuned migration of B cells between GC compartments seems to play a general role in the proper accumulation of somatic mutations and affinity maturation. Indeed, the analysis of somatic hypermutation in control and mutant mice revealed that the median number of mutations, the overall mutation frequency, and the number of mutations per sequence in GC B cells are significantly smaller in mutant mice. Furthermore, clones carrying the W33L substitution, characteristic of high-affinity anti-NP antibodies, were also significantly less represented among CXCL12gagtm GC B cells. Consequently, the reduced frequency of high-affinity B cells was reflected in impaired affinity maturation in the CXCL12gagtm mice. We propose that, in CXCL12gagtm mice, when LZ B cells up-regulate CXCR4, they are no longer able to efficiently return to the DZ to introduce more mutations in their Ig genes. Thus, when CXCL12 is not immobilized, B cells with higher affinity antibodies are no longer favored for further rounds of proliferation. Taken together, our results show that effective affinity maturation and optimal humoral immune responses require GC B-cell responsiveness to immobilized, but not free, CXCL12.
Materials and Methods
Mice and Immunizations.
CXCL12gagtm mice (8) and littermate controls (all backcrossed to C57BL/6 mice for at least seven generations) 8 to 12 wk old were bred at the Institut Pasteur. Mice were immunized i.p. with 300 μL of SRBC in alsever solution (Eurobio), or NP35-GCC (100 µg per mouse; Biosearch Technologies) mixed with 50% (vol/vol) alum (Thermo Scientific). All animal procedures were approved by the Pasteur Institute Safety Committee and conducted according to French and European Community institutional guidelines.
GC Analysis.
GC were analyzed by flow cytometry after staining splenocytes with antibodies to CD19, IgD, CD38, CD95, CD86, and CXCR4. Immunohistology was performed on 150-μm-thick spleen sections obtained using a vibration-blade microtome. Immunofluorescence images were acquired on a spinning-disk microscope and processed using ICY software (20, 31).
Antibodies.
All antibodies used in this work are listed in Table S1.
Table S1.
Antibodies used
| Molecule/antigen | Application | Species | Fluorochrome/ conjugate | Source | Clone | Manufacturer |
| B220 | FC | Mouse | FITC | Rat | RA3-6B2 | BD Pharmingen |
| B220 | IF | Mouse | APC | Rat | RA3-6B2 | BD Pharmingen |
| B220 | FC | Mouse | PE | Rat | RA3-6B3 | Biolegend |
| Bcl6 | IF | Mouse | AF647 | Mouse | K112-91 | BD Pharmingen |
| BrdU | IF | Mouse | Biotin | Mouse | MoBU-1 | Invitrogen |
| CD3ε | FC | Mouse | Biotin | Hamster | 145–2C11 | Sony Biotechnology |
| CD3ε | FC | Mouse | eFluor450 | Rat | 17A2 | eBioscience |
| CD4 | FC | Mouse | APCCy7 | Rat | GK1.5 | BD Pharmingen |
| CD8α | FC | Mouse | PE | Rat | 53–6.7 | BD Pharmingen |
| CD19 | FC | Mouse | APCCy7 | Rat | 6D5 | Biolegend |
| CD21/CD35 | IF | Mouse | FITC | Rat | 7E9 | Sony Biotechnology |
| CD38 | FC | Mouse | AF700 | Rat | 90 | eBioscience |
| CD45.2 | FC | Mouse | APC | Mouse | 104 | BD Pharmingen |
| CD86 | FC | Mouse | PE | Rat | GL1 | BD Pharmingen |
| CD95 | FC | Mouse | PE-Cy7 | Hamster | Jo2 | BD Pharmingen |
| CD138 | FC | Mouse | APC | Recombinant human IgG1 | REA104 | Miltenyi Biotec |
| CXCR4 | FC | Mouse | Biotin | Rat | 2B11 | eBioscience |
| FDC | IF | Mouse | — | Rat | FDC-M2 | Immunokontact |
| Histone H3 pS10 | FC, IF | Mouse | — | Rabbit | Polyclonal | Merck Millipore |
| Histone H3 pS28 | FC | Mouse | APC | Recombinant human IgG1 | REA379 | Miltenyi Biotec |
| Ig κ light chain | E | Mouse | — | Rat | 187.1 | BD Pharmingen |
| Ig κ light chain | E | Mouse | Biotin | Rat | 187.1 | BD Pharmingen |
| Ig κ light chain | FC | Mouse | FITC | Rat | 187.1 | BD Pharmingen |
| Ig, λ1, λ2, & λ3 light chain | FC | Mouse | FITC | Rat | R26-46 | BD Pharmingen |
| IgD | FC | Mouse | V450 | Rat | 11–26c.2a | BD Pharmingen |
| IgD | IF | Mouse | BV421 | Rat | 11–26c.2a | Biolegend |
| IgG1 | FC | Mouse | APC | Rat | X56 | BD Pharmingen |
| Ly5.1 | FC | Mouse | PECy7 | Mouse | A20 | Biolegend |
| Ly5.1 | FC | Mouse | FITC | Mouse | A20 | BD Pharmingen |
| Rabbit IgG (H+L) | FC, IF | Rabbit | AF647 | Donkey | Polyclonal | Invitrogen |
| Rabbit IgG (H+L) | IF | Rabbit | AF488 | Goat | Polyclonal | Invitrogen |
| Rat IgG (H+L) | IF | Rat | AF568 | Goat | Polyclonal | Invitrogen |
| Streptavidin | FC | — | APC | — | — | BD Pharmingen |
| Streptavidin | FC | — | FITC | — | — | BD Pharmingen |
| Streptavidin | FC | — | BV421 | — | — | Biolegend |
| Streptavidin | IF | — | AF633 | — | — | Invitrogen |
| TCRβ | FC | Mouse | Biotin | Hamster | H57-597 | Biolegend |
| TCRγδ | FC | Mouse | Biotin | Hamster | GL3 | BD Pharmingen |
E, ELISPOT; FC, flow cytometry; IF, immunofluorescence.
Sequence Analysis.
The VH186.2 gene was sequenced from the DNA of sorted splenic CD19+ IgD– CD95+ CD38– IgG1+ λ1+ cells on day 13 after NP35-CGG immunization. PCR products were gel-purified, cloned, and sequenced. The VH186.2 gene was identified by BLAST using the intron sequence upstream of the VH gene and confirmed by BLAST of the VH exon sequence to the mouse genome database and the ImMunoGeneTics (IMGT) database (mouse IMGT/V-QUEST; the alignment tool can be found at www.imgt.org/IMGT_vquest/vquest?livret=0&Option=mouseIg).
Affinity Maturation and Serum Antibody Analysis.
Serum from NP35-CGG–immunized mice was collected at several time points after immunization, and both high-affinity (anti-NP4) and low-affinity (anti-NP29) IgG1 antibody titers were determined by ELISA using standard procedures. The plates were standardized using pooled sera from hyperimmunized C57BL/6 mice.
Additional information can be found in SI Materials and Methods.
SI Materials and Methods
Mice and Immunizations.
CXCL12gagtm mice (8) and littermate controls (all backcrossed to C57BL/6 mice for at least seven generations) 8 to 12 wk old were bred at the Pasteur Institute. Mice were immunized i.p. with 300 μL of SRBC in alsever solution (Eurobio) or NP35-CGG (100 µg per mouse; Biosearch Technologies) mixed with 50% (vol/vol) alum (Thermo Scientific). All animal procedures were approved by the Pasteur Institute Safety Committee and conducted according to French and European Community institutional guidelines.
Flow Cytometry and Cell Depletion.
Spleen cell suspensions were stained on ice in HBSS supplemented with 2% (vol/vol) FCS with the antibodies listed in Table S1. Propidium iodide was used to exclude dead cells by gating. Stained cells were analyzed on a BD LSRFortessa (BD Biosciences). For cell-cycle analysis and PH3 staining, we used a modification of published protocols (25). Cells were labeled with surface antibodies before fixation with 1.6% (wt/vol) paraformaldehyde (PFA) [Inside Fix buffer containing 3.7% (wt/vol) PFA from Miltenyi Biotec] on ice for 12 min. Fixed cells were washed twice with PBS 2% (vol/vol) FCS and permeabilized by adding 500 μL of 70% (vol/vol) ice-cold EtOH dropwise while vortexing. Samples were stored at −20 °C overnight. The following day, cells were rehydrated in PBS 2% (vol/vol) FCS for 10 min and washed twice before staining with either directly coupled anti-histone H3 pS28-APC (Miltenyi Biotec) or purified rabbit anti-histone H3 pS10 (Merck Millipore) antibodies for 1 h at room temperature. In the latter case, secondary Ab staining (donkey anti-rabbit AF647; Invitrogen) was performed similarly. DAPI was added to a final concentration of 5 nM in PBS buffer before flow cytometry. For BrdU staining, mice were injected i.p. with 2.5 mg of BrdU on day 7 after SRBC immunization and were killed 5 h later. Anti-BrdU staining of spleen cell suspensions was performed using an FITC BrdU Flow Kit (BD Pharmingen) according to the manufacturer’s instructions. Samples were acquired and analyzed with a BD LSRFortessa (BD Biosciences). Data were analyzed using FlowJo software (Treestar).
VH186.2 Sequence Analysis of GC B Cells.
Splenic CD19+ IgD− CD95+ CD38− IgG1+ λ1+ GC B cells were sorted on day 13 after immunization with NP35-CGG and frozen on dry ice. DNA from sorted cells was extracted using a DNeasy Blood and Tissue DNA extraction kit (Qiagen), and a 489-bp fragment containing the VH186.2 gene was PCR-amplified using Phusion DNA polymerase (Thermo Fisher Scientific) with primers specific for VH186.2/JH2 and VH186.2/JH4 fragments: forward VH186.2 primer, 5′-CTCTTCTTGGCAGCAACAGC-3′; reverse JH2 primer, 5′-GAGGAGACTGTGAGAGTGGTGC-3′; reverse JH4 primer, 5′-GAGGAGACGGTGACTGAGGTTC-3′. Amplification bands were gel-purified using a Qiagen QIAquick Gel Extraction Kit (Qiagen). DNA was precipitated and cloned in the pCR-Blunt II-TOPO vector using a Zero Blunt TOPO PCR Cloning Kit (Invitrogen). Plasmid DNA from individual clones was extracted, and both strands of the insert were sequenced. Sequences were aligned and analyzed with Sequencher software (Gene Codes). The VH was identified by BLAST to the mouse genome database using the intron sequence upstream of the VH gene. The identification was confirmed by BLAST of the VH exon sequence to the mouse genome database and IMGT database (mouse IMGT/V-QUEST; the alignment tool can be found at www.imgt.org/IMGT_vquest/vquest?livret=0&Option=mouseIg.
Affinity Maturation, Serum Antibody Analysis, and Enzyme-Linked ImmunoSpot Assay.
Control and CXCL12gagtm mice were immunized with 100 µg of NP35-GCC in alum at day 0 and boosted with soluble NP35-GCC (10 µg per mouse; Biosearch Technologies) at day 28 and day 42. Peripheral blood serum was collected at days 0, 7, 15, 21, 28, 42, and 49. High-affinity (anti-NP4) and low affinity (anti-NP29) IgG1 antibody titers were determined by ELISA using standard procedures. Briefly, plates were coated with NP4-BSA and NP29-BSA (Biosearch Technologies) at 4 °C overnight. Diluted serum samples were incubated at 37 °C for 1 h, followed by 1-h incubation with biotinylated anti-mouse IgG1-specific antibody and 30 min of streptavidin-HRP. Then, 3,3′,5,5′-tetramethylbenzidine (TMB) was used as liquid substrate, and absorbance was read at 450 nm. Hyperimmune serum was used as standard. For the enzyme-linked immunospot (ELISPOT) assay, serial dilutions of bone marrow cells were added to a 96-well PVDF membrane plate (MSIPN4W; Millipore) coated with 10 µg/mL purified rat anti-mouse Ig kappa light chain antibody and incubated for at least 20 h at 37 °C and 5% CO2. Antibody-secreting cells were detected with biotinylated Ig kappa antibody and streptavidin conjugated to AP (Roche) and were visualized with 5-bromo-4-chloro-3'-indolyphosphate p-toluidine salt (BCIP)/nitro-blue tetrazolium chloride (NBT) substrate (Sigma-Aldrich). Images of membranes were captured using an Olympus SZ61 dissection microscope equipped with a USB camera and OPTIKA Vision lite software (OPTIKA SRL). Spots were counted manually.
Immunohistology and Immunofluorescence.
Paraformaldehyde-lysine-periodate solution (PLP)-fixed spleen sections (150 μm) were obtained using a Leica VT1000E Vibration-blade microtome. Staining with anti-mouse follicular dendritic cells (clone FDC-M2; Immunokontact) was performed overnight at 4 °C. Sections were washed three times with 1× PBS and stained with goat anti-rat IgG(H+L) coupled to AF568 antibody (Invitrogen) for 15 min at 37 °C. Sections were blocked with 2% (vol/vol) normal rat serum (eBioscience) in PBS for 15 min at room temperature and washed three times with 1× PBS. Finally, spleen sections were incubated with BV421 rat anti-mouse IgD (Biolegend) and APC rat anti-mouse CD45R/B220 (BD Pharmingen) antibodies, washed, and mounted in PBS. For the PH3 staining, spleen sections were permeabilized with the permeabilization buffer (0.5% Triton X-100 in 1× PBS) for 30 min at room temperature and incubated with anti-phospho-histone H3 (Ser-10) antibody (Merck Millipore) for 48 h at 4 °C. Sections were washed three times with permeabilization staining buffer (0.2% Triton X-100 in 1× PBS) and stained with the goat anti-rabbit antibody coupled to AF488 (Invitrogen) for 12 h at 4 °C. Spleen sections were washed twice with permeabilization staining buffer and with 1× PBS and were stained with the FDC-M2, IgD, and B220 antibodies as described previously. BrdU staining was performed using an adaptation of published protocols (11). Mice were injected i.p. with 2.5 mg of BrdU on day 7 after SRBC immunization, and, 5 h later, spleens were snap-frozen in Tissue-Tec OCT compound. Then, 10-μm spleen cryosections placed on SuperFrost Ultra Plus adhesion slides were air-dried for at least 2 h, fixed with ice-cold acetone for 5 min, and blocked with 10% (vol/vol) FCS and 0.1% Triton-X in PBS for 1 h. Slides were treated with 1 M HCl for 30 min and stained with IgD BV421, CD21/35 FITC, and anti-BrdU-biotin antibodies overnight at 4 °C. Sections were stained with streptavidin-AF633 for 2 h and mounted in Vectashield. Slides from immunized, and non-BrdU–injected mice were used as negative controls.
Microscopy and Image Analysis.
Immunofluorescence images were acquired on a Perkin-Elmer spinning-disk UltraView ERS microscope using Volocity software and were processed in ICY software (20). The color images were split into separate color channels to define the regions of interest (ROI): The GC ROI was delineated on the IgD channel, the ROI for the LZ was delineated on the FDC-M2 channel, and the DZ ROI was defined by the LZ ROI minus the GC ROI. Initial contours were extracted from the B220 channel inside LZ and DZ ROI. The initial contours were segmented based on the intensity image and filtered by their size and shape, and then these contours evolved under force constraints to converge to the cell contours of the image. Our active contour approach was able to cope with both issues of low signal-to-noise ratio (SNR) and cell contacts (31). For BrdU staining, spleen cryosections were imaged using a Zeiss LSM700 axioplan confocal microscope equipped with ZEN image acquisition software (Carl Zeiss Micro-Imaging) and were processed in ImageJ software (NIH).
Statistical Analysis.
All statistical analyses were carried out with Prism software version 5.0 (Graphpad).
Acknowledgments
We thank A. Cumano for critical reading of the manuscript, advice, and helpful discussions; H. Mouquet for critical reading of the manuscript; G. Millot and V. Rouilly for advice on the statistical analysis; L. Fiette, P. Flamant, and P. Ave for advice and help on histological preparations; the staff of the animal facility of the Pasteur Institute for mouse care; and the Imagopole platform for advice and access to microscopes. This work was supported by the Pasteur Institute, INSERM, and the Agence Nationale de Recherche (Grant “ChemImmun”).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611958114/-/DCSupplemental.
References
- 1.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286(5447):2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JJ, et al. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279(5349):381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 3.Laguri C, Arenzana-Seisdedos F, Lortat-Jacob H. Relationships between glycosaminoglycan and receptor binding sites in chemokines-the CXCL12 example. Carbohydr Res. 2008;343(12):2018–2023. doi: 10.1016/j.carres.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 4.Schumann K, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32(5):703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Laguri C, et al. The novel CXCL12gamma isoform encodes an unstructured cationic domain which regulates bioactivity and interaction with both glycosaminoglycans and CXCR4. PLoS One. 2007;2(10):e1110. doi: 10.1371/journal.pone.0001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rueda P, et al. The CXCL12gamma chemokine displays unprecedented structural and functional properties that make it a paradigm of chemoattractant proteins. PLoS One. 2008;3(7):e2543. doi: 10.1371/journal.pone.0002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Boyle G, Mellor P, Kirby JA, Ali S. Anti-inflammatory therapy by intravenous delivery of non-heparan sulfate-binding CXCL12. FASEB J. 2009;23(11):3906–3916. doi: 10.1096/fj.09-134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rueda P, et al. Homeostatic and tissue reparation defaults in mice carrying selective genetic invalidation of CXCL12/proteoglycan interactions. Circulation. 2012;126(15):1882–1895. doi: 10.1161/CIRCULATIONAHA.112.113290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Q, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95(16):9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 11.Allen CD, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5(9):943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 12.Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194:48–60. doi: 10.1034/j.1600-065x.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 13.Hargreaves DC, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194(1):45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247(1):52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 15.Pereira JP, Kelly LM, Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol. 2010;22(6):413–419. doi: 10.1093/intimm/dxq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crouch EE, et al. Regulation of AID expression in the immune response. J Exp Med. 2007;204(5):1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storck S, Aoufouchi S, Weill JC, Reynaud CA. AID and partners: For better and (not) for worse. Curr Opin Immunol. 2011;23(3):337–344. doi: 10.1016/j.coi.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Calado DP, et al. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol. 2012;13(11):1092–1100. doi: 10.1038/ni.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Chaumont F, et al. Icy: An open bioimage informatics platform for extended reproducible research. Nat Methods. 2012;9(7):690–696. doi: 10.1038/nmeth.2075. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 22.Nieuwenhuis P, Opstelten D. Functional anatomy of germinal centers. Am J Anat. 1984;170(3):421–435. doi: 10.1002/aja.1001700315. [DOI] [PubMed] [Google Scholar]
- 23.Goto H, et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem. 1999;274(36):25543–25549. doi: 10.1074/jbc.274.36.25543. [DOI] [PubMed] [Google Scholar]
- 24.Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J Cell Sci. 2003;116(Pt 18):3677–3685. doi: 10.1242/jcs.00735. [DOI] [PubMed] [Google Scholar]
- 25.Bannard O, et al. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 2013;39(5):912–924. doi: 10.1016/j.immuni.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen CDC, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315(5811):528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 27.Cumano A, Rajewsky K. Structure of primary anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur J Immunol. 1985;15(5):512–520. doi: 10.1002/eji.1830150517. [DOI] [PubMed] [Google Scholar]
- 28.Cumano A, Rajewsky K. Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP. EMBO J. 1986;5(10):2459–2468. doi: 10.1002/j.1460-2075.1986.tb04522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen D, Simon T, Sablitzky F, Rajewsky K, Cumano A. Antibody engineering for the analysis of affinity maturation of an anti-hapten response. EMBO J. 1988;7(7):1995–2001. doi: 10.1002/j.1460-2075.1988.tb03038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittenbrink N, Klein A, Weiser AA, Schuchhardt J, Or-Guil M. Is there a typical germinal center? A large-scale immunohistological study on the cellular composition of germinal centers during the hapten-carrier-driven primary immune response in mice. J Immunol. 2011;187(12):6185–6196. doi: 10.4049/jimmunol.1101440. [DOI] [PubMed] [Google Scholar]
- 31.Dufour A, Meas-Yedid V, Grassart A, Olivo-Marin J-C. Proceedings of the Nineteenth International Conference on Pattern Recognition. IEEE; New York: 2008. Automated quantification of cell endocytosis using active contours and wavelets; pp. 1–4. [Google Scholar]