Abstract
Background
Long-term follow-up data concerning isolated tricuspid valve pathology after replacement or reconstruction is limited. Current American Heart Association guidelines equally recommend repair and replacement when surgical intervention is indicated. Our aim was to investigate and compare operative mortality and long-term survival in patients undergoing isolated tricuspid valve repair surgery versus replacement.
Material/Methods
Between 1995 and 2011, 109 consecutive patients underwent surgical correction of tricuspid valve pathology at our institution for varying structural pathologies. A total of 41 (37.6%) patients underwent tricuspid annuloplasty/repair (TAP) with or without ring implantation, while 68 (62.3%) patients received tricuspid valve replacement (TVR) of whom 36 (53%) were mechanical and 32 (47%) were biological prostheses.
Results
Early survival at 30 days after surgery was 97.6% in the TAP group and 91.1% in the TVR group. After 6 months, 89.1% in the TAP group and 87.8% in the TVR group were alive. In terms of long-term survival, there was no further mortality observed after one year post surgery in both groups (Log Rank p=0.919, Breslow p=0.834, Tarone-Ware p=0.880) in the Kaplan-Meier Survival analysis. The 1-, 5-, and 8-year survival rates were 85.8% for TAP and 87.8% for TVR group.
Conclusions
Surgical repair of the tricuspid valve does not show survival benefit when compared to replacement. Hence valve replacement should be considered generously in patients with reasonable suspicion that regurgitation after repair will reoccur.
MeSH Keywords: Cardiac Valve Annuloplasty, Tricuspid Valve, Tricuspid Valve Insufficiency
Background
The indication for tricuspid valve (TV) surgery is mostly considered at the time of mitral or aortic valve surgery and most often because of tricuspid regurgitation (TR). Concomitant TV repair in this scenario does not significantly increase cross-clamping time and may reduce the morbidity associated with reoperation for isolated TR, leading to an increase in operative correction procedures over the past decade [1,2]. Hence most follow-up studies are concerned with comparing the difference between tricuspid annuloplasty/repair (TAP) and tricuspid valve replacement (TVR). However, few studies have investigated the outcome in patients with the need of tricuspid surgery because of isolated TV pathology [3,4]. The etiologies here for consist mostly for annular dilation, infective endocarditis (IE), congenital (structural) pathology of the leaflets, post-interventional destruction (e.g., post-biopsy or pacemaker implantation), and rarely rheumatic disorder. Severe TR is associated with poor prognosis and surgical treatment is known to provide a superior outcome than conservative medical treatment [5]. We present our follow-up data to evaluate long-term outcomes in patients undergoing isolated TAP or TVR.
Material and Methods
Study population
Patients were selected through review of our prospectively maintained TV registry. Data analysis was handled anonymously and without additional patient contact or examinations, hence our institutional review board waived the need of informed consent. Between February 1995 and June 2011, 109 consecutive patients underwent isolated TV procedures at University Hospital of Heidelberg: 41 (37.6%) received tricuspid valve annuloplasty/repair (TAP) and 68 (62.3%) underwent tricuspid valve replacement (TVR). The majority of patients suffered from tricuspid regurgitation due to structural pathology, annular dilation, or infective endocarditis. Detailed breakdown of pathologies revealed 32 patients suffered from isolated tricuspid endocarditis while 16 patients had prior pacemaker implantation causing valve defects in form of microbial vegetation, structural damage, or stenosis, 37 patients had tricuspid insufficiency (TI) because of annular dilation caused by right ventricular dilation or DCMP, while four patients had prior aortic or mitral valve replacements and developed severe TI in the further clinical course, whereas six patients suffered TI after cardiac transplantation. Six patients had congenital anomalies such as Ebstein’s malformation, RVOTO, or ASD-II. Furthermore, four patients suffered from mechanical TK-prosthesis thrombosis, four patients had primary tricuspid stenosis and one patient developed severe insufficiency after blunt trauma causing contusio cordis. Comprehensive data such as patient demographics, cardiovascular risk factors, cardiac function assessed by two-dimensional echocardiograms, intraoperative characteristics as well as the postoperative outcomes including long-term survival were compared (Tables 1–3).
Table 1.
Patient’s demographics and preoperative baseline characteristics.
| TAP | TVR | p-value | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 50.7±19.4 | 55.7±15.9 | 0.163 |
| Female | 18 (43.9%) | 37 (54.4%) | 0.305 |
| Height (cm) | 172.9±8.9 | 170.2±9.1 | 0.135 |
| Weight (kg) | 73.5±18.5 | 72.4±21.2 | 0.773 |
| BMI | 24.5±5.2 | 24.9±6.9 | 0.713 |
| Angina | |||
| Exertional angina | 4 (9.8%) | 13 (19.1%) | 0.283 |
| Rest angina | 0 | 5 (7.4%) | 0.157 |
| Unstable angina | 0 | 2 (2.9%) | 0.534 |
| Dyspnea | |||
| Exertional dyspnea | 26 (63.4%) | 58 (85.2%) | 0.053 |
| Rest dyspnea | 9 (22.0%) | 22 (32.4%) | 0.382 |
| Liver enlargement | 9 (22.0%) | 21 (30.9%) | 0.505 |
| Pulmonary congestion | 6 (14.6%) | 14 (20.6%) | 0.613 |
| Pulmonary edema | 2 (4.9%) | 8 (11.8%) | 0.324 |
| Peripheral edema | 15 (36.6%) | 26 (38.2%) | 1.000 |
| Previous MI | 1 (2.4%) | 5 (7.4%) | 0.408 |
| Previous syncope | 3 (7.3%) | 5 (7.4%) | 0.741 |
| Previous embolism | 3 (7.3%) | 3 (4.4%) | 0.602 |
| Previous decompensation | 7 (17.1%) | 19 (27.9%) | 0.252 |
| Previous CPR | 1 (2.4%) | 0 | 0.349 |
| Previous CVA | 0 | 7 (10.3%) | 0.089 |
| Mechanical ventilation | 2 (4.9%) | 0 | 0.119 |
| IABP | 0 | 1 (1.5%) | 1.000 |
| Cardiovascular risk factors | |||
| Family history | 11 (26.8%) | 9 (13.2%) | 0.178 |
| Hyperlipidemia | 10 (24.4%) | 21 (30.9%) | 0.559 |
| Hyperuricemia | 7 (17.1%) | 8 (11.8%) | 0.585 |
| Hypertension | 21 (51.2%) | 30 (44.1%) | 0.430 |
| Smoking | 19 (46.3%) | 27 (39.7%) | 0.548 |
| Pulmonary hypertension | 8 (19.5%) | 20 (29.4%) | 0.466 |
| Diabetes mellitus | 0.660 | ||
| Type I | 1 (2.4%) | 6 (8.8%) | |
| Type II not insulin dependent | 3 (7.3%) | 5 (7.4%) | |
| Type II insulin dependent | 3 (7.3%) | 4 (5.9%) | |
| Medication | |||
| Oral nitrates | 2 (4.9%) | 4 (5.9%) | 1.000 |
| i.v. nitrates | 1 (2.4%) | 2 (2.9%) | 1.000 |
| Beta-blockers | 13 (31.7%) | 31 (45.6%) | 0.165 |
| ACE-antagonists | 19 (46.3%) | 23 (33.8%) | 0.226 |
| Ca-antagonists | 5 (12.2%) | 11 (16.2%) | 0.781 |
| Glycosides | 11 (26.8%) | 28 (41.2%) | 0.152 |
| Diuretics | 24 (58.5%) | 43 (63.2%) | 0.216 |
| Inotropic agents | 3 (7.3%) | 2 (2.9%) | 0.359 |
| Antiarrhythmic agents | 4 (9.8%) | 5 (7.4%) | 0.726 |
| Vasodilators | 3 (7.3%) | 1 (1.5%) | 0.149 |
| Steroids | 1 (2.4%) | 0 | 0.354 |
| Immunosuppressive drugs | 2 (4.9%) | 2 (2.9%) | 0.612 |
| Antibiotics | 18 (43.9%) | 20 (29.4%) | 0.149 |
| Bronchodilators | 1 (2.4%) | 4 (5.9%) | 0.653 |
| Anticoagulants | 16 (39.0%) | 33 (48.5%) | 0.430 |
| Previous surgery | |||
| Coronary surgery | 2 (4.9%) | 7 (11.1%) | 0.481 |
| Aortic valve surgery | 2 (4.9%) | 4 (5.9%) | 1.000 |
| Mitral valve surgery | 3 (7.3%) | 9 (13.2%) | 0.530 |
| Tricuspid valve surgery | 0 | 10 (14.7%) | 0.013 |
| Pulmonary valve surgery | 0 | 1 (14.7%) | 1.000 |
| Surgery for congenital vitium | 1 (2.4%) | 6 (8.8%) | 0.257 |
| Cardiac transplantation | 3 (7.3%) | 6 (8.8%) | 1.000 |
| Infection | 21 (51.2%) | 22 (32.4%) | 0.068 |
| PVD | 0 | 4 (5.9%) | 0.292 |
| i.v. drug abuser | 1 (2.4%) | 2 (2.9%) | 1.000 |
| Malignancy | 2 (4.9%) | 4 (5.9%) | 1.000 |
TAP – tricuspid valve annuloplasty; TVR – tricuspid valve replacement; BMI – body mass index; MI – myocardial infarction; CPR – cardiopulmonary resuscitation; CVA – cerebrovascular event; IABP – intra-aortic balloon pump; ACE – angiotensin-converting enzyme; PVD – peripheral vascular disease.
Table 2.
Preoperative cardiac function and disease classification.
| TAP | TVR | p-value | |
|---|---|---|---|
| LV ejection fraction | 0.640 | ||
| >55% | 34 (83%) | 53 (78%) | |
| 41–55% | 4 (10%) | 10 (15%) | |
| 26–40% | 1 (2%) | 3 (4%) | |
| <25% | 2 (5%) | 2 (3%) | |
| Hypertrophy | 16 (39%) | 31 (46%) | 1.000 |
| Dilation | 14 (34%) | 33 (49%) | 0.401 |
| Anterior hypokinesia | 6 (15%) | 9 (13%) | 1.000 |
| Posterior hypokinesia | 1 (2%) | 2 (3%) | 1.000 |
| Septum hypokinesia | 6 (15%) | 11 (16%) | 1.000 |
| Apical hypokinesia | 6 (15%) | 11 (16%) | 1.000 |
| Anterior akinesia | 1 (2%) | 0 | 0.333 |
| Posterior akinesia | 1 (2%) | 2 (3%) | 1.000 |
| Septum akinesia | 1 (2%) | 0 | 1.000 |
| Septum aneurysm | 1 (2%) | 2 (3%) | 1.000 |
| Apical aneurysm | 2 (5%) | 0 | 0.333 |
| RV function | 0.73 | ||
| Mild | 12 (30%) | 31 (45%) | |
| Moderate | 23 (56%) | 24 (35%) | |
| Severe | 6 (14%) | 13 (20%) | |
| PH (mean >40 mmHg) | 23 (57%) | 31 (46%) | 1.000 |
| Tricuspid regurgitation | 0.721 | ||
| Severe | 35 (86%) | 31 (46%) | |
| Moderate | 4 (10%) | 12 (17%) | |
| Mild | 2 (4%) | 1 (2%) |
LV – left ventricle; RV – right ventricle; PH – pulmonary hypertension.
Table 3.
Intraoperative data.
| TAP | TVR | p-value | |
|---|---|---|---|
| Urgency of procedure | 0.93 | ||
| Elective | 26 (63%) | 45 (66%) | |
| Urgent | 9 (23%) | 16 (24%) | |
| Emergency | 5 (12%) | 5 (7%) | |
| Salvage | 1 (2%) | 2 (3%) | |
| Cross clamp time in min | 28.1±23.4 | 41±38.4 | 0.14 |
| Type of prosthesis | |||
| Biological | 36 (53%) | ||
| Mechanical | 32 (47%) |
Urgent procedure: requiring surgical intervention within 24 h; emergency procedure: requiring immediate surgical intervention within 2 hours because of increasing hemodynamic instability, salvage procedure: manifest significant hemodynamic instability.
Tricuspid valve annuloplasty/repair (TAP)
In most cases the procedures were performed through median sternotomy using cardio-pulmonary bypass (CPB) with cardioplegic arrest and standard cannulation of ascending aorta and venae cava superior and inferior (bicaval cannulation). However, some cases were performed through a transversal (n=1, 2%), parasternal (n=1, 2%), or antero-lateral (n=3, 7%) access. An alternative cannulation strategy through the femoral vessels was used in three patients (7%). There were several techniques applied depending on the morphological abnormalities of the valve including standard ring annuloplasties (n=11), ring reconstructions or tightening using the DeVega technique (n=6), valvuloplasty with pericardial patching or bicuscpidalization (n=20), commissurotomy (n=2), papillary muscle or chordae plasty or combination of them. The chest was closed in routine manner.
Tricuspid valve replacement (TVR)
In most cases the procedures were performed through median sternotomy except for two cases (3%) where the anterolateral access was used. All procedures were done under CPB with cardioplegic arrest and ascending aorta cannulation as well as bicaval cannulation. An alternative cannulation strategy through the femoral vessels was used in seven patients (10%). The choice of prosthesis was primarily based on patients’ age, hence patients up to the sixth decade received mechanical and patients in their sevenths decade received biological prosthesis. Exceptions were decided in respect to patients’ comorbidities, such as contraindications for continued anticoagulation and prosthesis were chosen in consent with the patient.
Anticoagulation protocol
Intravenous heparin infusion was started on the first postoperative day. A target activated partial thromboplastin time of 50–70 seconds was selected, which was measured. After removal of chest drains, warfarin medication was initiated to keep the international normalized ratio (INR) between 2.5–3.5. The heparin infusion was not discontinued before the target INR was achieved. Patients receiving biological prosthesis or annuloplasty/repair discontinued warfarin three months after surgery, if no other reason for anticoagulation existed. Patients with mechanical prosthesis needed to continue lifelong anticoagulation.
Statistics
All data were processed with the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) and are given as continuous or categorical variables. Continuous data were shown as mean ± standard deviation and analyzed with the Student t-test. Pearson’s χ2 or Fisher exact tests were utilized for categorical data. Kaplan-Meier survival estimation was used for patient’s survival investigation. The groups were compared using Log rank, Breslow and Tarone-Ware tests. A value of p<0.05 was considered statistically significant.
Results
Preoperative characteristics are shown in Table 1. There were no statistically significant differences between the two groups regarding age (50.7±19.4 years in the TAP group vs. 55.7±15.9 years in the TVR group, p=0.163), gender distribution (43.9% vs. 54.4% female patients in the TAP and TVR groups, respectively, p=0.305), and body mass index (BMI) (24.5±5.2 in the TAP group vs. 24.9±6.9 in the TVR group, p=0.713). Moreover, there were no significant differences regarding preoperative angina or dyspnea status, liver, renal and pulmonary function, infections, hemodynamic status, and cardiovascular risk factors (Table 1). Furthermore, the differences between the two groups in terms of previous coronary (p=0.481), aortic valve (p=1.000), mitral valve (p=0.530), or pulmonary valve surgery (p=1.000) was not statistically significant, whereas patients in the TVR group had a significantly higher rate of previous tricuspid valve repair (p=0.013). Preoperative cardiac function assessed by transesophageal echocardiography was also similar and the severity of the disease reflected by pulmonary hypertension and right ventricular function did not differ significantly in both groups (Table 2). Additionally, there was no significant difference in the intraoperative characteristics in terms of cross clamping time or urgency of the procedure (Table 3).
Postoperatively, patients from both groups had similar left ventricular and right ventricular function assessed by two-dimensional echocardiography. There were no statistically significant differences in terms of postoperative arrhythmias, the need for intra-aortic balloon pump or ventricular assist device implantations. Both groups were comparable in terms of inotropic support requirement, postoperatively, except for noradrenaline support which was needed more frequently in the replacement group (50.0% in the TVR group vs. 24.4% in the TAP group, p=0.014). The distribution of postoperative complications such as renal failure requiring conservative treatment, dialysis/hemofiltration, pleural effusions requiring negative balance, or drainage was similar in both groups (Table 4).
Table 4.
Postoperative characteristics.
| TAP | TVR | p-value | |
|---|---|---|---|
| Left ventricular function | 0.534 | ||
| Mild | 4 (9.8%) | 22 (32.4%) | |
| Moderate | 4 (9.8%) | 10 (14.7%) | |
| Severe | 0 | 1 (1.5%) | |
| Right ventricular function | 0.879 | ||
| Mild | 2 (4.9%) | 8 (11.8%) | |
| Moderate | 6 (14.6%) | 23 (33.8%) | |
| Severe | 0 | 1 (21.6%) | |
| Rhythm disturbance | |||
| AV-Block | 4 (9.8%) | 0 | 0.761 |
| Ventr. extrasystole | 1 (2.4%) | 12 (17.6%) | 1.000 |
| Supr. extrasystole | 9 (22.0%) | 7 (10.3%) | 0.811 |
| Atrial fibrillation | 0.820 | ||
| Paroxysmal | 4 (9.8%) | 9 (13.2%) | |
| Persistent | 3 (7.3%) | 5 (7.4%) | |
| Permanent | 1 (2.4%) | 4 (5.9%) | |
| Low output syndrome | 0.402 | ||
| Conservative treatment | 0 | 1 (1.5%) | |
| IABP | 1 (2.4%) | 4 (5.9%) | |
| Assist device | 1 (2.4%) | 0 | |
| Inotropic drugs | |||
| Noradrenaline | 10 (24.4%) | 34 (50.0%) | 0.014 |
| Dopamine | 7 (17.1%) | 14 (20.6%) | 0.804 |
| Dobutamine | 24 (58.5%) | 51 (75.0%) | 0.086 |
| Adrenaline | 11 (26.8%) | 14 (20.6%) | 0.189 |
| Renal failure | 0.477 | ||
| Conservative treatment | 7 (17.1%) | 18 (26.5%) | |
| Dialysis | 0 | 1 (1.5%) | |
| Hemofiltration | 2 (4.9%) | 2 (2.9%) | |
| Respiratory insufficiency | 0.233 | ||
| Forced respirat. therapy | 10 (23.3%) | 26 (38.2%) | |
| Re-intubation | 0 | 1 (1.5%) | |
| Pleural effusion | 0.693 | ||
| Conservative treatment | 0 | 1 (1.5%) | |
| Drainage | 1 (2.4%) | 1 (1.5%) | |
| Coagulation disorder | 1 (2.4%) | 6 (8.8%) | 0.412 |
| Myocardial infarction | 0 | 1 (1.5%) | 1.000 |
AV – atrioventricular; IABP – intra-aortic balloon pump.
Early survival at 30 days after surgery was 97.6% in the TAP group and 91.1% in the TVR group. After 6 months, 89.1% in the TAP group and 87.8% in the TVR group were alive. In terms of long-term survival, there was no further mortality observed after one year post-surgery in both groups (Log Rank p=0.919, Breslow p=0.834, Tarone-Ware p=0.880) in the Kaplan-Meier survival analysis. The 1-, 5-, and 8-year survival rates were 85.8% for TAP and 87.8% for TVR group (Figure 1).
Figure 1.
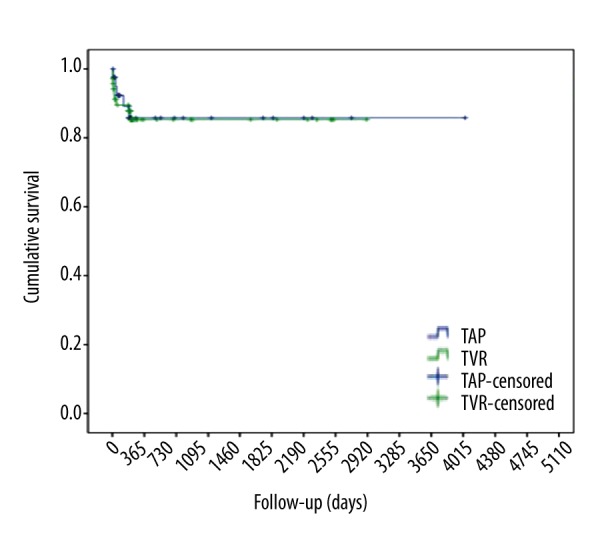
Kaplan-Meier Survival Curve for tricuspid valve repair (TAP) vs. tricuspid valve replacement surgery (TVR): Log Rank p=0.919, Breslow p=0.834, Tarone-Ware p=0.880.
Discussion
Only a few studies have investigated the outcome of isolated tricuspid surgery. Significant tricuspid disease requiring surgical correction is a challenging pathology, demanding critical decision-making regarding reconstruction versus replacement and timing of the procedure [6,7]. The reasons here for are the reported high mortality and the fact that TR is relatively well tolerated, even if severe. Hence patients present with complex morbidity such as manifest congestive heart failure, severe cardiac dilation, pulmonary hypertension or endocarditis. The presence of these serious preoperative conditions present highly confounding factors altering postoperative outcomes significantly and probably accounting for previously reported poor outcomes. Currently the indication for surgical correction vs. replacement remains at the surgeon’s discretion, although recently there is a growing tendency towards reconstructive strategies [8]. Theoretical benefits are the avoidance of inserting a rigid prosthesis into the thin-walled, low-pressure right ventricle, which can result further deterioration of right-ventricular dysfunction [9–11]. However, early and long-term outcomes after TAP show high rates of recurrent TR despite the use of annuloplasty rings [12]. In a large series reported by the Toronto group and the Cleveland Clinic group the recurrence of moderate to severe regurgitation was as high as 38% and 20% respectively [9,12]. This can account for diminished survival because deteriorating right ventricular function and redo tricuspid valve surgery in that case is associated with higher morbidity and mortality [13]. Hence outcome analysis of that patient cohort is of great interest. However, most studies include a variety of patients in their analysis, especially those in need of multiple cardiac interventions. In this study however, only isolated tricuspid valve pathologies were analyzed, thus our patient cohort was more uniform concerning preoperative characteristics and direct comparison between the test groups is feasible without the need for statistical alterations such as pair-matched analysis or propensity score matching. Age, gender, BMI, and the severity of the disease were comparable in both groups. Known preoperative risk factors like right ventricular function and pulmonary hypertension were also similar, as well as known operative confounding factors like cross clamping time or the urgency of the procedure.
The overall results of this study show that the outcome after tricuspid surgery is acceptable with 30-day survival of 97.6% in the TAP group and 91.1% in the TVR group and long-term survival of 85.8% in the TAP and 87.8% in the TVR group. These rates are in accordance with, if not better, than in previous published studies [8,14,15]. However, our promising results might be overestimated by a relatively small number of patients and therefore lower statistical power. Nonetheless the outcomes are somewhat disappointing when compared with survival after left sided valve replacement operations, even after exclusion of patients with complex cardiac pathologies needing multiple surgical corrections. The same observations were also reported by Moraca et al. with survival rates at 1-, 5-, and 10 years of 80%, 72%, and 66% for repair and 85%, 79%, and 49% respectively for the replacement group, without statistical significance [6]. The incidence of major adverse cardiac events was also similar in both groups, as were clinical surrogate parameters, except for the use of noradrenaline: which was significantly more needed in the tricuspid replacement group. This may be due to the fact that this patient group had a longer CPB time and thus suffered more vasoplegia in the early postoperative period.
Conclusions
Thus the main finding of this study is that there is no clear benefit when comparing tricuspid repair to replacement. Furthermore, freedom from reoperation was also non-significant. Hence the growing enthusiasm towards corrective procedures needs to be put into perspective and focus needs to be shifted towards the timing of the procedure to maintain right ventricular function. Current guidelines recommend surgical intervention when patients become symptomatic [1], which might lead to significant progression of the disease and further deterioration of right ventricular function. However, it has been shown that TR reduces exercise capacity and negatively affects long-term outcome, irrespective of pulmonary hypertension or left ventricular function [16–19]. Echocardiographic parameters of right ventricular function could be consulted when determining optimal timing of surgical intervention [4]. The decision should lean towards valve replacement in patients with reasonable suspicion of recurrent regurgitation.
Footnotes
Presented at the 44th Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery, Freiburg, Germany, 08–11 February 2015
Disclosures
None.
Source of support: Departmental sources
References
- 1.Nishimura RA, Otto CM, Bonow RO, et al. 2014 aha/acc guideline for the management of patients with valvular heart disease: Executive summary: A report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63(22):2438–88. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 2.Hwang HY, Kim KH, Kim KB, Ahn H. Reoperations after tricuspid valve repair: Re-repair versus replacement. J Thorac Dis. 2016;8(1):133–39. doi: 10.3978/j.issn.2072-1439.2016.01.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topilsky Y, Khanna AD, Oh JK, et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123(18):1929–39. doi: 10.1161/CIRCULATIONAHA.110.991018. [DOI] [PubMed] [Google Scholar]
- 4.Kim YJ, Kwon DA, Kim HK, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation. 2009;120(17):1672–78. doi: 10.1161/CIRCULATIONAHA.109.849448. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Song JM, Park JP, et al. Long-term prognosis of isolated significant tricuspid regurgitation. Circ J. 2010;74(2):375–80. doi: 10.1253/circj.cj-09-0679. [DOI] [PubMed] [Google Scholar]
- 6.Moraca RJ, Moon MR, Lawton JS, et al. Outcomes of tricuspid valve repair and replacement: A propensity analysis. Ann Thorac Surg. 2009;87(1):83–88. doi: 10.1016/j.athoracsur.2008.10.003. discussion 88–89. [DOI] [PubMed] [Google Scholar]
- 7.Kim JB, Jung SH, Choo SJ, et al. Clinical and echocardiographic outcomes after surgery for severe isolated tricuspid regurgitation. J Thorac Cardiovasc Surg. 2013;146(2):278–84. doi: 10.1016/j.jtcvs.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Hwang HY, Kim KH, Kim KB, Ahn H. Treatment for severe functional tricuspid regurgitation: Annuloplasty versus valve replacement. Eur J Cardiothorac Surg. 2014;46(2):e21–27. doi: 10.1093/ejcts/ezu224. [DOI] [PubMed] [Google Scholar]
- 9.Singh SK, Tang GH, Maganti MD, et al. Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease. Ann Thorac Surg. 2006;82(5):1735–41. doi: 10.1016/j.athoracsur.2006.06.016. discussion 1741. [DOI] [PubMed] [Google Scholar]
- 10.Bajzer CT, Stewart WJ, Cosgrove DM, et al. Tricuspid valve surgery and intraoperative echocardiography: Factors affecting survival, clinical outcome, and echocardiographic success. J Am Coll Cardiol. 1998;32(4):1023–31. doi: 10.1016/s0735-1097(98)00355-6. [DOI] [PubMed] [Google Scholar]
- 11.Ozpelit E, Akdeniz B, Ozpelit ME, Goldeli O. Severe tricuspid regurgitation mimicking constrictive pericarditis. Am J Case Rep. 2014;15:271–74. doi: 10.12659/AJCR.890092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: Durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127(3):674–85. doi: 10.1016/j.jtcvs.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Bernal JM, Morales D, Revuelta C, et al. Reoperations after tricuspid valve repair. J Thorac Cardiovasc Surg. 2005;130(2):498–503. doi: 10.1016/j.jtcvs.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy JT, Steckelberg JM. Infective endocarditis in patients receiving long-term hemodialysis. Mayo Clin Proc. 2000;75(10):1008–14. doi: 10.4065/75.10.1008. [DOI] [PubMed] [Google Scholar]
- 15.Oh TH, Wang TK, Sidhu K, Haydock DA. Isolated tricuspid valve surgery at a single centre: The 47-year auckland experience, 1965–2011. Interact Cardiovasc Thorac Surg. 2014;18(1):27–32. doi: 10.1093/icvts/ivt452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groves PH, Lewis NP, Ikram S, et al. Reduced exercise capacity in patients with tricuspid regurgitation after successful mitral valve replacement for rheumatic mitral valve disease. Br Heart J. 1991;66(4):295–301. doi: 10.1136/hrt.66.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koelling TM, Aaronson KD, Cody RJ, et al. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002;144(3):524–29. doi: 10.1067/mhj.2002.123575. [DOI] [PubMed] [Google Scholar]
- 18.Grabysa R, Wańkowicz Z. Can echocardiography, especially tricuspid annular plane systolic excursion measurement, predict pulmonary hypertension and improve prognosis in patients on long-term dialysis? Med Sci Monit. 2015;21:4015–22. doi: 10.12659/MSM.895033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Değirmenci H, Bakırcı EM, Demirtaş L, et al. Relationship of left atrial global peak systolic strain with left ventricular diastolic dysfunction and brain natriuretic peptide level in patients presenting with non-ST elevation myocardial infarction. Med Sci Monit. 2014;20:2013–19. doi: 10.12659/MSM.890951. [DOI] [PMC free article] [PubMed] [Google Scholar]


