Significance
Hydrogen sulfide is a universal bioactive molecule that functions in both prokaryotes and eukaryotes. However, little is known about intra- and extracellular sulfide-sensing mechanisms. Here we show that the sulfide-regulated repressor SqrR from a purple bacterium forms an intramolecular tetrasulfide bond in response to sulfide stress in vivo and organic persulfides in vitro, revealing the importance of this cysteine modification for sulfide sensing in cells. These findings provide new insights into bacterial sulfide homeostasis and its potential applications in synthetic biology. Given that purple bacteria retain characteristics of ancestral photosynthesis and photosynthetic electron transfer, the functional characterization of SqrR also provides new information on plausible mechanisms that regulated electron flow early in the evolution of photosynthesis.
Keywords: sulfide sensor, photosynthesis regulation, reactive sulfur species, purple bacteria, Rhodobacter
Abstract
Sulfide was used as an electron donor early in the evolution of photosynthesis, with many extant photosynthetic bacteria still capable of using sulfur compounds such as hydrogen sulfide (H2S) as a photosynthetic electron donor. Although enzymes involved in H2S oxidation have been characterized, mechanisms of regulation of sulfide-dependent photosynthesis have not been elucidated. In this study, we have identified a sulfide-responsive transcriptional repressor, SqrR, that functions as a master regulator of sulfide-dependent gene expression in the purple photosynthetic bacterium Rhodobacter capsulatus. SqrR has three cysteine residues, two of which, C41 and C107, are conserved in SqrR homologs from other bacteria. Analysis with liquid chromatography coupled with an electrospray-interface tandem-mass spectrometer reveals that SqrR forms an intramolecular tetrasulfide bond between C41 and C107 when incubated with the sulfur donor glutathione persulfide. SqrR is oxidized in sulfide-stressed cells, and tetrasulfide–cross-linked SqrR binds more weakly to a target promoter relative to unmodified SqrR. C41S and C107S R. capsulatus SqrRs lack the ability to respond to sulfide, and constitutively repress target gene expression in cells. These results establish that SqrR is a sensor of H2S-derived reactive sulfur species that maintain sulfide homeostasis in this photosynthetic bacterium and reveal the mechanism of sulfide-dependent transcriptional derepression of genes involved in sulfide metabolism.
The discovery of ∼550 deep-sea hydrothermal vents more than 30 y ago (1) has led to the theory that energy metabolism in early ancestral organisms may have arisen from deep-sea hydrothermal vents where simple inorganic molecules such as hydrogen sulfide or hydrogen gas, as well as methane, exist (2–4). Such ancient energy metabolism has been assumed to be similar to that of extant chemolithotrophs, which obtain energy from these molecules. Indeed, various chemolithoautotrophic microbes thrive in deep-sea hydrothermal vents and are capable of oxidizing sulfides, methane, and/or hydrogen gas for use as energy sources and electron donors (5). Some photosynthetic bacteria have also been isolated from deep-sea hydrothermal vents that can grow photosynthetically using sulfide as an electron donor and geothermal radiation as an energy source instead of solar radiation (6), as hypothesized for ancestral phototrophs.
Many purple photosynthetic bacteria have remarkable metabolic versatility required to meet the energy demands of sulfide-dependent and -independent photosynthesis as well as aerobic and anaerobic respiration. These bacteria tightly control the synthesis of their electron transfer proteins involved in each growth mode in response to a specific electron donor, oxygen tension, and light intensity (7, 8). Among these regulatory systems, oxygen- and light-sensing mechanisms have been well-studied; however, mechanisms used to sense hydrogen sulfide remain incompletely understood.
Understanding the general biological functions of hydrogen sulfide has emerged as a subject of topical interest since its recent description as a gasotransmitter in mammals (9–11). Despite an appreciation of sulfide signaling, the mechanisms of how H2S functions as a signaling molecule remain under investigation (12). Emerging evidence suggests that highly oxidized sulfur species, termed reactive sulfur species (RSS), are the actual sulfide-signaling species (13–16). These include HS•, cysteine sulfenic acids, cysteine persulfides, glutathione persulfides (GSSHs), and inorganic polysulfide species, which catalyze persulfidation of specific proteins and enzymes (13, 17–20), although the functional impact of this protein modification is as yet not generally clear (17).
Hydrogen sulfide inhibits respiration by poisoning cytochrome oxidase and binding and precipitating biologically required divalent transition metals, and is thus toxic at high concentrations (21). The ability to adapt to a sulfide-rich environment is therefore critical for the survival for some microorganisms. The universal first step in H2S detoxification and utilization of H2S as an electron donor includes the sulfide:cytochrome c reductases (SoxF and FccAB) or sulfide:quinone reductases (SQRs), the latter used to reduce quinones via the two-electron oxidation of sulfide to sulfane or zero-valent S0. In the case of SQRs, S0 is conjugated to a variety of nucleophilic acceptors, such as sulfite (SO32–), CN–, S2–, or a low-molecular-weight thiol, for example glutathione (22–24), the latter of which gives rise to glutathione persulfide. In the purple photosynthetic bacterium Rhodobacter capsulatus, SQR is the major enzyme responsible for catalyzing this first step in sulfide oxidation, with the level of SQR expression increasing after sulfide treatment (25). R. capsulatus can also grow photosynthetically, using sulfide as well as several inorganic and organic compounds as electron donors. Thus, R. capsulatus seems to use sophisticated regulatory strategies to control different photosynthetic growth modes in response to environmental changes. However, a sulfide-responsive sensor protein has not been identified in R. capsulatus.
To better understand sulfide sensing in photosynthetic bacteria, we aimed to identify a sulfide-responsive sensor protein from R. capsulatus. Genetic mutant screening resulted in the identification of the transcriptional regulator SqrR, which functions as a repressor of genes responsible for sulfide-dependent photosynthesis. We show that SqrR DNA-binding and transcriptional regulatory activity is regulated by persulfide-dependent cysteine modification, which is analogous to that previously described for the persulfide sensor in Staphylococcus aureus, CstR (26). However, SqrR and CstR are derived from distinct structural classes of transcriptional repressors, suggesting that SqrR functions as a novel sensor of sulfide-derived reactive sulfur species in bacterial cells.
Results
Identification of sqrR in R. capsulatus.
To identify a sulfide-regulated sensor protein in photosynthetic bacteria, we took advantage of the well-established genetic system of R. capsulatus (27). Because SQR expression in R. capsulatus increases upon sulfide treatment (25), we exploited changes in sqr expression as a means to isolate sulfide-insensitive mutants. To screen for mutants, we constructed a plasmid where the sqr promoter drives lacZ expression. To verify whether the sqr promoter responds to sulfide specifically, we carried out β-galactosidase assays with R. capsulatus containing the sqr::lacZ expression plasmid treated with sulfide under aerobic and anaerobic growth conditions. The sqr promoter specifically responds to sulfide under both conditions (Fig. 1). Primer extension analysis reveals the presence of a single transcriptional start site located 40 bp upstream of the sqr start codon (Fig. S1).
Fig. 1.
Sulfide responsiveness of the sqr promoter regions. (A) β-Galactosidase activity measurements of the sqr promoter region of 1,238 bp upstream of the start codon and lacZ fusion. Cells were grown to midlog phase under aerobic conditions and 0.6 mM Na2S (triangles) or nothing (squares) was added at t = 0. Cells were harvested at each time point and assayed for β-galactosidase activity. Error bars indicate SD of the mean. ONP, o-nitrophenyl-β-d-galactopyranoside. (B) Same as in A, except that cells were grown under anaerobic conditions.
Fig. S1.
Mapping of the transcriptional start site of the sqr gene by primer extension analysis. (A) BigDye Terminator (Thermo Fisher) sequence of a PCR product amplified from the target genomic region. (B) An example of ddCTP, ddTTP, ddGTP, and ddATP standard dideoxy-terminated product (blue) run with a 500 LIZ Size Standard (orange) to generate a reference sequence ladder. (C) Primer extension trace (blue) obtained with purified RNA. A–C were aligned by means of a 500 LIZ Size Standard and sequence data. The numbers below indicate the size of extension products. (D) Sequence of the promoter region containing a start codon (gray background), the putative −35 and −10 σ-subunit recognition sequences (boxed pink), and transcriptional start site (red).
Wild-type (WT) R. capsulatus cells cannot grow with lactose as a sole carbon source, because R. capsulatus does not encode the lac operon in its genome. However, R. capsulatus containing the sqr::lacZ fusion construct can grow on lactose-containing medium in the presence of sulfide but not in the absence of sulfide. This sulfide-dependent growth allowed the screening of sulfide-insensitive suppressor mutants. Specifically, R. capsulatus expressing the full-length sqr::lacZ gene fusion was treated with ethyl methanesulfonate to induce point mutations, with these mutagenized cells plated onto lactose plates for selective growth under aerobic conditions in the absence of sulfide. From this selection, we isolated ∼30 colonies from 108 mutagenized cells that exhibited growth independent of the presence of sulfide (Fig. S2A). We sequenced the reporter plasmids from these strains to confirm that there were no mutations present in the sqr promoter region, and then applied genome sequence analysis to identify suppressor mutations. Genomic suppressor mutations in several candidate genes were found (Dataset S1). Disruption of one candidate gene, which we named sulfide:quinone reductase repressor (sqrR) (GenBank accession no. ADE85198), yielded constitutively high sqr expression even in the absence of sulfide, suggesting that it likely encodes a transcriptional repressor (Fig. S2B). Oxidative stress resulting from H2O2 treatment does not significantly affect sqr expression in the WT or the sqrR mutant strain (Fig. S3A), with the sqrR gene constitutively expressed, although at a level slightly decreased by sulfide treatment (Fig. S3B). R. capsulatus encodes two homologs of the previously characterized homotetrameric persulfide sensor from S. aureus, CstR (26), and deletion strains of one or both cstR-like genes are fully sulfide-responsive (Fig. S2C). These two CstR paralogs are most closely related to the formaldehyde-sensing repressor FrmR (GenBank accession no. ADE84633) and the nickel-sensing repressor RcnR (GenBank accession no. ADE83853) (Fig. S4A), and are structurally unrelated to SqrR (28, 29). These data taken collectively reveal that SqrR is the primary sulfide sensor in R. capsulatus.
Fig. S2.
β-Galactosidase activity measurement. (A–C) Activity measurements were performed with the sqr–lacZ fusion in the isolated suppressor mutants (A), ΔsqrR mutant (B), and R. capsulatus mutants lacking one (∆cstR1, ∆cstR2) or both (∆cstR1,2) cstR-like genes (C). The control is the WT strain containing the same sqr–lacZ fusion. Aerobically grown cells that reached midlog phase were induced by sodium sulfide (gray bars) or not induced (open bars), and cells were harvested after 4 h. (D) Close-up data focusing on sqr promoter activity under noninduced conditions shown in Fig. 3B. Error bars indicate SD of the mean. *P < 0.05 (compared with WT).
Fig. S3.
Promoter activities of sqr and sqrR. (A) β-Galactosidase activity measurements with the sqr–lacZ fusion in WT and ΔsqrR cells. Aerobically grown cells were treated with 1 mM H2O2 (final concentration), and then cells were harvested after 0 (gray bar), 7 (light gray bar), and 30 min (dark gray bar). (B) β-Galactosidase activity measurements of the sqrR promoter–lacZ fusion construct consisting of the 1,200 bp upstream of the SqrR start codon. White and gray bars show activities in non–sulfide-induced and sulfide-induced cells (as in Fig. 3B), respectively. Error bars indicate SD of the mean.
Fig. S4.
Primary structure analysis of SqrR. (A) A phylogenetic tree based on amino acid sequences of SqrR, CstR, RcnR, and FrmR homologs. Red boxes indicate R. capsulatus genes. Phylogenetic analysis was performed using the programs CLUSTAL X (55) and MEGA (56). The tree was generated by the neighbor-joining method. (B) An amino acid sequence alignment of SqrR homologs. GenBank accession numbers of the homolog sequences are WP_023003595, AEI95148, WP_042464829, WP_011389407, WP_007163884, WP_035243515, WP_026177434, WP_045267543, ADC63927, WP_001329508, and WP_010893290, respectively. (C) A phylogenetic tree based on amino acid sequences of SqrR homologs and other ArsR/SmtB family proteins. α-, β-, and γ-proteobacteria are indicated by red, green, and blue backgrounds, respectively. Three sequences of ArsR/SmtB family proteins from other species were used as an outgroup.
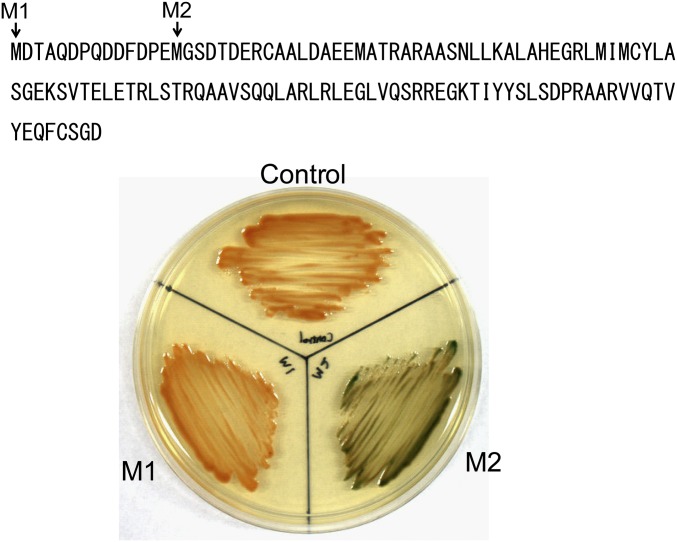
There are two possible AUG initiation codons separated by 13 residues in the putative sqrR ORF. To determine the exact start codon for SqrR translation, translational lacZ fusions to each start codon were constructed with subsequent β-galactosidase activity measurements; these experiments reveal that the more downstream initiation codon is the bona fide start of translation (Fig. S5). A bioinformatics analysis reveals that SqrR homologs are present in nearly all major classes of proteobacteria. A multiple-amino acid sequence alignment of SqrR homologs from α-, β-, and γ-proteobacteria reveals several conserved regions, including the helix-turn-helix motif and two highly conserved cysteines (Fig. S4B). SqrR is a member of the arsenic repressor (ArsR) family of prokaryotic repressors (30), and is evolutionarily related to BigR from the plant pathogens Xyella and Agrobacterium spp., described previously as mediating hydrogen sulfide detoxification (31). An unrooted phylogenetic tree of SqrR homologs indicates that some β- and γ-proteobacterial SqrR homologs are found within the α-proteobacterial clade, suggesting lateral gene transfer of sqrR among proteobacteria (Fig. S4C).
Fig. S5.
Determination of the SqrR start codon. The lacZ gene was fused to the first Met (M1) and/or the second Met (M2) with the 1,000-bp upstream region from the first Met. The two sqrR–lacZ translational fusions were separately introduced into WT R. capsulatus, and the lacZ expression in each strain was visualized on PYS medium containing X-gal.
Biochemical Properties of SqrR.
We next purified recombinant R. capsulatus SqrR for in vitro studies using an Escherichia coli overexpression system followed by affinity chromatography. DNase I footprint analysis was undertaken with purified SqrR to determine the site of SqrR binding to the sqr promoter region (Fig. 2A). Good protection was observed in the sqr promoter region around the −10 σ-subunit recognition sequences and transcriptional start site, consistent with the classification of SqrR as a transcriptional repressor. Gel mobility-shift analysis of purified SqrR was next carried out with a DNA probe that encompasses the SqrR-protected region in the sqr promoter. These assays were performed with untreated and fully reduced SqrR as well as with SqrR treated with a representative persulfide sulfur donor, GSSH. To avoid oxidation of Cys residues by molecular oxygen, all experiments were carried out under anaerobic conditions. SqrR binds to the DNA probe in a concentration-dependent manner (Fig. 2B), yielding an EC50 value (effective concentration of SqrR for 50% binding) (Fig. 2C) for untreated (reduced) SqrR of ∼56 nM, which is approximately twofold lower than that of GSSH-treated SqrR (∼110 nM). The DNA-binding affinity of untreated and fully reduced C9S SqrR is significantly higher than that of GSSH-treated C9S SqrR, suggesting that C9S SqrR, like WT SqrR, is fully capable of sensing persulfides (Fig. S6A). In striking contrast, the C41S and C107S SqrRs do not show a significant GSSH-dependent reduction in DNA affinity, with EC50 values (∼50 nM) similar to that of WT reduced SqrR (Fig. S6 B and C). Finally, aerobic gel mobility-shift experiments carried out in the absence or presence of the reductant DTT reveal that the affinity of SqrR is significantly higher in the presence of DTT relative to its absence (Fig. S6D). These experiments collectively reveal that the operator-promoter DNA-binding affinity of SqrR in vitro is sensitive to reversible thiol oxidation such as disulfide-bond formation, as previously observed for the SqrR homolog BigR (31).
Fig. 2.
Biochemical properties of SqrR. (A) DNase I footprint analysis of SqrR. Binding to the sqr promoter region under aerobic conditions. (A, Bottom) Regions corresponding to the DNase I protection regions are shown with a gray background. The −35 and −10 σ-subunit recognition sequences are in boxed letters, with the transcription start site indicated in red. (B) Gel mobility-shift assay using a DNA fragment of the sqr promoter region under anaerobic conditions with reduced SqrR (18 to 90 nM) or GSSH-treated SqrR (45 to 150 nM). (C) Binding isotherms of reduced (open circles and solid line) and GSSH-treated SqrR (filled circles and dashed line) plotted as a fraction of the shifted probe. (D) C9S SqrR reacts with glutathione persulfide but not glutathione, glutathione disulfide, or Na2S to form intramolecular cross-links. LC-ESI-MS spectra of reduced C9S SqrR, GSH-reacted C9S SqrR, GSSG-reacted C9S SqrR, Na2S-reacted C9S SqrR, and GSSH-reacted C9S SqrR. Monomeric C9S SqrR (12,296 Da) was found to be the dominant species in all samples except for GSSH-reacted C9S SqrR, in which the tetrasulfide–cross-linked C9S SqrR (12,358 Da) was found to dominate, accompanied by a small amount (≤5%) of trisulfide–cross-linked C9S SqrR (12,326 Da). Conditions: 25 mM Tris⋅HCl, 200 mM NaCl, 2 mM EDTA (pH 8.0).
Fig. S6.
Binding isotherms of the WT and point mutants of SqrR to the sqr promoter. DNA-binding isotherms were generated by measuring the levels of shifted probes. (A–C) Binding isotherms of reduced (open circles and solid line) and GSSH-treated (filled circles and dashed line) C9S (A), C41S (B), and C107S (C) SqrR under anaerobic conditions. (D) Binding isotherms of DTT-reduced (open circles and solid line) and oxidized (filled circles and dashed line) WT SqrR under aerobic conditions.
To define the chemical nature of the SqrR modification by GSSH, we measured the molecular mass distribution of C9S SqrR species following an anaerobic incubation with an excess of GSSH by liquid chromatography (LC) coupled with a mass spectrometer (MS) equipped with an electrospray interface (ESI). These mass spectra were compared with those obtained with untreated, reduced SqrR vs. those incubated with the corresponding thiol, glutathione (GSH), glutathione disulfide (GSSG), and Na2S (Fig. 2D). C9S SqrR is active in cells (vide infra), and was used for these experiments to minimize complications from oxidative chemistry occurring at Cys9. We find that untreated C9S SqrR is fully reduced, with no evidence of other modifications (Mr 12,296 Da). We observe no change in this mass spectrum in the presence of GSH, GSSG, or Na2S. In contrast, GSSH specifically shifts the mass distribution to that largely corresponding to a +62-Da species, a mass shift consistent with an intramolecular (intraprotomer) tetrasulfide cross-link between the remaining C41 and C107 residues (Fig. 2D). In addition, a small amount of +30-Da product is also observed, consistent with an intramolecular trisulfide cross-link (Fig. 2D). High-resolution tandem mass spectrometry of trypsin-digested GSSH-treated C9S SqrR confirms the presence of both intramolecular tri- and tetrasulfide cross-links linking C41 and C107 on the same subunit (Fig. S7).
Fig. S7.
Detection of cross-linked peptides of C9S SqrR. High-resolution LTQ Orbitrap tandem mass spectra of proteolytically digested peptides (trypsin) from GSSH-reacted C9S SqrR reveal the formation of a tetrasulfide–cross-linked peptide (+3 charge state) between C41 and C107, shown as (A) the fragmentation pattern and (B) the schematic interpretation of the fragmentation pattern. The C41-containing peptide, 37LMIMCYLASGEK48, is shown in red in the tetrasulfide–cross-linked peptide and purple in the persulfidated peptide, whereas the C107-containing peptide, 98VVQTVYEQFCSGD110, is shown in cyan in the tetrasulfide–cross-linked peptide and green in the persulfidated peptide. The high-resolution LTQ Orbitrap tandem mass spectra also reveal the formation of a trisulfide–cross-linked peptide (+3 charge state) between C41 and C107, shown as (C) the fragmentation pattern and (C, Inset) the schematic interpretation. The C41-containing peptide is shown in red in the trisulfide–cross-linked peptide, whereas the C107-containing peptide is shown in cyan.
Similar findings were obtained for the WT SqrR. Specifically, incubation with GSSH, but not GSH, GSSG, and Na2S, results in molecular mass shifts of +30 and +62 Da, with the latter the major product (Fig. S8A), consistent with intramolecular tri- and tetrasulfide cross-links between C41 and C107, as described for C9S SqrR. Tandem ESI-MS/MS analysis of the trisulfide–cross-linked species confirms this assignment in the +3 charge state (Fig. S8B). Both cross-links are fully reduced by the thiol-reducing agent Tris(2-carboxyethyl)phosphine (TCEP) (Fig. S8A), establishing the reversibility of the tri- and tetrasulfide bonds in SqrR. Interestingly, a number of S-glutathionylated adducts were also observed in GSSG- and GSSH-derivatized WT SqrR relative to C9S SqrR (Fig. S8C), but the significance of this S-glutathionylation is unknown, because C9S SqrR is fully functional in cells (vide infra). Intraprotomer cross-linking between C41 and C107 is supported by a model structure of SqrR derived from crystal structures of Xylella fastidiosa BigR, an SqrR homolog, in the disulfide-oxidized and thiol-reduced states (32) (Fig. 3A). Oxidized BigR is characterized by an intramolecular disulfide bond between two cysteines that correspond to C41 and C107 in R. capsulatus SqrR (31). These two cysteine residues must be in close physical proximity, as would be required for intramolecular di-, tri-, and tetrasulfide-bond formation in SqrR.
Fig. S8.
Detection of cross-linked peptides of WT SqrR. (A) LC-ESI-MS spectra of intact reduced and unreacted SqrR and SqrR following an anaerobic reaction with a fivefold molar S:SqrR cysteine excess of GSH, GSSG, Na2S, and GSSH. * and § correspond to the +30 and +62 mass adducts, consistent with tri- and tetrasulfide–cross-linked species. The GSSH-reacted sample was also treated with TCEP (GSSH+TCEP). (B) High-resolution LTQ Orbitrap tandem mass spectra of proteolytically digested peptides (trypsin) from the +30 adduct of the GSSH-reacted WT SqrR (*, A) reveal the formation of a trisulfide cross-link (+3 charge state) between C41 and C107, shown as the fragmentation pattern and schematic interpretation of the fragmentation pattern (B, Inset). The colors of each peptide are analogous to those shown in Fig. S7. (C) Full ESI-MS spectra of WT SqrR vs. C9S SqrR following anaerobic incubation with GSSG and GSSH, as indicated. The data on C9S SqrR are reproduced here (from Fig. 2) to allow ready comparison with the WT SqrR reactions (expanded from A). WT SqrR forms two S-glutathionylated adducts not found in fully functional C9S SqrR (see main text for details): (i) GSSG induces glutathionylation of WT SqrR (12618.0 Da, +306 Da), whereas (ii) GSSH treatment results in glutathione adduction (12680.0 Da, +306 Da) to tetrasulfide–cross-linked SqrR (12374.0 Da) only (third row). Both findings are consistent with a role of C9 in mediating S-glutathionylation of SqrR, the physiological impact of which is unknown.
Fig. 3.
Characterization of SqrR in vivo. (A) Structural model of SqrR based on the structure of Xylella BigR (32). Red and green are used to distinguish the two subunits (protomers) of the homodimer. Yellow spheres highlight the two cysteines (C41 and C107) separated by ∼9.5 Å in this model. (B) β-Galactosidase activity measurements are as in Fig. 1. The control is the WT strain containing the sqr–lacZ fusion. Aerobically grown cells that reached midlog phase were induced by sodium sulfide (gray bars) or not induced (open bars), and then cells were harvested after 4 h. A FLAG-tagged coding sequence was integrated into the genomic sqrR 3′ end in R. capsulatus (SqrR:FLAG). Each point mutant was constructed using the sqrR-FLAG mutant as background. Error bars indicate SD of the mean. (C) Detection of C-terminally FLAG-tagged WT and mutant SqrRs by Western blotting using an anti-FLAG antibody. (D) Nonreducing (−DTT) and reducing (+DTT) 2D electrophoresis coupled with Western blotting using an anti-FLAG antibody. WT (negative control) and SqrR-FLAG strains grown aerobically were treated with 0.6 mM Na2S for 4 h and then subjected to SDS/PAGE. An arrow indicates the specific band for the SqrR-FLAG monomer. Red asterisks indicate nonspecific bands. (E) Mobility shifts of SqrR caused by thiol modification on SDS/polyacrylamide gels. The SqrR-FLAG mutant was labeled with AMS. Cells were grown to midlog phase under aerobic conditions, followed by the addition of 0.6 mM sodium sulfide. Before harvesting, cells were treated with trichloroacetic acid (TCA) to rapidly quench thiol–disulfide exchange reactions.
We next constructed strains of R. capsulatus where each SqrR cysteine (C9, C41, and C107) was individually changed to a serine and recombined into its native genomic locus to determine the degree to which these residues impact the ability of SqrR to sense exogenous sulfide in vivo. As expected, the C41S and C107S mutants both lose the ability to derepress sqr expression in the presence of exogenous sulfide; in contrast, the C9S mutant retains WT transcriptional derepression activity (Fig. 3B). The C41S mutant was impaired in photoautotrophic growth using H2S as a sole electron donor (Fig. S9A), and ΔsqrR and C41S mutants exhibited a more pale or a darker color, respectively, than the WT strain on a sulfide-containing growth medium (Fig. S9B). Under these conditions, bacteriochlorophyll and carotenoid contents in the ΔsqrR strain were ∼30% lower than in WT. Photosynthetic growth profiles of WT and ΔsqrR were also analyzed. Cells precultured in the presence and absence of sulfide were transferred to sulfide-free fresh medium and growth rates were measured under anaerobic light conditions (Fig. S9C). When cells were transferred from sulfide-containing medium to sulfide-free medium, slow growth was observed before the logarithmic growth phase, although such delayed growth was not observed when cells were transferred from sulfide-free preculture. Notably, the delayed growth phenotype of ΔsqrR was longer (∼8 h) than that of WT (∼4 h). These experiments establish the physiological importance of the tetrasulfide-bond formation of SqrR for sulfide utilization and regulated photopigment synthesis.
Fig. S9.
Growth phenotypes of R. capsulatus WT, ΔsqrR, and sqrR C41S point mutant. (A) Anaerobic-light (photosynthetic) growth with or without malate as a carbon source in the presence or absence of hydrogen sulfide as an electron donor. (B) Aerobic growth in a high concentration of sulfide. Sterilized distilled water (SDW) and/or 0.6 M Na2S solution were spotted at the center of the plates. (C) Photosynthetic growth profiles of R. capsulatus WT (Left) and ΔsqrR (Right) strains. Cells precultured in PYS medium without (black squares) or with 0.1 mM Na2S (red circles) were transferred to sulfide-free PYS medium at time point 0.
To assess the stability of mutant SqrRs in cells, we performed Western blot analysis using C-terminally FLAG-tagged SqrRs and an anti-FLAG antibody. Given that the FLAG tag was integrated into the sqrR 3′-end coding sequence, the copy numbers should be similar to those of the WT. WT SqrR-FLAG could be specifically detected, with both C9S and C41S SqrRs also detected, albeit at levels modestly lower than WT SqrR (Fig. 3C). Although C107S SqrR was not detected in this experiment, this may be due to a lower stability and/or loss of the FLAG tag, because the sqr repressor activity of C107S SqrR is only modestly affected and readily distinguished from a ∆sqrR strain (Fig. S2D). Two-dimensional nonreducing and reducing electrophoresis coupled with Western blot detection reveals that SqrR does not form covalently cross-linked dimers in cells in the presence of sulfide stress (Fig. 3D), a finding consistent with the in vitro mass spectrometry experiments indicative of an intraprotomer cross-link (Fig. 2D and Fig. S8). To confirm the presence of an intraprotomer C41–C107 cross-link, total protein extracts were treated with the thiol-modifying agent 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS), which derivatizes only free Cys sulfhydryl groups, followed by analysis by SDS/PAGE and Western blotting. A tetrasulfide bond would be resistant to AMS modification, so any AMS modifications that retard electrophoretic mobility would reflect the status of free Cys sulfhydryls in SqrR. As shown in Fig. 3E, the molecular mass of SqrR-FLAG in non–sulfide-treated vs. sulfide-treated cells is increased by ∼2.4 and ∼0.8 kDa, respectively, after treatment with AMS relative to that of SqrR-FLAG that was not modified with AMS. Given that the increase in molecular mass of the non–sulfide-treated sample is approximately threefold that of the sulfide-treated sample, we conclude that the number of free Cys sulfhydryls is changed from three to one after treatment of cells with exogenous sulfide. Although the nature of the C41–C107 cross-link in SqrR isolated from cells can be defined by this experiment, these findings are fully consistent with the in vitro GSSH–cross-linking experiments (Fig. 2 and Fig. S8) in that there are two cysteine residues of SqrR that are cross-linked by exogenous sulfide-derived reactive sulfur species in vivo.
Identification of Genes Controlled by SqrR.
To identify SqrR-regulated genes, we performed an RNA-sequencing (RNA-seq) transcriptomic analysis of the R. capsulatus WT and sqrR-disrupted mutant in the absence and presence of exogenous sulfide. In one experiment, we identified sulfide-inducible genes in WT cells by comparing transcription levels in WT cells untreated vs. treated with sulfide. In a parallel experiment, we identified SqrR-regulated genes by comparing the expression levels in WT vs. sqrR mutant cells grown in the absence of sulfide (Dataset S2). These results reveal that 45% of all sulfide-responsive genes in the R. capsulatus genome are directly or indirectly regulated by SqrR. The identified sulfide-responsive genes include a number of genes known to function in sulfur metabolism or assimilation, including thiosulfate-sulfite oxidoreductases and thiosulfate sulfurtransferases or rhodanese homology domain proteins (33, 34), whose expression is strongly repressed by SqrR. Although originally described as functioning in cyanide detoxification (35), sulfurtransferases and structurally unrelated single-Cys–containing TusA domains (36) are generally involved in persulfide shuttling, and are capable of accepting sulfur from a variety of RSS, including thiosulfate and low-molecular-weight persulfides (37). Another major target of SqrR is a putative peroxiredoxin, a thiol peroxidase known to be involved in detoxification of reactive oxygen species (38); this finding suggests a novel role in detoxification of RSS. Interestingly, the expression of diguanylate cyclase/phosphodiesterase, which catalyzes bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) synthesis, and acetone carboxylase are also regulated by SqrR. A divergently transcribed operon encoding an RND family efflux transporter and acriflavin resistance protein located downstream of sqrR are also strongly repressed by SqrR. Although the repression level is not high, sulfite assimilation- and cysteine biosynthesis-related genes are also regulated by SqrR. Comparison of the promoter sequences of genes characterized by ≥10-fold changes by sqrR disruption reveals a strong consensus operator sequence for SqrR, namely ATTC-N8-GAAT (Fig. S10). Interestingly, the expression of most genes in the photosynthetic gene cluster (puf, puc, bch, and crt) are ∼0.5-fold repressed in the sqrR mutant relative to WT cells.
Fig. S10.
Putative consensus operator sequence for SqrR binding. (A) A nucleotide sequence alignment of promoter regions of sqr and genes showing over 10-fold changes by disruption of sqrR. A putative consensus sequence, ATTC-N8-GAAT, was found and is indicated by a gray background. The putative −35 and −10 σ-subunit recognition sequences are indicated. Numbers shown (Right) indicate nucleotides from the initial codon of each gene. (B) Visualization of the sequence motif of the consensus sequence for SqrR binding sites (weblogo.berkeley.edu).
Discussion
In this study, we have identified and characterized a novel sulfide-responsive transcriptional repressor, SqrR, that controls sulfide-dependent photosynthetic electron transfer in R. capsulatus. The expression level of sqr, which encodes a canonical sulfide:quinone oxidoreductase (39, 40), is induced by sulfide in WT cells and is elevated in the sqrR-disrupted mutant irrespective of the presence of sulfide (Fig. 3B). The DNA-binding activity of purified SqrR is significantly reduced upon anaerobic incubation of GSSH, revealing that negative regulation of DNA binding and transcriptional derepression in cells involves thiol modifications (Fig. 2C). Two cysteine residues, at positions 41 and 107, are conserved in SqrR homologs from other bacteria, and substitution of each with serine results in the loss of a GSSH-responsive decrease in DNA-binding affinity in vitro (Fig. S6 B and C) and sulfide-dependent regulation in vivo (Fig. 3B). LC-ESI-MS/MS analysis shows that GSSH reacts with purified and fully reduced WT and C9S SqrRs to increase the molecular mass by +30 or +62 Da, with the latter species predominating (Fig. 2D and Fig. S8A). Furthermore, in sulfide-treated relative to untreated cells, SqrR harbors two cysteine residues that cannot be modified by AMS (Fig. 3E). Our data collectively reveal that C41 and C107 form an intramolecular tetrasulfide bond upon sensing of sulfide-derived reactive sulfur species, such as glutathione persulfide, that are expected to accumulate in sulfide-treated bacterial cells (39) (Fig. 4).
Fig. 4.
Model for SqrR sulfide-responsive gene regulation. In the absence of hydrogen sulfide, C41 and C107 of SqrR are in the reduced form. This form of SqrR binds the promoter region and represses the expression of sulfide-responsive genes (SRGs). In the presence of hydrogen sulfide, the resultant cellular RSS promotes the formation of a di-, tri-, or tetrasulfide bond between C41 and C107, inhibiting the ability of SqrR to bind to the promoter region. RNA polymerase binds DNA and subsequently induces gene expression.
It was previously shown that the SqrR homolog BigR from X. fastidiosa is capable of forming an intramolecular disulfide bond in vitro via an unknown mechanism but that formation of this disulfide was proposed to be important in the detoxification of hydrogen sulfide, specifically under hypoxic, biofilm-promoting conditions (31). Here we show that purified R. capsulatus SqrR also forms an intramolecular di-, tri-, or tetrasulfide bond, which we show reduces its DNA-binding affinity in vitro (Fig. S6D). Consistent with a role in sensing reactive sulfur species rather than reactive oxygen species, SqrR-dependent transcriptional regulation in vivo is specifically sulfide-inducible and independent of oxygen and/or redox status of the cell (Fig. 1 and Fig. S3A). The findings parallel those previously described for the persulfide sensor CstR from S. aureus (26). These results reveal that SqrR in R. capsulatus senses sulfide and is not a simple redox sensor. The difference between SqrR and BigR functions may be due to their distinct metabolic features, as R. capsulatus is a facultative anaerobic bacterium whereas X. fastidiosa is strictly aerobic.
SqrR is a member of the ArsR family of bacterial repressors encoding a wide range of metal-, metalloid-, and non–metal-sensing proteins (41, 42). This structural classification contrasts sharply with that of the only other known sulfide-responsive, persulfide-sensing repressor, CstR, from S. aureus, which is a member of the CsoR/RcnR family of metalloregulatory proteins (43). Homotetrameric CstR forms intersubunit di-, tri-, and tetrasulfide bonds between two conserved cysteines in the presence of low-molecular-weight persulfides and inorganic polysulfides that negatively regulate DNA-binding affinity to target promoters in sulfide-stressed cells (26). Although R. capsulatus harbors two CstR-like proteins, they are true paralogs predicted to function in formaldehyde detoxification (FrmR) (29) and nickel homeostasis (RcnR) (Fig. S4A). Consistent with this, deletion of either or both genes in R. capsulatus has no effect on sqr expression (Fig. S2C). Our RNA-seq analysis indicates that SqrR contributes to the regulation of many (45%) of the sulfide-responsive genes. These SqrR-regulated genes include not only those anticipated RSS detoxification proteins and persulfide carriers but also c-di-GMP cyclase/phosphodiesterase and acetone carboxylase. C-di-GMP, known as a bacterial second messenger, affected sulfide homeostasis to motility and biofilm formation in X. fastidiosa (31); furthermore, the electrophilic C8 position of guanine nucleotides is reported to be a significant site of thiolation by RSS in mammalian cells (13). Acetone carboxylase, on the other hand, is involved in nonrubisco CO2 fixation (44), and may therefore function as an inducible electron sink. SqrR also regulates RND family efflux transporter genes, suggesting a role in reductase assembly. Furthermore, genes that show a ≥8-fold sulfide-dependent change in expression are clearly regulated by SqrR. Based on these observations, we conclude that SqrR is the master regulator of sulfide response in R. capsulatus.
Disruption of sqrR also influences the expression of photosynthesis-related genes in the photosynthesis gene cluster. The expression changes may be due to alteration of the redox status of the quinone pool, which would influence the phosphorylation activity of the sensor kinase RegB, known to control photosynthesis gene expression in response to alterations in the ubiquinone pool (45). These observations suggest that regulating the synthesis of the photosynthetic apparatus involves the synchronization of electron transfer activities among several components, with expression responding to different electron donors that are variable in nature. A non–sulfide-responsive mutant SqrR impacts sulfide-dependent pigmentation (Fig. S9), consistent with this hypothesis.
In mammalian cells, a small but significant fraction of the proteome is persulfidated as a result of the endogenous production of H2S by enzymes of the transsulfuration pathway, which potentially results in significant metabolic changes in the cell (18). The extent to which such intramolecular signaling by RSS and shifts in metabolism are operative in bacteria is currently unknown. These studies establish that the sulfide-regulated repressor SqrR allows R. capsulatus to adapt to changes in sulfide availability via thiol persulfidation chemistry on SqrR, which we hypothesize involves the intermediacy of RSS in cells. The functional characteristics of SqrR appear largely analogous to those of CstR in S. aureus (26), which also regulates an H2S oxidation and detoxification system (46), despite vastly distinct structural scaffolds (47). Further elucidation of the functional role(s) of persulfide trafficking and sulfide homeostasis in R. capsulatus promises a better understanding of how these processes impact photosynthesis in this industrially important bacterium.
Materials and Methods
Materials and methods are described in SI Materials and Methods. They include bacterial strains, growth conditions, mutagenesis, genetic sulfide-insensitive suppressor mutant screening, purification of recombinant SqrR, primer extension analysis, GSSH preparation, LC-ESI-MS analysis, LC-MS/MS analysis, gel mobility-shift analysis, DNase I footprint assay, β-galactosidase assay, Western blotting, extraction of photosynthetic pigments, and RNA-seq.
Data represent the mean of at least three independent experiments (error bars indicate SD of the mean). The P value and statistical significance of difference were analyzed by using unpaired t tests (P < 0.05, significant).
SI Materials and Methods
Bacterial Strains, Media, and Growth Conditions.
Rhodobacter capsulatus WT strain SB1003 and mutant strains were grown under aerobic-dark (aerobic) or anaerobic-light (photosynthetic) conditions at 30 °C in PYS, a rich medium, or RCV minimal medium, as described (27, 48). Illumination was provided by a light-emitting diode (λmax 860 nm) (CCS) for photosynthetic growth. For anaerobic growth, cultures in screw-capped test tubes were almost completely filled with medium. The strains were also grown on plates in an anaerobic jar with an AnaeroPack (Mitsubishi Gas Chemical) and a small tube containing 0.1 g of thioacetamide dissolved in 1 mL of aqueous 1 N HCl. Gentamycin, rifampicin, and spectinomycin were used at concentrations of 1.5, 75, and 10 µg/mL, respectively.
Escherichia coli strains were grown in Luria–Bertani medium at 37 °C. Kanamycin, gentamycin, trimethoprim, and spectinomycin were used at concentrations of 50, 10, 50, and 40 µg/mL, respectively.
Construction of lacZ Fusions and β-Galactosidase Assay.
The lacZ fusion plasmids were constructed with the pNM481 plasmid and the Ω-interposon gene, which were transferred into R. capsulatus with the conjugative E. coli strain XL1Blue/pDPT51, as described previously (27, 49). A DNA fragment consisting of 1,238 bp upstream and 12 bp downstream of the sqr start codon was amplified by PCR using PrimeSTAR HS Polymerase (TaKaRa) with a forward primer (5′-GGGGATCCGTCGACCCAGATCCTTCCTGAAAACCA-3′) and reverse primer (5′-GGGAGCAAGCTTGGCGATATGAGCCATCTGTCCCT-3′). The amplified DNA was cloned into the PstI-cut pNM481 vector by the In-Fusion HD Cloning Kit (Clontech). A DNA cassette containing the spectinomycin-resistance gene with Ω-interposon was then inserted into the plasmid upstream of the cloned fragment. The obtained plasmid, designated pNM::SqrRΩ, was introduced into R. capsulatus cells by conjugation with the mobilizing E. coli strain XL1Blue/pDPT51. For constructing sqrR–lacZ fusions, DNA fragments consisting of 1,500 bp upstream and 12 bp downstream of two putative sqrR start codons (Fig. S4) were separately amplified by PCR using PrimeSTAR HS Polymerase (TaKaRa) with a forward primer (5′-GGGGATCCGTCGACCGGATGTGACCCAGAAGCTCG-3′) and reverse primer (5′-GGGAGCAAGCTTGGCGGCAGTATCCATTGCCAAGT-3′) for the M1 fragment or 5′-GGGAGCAAGCTTGGCGTCGGACCCCATTTCGGGAT-3′ for the M2 fragment. The amplified DNA fragments were separately cloned into pNM481 as for the sqr–lacZ fusions described above.
R. capsulatus strains containing the fusion plasmids were grown aerobically or photosynthetically to midlog phase in PYS medium. For sulfide induction, a final 0.6 mM Na2S was added and cells were grown further for 4 h. For H2O2 treatment, 1 mM H2O2 was added and cells were further grown for 0, 7, and 30 min. A previous study showed that 1 mM H2O2 strongly induces expression of oxidative stress-related genes (50). After the induction, 10 mL cells was harvested, and β-galactosidase activity was determined essentially as described previously (49). Final results were obtained as the amount of o-nitrophenyl-β-d-galactopyranoside (ONP) hydrolyzed per min per mg protein.
Isolation and Genome-Resequencing Analysis of Sulfide-Insensitive Suppressor Mutants.
R. capsulatus WT strain containing pNM::SqrRΩ was grown aerobically to midlog phase in RCV medium. A final 500 or 100 mM ethyl methanesulfonate (EMS) (Sigma) was added to 10 mL culture. To induce mutation with EMS, the cells were grown aerobically at 37 °C for 2 h. The cells were harvested and washed with 0.1 M phosphate buffer (pH 7.4) twice. After washing with double-distilled H2O (ddH2O), the mutagenized cells were resuspended in 110 µL ddH2O and plated onto RCV-lactose plates (RCV containing 0.6% lactose instead of malate). Colonies grown on the selective plates were isolated as sulfide-insensitive suppressor mutants.
Genomic DNA of the WT and suppressor mutants was isolated and purified using the Puregene Yeast/Bact. Kit B (Qiagen) in accordance with the manufacturer’s instructions. The library was prepared following the protocol of the TruSeq DNA PCR-Free Library Preparation Kit (Illumina). Sequencing for a 69-bp single read was performed on the GAIIx platform (Illumina). Reads were mapped against the R. capsulatus SB1003 reference genome (National Center for Biotechnology Information reference sequence NC_014034) using BWA 0.7.12 (51), and pileups of the read alignments were produced by SAMtools version 1.2 (52). Identified mutation sites are summarized in Dataset S1. The sequencing data were deposited in the DNA Data Bank of Japan Sequence Read Archive (DRA) under accession nos. DRA004893 (WT), DRA004894 (line 1), DRA004895 (line 2), and DRA004896 (line 3) under BioProject accession no. PRJDB5013.
Cloning and Mutagenesis of SqrR.
Two 500-bp DNA fragments consisting of N- and C-terminal regions of sqrR were amplified by PCR with PrimeSTAR HS Polymerase (TaKaRa). Two sets of primer pairs were used for the amplification: a forward primer (5′-CAGCATGCGCCCTCGCAGGATGGGGCCGCCGGGAT-3′) and reverse primer (5′-TTTTGATATCTTGGGCAGTATCCATTGCCAAGTCGA-3′), and a forward primer (5′-TTTTGATATCTGCTCGGGCGACTGAAACGCGCGGC-3′) and reverse primer (5′-TTGGATCCATCTTCAAGACCTGAGGGCCCCGAAAG-3′); SphI, EcoRV, EcoRV, and BamHI restriction sites were designed at additional polynucleotide tails, respectively (underlined). The first PCR fragment was digested with SphI and EcoRV, and the second PCR fragment was digested with BamHI and EcoRV. After the digestion, these two fragments were mixed and ligated together with SphI-BamHI-cut pZJD29a (53). The obtained plasmid, designated pZJD29a::ΔsqrR, was introduced into R. capsulatus WT cells by conjugation with the mobilizing E. coli strain S17-1/λpir, as described (53). Cells undergoing a single cross-over event were selected by plating exconjugants on PYS plates containing gentamycin and rifampicin. After sequential cultivation in the absence of antibiotics, sucrose-resistant but gentamycin-sensitive cells were selected on PYS-agar plates containing 5% (wt/vol) sucrose to generate the ΔsqrR mutant. A deletion of sqrR was confirmed by PCR amplification followed by sequencing analysis.
For construction of R. capsulatus expressing FLAG-tagged SqrR, a nucleotide sequence encoding the FLAG peptide was fused at the 3′ end of the sqrR gene by PCR with a primer pair: a forward primer (5′-TTGGATCCCAGCATGCGCCCTCGCAGGATGGGGCC-3′) and reverse primer (5′-TTGAATTCTCACTTATCATCATCATCCTTATAGTCGCCCGAGCAGAACTGCTCGTAG-3′); BamHI and EcoRI restriction sites were designed at additional polynucleotide tails, respectively (underlined). The amplified DNA fragment, containing the full-length FLAG-tagged sqrR and the 500-bp upstream region of sqrR, was cloned into BamHI-EcoRI-cut pZJD3 (54) by ligation. The resulting plasmid was then introduced into R. capsulatus ΔsqrR mutant cells as described above. Subsequent single–cross-over recombinants were isolated as sqrR-FLAG–expressing strains. For cysteine mutant construction, the same processes were conducted with sqrR-FLAG plasmid clones containing each cysteine mutation, which were introduced by a standard PCR mutagenesis method.
Overexpression and Purification of WT and Mutant SqrR.
The SqrR overexpression plasmid was constructed as follows. First, a DNA fragment encoding full-length sqrR was amplified by PCR with a forward primer (5′-AGATTGGAGGACATATGGGGTCCGACACGGACGAG-3′) and reverse primer (5′-GTCCGCGGTACCATATCAGTCGCCCGAGCAGAACT-3′). The amplified DNA was cloned into NdeI-cut pSUMO vector (LifeSensors) by the In-Fusion HD Cloning Kit (Clontech). The obtained plasmid was designated pSUMO::SqrR. Cysteine mutations were subsequently introduced by a standard PCR mutagenesis method with pSUMO::SqrR plasmid used as a PCR template. pSUMO::SqrR was transferred into E. coli strain BL21 (DE3) (TaKaRa), and the recombinant protein SUMO-SqrR was overexpressed by induction with 0.2 mM isopropyl-β-d-thiogalactopyranoside at 16 °C overnight (12 to 16 h). Cells in a 500-mL culture were harvested and resuspended in 20 mL nickel column loading buffer composed of 20 mM Tris⋅HCl (pH 8.0), 500 mM NaCl, 5 mM imidazole, and 10% glycerol and lysed by sonication. The lysate was clarified by centrifugation at 30,000 × g for 30 min at 4 °C. The resultant supernatant was passed through a 45-µm membrane filter (Millipore) and loaded onto a 1-mL HisTrap column with the ÄKTAprime System (GE Healthcare), and washed with 30 column volumes of wash buffer containing 20 mM Tris⋅HCl (pH 8.0), 500 mM NaCl, 20 mM imidazole, and 10% glycerol. SUMO-SqrR was eluted with a gradient of 20 to 500 mM imidazole in loading buffer over a 15-column-volume total. The SUMO tag was then proteolytically cleaved at room temperature for 1 h at a 100:1 molar ratio of UlP1 protease. Tag-less SqrR was then isolated by passing through a 1-mL HisTrap column in wash buffer, followed by dialysis with buffer containing 20 mM Tris⋅HCl (pH 8.0), 500 mM NaCl, and 6% glycerol. Peak fractions were analyzed by SDS/PAGE, and SqrR-containing fractions were collected and concentrated. For cysteine mutant construction, the same processes were conducted with pSUMO::SqrR containing each cysteine mutation, which were introduced by a standard PCR mutagenesis method. Purification of proteins for LC-ESI-MS and LC-MS/MS analysis was performed anaerobically in an anaerobic glove box.
Primer Extension Analysis.
R. capsulatus WT cells were grown aerobically to midlog phase in PYS medium containing 0.6 mM Na2S. Total RNA was extracted from 1 mL cell culture using the RNeasy Mini Kit (Qiagen). Twenty micrograms of purified RNA and 100 µM (final concentration) 6-FAM–labeled primer (5′-TGATGACGGTGACCTTGTCT-3′) were mixed and incubated at 42 °C for 15 min. Reverse transcription was then performed with RNA PCR Kit (AMV) version 3.0 (TaKaRa). The reaction sample was purified by ethanol precipitation and resuspended in 20 µL Hi-Di formamide containing 0.5 µL 500 LIZ Size Standard (Applied Biosystems). For reference, the predicted promoter region of the sqr gene was amplified with a forward primer (5′-TCGCCTCGACGATCGACAAC-3′) and a reverse primer (5′-TGATGACGGTGACCTTGTCT-3′). The amplified fragment was used in each A, C, T, and G dideoxy chain sequence using 10 pmol FAM-labeled primer by the Taq Cycle Sequencing Kit (TaKaRa). The samples were analyzed by the 3730xl DNA Analyzer (Applied Biosystems) with Peak Scanner software version 1.0 (Applied Biosystems).
Preparation of Glutathione Persulfide.
Glutathione disulfide (GSSG) was obtained from a commercial source (Wako) and used without further purification. Glutathione persulfide (GSSH) was freshly prepared by mixing a fivefold molar excess of freshly dissolved Na2S with glutathione disulfide and incubating anaerobically at 30 °C for 30 min in degassed 300 mM sodium phosphate (pH 7.4). The concentration of the in situ generated GSSH was determined using a cold cyanolysis assay and used without further purification in the SqrR derivatization assays at the indicated final concentrations (46).
LC-ESI-MS Analysis of Derivatized SqrRs.
LC-ESI-MS analysis of the reduced WT and C9S SqrRs anaerobically incubated (30 min, room temperature) with a fivefold S:cysteine thiol excess of reduced glutathione (GSH), GSSG, Na2S, and GSSH was performed at the Laboratory for Biological Mass Spectrometry at Indiana University using a Waters/Micromass LCT Classic time-of-flight mass spectrometer with a CapLC inlet. Briefly, 5-µL protein samples were loaded onto a Thermo Scientific BioBasic C4 reversed-phase column (72305-050565; 0.5 mm × 50 mm, 5 μm, 300 Å) and chromatographed using an acetonitrile-based gradient (solvent A: 5% acetonitrile, 0.1% formic acid; solvent B: 90% acetonitrile, 0.1% formic acid). The elution protocol was as follows: 0 to 0.5 min, 10% B, isocratic; 0.5 to 14 min, 10–90% B, linear gradient; 14 to 17 min, 90% B, isocratic; and reequilibration to 10% B. The elution was monitored at 215 nm. Data were collected and analyzed using MassLynx software (Waters).
LC-MS/MS Analysis of Proteolytically Digested SqrR.
In a 200-μL reaction mixture, 25 μM reduced WT and C9S SqrR, and GSSH-reacted WT and C9S SqrR, were alkylated by addition of 200 μL of alkylating buffer containing 100 mM Tris⋅HCl, 200 mM iodoacetamide, and 8 M urea (pH 8.0) and incubated for 30 min in the dark. Following alkylation, proteins were precipitated by addition of 100% trichloroacetic acid (TCA), with the supernatant removed by centrifugation at 17,000 × g for 15 min at 4 °C. The protein pellets were then washed with 500 μL ice-cold acetone three times to remove the TCA and dried in a SpeedVac concentrator. Dried pellets were resuspended in 20 μL of digestion buffer containing 100 mM NH4HCO3 and 2 M urea. One microliter of 40 ng/μL sequencing-grade trypsin was added to each sample and digested overnight at 37 °C. The digestion was terminated by the addition of 1 μL 10% TFA. Peptides were enriched from the digestion mixture by a C18 ZipTip using a standard protocol, dried in a SpeedVac concentrator, and resuspended in 10 μL 0.1% formic acid, with the analysis performed at the Laboratory for Biological Mass Spectrometry at Indiana University using a Thermo Finnigan LTQ Orbitrap XL mass spectrometer equipped with the Eksigent NanoLC Ultra 2D Plus System. Briefly, 2 μg of peptides was loaded onto a self-packed C18 reversed-phase trapping column (100 μm × 50 mm, 5 μm, 200 Å Magic C18AQ) for 6 min in 0.1% formic acid and further chromatographed on a self-packed C18 reversed-phase analytical column (75 μm × 100 mm, 5 μm, 100 Å Magic C18AQ) using an acetonitrile-based gradient (solvent A: 0% acetonitrile, 0.1% formic acid; solvent B: 100% acetonitrile, 0.1% formic acid). The elution protocol was as follows: 0 to 1 min, 3 to 7% B, linear gradient; 1 to 19 min, 7 to 40% B, linear gradient; 19 to 21 min, 40 to 50% B, linear gradient; 21 to 21.5 min, 50 to 90% B, linear gradient; 21.5 to 25 min, 90% B, isocratic; and reequilibration to 3% B. Data were collected by the Xcalibur System (ThermoFisher) and converted into Mascot Generic Format by the Proteomics Tools Suite. The extracted peak list was analyzed using ProteinProspector.
Gel Mobility-Shift Analysis.
A Cy5-labeled 200-bp DNA probe corresponding to the sqr promoter region was prepared by PCR amplification with a forward primer (5′-TTGAATTCACGGCTCGGACTGGCTGGCCGAGGGCA-3′) and a reverse primer (5′-TTAAGCTTGCAACCTGTCAAGCGGGAAGATCGGTC-3′); EcoRI and HindIII restriction sites were designed at additional polynucleotides, respectively (underlined). The amplified fragment was cloned into EcoRI-HindIII-cut pUC118 vector (TaKaRa); the resulting plasmid was designated pUCsqr. The inserted DNA of pUCsqr was amplified by PCR with a Cy5-labeled primer as described previously (48). The amplified DNA was purified and used as a probe for the gel mobility-shift analysis.
DNA probe (5 nM) was incubated for 15 min at room temperature in 7 µL binding reaction buffer composed of 25 mM Tris⋅HCl (pH 8.0), 100 mM NaCl, 2 mM MgCl2, 6% glycerol, and 50 µg/mL heparin. The mixture was incubated with various amounts of purified protein for 30 min at room temperature and then subjected to 7% polyacrylamide gel electrophoresis at room temperature in a buffer composed of 25 mM Tris⋅HCl (pH 8.0), 1 mM EDTA, and 144 mM glycine. After electrophoresis, the gel was analyzed using the Fluoro Image Analyzer (Fujifilm; FLA-9500). When reducing conditions were required, 5 mM DTT (final concentration) (Wako) was added to all buffers and reaction mixtures. When anaerobic conditions were required, all buffers were degassed and all processes were carried out in an anaerobic glove box. Reduced SqrR was prepared by treating with 5 mM DTT, followed by gel filtration (to remove DTT) under anaerobic conditions.
DNase I Footprint Assay.
The fluorescently labeled DNA probe consisting of the sqr promoter region was prepared by PCR. Specifically, a 300-bp sqr promoter region was amplified by PCR with a forward primer (5′-CTTCGTCGAGGGCATCAAGGCCGAA-3′) and a 6-FAM–labeled reverse primer (5′-TGATGACGGTGACCTTGTCT-3′). Footprint analysis was performed with the PCR fragment as described (48).
Western Blotting.
R. capsulatus WT- and SqrR-FLAG–expressing recombinants were grown aerobically to midlog phase in PYS medium. For sulfide induction, 0.6 mM Na2S (final concentration) was added and cells were grown further for 4 h. Ten milliliters of cells was taken and mixed with 1 mL of 100% TCA followed by incubation on ice for 20 min. Protein precipitates were collected by centrifugation at 17,000 × g and then washed with cold acetone to remove the TCA. Precipitates were resuspended in 100 µL of buffer containing 1% SDS, 50 mM Tris⋅HCl (pH 7.5), and 15 mM 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) (with the exception of the non–AMS-modified sample, which was resuspended in the same buffer without AMS). AMS modifications were performed at 30 °C for 2 h. Proteins were then separated by 10% SDS/polyacrylamide gels. Gels were made with WIDE RANGE Gel Preparation Buffer (Nacalai Tesque). After electrophoresis, proteins were blotted onto PVDF membrane (FluoroTrans Transfer Membranes; Pall) and probed with an anti-FLAG M2 monoclonal antibody (Sigma) according to product instructions. The secondary antibody was visualized by SuperSignal West Femto Maximum Sensitivity Substrate (Thermo).
For 2D electrophoresis analysis of SqrR, WT- (negative control) and SqrR-FLAG–expressing strains were grown aerobically in the presence of Na2S as indicated above, and whole proteins were precipitated by TCA as described above. Precipitates were resuspended and subjected to SDS/polyacrylamide gels in the absence of any reducing reagents such as 2-mercaptoethanol or DTT. After the first electrophoresis, the lane was cut out and incubated with 1.2 M 2-mercaptoethanol (Nacalai Tesque) for 10 min at room temperature. The gel was set on a 12.5% SDS/polyacrylamide gel containing 5 mM DTT, and the second electrophoresis was performed with buffer containing 1 mM DTT.
Extraction and Quantification of Photosynthetic Pigments.
R. capsulatus WT and ΔsqrR grown on a sulfide-containing PYS plate (Fig. S9B) were harvested and washed with 10 mM Tris⋅HCl (pH 8.0). After washing, cells were resuspended in 10 mM Tris⋅HCl (pH 8.0) and cell densities were adjusted to OD660 1.5. Cells were harvested from a 0.5-mL suspension and pigments were extracted with acetone:methanol (7:2). After sonication and centrifugation at 17,000 × g, absorption spectra of the supernatants were recorded using a UV-1800 spectrophotometer (Shimadzu). Bacteriochlorophyll a and carotenoid contents were calculated based on the absorbance at 770 and 485 nm, respectively.
RNA Isolation and RNA-Seq.
R. capsulatus WT and the sqrR-disrupted mutant were grown and harvested in the same way as the preparation of samples for Western blotting described above. Cell culture (0.5 mL) was harvested and stored at −80 °C until needed. Under each condition, three biological replicates were provided for each strain. Total RNA was extracted using the ISOLATE II RNA Mini Kit (Bioline) followed by TURBO DNase (Ambion) treatment to remove genomic DNA contamination. The reaction mixture was cleaned up and concentrated by the RNeasy MinElute Cleanup Kit (Qiagen). Final RNA concentrations were measured by NanoDrop (Thermo Scientific). Further quality control was performed on the 2200 TapeStation using RNA ScreenTape (Agilent Technologies). Library construction and RNA sequencing were performed by the Center for Genomics and Bioinformatics at Indiana University. Generally, ribosomal RNA was depleted from 2 μg total RNA using the Epicentre Ribo-Zero Magnetic (Bacteria) Kit. Illumina mRNA-seq libraries were constructed using the TruSeq RNA Sample Prep Kit (Illumina) according to the manufacturer’s protocol. Single-end sequencing reactions (75×) were performed on the Illumina NextSeq 500 sequencer. The raw sequence read files have been deposited in the Sequence Read Archive under accession no. SRP077723.
The raw reads were trimmed with Trimmomatic (version 0.32) (www.usadellab.org/cms/?page=trimmomatic) and aligned to the R. capsulatus SB1003 genome (GenBank accession no. CP001312.1) with Bowtie 2 (version 2.1.0) (bowtie-bio.sourceforge.net/bowtie2/index.shtml). HTSeq-count (version 0.6.0) (www-huber.embl.de/HTSeq/doc/index.html) was used to count read numbers in each gene followed by differential expression analysis using the DESeq2 package in R (https://bioconductor.org/packages/release/bioc/html/DESeq2.html). Genes that showed constant expression changes with a twofold increase between different biological replicates were selected (an adjusted P value with a cut of <0.01) (Dataset S2).
Supplementary Material
Acknowledgments
We thank Dr. Hengyao Niu (Indiana University) for permitting J.S. to perform the experiments outlined here using his laboratory resources. We also thank Dr. Nobuhisa Furuya (Tokyo Metropolitan University) for valuable advice on primer extension, Dr. Masato Nikaido (Tokyo Institute of Technology) for providing the 500 LIZ Size Standard, and Dr. Lucy Kwok (Tokyo Institute of Technology) for critical reading of the manuscript. Funding for this study was provided in part by the Japan Science Society Sasagawa Fellowship (to T.S.), Grants-in-Aid for Scientific Research 16K14694 and 16H03280 (to S.M.) and NIH Grants GM040941 (to C.E.B.) and GM097225 and GM118157 (to D.P.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The resequencing data reported in this paper have been deposited in the DNA Data Bank of Japan Sequence Read Archive (DRA) (accession nos. DRA004893–DRA004896; BioProject accession no. PRJDB5013). The RNA-seq raw sequence read files reported in this paper have been deposited in the National Center for Biotechnology Information Sequence Read Archive (accession no. SRP077723).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614133114/-/DCSupplemental.
References
- 1.Godet L, Zelnio KA, Van Dover CL. Scientists as stakeholders in conservation of hydrothermal vents. Conserv Biol. 2011;25(2):214–222. doi: 10.1111/j.1523-1739.2010.01642.x. [DOI] [PubMed] [Google Scholar]
- 2.Baross JA, Hoffman SE. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig Life Evol Biosph. 1985;15(4):327–345. [Google Scholar]
- 3.Corliss JB, Baross JA, Hoffman SE. An hypothesis concerning the relationship between submarine hot springs and the origin of life on Earth. Oceanol Acta. 1981;1980:59–69. [Google Scholar]
- 4.Ishibashi J, Okino K, Sunamura M, editors. Subseafloor Biosphere Linked to Hydrothermal Systems: TAIGA Concept. Springer; Tokyo: 2015. [Google Scholar]
- 5.Fisher C, Takai K, Le Bris N. Hydrothermal vent ecosystems. Oceanography. 2007;20(1):14–23. [Google Scholar]
- 6.Beatty JT, et al. An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. Proc Natl Acad Sci USA. 2005;102(26):9306–9310. doi: 10.1073/pnas.0503674102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer C, Elsen S, Swem LR, Swem DL, Masuda S. Redox and light regulation of gene expression in photosynthetic prokaryotes. Philos Trans R Soc Lond B Biol Sci. 2003;358(1429):147–153. doi: 10.1098/rstb.2002.1189. discussion 153–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeilstra-Ryalls JH, Kaplan S. Oxygen intervention in the regulation of gene expression: The photosynthetic bacterial paradigm. Cell Mol Life Sci. 2004;61(4):417–436. doi: 10.1007/s00018-003-3242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneko Y, Kimura Y, Kimura H, Niki I. L-cysteine inhibits insulin release from the pancreatic beta-cell: Possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes. 2006;55(5):1391–1397. doi: 10.2337/db05-1082. [DOI] [PubMed] [Google Scholar]
- 10.Kimura H. Hydrogen sulfide: From brain to gut. Antioxid Redox Signal. 2010;12(9):1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 11.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D, et al. Detection of protein S-sulfhydration by a tag-switch technique. Angew Chem Int Ed Engl. 2014;53(2):575–581. doi: 10.1002/anie.201305876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ida T, et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci USA. 2014;111(21):7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishida M, et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol. 2012;8(8):714–724. doi: 10.1038/nchembio.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuevasanta E, et al. Reaction of hydrogen sulfide with disulfide and sulfenic acid to form the strongly nucleophilic persulfide. J Biol Chem. 2015;290(45):26866–26880. doi: 10.1074/jbc.M115.672816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav PK, et al. Biosynthesis and reactivity of cysteine persulfides in signaling. J Am Chem Soc. 2016;138(1):289–299. doi: 10.1021/jacs.5b10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustafa AK, et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2(96):ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao XH, et al. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. eLife. 2015;4:e10067. doi: 10.7554/eLife.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dóka É, et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci Adv. 2016;2(1):e1500968. doi: 10.1126/sciadv.1500968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wedmann R, et al. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem Sci (Camb) 2016;7(5):3414–3426. doi: 10.1039/c5sc04818d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40(5):533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 22.Meyer TE, Cusanovich MA. Discovery and characterization of electron transfer proteins in the photosynthetic bacteria. Photosynth Res. 2003;76(1–3):111–126. doi: 10.1023/A:1024910323089. [DOI] [PubMed] [Google Scholar]
- 23.Gregersen LH, Bryant DA, Frigaard N-U. Mechanisms and evolution of oxidative sulfur metabolism in green sulfur bacteria. Front Microbiol. 2011;2:116. doi: 10.3389/fmicb.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libiad M, Yadav PK, Vitvitsky V, Martinov M, Banerjee R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J Biol Chem. 2014;289(45):30901–30910. doi: 10.1074/jbc.M114.602664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griesbeck C, et al. Mechanism of sulfide-quinone reductase investigated using site-directed mutagenesis and sulfur analysis. Biochemistry. 2002;41(39):11552–11565. doi: 10.1021/bi026032b. [DOI] [PubMed] [Google Scholar]
- 26.Luebke JL, et al. The CsoR-like sulfurtransferase repressor (CstR) is a persulfide sensor in Staphylococcus aureus. Mol Microbiol. 2014;94(6):1343–1360. doi: 10.1111/mmi.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sganga MW, Bauer CE. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell. 1992;68(5):945–954. doi: 10.1016/0092-8674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- 28.Higgins KA, Giedroc D. Insights into protein allostery in the CsoR/RcnR family of transcriptional repressors. Chem Lett. 2014;43(1):20–25. doi: 10.1246/cl.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osman D, et al. The effectors and sensory sites of formaldehyde-responsive regulator FrmR and metal-sensing variant. J Biol Chem. 2016;291(37):19502–19516. doi: 10.1074/jbc.M116.745174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109(10):4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guimarães BG, et al. Plant pathogenic bacteria utilize biofilm growth-associated repressor (BigR), a novel winged-helix redox switch, to control hydrogen sulfide detoxification under hypoxia. J Biol Chem. 2011;286(29):26148–26157. doi: 10.1074/jbc.M111.234039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbosa RL, Rinaldi FC, Guimarães BG, Benedetti CE. Crystallization and preliminary X-ray analysis of BigR, a transcription repressor from Xylella fastidiosa involved in biofilm formation. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63(Pt 7):596–598. doi: 10.1107/S1744309107028722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins KA, Peng H, Luebke JL, Chang FMJ, Giedroc DP. Conformational analysis and chemical reactivity of the multidomain sulfurtransferase, Staphylococcus aureus CstA. Biochemistry. 2015;54(14):2385–2398. doi: 10.1021/acs.biochem.5b00056. [DOI] [PubMed] [Google Scholar]
- 34.Cipollone R, Ascenzi P, Visca P. Common themes and variations in the rhodanese superfamily. IUBMB Life. 2007;59(2):51–59. doi: 10.1080/15216540701206859. [DOI] [PubMed] [Google Scholar]
- 35.Roy AB, Trudinger PA. The Biochemistry of Inorganic Compounds of Sulphur. Cambridge Univ Press; Cambridge, United Kingdom: 2010. [Google Scholar]
- 36.Shi R, et al. Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol. 2010;8(4):e1000354. doi: 10.1371/journal.pbio.1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller EG. Trafficking in persulfides: Delivering sulfur in biosynthetic pathways. Nat Chem Biol. 2006;2(4):185–194. doi: 10.1038/nchembio779. [DOI] [PubMed] [Google Scholar]
- 38.Perkins A, Poole LB, Karplus PA. Tuning of peroxiredoxin catalysis for various physiological roles. Biochemistry. 2014;53(49):7693–7705. doi: 10.1021/bi5013222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen J, Peng H, Zhang Y, Trinidad JC, Giedroc DP. Staphylococcus aureus sqr encodes a type II sulfide:quinone oxidoreductase and impacts reactive sulfur speciation in cells. Biochemistry. 2016;55(47):6524–6534. doi: 10.1021/acs.biochem.6b00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcia M, Ermler U, Peng G, Michel H. A new structure-based classification of sulfide:quinone oxidoreductases. Proteins. 2010;78(5):1073–1083. doi: 10.1002/prot.22665. [DOI] [PubMed] [Google Scholar]
- 41.Busenlehner LS, Pennella MA, Giedroc DP. The SmtB/ArsR family of metalloregulatory transcriptional repressors: Structural insights into prokaryotic metal resistance. FEMS Microbiol Rev. 2003;27(2–3):131–143. doi: 10.1016/S0168-6445(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 42.Campbell DR, et al. Mycobacterial cells have dual nickel-cobalt sensors: Sequence relationships and metal sites of metal-responsive repressors are not congruent. J Biol Chem. 2007;282(44):32298–32310. doi: 10.1074/jbc.M703451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang FMJ, et al. Cu(I)-mediated allosteric switching in a copper-sensing operon repressor (CsoR) J Biol Chem. 2014;289(27):19204–19217. doi: 10.1074/jbc.M114.556704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birks SJ, Kelly DJ. Assay and properties of acetone carboxylase, a novel enzyme involved in acetone-dependent growth and CO2 fixation in Rhodobacter capsulatus and other photosynthetic and denitrifying bacteria. Microbiology. 1997;143:755–766. doi: 10.1099/00221287-143-3-755. [DOI] [PubMed] [Google Scholar]
- 45.Wu J, Bauer CE. RegB/RegA, a global redox-responding two-component system. Adv Exp Med Biol. 2008;631:131–148. doi: 10.1007/978-0-387-78885-2_9. [DOI] [PubMed] [Google Scholar]
- 46.Shen J, et al. Staphylococcus aureus CstB is a novel multidomain persulfide dioxygenase-sulfurtransferase involved in hydrogen sulfide detoxification. Biochemistry. 2015;54(29):4542–4554. doi: 10.1021/acs.biochem.5b00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaturvedi KS, et al. Cupric yersiniabactin is a virulence-associated superoxide dismutase mimic. ACS Chem Biol. 2014;9(2):551–561. doi: 10.1021/cb400658k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu T, Cheng Z, Matsuura K, Masuda S, Bauer CE. Evidence that altered cis element spacing affects PpsR mediated redox control of photosynthesis gene expression in Rubrivivax gelatinosus. PLoS One. 2015;10(6):e0128446. doi: 10.1371/journal.pone.0128446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young DA, Bauer CE, Williams JC, Marrs BL. Genetic evidence for superoperonal organization of genes for photosynthetic pigments and pigment-binding proteins in Rhodobacter capsulatus. Mol Gen Genet. 1989;218(1):1–12. doi: 10.1007/BF00330558. [DOI] [PubMed] [Google Scholar]
- 50.Zeller T, et al. Regulation of hydrogen peroxide-dependent gene expression in Rhodobacter sphaeroides: Regulatory functions of OxyR. J Bacteriol. 2007;189(10):3784–3792. doi: 10.1128/JB.01795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, et al. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masuda S, Bauer CE. Null mutation of HvrA compensates for loss of an essential relA/spoT-like gene in Rhodobacter capsulatus. J Bacteriol. 2004;186(1):235–239. doi: 10.1128/JB.186.1.235-239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang ZY, Gest H, Bauer CE. Chemosensory and photosensory perception in purple photosynthetic bacteria utilize common signal transduction components. J Bacteriol. 1997;179(18):5720–5727. doi: 10.1128/jb.179.18.5720-5727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar S, Tamura K, Nei M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5(2):150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.